Abstract
Nanotechnology is an innovative approach that has potential applications in nutraceutical research. Phytochemicals have promising potential for maintaining and promoting health, as well as preventing and potentially treating some diseases. However, the generally low solubility, stability, bioavailability and target specificity, together with the side-effects seen when used at high levels, have limited their application. Indeed, nanoparticles can increase solubility and stability of phytochemicals, enhance their absorption, protect them from premature degradation in the body, and prolong their circulation time. Moreover, these nanoparticles exhibit high differential uptake efficiency in the target cells (or tissue) over normal cells (or tissue)through preventing them from prematurely interacting with the biological environment, enhanced permeation and retention effect in disease tissues, and improving their cellular uptake, resulting in decreased toxicity, In this review we outline the commonly used biocompatible and biodegradable nanoparticles including liposomes, emulsions, solid lipid nanoparticles, nanostructured lipid carriers, micelles and poly (lactic-co-glycolic acid) (PLGA) nanoparticles. We then summarize studies that have used these nanoparticles as carriers for EGCG, quercetin, resveratrol and curcuminadministration to enhance their aqueous solubility, stability, bioavailability, target specificity, and bioactivities.
Keywords: Nanotechnology, Phytochemicals, Nanoparticles, Bioactivities, Biocompatible and biodegradable
1. Introduction
Nanotechnology is the study of the control of matter generally in the size range of 100 nm or smaller (1). As a comparison, an H atom has a size of 0.1 nm in diameter, a lysosome is between 200 to 500 nm, an E. Coli bacterium is about 2 µm in length, and most of eukaryotic cells have a size between 8 and 30 µmin diameter or larger [1]. The size of proteins is in a range between 3 and 90 nm, therefore, many enzymes, signaling molecules and receptors are in the nanoscalerange [1]. Since most of the biological processes occur at the nanoscale, nanoparticulate technology has a promising future in developing novel preventive, diagnostic, and therapeutic agents [2]. Such an application, often called the nanomedicine, has recently gained tremendous attention in pharmaceutical sciences[3]. In contrast, the application of nanotechnology in nutraceutics is far behind. Many nutrients, phytochemicals, and other natural compounds can be loaded into biocompatible and biodegradable nanoparticles, which will improve their aqueous solubility, stability, bioavailability, circulation time and target specificity, i.e., more nanoparticles enter disease tissues, due to leaky vasculature, but less to normal tissues[4].
2. Biocompatible and biodegradable nanoparticles
The common biocompatible and biodegradable nanoparticles include nanoliposomes, nanoemulsions, lipid nanocarries, micelles and poly(lactic-co-glycolic acid) (PLGA) nanoparticles.
2.1. Liposomes
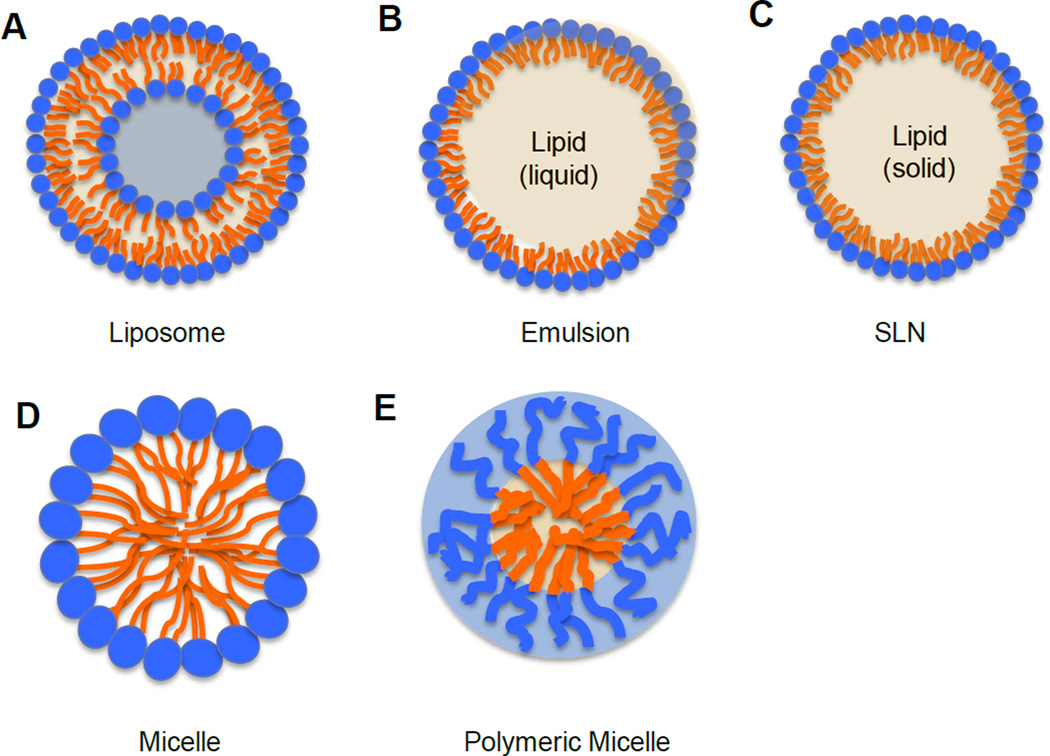
Liposomes have lipid bilayed membrane structures composed of phospholipids, which have hydrophilic heads and hydrophobic fatty acid tails(Figure 1A). Initially, they were used to study biological membranes in the mid-1960s[5–7]. Since then, their application has been extended to a variety of areas such as in drug delivery, cosmetic formulations, diagnostic agents, and food industry[6,8–10]. Some liposome-based drugs have been approved by Food and Drug Administration (FDA) and they are available in the market for treating different diseases[11]. Due to its biphasic character, liposomes can serve as carriers for both hydrophilic(in the central aqueous compartment) and hydrophobic (in lipid bilayers)compounds[8].
Figure 1.
Schematic structure of nanoparticles.
