Abstract
After spinal cord transection, lampreys recover functionally and axons regenerate. It is not known whether this is accompanied by neurogenesis. Previous studies suggested a baseline level of non-neuronal cell proliferation in the spinal cord and rhombencephalon (where most supraspinal projecting neurons are located). To determine whether cell proliferation increases after injury and whether this includes neurogenesis, larval lampreys were spinally transected and injected with BrdU at 0-3 weeks post-transection. Labeled cells were counted in the lesion site, within 0.5 mm rostral and caudal to the lesion, and in the rhombencephalon. One group of animals was processed in the winter, and a second group was processed in the summer. The number of labeled cells was greater in winter than in summer. The lesion site had the most BrdU labeling at all times, correlating with an increase in the number of cells. In the adjacent spinal cord the percentage of BrdU labeling was higher in the ependymal than in non-ependymal regions. This was also true in the rhombencephalon but only in summer. In winter, BrdU labeling was seen primarily in the subventricular and peripheral zones. Some BrdU-labeled cells were also double-labeled by antibodies to glial-specific (anti-keratin) as well as to neuron-specific (anti-Hu) antigens, indicating that both gliogenesis and neurogenesis occurred after spinal cord transection. However, the new neurons were restricted to the ependymal zone, were never labeled by anti-neurofilament antibodies, and never migrated away from the ependyma, even at 5 weeks after BrdU injection. They would appear to be CSF-contacting neurons.
Keywords: spinal cord injury, lamprey, BrdU, neurogenesis, gliogenesis, proliferation
INTRODUCTION
Spinal cord transection (TX) in mammals is followed by permanent paralysis below the level of injury due to a failure of axons to regenerate. Glial cells in the injured mammalian CNS respond by hypertrophy, an increase in glial fibrillary acidic protein, migration, and proliferation (see Schwab and Bartholdi, 1996 for a review). In the spinal cord, if the central canal is injured, ependymal cell proliferation is further increased (Adrian and Walker, 1962). Although the relationship between the glial reaction to injury and failure of axonal regeneration is not known, reactive gliosis, including glial proliferation and scar formation, has been cited as important disrupting features that block axonal regeneration in the CNS. Proliferation of neural progenitors leads to generation of mature astrocytes and oligodendrocytes in the uninjured and injured postnatal mammalian spinal cord (Barnabe-Heider et al., 2010; Horner et al., 2000; Yang et al., 2006; Zhu et al., 2008). However, the spinal cord environment is thought to be inhibitory to neurogenesis (Shihabuddin et al., 2000). Unlike mammals, lampreys recover behaviorally from complete spinal TX. Recovery is accompanied by directionally specific axonal regeneration (Mackler et al., 1986; Yin et al., 1984; Yin and Selzer, 1983) and the formation of synaptic contacts with appropriate neurons distal to the lesion (Mackler and Selzer, 1987). It is not known to what degree the regeneration is accompanied by cell proliferation or whether new neurons are generated.
Previous work in this laboratory has demonstrated that lamprey astrocytes near the lesion do not appear to increase their cell body size. Rather, glial cells extend thickened, keratin-containing processes into the lesion. The glial filaments are not more densely packed than those of normal astrocytes. Instead, the thickened glial processes contain larger than normal concentrations of microtubules. The processes are oriented longitudinally within the lesion, parallel to the regenerating axons, whereas they are normally oriented transversely (Lurie et al., 1994). Thus, the lamprey CNS shows few features of reactive gliosis seen in higher species and this may contribute to its ability to regenerate. Following tail amputation in adult newt, Neuron-Specific Enolase-staining cells appear in the ependymal layer of the ependymal tube (Benraiss et al., 1999). Consequently, there remains a possibility for neurogenesis to play a role in spinal cord regeneration in cold-blooded vertebrates such as the lamprey. Previous studies demonstrated that the lamprey CNS has endogenous cell proliferation and that this undergoes seasonal variation, with increased proliferation in the summer and decreased in the winter (Vidal Pizarro et al., 2004). However, based on neurofilament immuno-labeling, there was no evidence for neurogenesis. At the time, other available neuron-specific antibodies were found not reliable in lamprey. Because it is not known whether cell proliferation, neuronal or glial, accompanies axonal regeneration in the spinal cord, in the current study, we examined the profile of BrdU labeling in lamprey CNS after spinal cord TX, using anti-Hu antibodies, which proved to be reliably neuron-specific in lamprey, and LCM29, a lamprey glia-specific antibody. In addition to gliogenesis (Vidal Pizarro et al., 2004), evidence for proliferation of neuron-like ependymal cells was found.
MATERIALS AND METHODS
Surgical Procedure
The larval sea lamprey (Petromyzon marinus) 10–15 cm in length (4–5 years old) and in a steady state of neural development were obtained from fresh water streams feeding the Delaware River (NJ) or from streams feeding Lake Michigan (MI). They were maintained in fresh water tanks at 16° C. Embryos of P. marinus hatch at 10-13 days, after which they become filter-feeding larvae (ammocoetes) and burrow in streambeds for approximately 5 years. As described by Hardisty and Potter, “Most of the profound anatomical and physiological changes involved in the transformation of the ammocoete into the adult lamprey are heralded by the more obvious changes in external morphology, including the development of the oral disc, extension of the preorbital region, modifications in the structure of the gill openings, the appearance of teeth, eruption of the eyes, enlargement of the fins and changes in pigmentation (Hardisty, 1979). These changes take place over the course of approximately 4–5 weeks during the 6th summer of life, after which the lamprey enters the ocean (or the great lakes, in the case of the land-locked specimens) and lives as a parasite on the surfaces of fish. For a more detailed description developmental stages of the lamprey, particularly metamorphosis from larva to adult, the reader is referred to (Potter, 1982; Potter et al., 1978).
Lampreys were anesthetized by immersion in a saturated aqueous benzocaine solution (Sigma, St. Louis, MO) and pinned to a Sylgard (184 silicone elastomer, Dow Corning) plate containing lamprey Ringer. The spinal cord was exposed from the dorsal midline at the level of the ninth segment caudal to the last gill and transected with Castroviejo scissors. Completeness of TX was confirmed by visual inspection of the cut ends. Spinally transected lampreys recovered in fresh water tanks at room temperature for 1, 2, or 3 weeks before bromodeoxyuridine (BrdU) was injected and incorporated for 4 hours (see below). All procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Institutional Animal Care and Research Committee.
In order to determine whether the effects of season or TX would set a limit to the extent of cellular proliferation, two groups of animals were tested. One group was spinally transected in February and the other in June/July. The numbers of animals used at different recovery times and different seasons is shown in the relevant figures. In addition, to determine whether nascent cells became neurons in spinally transected lamprey, 4 summer animals were injected with BrdU at 2 weeks post-TX, when BrdU labeling is rapid, and allowed to survive for 5 more weeks. Four non-transected animals were used as controls.
Bromodeoxyuridine Injection
After a recovery period from spinal cord TX, larval Petromyzon marinus were anesthetized with benzocaine. Twenty μ 1/gram body weight of 10 mM 5-Bromo-2'-deoxyuridine (BrdU, Roche Applied Science, Indianapolis, IN) in phosphate buffered saline (PBS) was injected into the coelomic body cavity 1.5 cm caudal to the last gill. Animals were allowed to survive either 4 hours or 5 weeks post-BrdU injection.
Immunohistochemistry
Animals were over-anesthetized in benzocaine. The tissue was fixed in 4% paraformaldehyde in PBS (pH 7.2) or in a modified Carnoy’s fixative consisting of ethanol, chloroform, glacial acetic acid, and 10 X PBS in a ratio of 6:2:1:1 as previously described (Lurie et al., 1994), then washed, dehydrated and embedded in paraffin. Avidin-Biotin Complex (ABC) immunohistochemistry was performed on deparaffinized 8 μm thick cross sections through the brain and spinal cord. Sections were either autoclaved in 10 mM citric acid buffer (pH 6.0) for ten minutes or treated in 2N HCl at 50° C for 1 hour (followed by washes in 10 mM borate buffer, pH 8.5) to denature the DNA. Anti-BrdU mouse monoclonal antibody (Chemicon, Temecula, CA) in PBS with 0.1% BSA and 0.2% Triton-X was applied, followed by a biotinylated anti-mouse secondary antibody (Biomeda Biostain Super ABC-Alkaline Phosphatase Kit, Biomeda, Foster City, CA). Labeled cells were revealed colorimetrically by an intense blue color, using BCIP/NBT chromogen substrate (Roche Applied Science).