The term nanoliposome has been introduced recently to exclusively refer to nanometricsize of liposomes[12]. Although, in a broad sense, liposomes and nanoliposomes have the same chemical, structural and thermodynamic properties, the smaller size of nanoliposomes could produce larger interfacial area of encapsulated compounds with biological tissues and thus provide higher potential to increase the bioavailability of encapsulated compounds[12]. Especially for solid tumor treatment, nanoliposomes can accumulate more in tumors because of the enhanced permeation and retention (EPR) effect[12,13]. Higher energy input is required to produce nanoliposomes in the aqueous solution[9]. The commonly used methods for nanoliposome synthesis include sonication, extrusion, freeze-thawing, ether injection, and microfluidization. Sonication and extrusion are widely used in the laboratory scale[9,14]. High power, a long period and small pore size of the extruder filtration can generate small size of nanoliposomes. Microfluidization method is a commonly used technique for industrial manufacturers, which involves high pressure and high force technologies using a device called a microfluidizer to produce a flow stream passing through a fine orifice in order to reduce particle sizes of liposomes[9,14]. The notable advantages of this method are the adjustable size, high reproducibility for large scale of nanoliposome preparation, and noexposure to toxic organic solvent [14].
Nanoliposomes can be administeredparenterally, orally, topically, or nasally[12,15,16]. Nanoliposomesin the circulatory system are recognized as foreign particles and are rapidly cleared by the reticuloendothelial system (RES)[17]. Additionally, electrostatic, hydrophobic, and van der Waals forces can disintegrate nanoliposomes[18,19]. Therefore, steric stabilization is required and can be achieved by coating the nanoliposomes with inert polymers[20,21]. The polymer coating reduces adsorption of opsonins and avoids rapid RES clearance[20]. Poly(ethylene glycol) (PEG)or poloxamer can form a sterically stabilized corona on nanoliposomes[17]. This “STEALTH” technology increases circulation time of nanoliposomes[20]. In 1995, the FDA approved the first liposomal drug, a PEG-lated liposomal formulation of doxorubicin (Doxil in the U.S. and Caelyx outside the U.S.), for the treatment of Kaposi’s sarcoma[11]. Doxil is a liquid suspension of 80-100nm sterically stabilized nanoliposomes containing doxorubicin HCl at 2mg/ml[11]. PEG-lated liposomes significantly decrease doxorubicin’scardiotoxicity and increase the circulation half-life of doxorubicin from several minutes to more than 20hours[22,23]. Due to the success of liposomal doxorubicin, many liposomal formulations have been developed and are currently under test in clinical trials.
2.2. Emulsions
An emulsion is a mixture composed of two immiscible liquids. When oil is dispersed it will form into droplets through the aqueous phase; this is referred to as oil-in-water (O/W) emulsions (Figure 1B). On the contrary an aqueous solution dispersed in oil phase is referred to as water-in-oil (W/O) emulsions[24]. In order to disperse two immiscible liquids and to stabilize the emulsion structure, a surfactant or emulsifier is required, which has the amphiphilic structure with one fragment being hydrophilic and the other one being hydrophobic[25,26]. Emulsifiers can reduce the interfacial tension, create a film over one phase to repel the other phase, maintain and stabilize the emulsion structure, and increase the viscosity of the medium. Most of emulsions are of the O/W type, especially those designed for parenteral or oral administration[26]. In 1972, FDA approved the first intravenous fat emulsion, Intralipid®, which was composed of egg phospholipids, soy bean oil, and glycerin. Intralipid® is used to deliver essential fatty acids through intravenous injection for the patients who are unable to absorb those nutrients through diet[27]. The success of clinical application of this emulsion has paved the road for encapsulating and dissolving hydrophobic compounds into the internal oil core of emulsions for treating other diseases and disorders[28]. Several commercially available products, such as diazepam (Diazemuls®), propofol (Diprivan®), vitamin A,D,E,K (Vitalipid®), are developed using the O/W emulsion technique. Emulsion can be used to deliver many bioactive lipids and hydrophobic components including omega-3 fatty acids, carotenoids, phytosterols, flavonoids and other phytochemicals[26]. Nanoemulsions, having a size less than 100 nm in diameter, require more surfactants or co-surfactants and high energy input to lower the surface tension in order to be small and thermodynamically stable[29]. Sonication and homogenization are common methods for making nanoemulsions in the laboratory scale[30]. High-pressure homogenization and microfluidization can be used to produce nanoemulsions on a large scale [31].
Oral route is the easiest and most convenient route for the chronic delivery of preventive and therapeutic nutrients and dietary supplements, however, many phytochemicals have extremely low levels of oral bioavailability [32–35]. Novel self-emulsifying drug delivery systems (SEDDS) have received considerable attention due to their capability of improving oral absorption of highly hydrophobic compounds [36,37]. SEDDS formulations are isotropic mixture of oils, surfactants, nutrients (or drugs), usually with one or more of co-surfactants or co-emulsifiers [32,36]. When oral administration of SEDDS is in solid, liquid, or semi-liquid form, the mixture is dispersed into gastrointestinal fluids, yielding fine O/W emulsions containing hydrophobic compounds upon gentle agitation in gastrointestinal tract [36]. The SEDDS have small size and large surface areas, and thus enhance aqueous solubility of hydrophobic compounds, which in turn contributes to improved oral bioavailability [32].
2.3. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs)
SLNs have a similar structure of the O/W nanoemulsion including a hydrophilic shell and a hydrophobic lipid core, which is solid at room temperature(Figure 1C)[38]. SLNs were developed in the early 1990s as an alternative novel carrier system to traditional nanocarriers such as polymeric nanoparticles, nanoemulsions, and nanoliposomes[39]. The hydrophobic compounds can be encapsulated into the solid lipid core, resulting in an increased stability, reduced degradation, sustained and prolonged release. This process can minimize toxicity, and improve target specificity of the compounds to disease tissues[40]. To achieve and to maintain a solid lipid nanocarrier structure, lipids in SLNs have relatively high melting temperature. SLNs are usually composed of solid lipids, surfactants, water, with or without co-surfactants[41]. The commonly used lipids include fatty acids(stearic acid), triglycerides (e.g. tristearine), waxes (e.g. cetylpalmitate), or a mixture of the above lipids[41,42]. Surfactants are used to stabilize the lipid dispersion. The typical surfactants used include bile salts, phospholipids, sorbitan esters, fatty acid ethoxylates, or a mixture of these components [41]. SLNs are favored because of their high biocompatibility, avoidance of organic solvent, excellent reproducibility even using different preparation methods, easily scaled-up synthesis processes [38]. Although SLNs are one of the most useful lipid based nanocarriers in nutraceutical and pharmaceutical research, some limitations exist, such as low compound loading capacity and leakage during storage[42]. These limitations are overcome by the recently developed NLCs, an improved, new generation of lipid nanocarriers[42]. NLCs are favorable because they are small in size, stable, biocompatible and biodegradable, and have high loading capacity [38]. In order to enhance the loading capacity, more complex lipid mixtures are employed in the hydrophobic core. For example, a mixture of mono-, di-, or triglycerides with fatty acids of different chain length forms less perfect crystals, which can accommodate more hydrophobic drugs, nutrients, phytochemicals, or other compounds to avoid expulsion[43,44]. NLCs represent a new delivery system for poorly soluble compounds, such as phytochemicals [45,46].
Hydrophobicphy to chemicals, such as quercetin and resveratrol, can be easily encapsulated into the lipid core and are stable in the lipid core [38,47]. The common methods for making SLNs and NLCs include high pressure homogenization, cold homogenization, hot homogenization/ultra sonication, phase inversion and solvent evaporation/emulsification[44]. The most cost-effective and relatively simple way for the larger scale production is a high pressure homogenization method[48].
Emulsification/ultra sonication technique is a common laboratory approach for making both SLNs and NLCs. To begin this procedure the solid lipid (for making SLNs) or solid-liquid lipid mixture(for making NLCs) is melted, and the hydrophobic nutrients or phytochemicals are dissolved in melted lipid phase. Next, the melted lipid and hydrophobic compound mixture is dispersed in a hot aqueous surfactant/stabilizer solution, and they are stirred at a high speed at the equivalent melting temperature. Lastly, obtained pre-emulsion is homogenized or sonicated yielding a hot O/W nanoemulsion. After cooling, SLNs or NLCs are formed[44]. SLNs and NLCs have been extensively investigated for application in pharmaceutics, cosmetics, food, and agricultural products[38,49]. They can be administrated through oral, parenteral, dermal, rectal, nasal, ocular and pulmonary routes[50]. Oral administration is the most preferable route for the application of SLNs and NLCs [50]. Both SLNs and NLCs are widely used to improve bioavailability and to achieve sustained release for hydrophobic nutrients and phytochemicals. The encapsulated hydrophobic compounds are absorbed into lacteals after oral administration of SLNs or NLCs[51]. Then, these compounds are transported from intestinal lymphatic vessels to the thoracic lymph duct, and eventually into the system circulation at the junction of the jugular and left subclavian vein[52]. The transport can avoid the first pass effect, which can metabolize the encapsulated compound in liver before it release into the circulation system, and therefore enhances bioavailability.
2.4. Micelles
Micelles belong to a group of amphiphilic colloids, which are composed of amphiphilic monomers including phospholipids and some polymers. Micelles usually have a size between 20 and 80 nm in diameter. The traditional micelles are lipid based[53]. When amphiphilic phospholipid concentrations reach the critical micelle concentration (CMC) and temperatures reach the critical micellization temperature, micelles are formed [53,54]. Hydrophilic heads of phospholipids form the shell of micelles, while fatty acid tails of phospholipids form a hydrophobic core, which can accommodate phytochemicals and other hydrophobic compounds (Figure 1D). More recently, polymeric micelles are of great interest to the investigators. Polymeric micelles are made of block-copolymers that consist of hydrophobic and hydrophilic monomer units [55]. Two monomers with different hydrophobicity can be conjugated and form a core-shell micelle structure, where the hydrophilic and hydrophobic blocks form the micelle shell and core, respectively (Figure 1E). The commonly used hydrophilic monomer is polyethylene glycol (PEG), and the widely used core-forming blocks are poly(propyleneoxide), poly(caprolactone), poly(D, L-lactic acid) and poly(L-aspartic acid)[55,56]. Micelles have been used as various hydrophobic compound carriers for oral, nasal, topical, parenteral and ocular application [55]. Micelles can increase aqueous solubility of hydrophobic compounds, extend their blood circulation time, increase target specificity to disease tissues through enhanced EPR effect, and lower toxicity of compounds to normal tissues[57,58].
2.5. PLGA nanoparticles
PLGA is a widely used biocompatible and biodegradable polymers[59]. Due to minimal systemic toxicity, PLGA has been approved by FDA for developing therapeutic device[59]. PLGA can be hydrolyzed in the body and yield biodegradable lactic acid and glycolic acid. PLGA nanoparticles have been used as carriers for many phytochemicals such as quercetin and curcumin[60–62]. Those phytochemical can either entrapped inside or adsorbed on the surface of PLGA nanoparticle [63]. The solvent evaporation, the emulsification-diffusion, and the nanoprecipation methods can be used to synthesize PLGA nanoparticles [59,64,65].
2.6. Characteristics of nanoparticles
Characteristics of nanoparticles determine their functions. The major characteristics include, as follows: 1) nanoparticle size, which can be measured using a dynamic light scattering method, or a transmission electron microscope (TEM) or a scanning electron microscope (SEM); 2) Zeta potential, which indicates the surface charge of nanoparticles and can be measured using a Zeta potential analyzer; 3) polydispersity index, which indicates nanoparticle size distribution and can be measured by a dynamic light scattering method; 4) physical and chemical stability, which indicates the stability of nanoparticles and loaded compounds, respectively;5)encapsulation efficiency, which is determined as mass of encapsulated compound divided by mass of total compound ×100%; 6) loading capacity, which is determined as mass of encapsulated compound divided by mass of nanoparticles ×100%.
While in most cases, smaller size is preferable for enhancing absorption of encapsulated compounds into target tissues, it is not always true because the small nanoparticles also easily move in and out of the target tissues[66]. Additionally, smaller size requires a larger amount of surfactants, which may introduce toxicity, and may also hinder drug absorption under certain circumstances. However, the nanoscale is not well defined as many particles have a size larger than 100 nm in diameter and are still referred to as nanoparticles. Nanoparticles can be modified to improve their stability and functions. For example, an uptake enhancer can be coated on the surface of nanoparticles to increase uptake and bioavailability of loaded compounds [67,68]. Poly(ethylene glycerol) (PEG) can be incorporated on the surface of nanoparticles to maintain their integrity and stability [69–71] and protect them from degradation by enzymes. PEG can also prolong the circulation of nanoparticles by stabilizing them against opsonization[2,3]. Target ligands can be incorporated on the surface of nanoparticles to increase the target specificity. Target ligands including antibodies, small peptides, and receptor binding compounds can be incorporated on the surface of nanoparticles [23,72]. Increased target specificity can improve bioactivities of encapsulated compounds, decrease their side-effects, and reduce administration dose and frequency [23,72].
3. Improvement of characteristics and bioactivities of phytochemicals by nanotechnology
Many natural compounds, especially phytochemicals, may have preventive and therapeutic potential for diseases. However, most of these compounds have low levels of solubility, stability, bioavailability, and target specificity in the body, which makes it unrealistic for these compounds to be present at their effective levels in the target tissues. This is particularly true for (−)-epigallocatechingallate (EGCG) found in green tea, resveratrol found in grapes, curcumin found in turmeric, and quercetin found in red onions, that are valuable for prevention and treatment of many diseases. Therefore, this represents an excellent opportunity for introducing nanoparticle technology to help resolve this issue.