In order to determine the identities of the BrdU-labeled cells as either neurons or glial cells, the sections were double-labeled with sheep polyclonal antibody to BrdU (5μg/ml, ab1893, Abcam) and one of three monoclonal antibodies: 1) the general neuronal marker, anti-Hu antibody (1:100; mAb 16A11, Molecular Probes), which recognizes a regulatory RNA binding protein that functions early in neuronal differentiation (Barami et al., 1995; Marusich et al., 1994; Szabo et al., 1991); or 2) LCM 3 (1:100), a mAb that is specific for all 4 known lamprey NFs (Jin et al., 2011), including NF-180 (Merrick et al., 1995; Pleasure et al., 1989) and all three of the more recently identified NF subunits, L-NFL, NF95 and NF132, on Western blots (unpublished); or 3) LCM 29 (1:100), a mAb that recognizes all three identified glial cytokeratins and has been characterized previously with regard to specificity in glia of lamprey CNS (Merrick et al., 1995; Pleasure et al., 1989). Then the sections were visualized by secondary antibodies of Alexa Fluor 594 donkey anti-sheep IgG and Alexa Fluor 488 donkey anti-mouse IgG (1:500; A21202 and A11016, Molecular Probes) and mounted in Fluoromount G (NC9642668, Fisher Scientific). The specificities of the antibodies were tested in spinal cord sections from a normal animal. The sections were stained with a Biotin-conjugated anti-Hu antibody purified from the serum of a patient with paraneoplastic encephalomyelitis (kindly provided by Dr. Joseph Dalmau, Department of Neurology, University of Pennsylvania) and LCM29, and visualized with streptavidin, Alexa Fluor 594 (S32356, Molecular Probes) and Alexa Fluor 488 donkey anti-mouse IgG as above. The neuronal nature of the Hu positive cells in the ependymal region were also confirmed with an anti-acetylated tubulin antibody (1:500, clone: 6-11B-1, Sigma,). The antibodies used in this study are characterized in Table 1. Images were captured by an Axiocam REC camera connected to a Zeiss Axioscop fluorescence microscope. Photoshop software (CS4, Adobe System, San Jose, CA) was used to adjust contrast and brightness, and to add labels.
Table 1.
Primary antibodies used. LCM 29 and LCM3 are mAbs that recognize all lamprey glial keratin and all lamprey neurofilament subunits, respectively (see Methods). They have been characterized previously with regard to specificity in lamprey CNS (Merrick et al., 1995; Pleasure et al., 1989). The biotin-conjugated anti-HuD antibody was purified from the serum of a patient with paraneoplastic encephalomyelitis, and was kindly provided by Dr. Joseph Dalmau, Department of Neurology, University of Pennsylvania, now at University of Barcelona Hospital Clinic.
| Antigen | Immunogen | Host and Type | Manufacturer | Dilution |
|---|---|---|---|---|
| Bromodeoxyuridine | Bromodeoxyuridine-bovine serum albumin |
Mouse monoclonal |
Chemicon (Temecula, CA), MAB3424 |
1:200 |
| Bromodeoxyuridine | Bromodeoxyuridine coupled to keyhole limpet hemocyanin |
Sheep polyclonal |
Abcam, ab1893 | 5 μg/ml |
| Neuronal Protein HuC/HuD and Hel- N1 |
Human neuronal protein | Mouse monoclonal |
Molecular Probes, 16A11 |
1:100 |
| Cytokeratin | Lamprey cytoskeleton | Mouse monoclonal | Selzer lab, LCM29 | 1:100 |
| Neuronal protein HuD |
Recombinant Human HuD protein |
Human polyclonal | Dr. Josep Dalmau | 1:500 |
| Neurofilaments | Lamprey cytoskeleton | Mouse monoclonal | Selzer lab, LCM 3 | 1:100 |
| Acetylated α - tubulins |
Outer arm of sea urchin | Mouse monoclonal |
Sigma-Aldrich, 6-11B-1 |
1:500 |
Light microscopic analysis
Serial sections were examined with a Zeiss Axioscop light microscope using Nomarski optics. Both the BrdU–positive and BrdU-negative cells were counted in the rhombencephalon and spinal cord. The rhombencephalon was examined because this is where most supraspinal projecting neurons reside. For counts in the rhombencephalon, the boundaries were set from the point at which the cerebral aqueduct opens into the fourth ventricle rostrally to the obex caudally. The rhombencephalon was divided into three discrete regions: the ependyma, a monolayer of cells that line the ventricle; the subventricular layers, a region of densely packed neuronal and glial cell bodies subjacent to the ependyma; and the peripheral zone, the very sparsely celled region peripheral to the subventricular layers. For counts in the spinal cord, 0.5 mm lengths of tissue immediately rostral and caudal to the lesion site were studied. The general cellular organization of these adjacent regions appeared virtually unchanged. The lesion site itself showed a dramatic change in the size and shape of the spinal cord, particularly an enlargement of the central canal and a loss of right-left symmetry. The boundaries of the lesion site were defined arbitrarily as the locations rostral and caudal to the point of maximum enlargement of the central canal where the central canal tapered to less than 25 μm. An uninjured spinal cord has a central canal that is approximately 10 μm in diameter. Although the ependyma is easy to define in an injured spinal cord, the anatomical distinctions between the gray and white matter are not distinct within the lesion site. For example, the Mauthner axon is atrophied and there are few, if any, other giant axons. Consequently, the spinal cord cross section was divided into two regions, the ependyma and the non-ependyma. In uninjured cord, the ependyma is a diamond shaped, pseudostratified collection of cells layered 2-3 nuclei deep that surround the central canal. The non-ependyma includes both the gray matter, which extends laterally from the ependyma to the Mauthner axons, and the “white matter,” the region of axon tracts that surrounds the gray matter (although all axons in the lamprey are un-myelinated). The method of Abercrombie (Abercrombie, 1946) was used to correct for double-counting of nuclei in densely-labeled adjacent sections. In addition to raw counts of labeled cells, a BrdU labeling index (BLI) was calculated as the percentage of cells within category that were labeled in a given region. For example, the BLI for ependymal cells = (BrdU-labeled ependymal cells) × 100 ÷ (BrdU-labeled ependymal cells + unlabeled ependymal cells). Because the labeled cell counts often did not fit a normal distribution, the data were analyzed by the Kruskal-Wallis one-way analysis of variance, followed by Dunnett's multiple comparisons test, comparing labeling at different times post-TX with that in control animals.
RESULTS
The cytoarchitectonic structure of the brainstem and spinal cord are shown in figure 1A and B, respectively. At the site of injury there was disruption of the normal architecture with an increase in cellularity (Fig. 1C), which could be seen even 500 μm away from the lesion (Fig. 1D). Proliferative responses to spinal cord TX showed seasonal variations. Therefore, results for animals studied in winter (January/February) and summer (June/July) are presented separately.
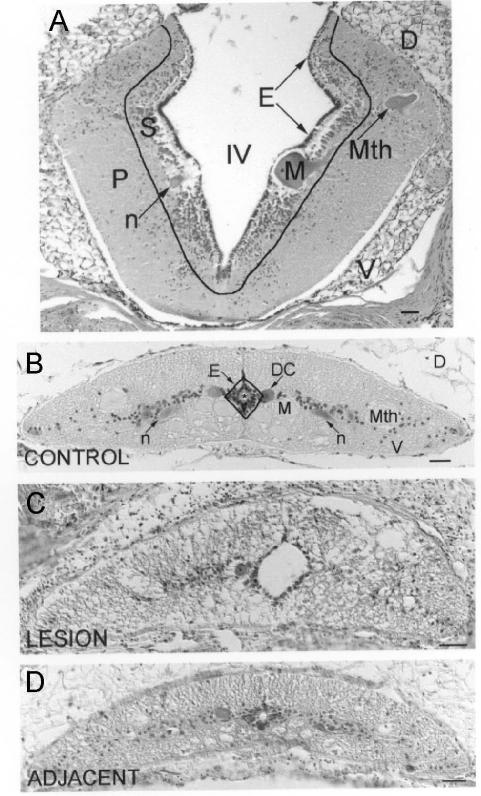
Fig. 1. Cytoarchitecture of the lamprey rhombencephalon and spinal cord.
Hematoxylin-stained CNS cross-sections are shown with outlines indicating boundaries between adjacent cytoarchitectonic regions. A, the rhombencephalon is divided into ependyma (E), subventricular zone (S), and peripheral region (P). IV = fourth ventricle ; D = Dorsal; V = ventral; Mth = Mauthner neuron ; M = Müller neuron ; n = neuron. B, the spinal cord is divided into ependyma (E), a diamond shaped, pseudostratified layer of cells 2 – 3 nuclei deep that surround the central canal, and the non-ependyma. Mth = Mauthner axon; M = Müller axon; n = neuron; DC = dorsal cell. C, lesioned spinal cord. Note the enlarged central canal, the asymmetrical shape of the spinal cord in comparison with the control, and the absence of landmarks such as the Mauthner axons in the lateral spinal cord. D, spinal cord adjacent to the lesion. * = central canal; D = dorsal; V = ventral; scale = 50 μm.
Spinal cord
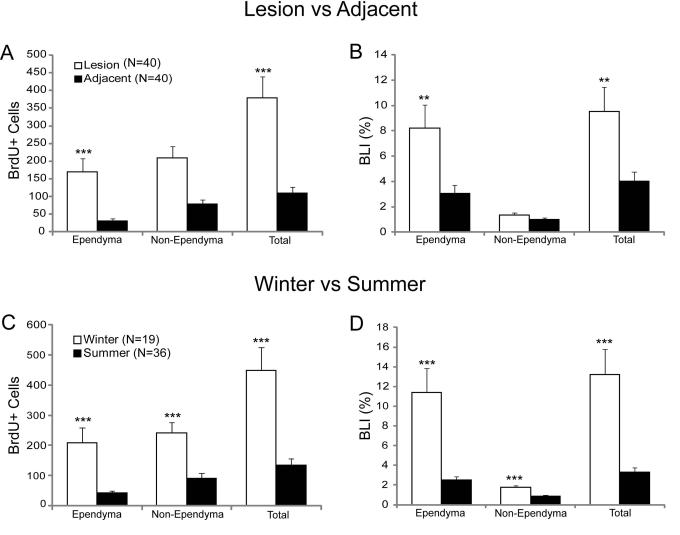
There was no statistically significant difference in BrdU labeling rate between spinal cord regions immediately rostral and caudal to the lesion site. Therefore, the data from these adjacent regions were pooled. Spinal cord TX-induced cell proliferation was higher in the lesion site than in the adjacent spinal cord, particularly in the ependymal zone (p<0.01; Figs. 2, 3A, B). The average number of BrdU-labeled cells in all animals reached 380 in the lesion site, vs. 109 in the adjacent cord (p<0.001; Fig. 3A). The rate of BrdU labeling was much higher among ependymal than non-ependymal cells in both the lesion and the adjacent sites (Fig. 3B), although the non-ependymal regions contributed a larger number of BrdU-labeled cells (Fig. 3A). This is because there were 7 times as many non-ependymal cells as ependymal cells (15,800 vs. 2,127 in the lesion site and 6,983 vs. 1,012 in the adjacent cord).