3.1. (−)-Epigallocatechingallate (EGCG)
Green tea (Camellia sinensis) is a popular beverage. Green tea is prepared by drying the leaves under hot steam and air to inactivate the polyphenol oxidases, which prevents fermentation and gives green tea its distinctive color compared to black and oolong tea[73,74]. Green teacatechins constitute about 8–15% of total dry tea weight [74]. The most abundant, and also most bioactive, catechin is EGCG, which accounts for25–55% of total catechins. One cup of green tea made using a 2.5 g tea bag contains about 100 mg of EGCG [75]. Consumption of EGCG has been reported to have several health benefits including antioxidant, anti-inflammatory, antitumorigenic, and antiangiogenic properties [76].
EGCG is stable in an acidic solution, but is rapidly degraded in body fluids (at pH 7.4) [77]. Depending on the type of nanoparticles, incorporated EGCG can be partially or completely sequestered in the nanoparticles, resulting in high stability. We loaded EGCG into liposomes and chitosan-coated liposomes, with size less than 100 nm in diameter[78]. Nanoliposomes dramatically enhanced the EGCG stability in both 1 × phosphate buffered saline (PBS) and Eagle’s minimum essential (EME) cell culture medium [78]. At 4°C, 0.5 mM of free EGCG in EME medium was completely degraded after eight days. However, EGCG loaded into liposomes and chitosan-coated liposomes was degraded 62% and 38%, respectively, at the same conditions and initial concentrations[78]. After 1-hour incubation at 37°C, the EGCG degradation rates of 0.5 mM of free EGCG, EGCG loaded liposomes and chitosan-coated EGCG loaded liposomes in EME medium were 100%, 46% and 32%, respectively[78]. Barras A et al. demonstrated that free EGCG and EGCG loaded SLNs in water exhibited 100% degradation within four hours and over four weeks, respectively [79]. In addition, free and nano-EGCG exhibit burst and sustained release properties, respectively [80,81]. Sustained release creates a steady EGCG release pattern resulting in prolonged EGCG availability after administration. The advantages of sustained release include reduction in administration frequency, doses and side effects, and improvement of compliance[78,82].
EGCG is not readily absorbed in humans and research animals [83–85]. Scientists have conducted pharmacokinetic and bioavailability studies of green tea catechins in rats [86]. The blood peak concentrations of green tea catechins appear at 2 to 4 hours after oral administration. The absolute bioavailability of EGCG after intra gastric administration of decaffeinated green tea is about 0.1% in rats [86]. Consistent with this result, the bioavailability of EGCG is 0.14% in men and women after drinking tea containing 400 mg catechins throughout the day [84]. The peak plasma EGCG concentration is around 0.15 µM after drinking 2 cups of green tea [85]. A majority of published cell culture studies have used EGCG at physiologically irrelevant concentrations in the range of 10 to 200 µM[87,88]. Since EGCG at lower, and physiological relevant (achievable by oral intake) concentrations has little or very limited effect, it is important to increase EGCG bioavailability, and the nanotechnology appears to be an appropriate approach to meet this need. In fact, studies along this line have shown that nanoencapsulation significantly increases EGCG stability and improves its sustained release, which may partially contribute to the increased cellular uptake of EGCG [80,89]. Chitosan nanoparticles can significantly enhance EGCG bioavailability [90,91]. Since chitosan, a biocompatible polysaccharide, confers a positive charge to the surface of nanoparticles, it has been used as an absorption enhancer [92,93]. Hu et al. encapsulated EGCG into food grade peptide/chitosan nanoparticles, and they found the apparent permeation rate across the Caco-2 monolayers was increased more than two-fold by nanoencapsulation[90]. Dube A et al. compared the intestinal absorption of free EGCG and nano-EGCG (chitosan nanoparticles) using excised mouse jejunum[91]. They added 50 µM of freeornano-EGCG in the mucosal chambers and collected the transported EGCG in the serosal chambers over a three-hour period, and found that nano-EGCG had about two-fold higher accumulative transported amounts than free EGCG[91]. Enhanced EGCG stability and trans cellular transport process by nanoparticles, but not paracellulartransport process, may partially contribute to the enhanced intestinal absorption[91]. After reaching the apical membrane of intestinal epithelial cells, most nanoparticles cross enterocytes via transcellular transport[91]. Nanoparticles are internalized into enterocytes and then transported across enterocytes[91]. Finally, nanoparticles are moved out of the basolateral membrane through exocytosis to enter the bloodstream or lymphatic vessels. Particles less than 500 nm in diameter are internalized through both clathrin- and caveolae-mediated endocytosis [94]. The process of endocytosis can be enhanced by modifying nanoparticles, e.g., by adding PEG or positive charges on the surface of nanoparticles[95]. Moreover, coating nanoparticles with cationic chitosan or ions protects them from endolysosomal degradation in enterocytes[96]. Therefore, chitosan nanoparticles are capable of enhancing EGCG oral bioavailability. Dube A et al. further measured the plasma concentrations of EGCG in mice after oral administration of either free EGCG or EGCG encapsulated chitosan nanoparticles[89]. Compared to free EGCG, EGCG loaded chitosan nanoparticles increased plasma EGCG concentrations by a factor of 1.5 [89]. Consistently, EGCG nanolipidic particles increased its oral bioavailability by more than two-fold compared to free EGCG in rats [97] (Table 1).
Table 1.
The characteristics, functions and application of EGCG nanoparticles
| NP type | NP characteristics |
Experiment model/dose/route | Functions | Application | Year reference |
|---|---|---|---|---|---|
| Chitosan- caseinophos phopeptide NPs |
SZ: around 143 nm ZP: 31 mV EE: 70.5– 81.7% |
HepG2 cells; dose: 0.125 mg/mL; duration: 2 hours. |
↑ Sustained release ↑ Cellular uptake ↑ Antioxidant activity |
Antioxidant | 2013 [80] |
| Chitosan- caseinophos phopeptide NPs |
SZ: around 150 nm ZP: 32 mV |
Caco-2 cells. | ↑EGCG intestinal permeability and absorption through Caco-2 cells |
Enhance bioavailability |
2012 [90] |
| Chitosan NPs |
SZ: around 440 nm ZP: 25 mV |
Male Swiss Outbred mice; dose: 0.76 mg/kg body weight of free EGCG or nano-EGCG; route: oral gavage; blood collection at minute 30, 60, 90, 150, 210, 300 and 360. |
↑EGCG stability ↑EGCG bioavailability |
Enhance bioavailability |
2011 [89] |
| PLGA-PEG | SZ: around 80 nm EE: around 8% |
PSMA positive Prostate cancer (LNCaP) cells; dose: 30 µM of free EGCG or nano-EGCG; duration: 48 h and 72 hours. |
↑ Sustained release ↓ viability of LNCaP cells No effect on viability of normal cells |
Prostate Cancer | 2011 [81] |
| Gum arabic and maltodextrin NPs |
SZ: around 100 nm ZP: −12 mV EE: >80% |
Human prostate carcinoma Du145 cells; dose: 0–10 µM. |
Retain EGCG anticancer activity |
Prostate cancer | 2011 [156] |
| Chitosan NPs |
SZ: 440 nm ZP: 25 mV |
Excised jejunum from male, Swiss outbred mice. |
↑EGCG stability ↑EGCG intestinal absorption |
Enhance bioavailability |
2010 [91] |
| nanolipidic EGCG NPs |
SZ: 30 to 80 nm. |
Male Sprague Dawley rats; dose: 100 mg /kg body weight; route: oral gavage; blood collection at minute 0, 5, 10, 30, 60, 120, 240, and 480. |
↑EGCG bioavailability by more than 2-fold. ↓Brain beta amyloid plaque formation |
Alzheimer’s disease (AD) |
2010 [97] |
| NLCs | SZ: 30 to 260nm EE: 95% |
N/A | ↑EGCG stability | N/A | 2009 [79] |
| Polylactic acid- polyethylene glycol (PLA- PEG) NPs |
SZ: 285nm ZP: −7.92mV |
Prostate cancer (PC3) cells; dose: 1- 80µM of free or nano-EGCG; duration: 24 and 48h. Tumor xenograft mice; dose: 1 mg and 100 µg of free or nano-EGCG, respectively; route: intraperitoneal injection three times per week. |
↑ Apoptosis ↓ Cell viability ↓ Colony formation ↓ Tumor size ↓ Angiogenesis Nano-EGCG exhibits > 10- fold dose advantage over |
Prostate cancer | 2009 [98] |
EE: Encapsulation efficiency; N/A: not applicable; NLC: nanostructured lipid carrier; NP: Nanoparticle; PEG: polyethyleneglycol; PLGA: poly (D,L-lactide-co-glycolide); PSMA: Prostate-specific membrane antigen; SZ: Size; ZP: Zeta-potential; ↑, increase; ↓, decrease.
Another advantage is that nanoencapsulation can increase EGCG bioactivities, in particular its antioxidant, antitumorigenic, and antiangiogenic properties. Hu B et al. reported that treating HepG2 cells with 26 ∼ 37 µM of nano-EGCG, resulted in a higher cellular antioxidant activity compared to free EGCG at the same concentrations [80]. Siddiqui IA et al. demonstrated that, compared to free EGCG, nano-EGCG (PLGA nanoparticles) exhibited more than ten-fold dose advantage in inducing apoptosis, decreasing viability, and inhibiting colony formation of prostate cancer cells[98]. The IC50 values of free and nano-EGCG are 43.6 and 3.74 µM, respectively. They also gave tumor xenograft mice either 100 µg of nano-EGCG or 1 mg of free EGCG three times per week through intra peritoneal injection and found that nano-EGCG, even at a dose ten-fold lower, significantly reduced prostate tumor size [98]. Moreover, when incorporating target ligands on the surface of nanoparticles, to specifically target an antigen on prostate cancer cells, the EGCG nanoparticles can reduce the viability of prostate cancer cells to a significantly larger degree compared to EGCG nanoparticles without target ligands [81] (Table 1).
3.2. Quercetin
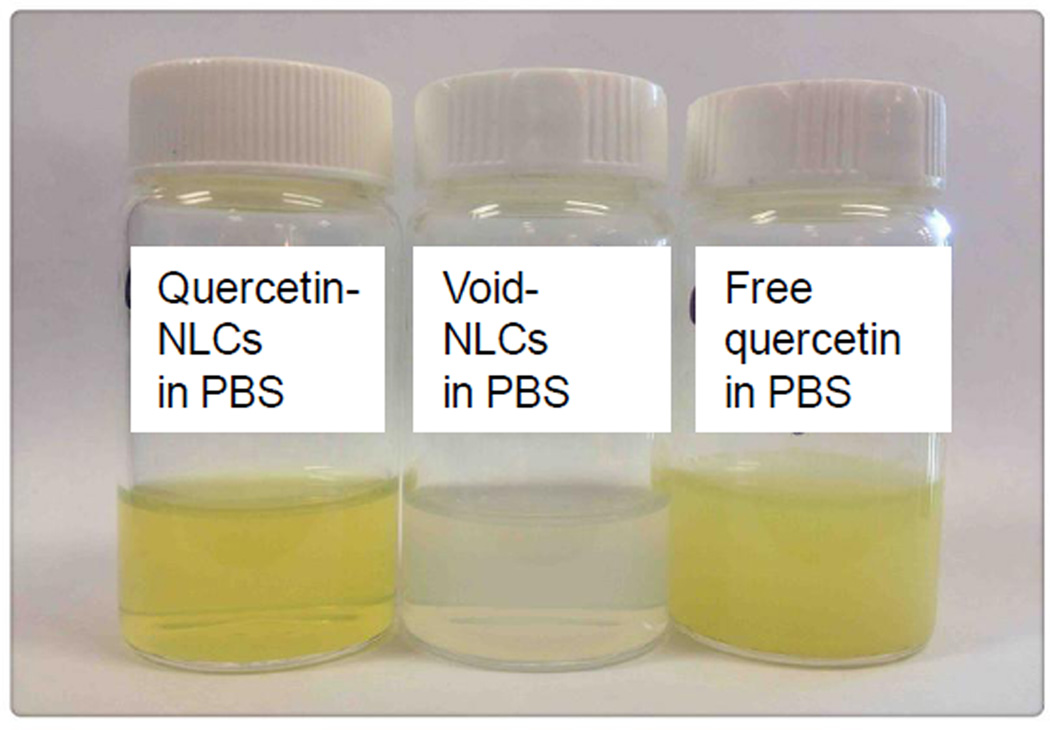
Quercetin (3,3’,4’,5’-7-pentahydroxy flavone) is a plant-derived flavonol and it is abundant in caper, berries, buckwheat, black and green tea leaves, apple, onion, broccoli and other leafy green vegetables[99]. Studies have demonstrated that quercetin has antioxidant, antiviral, anti-inflammatory and antitumorigenic properties[100–102]. Quercetin has low aqueous solubility and bioavailability and is quickly metabolized in the body, which may reduce its efficacy as an application in preventing or treating diseases [103]. Quercetin is a hydrophobic compound and its solubility in an aqueous solution varies from 0.00215 g/L at 25 °C to 0.665 g/L at 140 °C [104]. Quercetin has a high solubility in organic solvents such as ethanol, dimethylsulfoxide (DMSO), and dimethyl form amide (DMF). The solubility of quercetin is approximately 2 g/L in ethanol and 30 g/L in DMSO at 25 °C [104]. Using nanomicelles, the aqueous solubility of quercetin can increase by 110fold [105]. We have successfully synthesized quercetin encapsulated NLCs and found that the aqueous solubility of quercetin increased about 1000-fold (Figure 2). Additionally, a sustained release pattern was observed in quercetin loaded nanoparticles including poly(lactic acid) or PLGA nanoparticles [60,106,107], SLNs and NLCs [108,109].