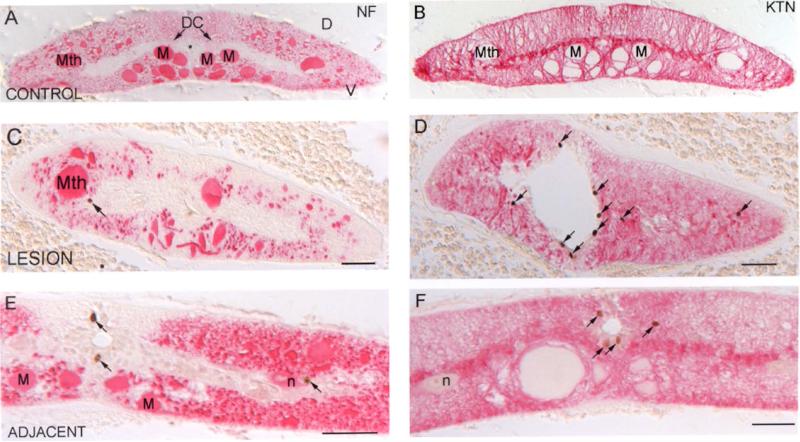
Fig. 2. BrdU label in the spinal cord is found in small cells, some labeling for glial keratin (KTN) but none for neurofilaments (NFs).
A and B, control lamprey spinal cord stained with anti-lamprey-NF (A) and anti-glial-keratin (B) antibodies. * = central canal; D = dorsal; V = ventral; Mth = Mauthner axon; M = Müller axon; DC = dorsal cell. C and D, lamprey spinal cord at 2 weeks post-TX and injected with BrdU 4 hours before sacrifice, stained with the anti-lamprey-NF antibody LCM3 (C) and the anti-lamprey-glial keratin antibody LCM29 (D). E and F, spinal cord 5 mm rostral (“adjacent”) to a TX, and processed as in C and D, stained with the same anti-lamprey-NF (E) and anti-lamprey-glial keratin (F) antibodies. Arrows point to BrdU-positive cells; scale bar = 50 μm. At this magnification, most glial cells are too small to definitively identify their nuclei with specific keratin-containing processes.
Fig. 3. Effect of spinal cord transection on BrdU labeling.
A and B: BrdU labeling in the lesion site and adjacent cord in all animals (winter + summer). C and D show BrdU labeling in winter and summer of total SC transected animals (1 week to 3 weeks). Graphs show mean ±SEM, ** p≤0.01, *** p≤0.001. N’s in A refer to the number of animals used in A and B. N’s in C are the number of animals in C and D.
Winter Animals
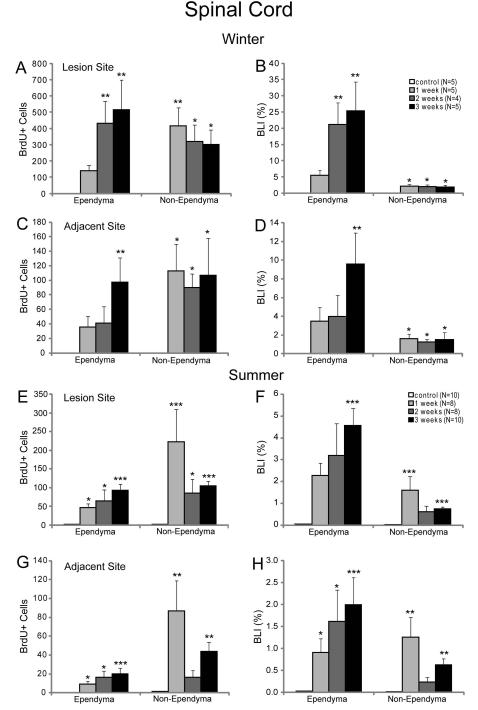
There were no BrdU-positive cells in the spinal cords of control animals in winter (Fig. 2A, B), but spinally transected animals had substantial BrdU labeling, which was more frequent in the lesion site than in the adjacent spinal cord (Fig. 2C-F). The TX-induced increase in BrdU labeling was much greater in winter than in summer (Fig. 3C, D). During the first 3 weeks post-TX, BrdU labeling increased progressively in the ependymal zone, reaching 514 cells/mm length of cord in the lesion and 97 cells/mm in the adjacent cord (Fig. 4A, C), whereas non-ependymal labeling was already maximal at 1 week post-TX (415 cells/mm). The rate of BrdU labeling was much higher in the ependymal than the non-ependymal zone, both in the lesion site (Fig. 4B) and the adjacent regions (Fig. 4D), although in this difference was not apparent when considering only absolute numbers of labeled cells (Fig. 4A, C).
Fig. 4. Time course of BrdU labeling after spinal cord transection in winter and summer.
Graphs A, C, E and G show the number of BrdU-labeled cells (mean ± SEM) per mm length of spinal cord. Graphs B, D, F and H show the BLI (mean ± SEM), which is the percent of cells that are labeled. This is calculated as (BrdU-labeled cells) ×100 ÷ (labeled + unlabeled cells). A and B are in the lesion of animals transected in winter, while C and D are in the adjacent spinal cord of winter animals. Frames E-H are comparable data for summer animals. There was no BrdU labeling in the spinal cord of the winter control animals but some labeling in summer. BrdU labeling was dramatically induced by spinal cord transection. There was more BrdU labeling in the lesion site than in the adjacent spinal cord, and the highest rates of BrdU labeling occurred in the ependymal zone. Statistical significance compared to control (Kruskal-Wallis one-way ANOVA followed by Dunnett’s test for multiple comparisons) * p≤0.05; ** p≤0.01; *** p≤0.001. N’s in B are the number of animals used in A-D. N’s in F are the numbers of animals used in E-H. The greater availability of animals in summer than in winter resulted in higher N values and greater calculated statistical significances than seen in the winter data.
Thus for animals spinally transected in winter, the spinal cords showed more BrdU labeling than in controls, the lesion site had more apparent mitotic activity than the adjacent spinal cord, and the ependyma showed a larger proportional increase in BrdU labeling than did non-ependymal regions.
Summer Animals
In contrast with control animals, in which BrdU labeling was far more frequent in summer than in winter, TXed animals showed a smaller BrdU-labeling response in summer than in winter (Fig. 4). As in winter animals, there was a progressive increase in BrdU labeling in the ependyma zone over the first three weeks post-TX, while labeling in non-ependymal regions was maximal by 1 week post-TX (Fig. 4E-H). Nevertheless, as in the winter animals, animals spinally transected in the summer had more BrdU labeling than controls and the lesion site had more labeling than the adjacent tissue. The rate of BrdU labeling (BLI) in the ependyma was much higher than in non-ependymal regions (Figs. 3D, 4F, H).
Rhombencephalon
Winter Animals
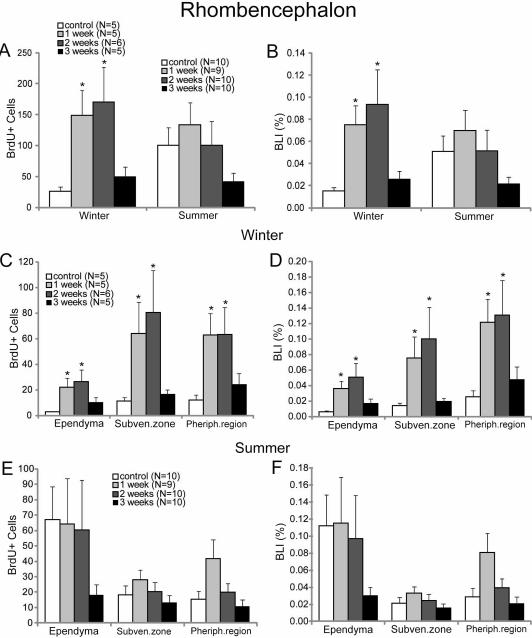
A proliferative response to spinal cord TX could be seen in winter animals even in the rhombencephalon (Figs. 5, 6). In control animals, the mean BLI was 0.015%. There was a peak in BLI at 2 weeks post-TX (0.094%), but even at one week, the BLI was more than four times that seen in untransected controls (p<0.05; Fig. 6B). All three cytoarchitectonic regions (ependyma, subventricular zone and peripheral region) participated in this increase, but most of the BrdU labeling in both control and spinally transected animals was in the subventricular and peripheral zones (Fig. 6C, D).