Figure 2.
Visual observation of void NLCs, and 20 mg of quercetin encapsulated in NLCs and free quercetin dissolved in 2 mL of phosphate buffered saline(PBS).
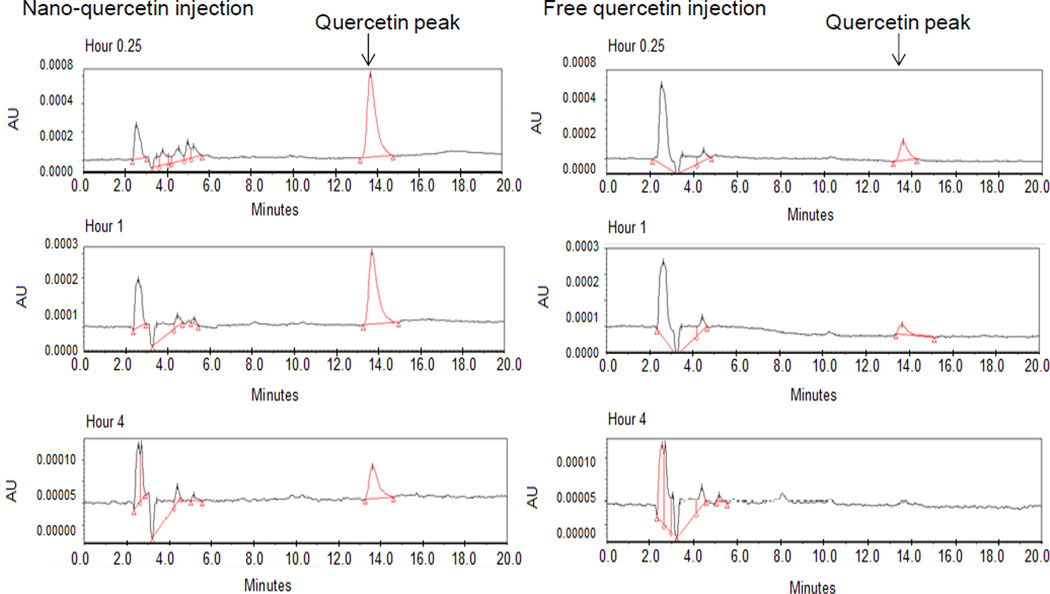
Consumption of quercetin rich food or supplements can increase the plasma quercetin concentrations up to low µM levels [33,110]. Quercetin in human bodies is quickly metabolized by enzymes in the liver as well as the other organs or tissues. Hong Y et al. found that at least 21 metabolites of quercetin are detected in human urine after ingestion of quercetin glycosides from onions[111]. Blood quercetin includes not only free quercetin (low concentrations), but also its conjugated forms(high concentrations) including glucuronide or sulfate forms of quercetin, and O-methylated forms, among others[112]. Encapsulating quercetin into biodegradable and biocompatible nanoparticles may help delay its metabolism and maintain free quercetin levels in blood and other tissues for a prolonged period. Li W et al. developed a SNEDDS of persimmon leaf extract rich in flavonoids, especially quercetin. Persimmon leaf extract tablets and persimmon leaf extract-loaded SNEDDS at 5.3 mg/kg body weight were given to two groups of beagle dogs through oral administration. Compared to the tablets, persimmon leaf extract-loaded SNEDDS increased the bioavailability (area under curve) of plasma quercetin by 1.5 fold [113]. Li H et al. made quercetin encapsulated SLNs, which exhibited sustained release. They gave male Wistar rats free or nanoencapsulated quercetin solution at a dose of 50 mg/ kg body weight and collected blood at different time points for up to 48 hours. They found that the quercetin encapsulated SLNs increased oral bioavailability by a factor of five compared to free quercetin. They also demonstrated that the absorption sites were primarily ileum and colon, but not stomach and duodenum [109]. Sprague-Dawley rats are widely used animal model for pharmacokinetic study. After intravenous (10 mg/ kg body weight) or oral (50 mg/ kg body weight) administration of free and nano-quercetin (quercetin-NLCs) to male Sprague-Dawley rats, we found that compared to free quercetin solution, quercetin-NLCs increased bioavailability (area under the curve) of plasma total quercetin concentrations by 2.8 and 2.0 fold after intravenous and oral administration, respectively (unpublished data). We also measured blood concentrations of unmodified (free) quercetin after intravenous administration. Quercetin-NLCs significantly increased the blood unmodified (free)quercetin concentrations and prolonged its circulation time (Figure 3).
Figure 3.
High performance liquid chromatography peaks of unmodifiedquercetin in blood isolated from male SD rats after single intravenous administration of 10 mg/kg body weight of free or nanoencapsulated quercetin in NLCs for 0.25 hours, 1 hour and 4 hours.