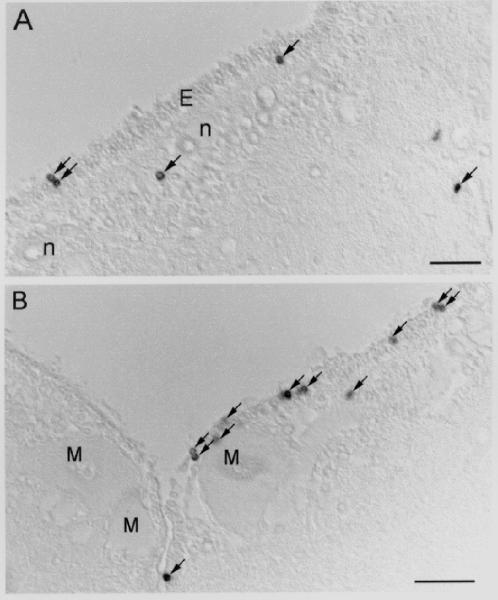
Fig. 5. BrdU-labeling of cells in the rhombencephalon after spinal cord TX.
Two sections through the rhombencephalon of an animal two weeks post-TX, viewed with Nomarski optics. A, BrdU-labeled cells in the ependyma (E), the subependyma, and the peripheral zone of the rhombencephalon of a winter animal. n = neuron. B, BrdU-labeled cells in the ependyma and the subventricular zone of the rhombencephalon of a summer animal. M = Müller neuron. Arrows point to BrdU-positive cells; scale bar = 50 μm.
Fig. 6. Regional distribution of BrdU labeling in the rhombencephalon.
Graphs A, C and E show the number of BrdU-labeled cells in the whole rhombencephalon (mean ± SEM). Graphs B, D, and F give the BLIs for cells of the rhombencephalon as in figure 5. A and B show the data for all cells in winter and summer. C and D give data by zone for winter animals, while E and F give analogous data for summer animals. * = statistically significant (p<0.05; Kruskal-Wallis one-way ANOVA followed by Dunnett’s test for multiple comparisons). N’s shown in A are the number of animals used in winter and summer in A and B. N’s in C are the number of animals used in C and D. N’s in E are the numbers of animals used in E and F.
Summer Animals
The BLI in the rhombencephalon of control animals studied in summer was more than three times that of animals studied in winter. However, summer animals showed a smaller and statistically not significant BrdU labeling response following spinal cord TX (Fig. 6A, B, E, F). Moreover, in both control and TX animals, BrdU labeling was greater in the ependyma than in the subventricular zone and peripheral region. In contrast to other regions, the BrdU labeling decreased in the ependyma post-TX. Furthermore, an apparent decrease in BrdU labeling was seen in all regions over the 3 weeks post-TX in all the regions, although none of these differences reached statistical significance, despite a two-fold larger sample size (number of animals) compared to the winter group.
Identification of the Proliferating Cell Types
As shown in figure 7, in control animals, cells labeled by the neuronal marker, anti-Hu antibody (arrows in Fig. 7B), were never labeled by the anti-lamprey-glial keratin antibody LCM29 (arrows in Fig. 7A), indicating that the anti-Hu antibody is specific for neurons. While there was an indication of both gliogenesis and neurogenesis in the spinal cord and rhombencephalon, evidence for neurogenesis was restricted to small cells that remained near the central canal or 4th ventricle, and did not appear to migrate peripherally or to differentiate into other neuron types. These cells were also labeled by anti-acetylated tubulin antibodies (Fig. 7E) but never by anti-NF antibodies (Fig. 7F) and were previously assumed to be glial/ependymal in nature (Merrick et al., 1995; Rovainen, 1979). Despite their small size and ependymal location, the Hu-positive cells did not contain glial keratin immunoreactivity (as indicated by confocal microscopy; Fig. 7D). However, many BrdU-positive cells were not double-labeled by either the anti-keratin, the anti-NF, or the anti-Hu antibodies.
Fig. 7. Specificity of anti-keratin and anti-Hu antibodies.
Control lamprey spinal cord stained with anti-glial keratin antibody LCM 29 (magenta in A) or the anti-Hu antibody (green in B) imaged with an epifluorescence microscope. D = dorsal; V = ventral; * = the giant axons of Müller neurons. Arrows point to neurons stained with anti-Hu but not with the anti-keratin antibody. C, images in A and B merged, showing segregation of stained profiles. Scale bar = 20 μm. The small amount of pale labeling in a few cells is due to overlap of different cells in the section, and not to co-expression of Hu and keratin in the same cell. This is shown by the absence of pale label on confocal imaging in the region of the central canal (cc) in D. Two large Müller axons are indicated by *. E, cc region stained with anti-acetylated tubulin antibody showing subependymal cells sending processes to the cc. These are the CSF-contacting neurons described by others (see text). F, a section double-labeled with antibodies to BrdU (brown) and neurofilament (red). N = dorsal cells (primary sensory neurons). Note the absence of NF stain in the ependymal and subependymal cells surrounding the central canal, even though some of these cells would be labeled by anti-Hu, as in D. The arrowhead points to a BrdU-positive cell that has no NF stain, at the base of the dorsal column.
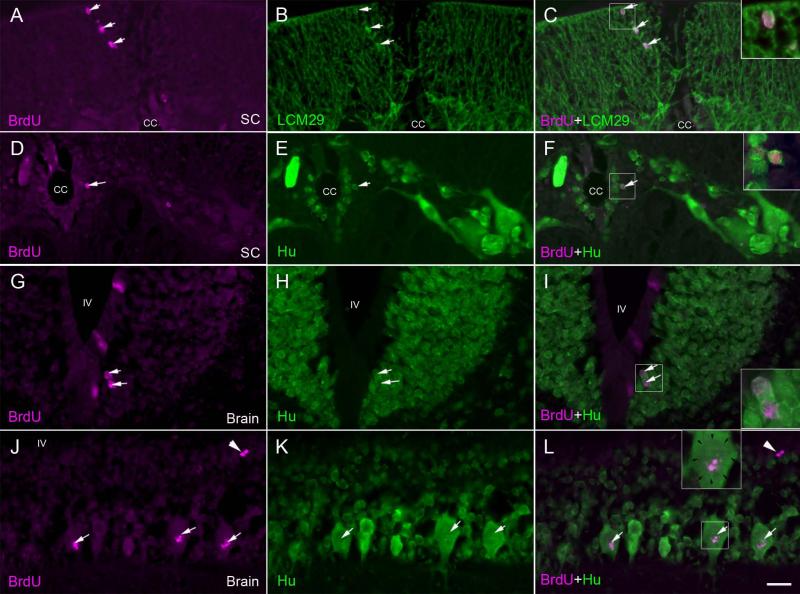
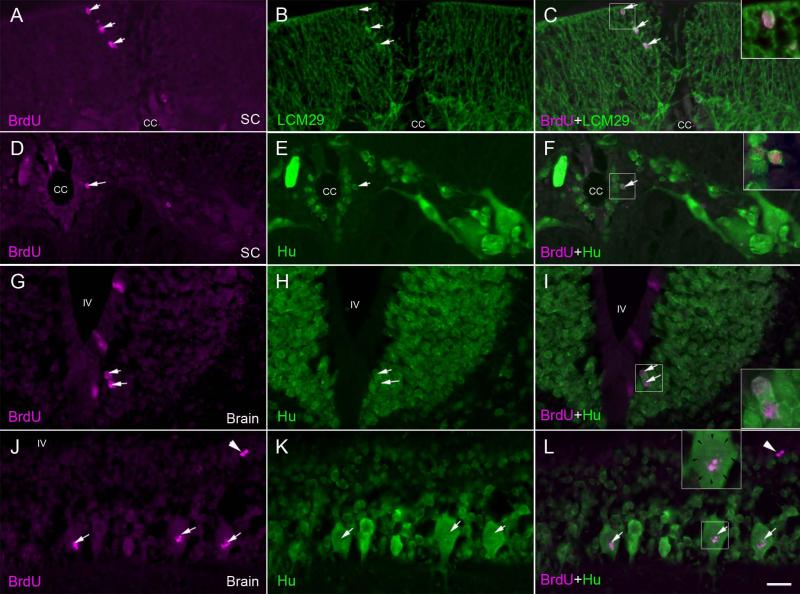
In order to determine whether the cells proliferating after spinal cord TX were maturing into neurons or glial cells, double labeling for BrdU and either anti-Hu, LCM3 (anti-lamprey-NF) or LCM29 (anti-glial-keratin) antibody was performed in animals that had been injected with BrdU at two weeks post-TX, when BrdU labeling was already vigorous, and permitted to recover an additional 5 weeks. Thus these animals survived a total of 7 weeks post-TX. No BrdU-labeled cells in these or any other animals were ever labeled by the anti-NF antibody LCM3 (c.f., Fig. 2C, E). In both the spinal cord and rhombencephalon, most BrdU-labeled nuclei were found in cells that were also labeled with anti-keratin. Those double-labeled cells were located in both the ependymal and non-ependymal regions (Fig. 8C), although the cells in the ependymal zone tended to be larger and stained less intensely than glial cells in the periphery (see Figs. 2D, F, 8C). Some BrdU-labeled cells were also labeled with anti-Hu. These were located exclusively in the ependymal zone (2nd or 3rd cell layers from the central canal) of the spinal cord (Fig. 8F) and in the subependyma of the rhombencephalon (Fig. 8I). A few Hu-BrdU double-labeled reticulospinal neurons were noted in the subventricular zones of the rhombencephalon. However, in these neurons, the BrdU label was fragmentary, and did not fill the nucleus (Fig. 8L). This suggested that the label was due to DNA damage or repair, rather than mitosis. It corresponds to the TUNEL (Shifman et al., 2008) and caspase (Barreiro-Iglesias and Shifman, 2012) positivity seen at early times in the course of very delayed retrograde neuronal death after spinal cord TX. In the present study, there was no evidence for the addition of new neurons in the peripheral regions, even in those animals allowed to survive for 5 weeks after BrdU injection. Of all the BrdU-positive cells stained with additional antibodies, 7.0% were also Hu-positive, 61.3% were keratin-positive and 31.7% were not positive for any of the three labels (NF, Hu or keratin). The percentage of keratin positive cells should be viewed as approximate because the glial cells have very little cytoplasm and at the light microscopic level, it was not always possible to determine whether a BrdU-labeled nucleus belonged to the same cell as an adjacent keratin-labeled process.
Fig. 8. Gliogenesis and neurogenesis after spinal cord transection.
At 2 weeks post-TX, animals were injected with BrdU and sacrificed 5 weeks later, in order to allow enough time for any post-mitotic cells to differentiate. Sections of the spinal cord and rhombencephalon were double stained for BrdU (A, D, G, J) and either keratin (B) or anti-Hu antibody (E, H, K). A, spinal cord at the TX site imaged for BrdU. B, same section imaged for keratin. C, superimposed. Note the co-labeling in glial cells (arrows) in the dorsal column. D, spinal cord imaged for BrdU. E, same section imaged for Hu. F, superimposed. Note the co-labeling in a cell (arrow; enlarged in inset) immediately subjacent to the cell layer lining the central canal (cc). Note the absence of co-labeling in the larger neurons away from the ependymal zone. G, Brainstem imaged for BrdU. H, same section imaged for Hu. I, superimposed. Note the co-labeling in ependymal cells (arrows; enlarged in inset). Label was also seen in cells of the subventricular zone (not shown here). J, Brain imaged for BrdU. K, same section imaged for Hu. L, superimposed. In a few larger neurons of the peripheral region, there is some BrdU labeling, but this does not occupy the entire nucleus (margins of the nucleus are indicated by arrows in the inset enlargement). It probably represents DNA repair (white arrows). Compare this pattern to that of the cells shown in the insets of C, F and I. White arrowheads point to a pair of BrdU-positive Hu-negative cells. Cc = central canal, SC = spinal cord, IV = fourth ventricle, scale bar = 20 μm.
DISCUSSION
Transection induces gliogenesis
BrdU labeling is conventionally interpreted as a sign of mitosis, although strictly speaking, it indicates only that at the time of injection, the cell was in S-phase (synthesizing DNA), which can occur without cell division. Nevertheless, when combined with the large increase in cell counts seen in and adjacent to the TX, this study suggests that at the site of a SCI in the lamprey, glial cells or their progenitors undergo extensive proliferation. In fact, the majority (61%) of BrdU-labeled cells also appeared to be labeled by an antibody specific for glial keratin, although the small sizes of glial cells made this figure only approximate. The cells in the ependymal zone are slightly larger, but in these cells, the keratin staining was less dark, suggesting that they might be immature. Injury-induced gliogenesis might have been expected based on the cellularity of the enlarged central canal (Rovainen, 1976; Selzer, 1978). However, mitotic figures were not noted in previous studies (Lurie et al., 1994). Nor had 3H-thymidine incorporation studies suggested large-scale mitosis (unpublished). Indeed, apart from the central canal, the TX scar has relatively few cells, but consists mainly of axons and glial fibers that arise from glial cells at the margins of the injury (Lurie et al., 1994). Based on these observations and the interconnection of glial processes by desmosomes, it was proposed that glial desmosomes prevent glial proliferation and migration, so that their thickened, longitudinally oriented fibers could serve as a scaffold for regenerating axons to grow along (Lurie et al., 1994). In part, this hypothesis was proposed to explain the previously documented directional specificity of axon regeneration in the lamprey spinal cord (Mackler et al., 1986; Yin et al., 1984). However, the extensive BrdU labeling activity demonstrated in the present study, and the evidence that many of the dividing cells are glia or glial projenitors, calls this hypothesis into question. It remains to be determined whether glial cells at the margin of a spinal cord lesion in the lamprey migrate less than those of species in which glial processes are not interconnected by desmosomes.
Transection induces neurogenesis
In the present study, following spinal cord injury, evidence for both gliogenesis and neurogenesis was found in the spinal cord and brainstem. In mammals, neural precursors in the spinal cord are thought to generate only glial cells (Kang et al., 2010; Zhu et al., 2008) and to differentiate into neurons only in vitro (Kondo and Raff, 2000) or if transplanted into neuronogenic areas of the brain such as the dentate gyrus (Aguirre et al., 2004; Shihabuddin et al., 2000). Unlike in lamprey, ependymal cells in the mouse spinal cord do not appear to generate neurons after injury, as judged by NeuN staining (Barnabé-Heider et al., 2010). However, injury-induced neurogenesis has been seen in other CNS areas: in the rat hippocampus (Dash et al., 2001; Farel and Boyer, 1999; Kernie et al., 2001; Snyder et al., 2001; Yagita et al., 2001), the olfactory epithelium (Barber, 1982), and the neocortex (Magavi and Macklis, 2001). Neurogenesis also occurs in the injured brains of lizards (Font et al., 1997; Font et al., 2001; Ramirez-Castillejo et al., 2002), and among fish retinal cone photoreceptors (Cameron and Easter, 1995).
In both fish (Anderson and Waxman, 1985) and newts (Benraiss et al., 1999; Nordlander and Singer, 1978) glial cells and neurons derived from the proliferative ependymal layer migrate to regenerate the entire missing spinal cord. We have not seen lamprey larvae the ages of those used in these experiments survive tail amputation (unpublished), although at earlier stages, it may be possible that they can regenerate a 2-3 mm stretch of excised spinal cord (Marón, 1959). Some neurons differentiate from radial glial cells during embryogenesis of the cortex (Malatesta et al., 2003; Noctor et al., 2001). In adult mice, neurons can arise from astrocytes in the subgranular layer of the dentate gyrus (Seri et al., 2001). How the newly proliferated neurons participate in spinal cord regeneration, is unknown. In the tail-amputated newt, neurons derived from the proliferative ependymal layer migrate to regenerate the spinal cord (Zhang et al., 2003) or become interneurons to help re-build neuronal circuits. However, in the present study, newly formed neurons remained clustered around the central canal, even at 7 weeks post-TX and 5 weeks after injection. These may be the CSF-contacting neurons as described by other authors (Rodicio et al., 2008). A similar phenomenon was reported in the eel (Dervan and Roberts, 2003).
In the present study, approximately 32% of BrdU-labeled cells did not stain with either the anti-Hu or anti-keratin antibodies and their phenotypes are unknown. From their locations and sizes they most likely are non-neuronal ependymal cells. The evidence from the present study and from previous studies describing CSF-contacting neurons suggests that the ependymal cells of lamprey are a heterogeneous population. Some express neuronal markers and some do not. Those that are neurons have varying transmitter phenotypes (Brodin et al., 1990; Rodicio et al., 2008; Shupliakov et al., 1996). Of those that do not label with neuronal markers, not all stain with anti-keratin antibodies. Many (but not necessarily all) ependymal cells surrounding the central canal of the lamprey spinal cord are tanycytes (Tretjakoff, 1909), meaning that they have processes projecting to the spinal cord surface. These may contribute to scaffolding within the lesion cavity that provides the substrate for cellular infiltration and axonal regeneration, as has been suggested in the lesioned rat spinal cord (Beattie et al., 1997), or that accompanies the outgrowth of elongating axons, as in injured tadpole spinal cords (Michel and Reier, 1979). In the large lamprey larva, regenerating giant reticulospinal axons contact neuronal and glial elements and do not grow through acellular, fluid-filled open channels (Lurie and Selzer, 1991). Rather, keratin-containing glial processes form a bridge across the TX in advance of the axons (Lurie et al., 1994). Recently, BrdU labeling experiments showed that tanycyte-derived cells in the mouse hypothalamus develop into Hu+ neurons (Lee et al., 2012). Whether the Hu-positive cells in the present study also are tanycytes remains to be determined.