The increased solubility and bioavailability and improved sustained release by nanoencapsulation may elevate the bioactivities of quercetin. Some experimental results suggest that quercetin may have an anti-cancer potential[114,115]. However, there is no consistent clinical evidence to substantiate this proposed benefit[116,117]. One of the reasons may be that the tissue quercetin concentrations after consumption of quercetin may not be adequate enough to allow it to exert its effect to the fullest. Utilizing nanoparticles to increase the bioavailability and biopotency may open an area of research toward this direction. Tan BJ et al. synthesized quercetin loaded nanomicelles, and found that they were stable in gastric and intestinal fluids, and had no toxic effects on Caco-2 cells. Both free quercetin and quercetin loaded nanomicelles decreased the viability of A549 lung cancer cells in vitro, but 100 µM of free and nano-quercetin decreased the cell viability to 60% and 100%, respectively. Oral administration of 30 mg/kg of free and nano-quercetin three times per week at week 3, 4, 5 post tumor inoculation to A549 lung tumor xenograft mice decreased the tumor size 20% and 40%, respectively, but they did not change animal body weight [105]. Wang G et al. demonstrated that quercetin nanoliposomes decreased the viability of C6 glioma cells, and induced necrotic death of those cells [118]. Dhawan S et al. found that the intravenous administration of quercetin improved memory retention and increased brain antioxidant capacity in rats with aluminium-induced dementia, and quercetin encapsulated SLNs significantly improved those beneficial effects by more than two-fold [119]. Using quercetin liposomes to prevent Arsenic-induced acute liver toxicity, Ghosh D et al. injected either free or nano-quercetin at 2.71 mg/ kg body weight to rats via tail veinsone hour after oral administration of Arsenic salt. Compared to control, free and nano-quercetin decreased liver Arsenic concentrations by 20% and 40%, increased liver quercetin concentrations by 20 and 40 fold, enhanced antioxidant capacity by 1.2 and 1.6 fold, and decreased blood aspartate aminotransferase concentrations by10% and 33%, respectively [120]. Nano-quercetin(PLGA nanoparticles) increased anti-oxidant capacity and decreased inflammatory responses in the stomach, which might contribute to the high preventive efficacy of gastric ulcer by a factor of 20 in rats compared to free quercetin[61]. Quercetin nanoparticles have also been used for topical delivery and treating skin problems. Chen-Yu G et al. demonstrated that quercetin encapsulated NLCs resulted in a two-fold increase in quercetin concentrations in epidermis and dermis of Kunming mice [121]; they also showed that the quercetin encapsulated NLCs decreased inflammatory response in inflamed skin. Consistent with this, quercetin encapsulated NLCs are shown to improve quercetin skin concentrations by more than two-fold in an in vitro permeation study using human skin [108](Table 2). Together, these studies suggest that nanoencapsulation may increase quercetin aqueous solubility, improve its sustained release, prevent it from modification and metabolism, enhance its bioavailability and bioactivity, and lower its toxicity.
Table 2.
The characteristics, functions and application of quercetin nanoparticles
| NP type | NP characteristics |
Experiment model/dose/route | Functions | Application | Year reference |
|---|---|---|---|---|---|
| SLNs and NLCs |
SZ: around 282 nm ZP: −36.57 mV LC: 0.05% and 0.025% |
In vitro permeation study: full thickness human skin |
↑ Stability ↑ Sustained release ↑ Quercetin concentrations in skin |
Improve topical delivery |
2012 [108] |
| PLGA NPs | SZ: 41.3 nm ZP: −47.3 mV EE: 92% |
Measure biocompatibility in human fibroblasts (FY11 cells) |
↑ Stability ↑ Sustained release ↑ Biocompatibility |
Improve transdermal delivery |
2012 [60] |
| PLA NPs | SZ: 70– 143 nm ZP: −5.4 to −53.6 mV EE: 100 % LC: 13.91 % |
N/A | ↑ Sustained release | Enhance therapeutic efficacy |
2012 [106] |
| Lipid-core nanocapsules |
SZ: 212 nm ZP: −11 mV |
Evaluation of antioxidant capacity and toxicity in Yeast Cells |
↑ Photostability ↑ Antioxidant activity ↓ Cytotoxicity |
Antioxidant | 2012 [157] |
| NLCs | SZ: 215.2 nm ZP: −20.10 mV EE: 89.95 % LC: 3.05 % |
In vitro skin permeation studies: Franz diffusion cells: dose: 1mg/mL; duration: 1, 3, 6, 9 and 12 hours. In vivo permeation study male Kunming mice; dose: 1.0 mg/mL; route: topical application; duration: 3, 6, 9 and 12 hours. |
↑ Quercetin concentrations in epidermis and dermis ↑ Anti-oxidant and anti- inflammation |
Improve topical delivery |
2012 [121] |
| Nanomicelles | SZ: around 16 nm ZP: −14.8 mV |
Lung tumor mice; dose: 30 mg/kg body weight; route: oral gavage; duration: three times per week for |
↑ Stability ↓ Viability of cancer cells ↓ Tumor size |
Lung cancer | 2012 [105] |
| EE: ≥88.9% | three weeks. | ||||
| Nanoliposom Es |
SZ: 62.3 – 191.5 Nm |
C6 glioma cells; dose: 0, 50, 100, 200 and 400 µM of free quercetin or nanoe-quercetin for 12, 24, 36, and 48 hours. |
↓ Viability of C6 glioma cells ↑ Necrotic cell death |
Cancer | 2012 [118] |
| PLGA | SZ: 15 nm EE: 66% |
Male Sprague Dawley rats; dose: 2.5 and 50 mg/kg body weight |
↓ Inflammation ↓ Oxidative stress Prevent gastric ulcer Formation |
Gastric ulcer | 2012 [61] |
| SNEDDS | SZ: 34.85 nm ZP: −6.18 mV |
Beagle dogs; dose: 45 mg of free or SNEDDS flavonoids containing quercetion; route: oral administration. |
↑ Plasma quercetin concentrations |
Improve oral bioavailability |
2011 [113] |
| SLNs | SZ: < 200 nm ZP: 21.05 mV EE: 85.73% |
Male Wistar rats; dose: aluminium chloride (100 mg/kg) in combination with either free or nano-quercetin (equivalent to 4.41 mg/kg body weight of quercetin); route: through oral administration; duration: 8 weeks. |
↑ Brain anti-oxidant capacity ↑ Memory retention ↓ Aluminium-induced neurotoxicity |
Alzheimer’s disease |
2011 [119] |
| Liposomes and PLA NPs |
SZ: 100 to 200 nm |
Adult male Swiss Albino rats; dose: NaAsO2 (13 mg/kg body weight) through oral administration in combination with either free or nano-quercetin (2.7 mg/kg body weight) through intravenous injection. |
↑ Liver quercetin concentrations ↑ Liver anti-oxidant capacity ↓ Arsenic-induced liver fibrosis |
Reduce arsenic- induced acute liver toxicity |
2010 [120] |
| SLNs | SZ: 155.3 nm ZP: −32.2 mV EE: 91.1% LC: 13.2% |
Male Waster rats; dose: 50 mg/kg body weight; route: intragastric administration. |
↑ Sustained release ↑ Blood quercetin concentrations |
Improve bioavailability |
2009 [109] |
EE: encapsulation efficiency; LC: loading capacity; N/A: not applicable; NP: nanoparticle; NLC: nanostructured lipid carriers; PLA: poly (D,L-lactide); PLGA: poly (D,L-lactide-co-glycolide) ; SLN: Solid lipid nanoparticle; SNEDDS: self-nanoemulsifying drug delivery system; SZ: size; ZP: Zeta-potential; ↑, increase; ↓, decrease.