A previous study on control lamprey failed to find BrdU-positive cells that were double-labeled by anti-NF antibodies, even after 4-5 weeks of BrdU incorporation. This suggested that only gliogenesis was occurring but not neurogenesis. However, since some neurons do not stain positively for NF (Jacobs et al., 1996; Swain et al., 1994), this did not prove that no neurogenesis occurred. In that study, several other neuronal markers were tested, including commercially available antibodies to nestin, NeuN, neuron specific enolase, TUJ1, and MAP-2, but these proved either insufficiently specific or insufficiently intense in their immunohistochemical labeling to be reliable neuronal markers in lamprey CNS (Vidal Pizarro et al., 2004). In the present study, the anti-Hu antibody was found to be a reliable neuronal marker. This antibody recognizes a regulatory RNA binding protein that functions early in neuronal differentiation (Marusich et al., 1994; Szabo et al., 1991) and has been used as a general neuronal marker in many species, including zebrafish (Byrd and Brunjes, 2001). Approximately 7% of BrdU-positive cells were also labeled by the anti-Hu antibody in lamprey spinal cord and brain, indicating that neurogenesis occurred in control animals and was increased after spinal cord TX. Although not quantified, a similar pattern was seen with a second neuronal marker, anti-acetylated tubulin antibody. Whenever Hu-positive proliferating cells were found, they were in the ependymal or immediately subependymal region. Moreover, none of the BrdU-positive cells appeared to contain neurofilament, which is developmentally regulated, so that its expression increases with age in the larvae and with metamorphosis (Jacobs et al., 1996). This suggests that the BrdU-labeled neurons have a restricted phenotype. Since anti-Hu stains cell bodies but not their processes, the projections of these neurons could not be determined, although they were similar in size to other ependymal cells. In preparations stained with anti-acetylated tubulin, many of these cells had projections that contacted the CSF (central canal). This suggests that they are the GABAergic and monoaminergic CSF-contacting neurons as described by others in the lamprey (Brodin et al., 1990; Melendez-Ferro et al., 2002; Rodicio et al., 2008) and other species (Barber, 1982; Franzoni and Morino, 1989; Sueiro et al., 2004; Vigh et al., 2004). Although it is possible that with even longer incubation times, these cells would have migrated away from the ependymal region and/or differentiated into other neuron types, the present results show that as much as 7 weeks post-transection, when many axons have regenerated across the TX (Lurie et al., 1994; Yin and Selzer, 1983), and 5 weeks after BrdU incorporation, there are Hu-positive BrdU-labeled cells that are restricted to the ependymal region. We cannot know to what extent they migrate away from this region, but if they do, they do not maintain a neuronal phenotype.
BrdU-positive cells may become glial cells, since most of the BrdU-positive cells, especially in peripheral regions, are surrounded by glial-keratin-containing processes. Although a few larger, non-ependymal neurons in the rhombencephalon contained BrdU label, the labeling was speckled and did not fill the nuclei. BrdU may be incorporated into the DNA of non-dividing neurons that are dying or during DNA repair (Kuan et al., 2004; Yang et al., 2006). Delayed apoptosis was recently reported in reticulospinal neurons after spinal cord TX in the lamprey (Shifman et al., 2008). In these neurons, TUNEL labeling could be detected weeks before the neurons died, and this could account for spurious BrdU labeling.
Spinal Cord
After spinal cord TX, the lesion site had more BrdU labeling than the adjacent tissue, but at both locations, most of the BrdU labeling took place in the ependyma. The BLI in spinal-transected animals was extraordinarily high, reaching a peak of 25% at 3 weeks post-TX in winter animals. Since the calculation of BLI was based on the ratio of labeled cells to the number of cells in a standard length of control spinal cord, a cumulative increase in the number of cells in the lesion could mean that the actual ratio is smaller than calculated. There were 3.5 times as many ependymal cells as in control cord, whereas the number of non-ependymal cells was about the same as in control cord. Since the BLI for the ependyma was generally greater than for the non-ependyma, the very large apparent BLI within the lesion would seem to be partly an artifact of the increase in the proportion of ependymal cells. Neurogenesis has been reported after spinal cord hemisection in goldfish (Takeda et al., 2008), with at least some of the neurons staining for 5-HT, but how well the neurons integrated into the spinal circuitry and how many survived subsequent apoptosis is not clear.
The injury-induced cell proliferation in the spinal cord and the localized BrdU labeling of the ependyma is consistent with the findings in injured mammalian spinal cord, where more cell division was reported in the injury site than in the adjacent tissue (Adrian and Walker, 1962; Adrian and Williams, 1973; Beattie et al., 1997; Bruni and Anderson, 1987; Gilmore and Leiting, 1980; Namiki and Tator, 1999; Zhang et al., 2000). In injured adult mouse spinal cord, most of the proliferation appeared to be in the ependyma, particularly when the central canal was injured, whereas most of the proliferation in uninjured adult rat spinal cord was in the peripheral zone (Horner et al., 2000; Kojima and Tator, 2000).
Origins of the Reconstituted Ependyma of the Enlarged Central Canal in the Lesion Zone
Clearly the cells filling in the lesion must originate from somewhere, presumably from the adjacent cord. At least some of the BrdU labeling in the ependymal cells of the injury zone probably represented actual cell proliferation, since the total number of cells was increased 2.5-fold in the TX site compared to either the untransected spinal cord or the cord adjacent to the lesion. And despite an increase in BrdU labeling, there was no change in the number of cells in the adjacent cord. This suggests that cells from the adjacent cord contributed to making up the cord in the injury zone, while the number of cells in the adjacent cord was kept constant by proliferation. However, alternate explanations are possible. The observed dilation of the ependyma concurrently with a retraction of the transected cord could result in a high number of ependymal cells in the length examined, without requiring ependymal cell proliferation.
Seasonal Variation in the Spinal Cord
Our previous study suggested that there was a seasonal variation in the baseline cell proliferation, such that the summer animals had more BrdU labeling than those in the winter (Vidal Pizarro et al., 2004). Thus we expected to find a larger proliferative response to injury in summer than in winter. However, while TX of the spinal cord did result in an increase in BrdU labeling year round, the apparent increase in proliferation was greater in winter than in summer. Moreover, although there was more BrdU labeling in the ependyma than in the non-ependyma, regardless of season, animals transected in winter had greater ependymal BLIs than summer animals. The cause of the seasonal variation was not examined in this study. High BLIs observed in winter might be due to longer S-phases, and thus higher efficiency in BrdU incorporation, compared to those in summer larvae, as opposed to greater proliferation. This seems unlikely because during the winter, BLIs in non-transected animals were much lower than those in summer (see Vidal Pizarro et al., 2004). Alternatively, there might be seasonal variations in the vulnerability of cells to injury, resulting in differences in the number of surviving labeled cells. Another possible explanation is that proliferation could slow (fatigue?) with repeated divisions. If the rate of mitosis immediately after injury is much greater in summer than winter, then by 1 week post-TX, ependymal cells in summer animals might have slowed their proliferation, while proliferation in the winter cells was still vigorous. In preliminary experiments to select times for injection, we did not notice such an early effect, but additional studies may be necessary to clarify this issue.
The cause for season-dependent cell proliferation in lamprey is not known; several hormones such as gonadal, thyroid, and/or adrenal hormones might affect cell division. Gonadal hormones are unlikely candidates because larval lampreys have low levels of gonadotropin releasing hormone (Youson and Sower, 2001). A decline in thyroid hormones (TH) marks the initiation of metamorphosis (Kao et al., 1999; Manzon and Youson, 1999; Wright and Youson, 1977; Youson et al., 1994), but seasonal variations in TH concentration appear to be related to environmental temperature (Lintlop and Youson, 1983), which would not affect animals in the present study. Corticosterone reduces cell proliferation (Alonso, 2000; 2001; Cameron and Gould, 1994) and corticosterone levels exhibit seasonal fluctuations in mammals (Galea and McEwen, 1999; Ormerod and Galea, 2001), birds (Wada et al., 1999), reptiles (Lance et al., 2001; Munoz et al., 2000; Schramm et al., 1999), and amphibians (Zerani and Gobbetti, 1993), mostly peaking in the spring and summer. Whether lamprey corticosterone levels undergo seasonal variations or affect cell division is unknown.
Rhombencephalon
Most mammalian studies of cell proliferation following spinal cord injury have focused exclusively on the spinal cord, overlooking the brain regions where the cell bodies of spinal projecting axons reside and where degenerating rostral projections would terminate. Bruni and Anderson (Bruni and Anderson, 1987) examined the proliferative response in the ependyma of the fourth ventricle after puncturing the dorsolateral medulla, and the ependyma of the central canal after creating a unilateral incision in the lateral funiculus of the thoracolumbar cord in the rat. The proliferative response remote from a spinal cord injury was not investigated.
In the present study, BrdU labeling in the rhombencephalon seemed to be regulated by two effects – seasonal variation and TX. There was an increased BLI in the ependyma of the rhombencephalon in the summer. The winter-transected animals had more apparent cell proliferation than their season-matched controls, which suggested that spinal cord TX stimulated cellular proliferation in the rhombencephalon. However, there was no similar increase in the summer. Since the post-TX BrdU labeling response in winter animals reached approximately the same level as the rate in control animals in summer, it may be that the summer level of proliferation represents a ceiling. If so, the mechanism is not clear, since the BLIs in the rhombencephalon never exceeded 0.10%, whereas BLIs in the spinal cord were much higher.
Cellular Mechanisms of Sustaining Axonal Outgrowth
There are a number of ways in which cell proliferation could contribute to the repair process that sustains axon outgrowth. First, nascent neurons might replace injured or dead neurons or serve as interneurons to relay neuronal information across the injury, as in the newt (Benraiss et al., 1999). Second, with gliogenesis or ependymogenesis, the new cells could provide physical and trophic support to regenerating axons. Following spinal cord TX in lamprey, the processes of glial cells bridge the lesion in advance of regenerating axons (Lurie et al., 1994).
Glial cells could enrich the environment for axon growth by producing trophic factors (Bohn, 2004). Glial cells in the hippocampus of mammals may switch from a glial to neuronal phenotype (Seri et al., 2001) or serve as neuronal precursors as do radial glial cells in the mammalian brain (Malatesta et al., 2003; Noctor et al., 2001). Although lamprey glial cells are similar to radial glia in that their processes span the entire thickness of the spinal cord (or brain), it is not known whether glia in the lamprey CNS can generate neuronal progeny or be converted into neurons. However, the evidence discussed above suggests that neurogenesis after injury in the lamprey CNS derives from ependymal cells.
CONCLUSIONS
Spinal cord TX in the lamprey induced massive BrdU labeling, which was most intense at the site of injury, became less intense centrifugally, but was still detectable as far as the rhombencephalon. The mechanism for this remote proliferation is unknown. In the spinal cord, BrdU labeling occurred primarily in the ependymal zone, where many of the labeled cells had a neuronal phenotype, like the CSF-contacting neurons previously described in several species, including lampreys. However, none of the newly formed neurons were labeled by anti-NF antibodies, and there was no evidence for neurogenesis away from the ependymal zone, or for migration of these csf-contacting neurons away from the ependymal zone. Thus neurogenesis may be limited to a very restricted neuron type. What role such neurons play in restoring function to the injured spinal cord is not known. Further insights may be derived from using phenotype specificity markers to identify neuronal subtypes, from retrograde labeling studies to determine where the new neurons send their axons, if any, and from anterograde labeling studies to determine whether and from where the new neurons receive afferent inputs. Cell proliferation showed seasonal variation such that in uninjured animals, BrdU labeling was more prevalent in summer than in winter. Yet both the relative and absolute levels of proliferative response to spinal cord injury were much greater in winter than in summer. In the rhombencephalon, an increase in the BrdU labeling after spinal cord TX was seen only in winter, and this reactive labeling was approximately equal to the baseline level of labeling in summer, suggesting that this level might reflect a ceiling effect. The role of cell proliferation in axonal regeneration is not known, but glial and/or ependymal cells appear to play a role in supporting and guiding regenerating axons across a lesion.
ACKNOWLEDGEMENTS
This study was supported by NSF grant IBN9319702, NIH grants RO1 NS-14837, RO1 NS-25581 to MES, by NRSA Grant NS-11009 to IVP, and by Shriners Hospitals for Children.
Grant information:
NSF
Grant IBN9319702
NIH
Grants RO1 NS-14837, RO1 NS-25581, R24 HD050838
Shriners Hospitals for Children (GKZ, SK, and MES), and NRSA
Grant NS-11009 (to IVP).
Footnotes
CONFLICT OF INTEREST
There is no known or potential conflict of interest.
BIBLIOGRAPHY
- Abercrombie M. Estimation of nuclear population from microtome sections. Anatomical Records. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Adrian EK, Jr., Walker BE. Incorporation of thymidine-H3 by cells in normal and injured mouse spinal cord. J Neuropathol Exp Neurol. 1962;21:597–609. doi: 10.1097/00005072-196210000-00007. [DOI] [PubMed] [Google Scholar]
- Adrian EK, Jr., Williams MG. Cell proliferation in injured spinal cord. An electron microscopic study. J Comp Neurol. 1973;151(1):1–24. doi: 10.1002/cne.901510102. [DOI] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165(4):575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31(3):219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Alonso G. Proliferation of progenitor cells in the adult rat brain correlates with the presence of vimentin-expressing astrocytes. Glia. 2001;34(4):253–266. doi: 10.1002/glia.1059. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Waxman SG. Neurogenesis in adult vertebrate spinal cord in situ and in vitro: a new model system. Ann N Y Acad Sci. 1985;457:213–233. doi: 10.1111/j.1749-6632.1985.tb20807.x. [DOI] [PubMed] [Google Scholar]
- Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28(1):82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- Barber PC. Neurogenesis and regeneration in the primary olfactory pathway of mammals. Bibl Anat. 1982;(23):12–25. [PubMed] [Google Scholar]
- Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7(4):470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148(2):453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Arsanto JP, Coulon J, Thouveny Y. Neurogenesis during caudal spinal cord regeneration in adult newts. Dev Genes Evol. 1999;209(6):363–369. doi: 10.1007/s004270050265. [DOI] [PubMed] [Google Scholar]
- Bohn MC. Motoneurons crave glial cell line-derived neurotrophic factor. Exp Neurol. 2004;190(2):263–275. doi: 10.1016/j.expneurol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Brodin L, Dale N, Christenson J, Storm MJ, H:okfelt T, Grillner S. Three types of GABA-immunoreactive cells in the lamprey spinal cord. Brain Res. 1990;508(1):172–175. doi: 10.1016/0006-8993(90)91134-3. [DOI] [PubMed] [Google Scholar]
- Bruni JE, Anderson WA. Ependyma of the rat fourth ventricle and central canal: response to injury. Acta Anat (Basel) 1987;128(4):265–273. doi: 10.1159/000146352. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105(4):793–801. doi: 10.1016/s0306-4522(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Cameron DA, Easter SS., Jr Cone photoreceptor regeneration in adult fish retina: phenotypic determination and mosaic pattern formation. J Neurosci. 1995;15:2255–2271. doi: 10.1523/JNEUROSCI.15-03-02255.1995. 3 Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61(2):203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63(4):313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dervan AG, Roberts BL. Reaction of spinal cord central canal cells to cord transection and their contribution to cord regeneration. J Comp Neurol. 2003;458(3):293–306. doi: 10.1002/cne.10594. [DOI] [PubMed] [Google Scholar]
- Farel PB, Boyer A. Transient effects of nerve injury on estimates of sensory neuron number in juvenile bullfrog. J Comp Neurol. 1999;410(2):171–177. [PubMed] [Google Scholar]
- Font E, Desfilis E, Perez-Canellas M, Alcantara S, Garcia-Verdugo JM. 3-Acetylpyridine-induced degeneration and regeneration in the adult lizard brain: a qualitative and quantitative analysis. Brain Res. 1997;754(1-2):245–259. doi: 10.1016/s0006-8993(97)00085-1. [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E, Perez-Canellas MM, Garcia-Verdugo JM. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav Evol. 2001;58(5):276–295. doi: 10.1159/000057570. [DOI] [PubMed] [Google Scholar]
- Franzoni MF, Morino P. The distribution of GABA-like-immunoreactive neurons in the brain of the newt, Triturus cristatus carnifex, and the green frog, Rana esculenta. Cell Tissue Res. 1989;255(1):155–166. doi: 10.1007/BF00229077. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89(3):955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Gilmore SA, Leiting JE. Changes in the central canal area of immature rats following spinal cord injury. Brain Res. 1980;201(1):185–189. doi: 10.1016/0006-8993(80)90783-0. [DOI] [PubMed] [Google Scholar]
- Hardisty M. Biology of the cyclostomes. Chapman and Hall; distributed by Halsted Press; London & New York: 1979. [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20(6):2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AJ, Swain GP, Selzer ME. Developmental increases in expression of neurofilament mRNA selectively in projection neurons of the lamprey CNS. J Comp Neurol. 1996;364(3):383–401. doi: 10.1002/(SICI)1096-9861(19960115)364:3<383::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Zhang G, Pennicooke B, Laramore C, Selzer ME. Multiple neurofilament subunits are present in lamprey CNS. Brain Research. 2011;1370:16–33. doi: 10.1016/j.brainres.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68(4):668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y, Manzon RG, Sheridan MA, Youson JH. Study of the relationship between thyroid hormones and lipid metabolism during KClO4-induced metamorphosis of landlocked lamprey, Petromyzon marinus. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;122(3):363–373. doi: 10.1016/s0742-8413(99)00004-3. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66(3):317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Kojima A, Tator CH. Epidermal growth factor and fibroblast growth factor 2 cause proliferation of ependymal precursor cells in the adult rat spinal cord in vivo. J Neuropathol Exp Neurol. 2000;59(8):687–697. doi: 10.1093/jnen/59.8.687. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289(5485):1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci. 2004;24(47):10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance VA, Grumbles JS, Rostal DC. Sex differences in plasma corticosterone in desert tortoises, Gopherus agassizii, during the reproductive cycle. J Exp Zool. 2001;289(5):285–289. [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15(5):700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintlop SP, Youson JH. Concentration of triiodothyronine in the sera of the sea lamprey, Petromyzon marinus, and the brook lamprey, Lampetra lamottenii, at various phases of the life cycle. Gen Comp Endocrinol. 1983;49(2):187–194. doi: 10.1016/0016-6480(83)90135-1. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Pijak DS, Selzer ME. Structure of reticulospinal axon growth cones and their cellular environment during regeneration in the lamprey spinal cord. J Comp Neurol. 1994;344(4):559–580. doi: 10.1002/cne.903440406. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. Preferential regeneration of spinal axons through the scar in hemisected lamprey spinal cord. J Comp Neurol. 1991;313(4):669–679. doi: 10.1002/cne.903130410. [DOI] [PubMed] [Google Scholar]
- Mackler SA, Selzer ME. Specificity of synaptic regeneration in the spinal cord of the larval sea lamprey. J Physiol. 1987;388:183–198. doi: 10.1113/jphysiol.1987.sp016609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler SA, Yin HS, Selzer ME. Determinants of directional specificity in the regeneration of lamprey spinal axons. J Neurosci. 1986;6(6):1814–1821. doi: 10.1523/JNEUROSCI.06-06-01814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magavi SS, Macklis JD. Manipulation of neural precursors in situ: induction of neurogenesis in the neocortex of adult mice. Neuropsychopharmacology. 2001;25(6):816–835. doi: 10.1016/S0893-133X(01)00357-8. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37(5):751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Manzon RG, Youson JH. Temperature and KClO(4)-induced metamorphosis in the sea lamprey (Petromyzon marinus) Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124(3):253–257. doi: 10.1016/s0742-8413(99)00072-9. [DOI] [PubMed] [Google Scholar]
- Marón K. Regeneration capacity of the spinal cord in Lampetra fluviatilis larvae. Folia Biol. 1959;7:179–189. [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25(2):143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Melendez-Ferro M, Perez-Costas E, Villar-Cheda B, Abalo XM, Rodriguez-Munoz R, Rodicio MC, Anadon R. Ontogeny of gamma-aminobutyric acid-immunoreactive neuronal populations in the forebrain and midbrain of the sea lamprey. J Comp Neurol. 2002;446(4):360–376. doi: 10.1002/cne.10209. [DOI] [PubMed] [Google Scholar]
- Merrick SE, Pleasure SJ, Lurie DI, Pijak DS, Selzer ME, Lee VM. Glial cells of the lamprey nervous system contain keratin-like proteins. J Comp Neurol. 1995;355(2):199–210. doi: 10.1002/cne.903550204. [DOI] [PubMed] [Google Scholar]
- Michel ME, Reier PJ. Axonal-ependymal associations during early regeneration of the transected spinal cord in Xenopus laevis tadpoles. J Neurocytol. 1979;8(5):529–548. doi: 10.1007/BF01208508. [DOI] [PubMed] [Google Scholar]
- Munoz FJ, Galvan A, Lerma M, De la Fuente M. Seasonal changes in peripheral blood leukocyte functions of the turtle Mauremys caspica and their relationship with corticosterone, 17-beta-estradiol and testosterone serum levels. Vet Immunol Immunopathol. 2000;77(1-2):27–42. doi: 10.1016/s0165-2427(00)00228-2. [DOI] [PubMed] [Google Scholar]
- Namiki J, Tator CH. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J Neuropathol Exp Neurol. 1999;58(5):489–498. doi: 10.1097/00005072-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409(6821):714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Nordlander RH, Singer M. The role of ependyma in regeneration of the spinal cord in the urodele amphibian tail. J Comp Neurol. 1978;180(2):349–374. doi: 10.1002/cne.901800211. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102(2):369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Selzer ME, Lee VM. Lamprey neurofilaments combine in one subunit the features of each mammalian NF triplet protein but are highly phosphorylated only in large axons. J Neurosci. 1989;9(2):698–709. doi: 10.1523/JNEUROSCI.09-02-00698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter IC, Hillard RW, Bird DJ. The Biology of Lampreys. Academic Press; New York: 1982. Stages in Metamorphosis In: Hardisty MW, Potter IC, Editors; pp. 137–164. editor. [Google Scholar]
- Potter IC, Wright GM, Youson JH. Metamorphosis in the anadromous sea lamprey, Petromyzon marinus L. Can J Zool. 1978;56(4):561–570. doi: 10.1139/z78-080. [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Nacher J, Molowny A, Ponsoda X, Lopez-Garcia C. PSA-NCAM immunocytochemistry in the cerebral cortex and other telencephalic areas of the lizard Podarcis hispanica: differential expression during medial cortex neuronal regeneration. J Comp Neurol. 2002;453(2):145–156. doi: 10.1002/cne.10390. [DOI] [PubMed] [Google Scholar]
- Rodicio MC, Villar-Cervino V, Barreiro-Iglesias A, Anadon R. Colocalization of dopamine and GABA in spinal cord neurones in the sea lamprey. Brain Res Bull. 2008;76(1-2):45–49. doi: 10.1016/j.brainresbull.2007.10.062. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Regeneration of Muller and Mauthner axons after spinal transection in larval lampreys. J Comp Neurol. 1976;168(4):545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Neurobiology of lampreys. Physiol Rev. 1979;59(4):1007–1077. doi: 10.1152/physrev.1979.59.4.1007. [DOI] [PubMed] [Google Scholar]
- Schramm BG, Casares M, Lance VA. Steroid levels and reproductive cycle of the Galapagos tortoise, Geochelone nigra, living under seminatural conditions on Santa Cruz Island (Galapagos) Gen Comp Endocrinol. 1999;114(1):108–120. doi: 10.1006/gcen.1998.7240. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76(2):319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Selzer ME. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol. 1978;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Zhang G, Selzer ME. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J Comp Neurol. 2008;510(3):269–282. doi: 10.1002/cne.21789. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20(23):8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Fagerstedt P, Ottersen OP, Storm-Mathiesen J, Grillner S, Brodin L. Immunocytochemical localization of glycine in the lamprey spinal cord with reference to GABAergic and glutamatergic synapses: a light and electron microscopic study. Acta Biol Hung. 1996;47(1-4):393–410. [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85(6):2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Sueiro C, Carrera I, Molist P, Rodriguez-Moldes I, Anadon R. Distribution and development of glutamic acid decarboxylase immunoreactivity in the spinal cord of the dogfish Scyliorhinus canicula (elasmobranchs) J Comp Neurol. 2004;478(2):189–206. doi: 10.1002/cne.20285. [DOI] [PubMed] [Google Scholar]
- Swain GP, Jacobs AJ, Frei E, Selzer ME. A method for in situ hybridization in wholemounted lamprey brain: neurofilament expression in larvae and adults. Exp Neurol. 1994;126(2):256–269. doi: 10.1006/exnr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell. 1991;67(2):325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- Takeda A, Nakano M, Goris RC, Funakoshi K. Adult neurogenesis with 5-HT expression in lesioned goldfish spinal cord. Neuroscience. 2008;151(4):1132–1141. doi: 10.1016/j.neuroscience.2007.10.059. [DOI] [PubMed] [Google Scholar]
- Tretjakoff D. Das nervensystem von Ammocoetes II. Gehirn Arch Mikrosk Anat Entwicklungsmech. 1909;74:636–779. [Google Scholar]
- Vidal Pizarro I, Swain GP, Selzer ME. Cell proliferation in the lamprey central nervous system. J Comp Neurol. 2004;469(2):298–310. doi: 10.1002/cne.11013. [DOI] [PubMed] [Google Scholar]
- Vigh B, Manzano e Silva MJ, Frank CL, Vincze C, Czirok SJ, Szabo A, Lukats A, Szel A. The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histol Histopathol. 2004;19(2):607–628. doi: 10.14670/HH-19.607. [DOI] [PubMed] [Google Scholar]
- Wada M, Shimizu T, Kobayashi S, Yatani A, Sandaiji Y, Ishikawa T, Takemure E. Behavioral and hormonal basis of polygynous breeding in male bush warblers (Cettia diphone) Gen Comp Endocrinol. 1999;116(3):422–432. doi: 10.1006/gcen.1999.7381. [DOI] [PubMed] [Google Scholar]
- Wright GM, Youson JH. Serum thyroxine concentrations in larval and metamorphosing anadromous sea lamprey, Petromyzon marinus L. J Exp Zool. 1977;(1):27–32. doi: 10.1002/jez.1402020104. [DOI] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32(8):1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu P, McKay HM, Bernot T, Keirstead H, Steward O, Gage FH, Edgerton VR, Tuszynski MH. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci. 2006;26(8):2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HS, Mackler SA, Selzer ME. Directional specificity in the regeneration of lamprey spinal axons. Science. 1984;224(4651):894–896. doi: 10.1126/science.6719120. [DOI] [PubMed] [Google Scholar]
- Yin HS, Selzer ME. Axonal regeneration in lamprey spinal cord. J Neurosci. 1983;3(6):1135–1144. doi: 10.1523/JNEUROSCI.03-06-01135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youson JH, Plisetskaya EM, Leatherland JF. Concentrations of insulin and thyroid hormones in the serum of landlocked sea lampreys (Petromyzon marinus) of three larval year classes, in larvae exposed to two temperature regimes, and in individuals during and after metamorphosis. Gen Comp Endocrinol. 1994;94(3):294–304. doi: 10.1006/gcen.1994.1086. [DOI] [PubMed] [Google Scholar]
- Youson JH, Sower SA. Theory on the evolutionary history of lamprey metamorphosis: role of reproductive and thyroid axes. Comp Biochem Physiol B Biochem Mol Biol. 2001;129(2-3):337–345. doi: 10.1016/s1096-4959(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Zerani M, Gobbetti A. Corticosterone during the annual reproductive cycle and in sexual behavior in the crested newt, Triturus carnifex. Horm Behav. 1993;27(1):29–37. doi: 10.1006/hbeh.1993.1003. [DOI] [PubMed] [Google Scholar]
- Zhang F, Clarke JD, Ferretti P. FGF-2 Up-regulation and proliferation of neural progenitors in the regenerating amphibian spinal cord in vivo. Dev Biol. 2000;225(2):381–391. doi: 10.1006/dbio.2000.9843. [DOI] [PubMed] [Google Scholar]
- Zhang F, Ferretti P, Clarke JD. Recruitment of postmitotic neurons into the regenerating spinal cord of urodeles. Dev Dyn. 2003;226(2):341–348. doi: 10.1002/dvdy.10230. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008;4(1):19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]