3.3. Resveratrol
Resveratrol (trans-3, 4’,-5-trihydroxystilebene) is a type of natural polyphenol abundant in the skin of red grapes and other fruits such as berries. Resveratrol possess two structural isomers: cis- and trans-resveratrol. Under UV exposure, tran-resveratrol is converted into cis-resveratrol [122]. Nanoencapsulation protects trans-resveratrol against light-exposure degradation and hence increases its stability [123,124]. Detoni CB et al. compared photostability of different trans-resveratrol incorporated nanoparticles including liposomes, SLNs, nanospheres, and polymeric lipid-core nanocapsules[124]. All tested nanoparticles increased photostability of trans-resveratrol and liposomes maintained trans-resveratrol concentrations for the longest time [124]. After applying the free or nanoencapsulatedtrans-resveratrol to the porcine skins and then exposed to UVA radiation, they found that nanoencapsulation resulted in a larger increase in the trans-resveratrol concentrations in epidermis and dermis compared to free trans-resveratrol solution [124]. Consistent with these findings, PLGA nanoparticles significantly increased the photostability of trans-resveratrol when exposed to UVA for two hours [123]. Since trans-resveratrol is more stable than cis-resveratrol and thus has been extensively used in the studies, it is the subject of this review and is referred as resveratrol in the rest of article[122].
The aqueous solubility of resveratrol is extremely low. Sigma-Aldrich company reports that resveratrol solubility in water is about 3 mg/100 mL. Resveratrol is much more soluble in organic solvents such as ethanol and dimethyl sulfoxide (DMSO), with solubility at 50 mg/mL and at least 16 mg/mL, respectively. Resveratrol can be encapsulated or incorporated in the lipid compartment of nanoparticles, especially SLNs and NLCs, resulting in enhanced aqueous solubility. We have successfully encapsulated resveratrol into the hydrophobic core of lipid nanocarriers. The resveratrol aqueous solubility was improved more than 100 times (unpublished data). Other nanoparticles including SLNs, NLCs and liposomes can also increase resveratrol aqueous solubility [124–126](Table 3).
Table 3.
The characteristics, functions and application of resveratrol nanoparticles
| NP type | NP characteristics | Experiment model/dose/route | Functions | Application | Year reference |
|---|---|---|---|---|---|
| SLNs and NLCs. |
SZ: 150–250 nm ZP: around −30 mV EE: about70% PI: about 0.2 |
In vitro release and stability study. | ↑Stability ↑ Sustained release |
Improve oral bioavailability |
2013 [126] |
| Poly(D,L- lactide-co -glycolide) NPs |
SZ: 135–580 nm EE: 18% − 24% ZP: around −20 mV or +20 mV |
In vitro release and stability study. | ↑Stability ↑ Sustained release |
Nanochemoprevent ion |
2012 [123] |
| Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) |
SZ: 110–280 nm ZP: around −14 mV EE: 73%–91% |
Normal Human Dermal Fibroblasts (NHDF) from juvenile foreskins and rat abdominal skin; dose: 10, 25, 50, 100, 250, and 500 µM of free or nano- resveratrol. . |
↓ Reactive oxygen species production ↑ Resveratrol concentrations in dermis |
Dermal applications. |
2012 [45] |
| Eudragit E100 (EE100, aminoalkyl methacrylate copolymers) and polyvinyl alcohol (PVA) NPs |
SZ: around 73.8 nm EE: 99.5% PI: around 0.16 |
Male Wistar rats were administrated orally with water, or void nanoparticles, or 20 mg/kg of free or nano-resveratrol for 3 days. Day 4, rats were given carbon tetrachloride (CCl4) through intraperitoneal injection to induce acute hepatotoxicity. Rats were sarcrificed at day 5. |
↓ Oxidative stress ↓ Inflammatory response ↑ Hepatoprotective effects |
Prevent chronic liver diseases |
2012 [132] |
| Lipid-core nanocapsules, nanospheres, liposomes and NLCs |
SZ (nm): 170– 266 nm EE (%): 98– 99% PI: 0.1–0.4 |
Automated Franz cells and porcine skin were treated for 8 hours; dose: 1 mg/mL total 0.2 mL. |
↑ Resveratrol chemical photostability ↑ Resveratrol skin penetration |
Skin cancer | 2012 [124] |
| Lipid-core nanocapsules |
SZ: around 241nm ZP: −15mV EE: 99.9% PI: 0.2 |
Male Wistar rats; dose: 5mg/kg body weight of free or nano- resveratrol; route: intraperitoneal or oral administration; duration: 14 days. |
↑ Resveratrol concentrations in liver, kidney, and brain. ↑ Gastrointestinal safety |
Alzheimer’s disease |
2010 [129] |
| Bovine serum albumin nanoparticles |
SZ: 400 to 500 nm EE: 34% |
Xenograft ovarian cancer nude mice; dose: 200, 100, and 50 mg/kg body weight of free or nano-resveratrol; route: intraperitoneal injections once a week; duration: 4 weeks. |
↑ Resveratrol concentrations in the liver ↓ Tumor size ↑ Caspase-9 and caspase-3 expression |
Ovarian cancer | 2010 [133] |
| SLNs | SZ: around 180 nm ZP: −38 mV |
Human keratinocyte cell line NCTC2544; dose: 100 µM of free or nano-resveratrol; duration: 24 hours. |
↑ Cellular uptake ↓ Keratinocyte proliferation |
Skin cancer | 2010 [125] |
| PCL-PEG polymeric micelles |
SZ: 100 nm EE: 89% LC: 20% |
PC12 cells; dose: 2, 5, and 10 uM of free or nano-resveratrol; duration: 48 hours |
↑ Sustained release ↓Reactive oxygen species accumulation ↑ Improve Aβ-induced PC12 cell viability |
Alzheimer’s disease |
2009 [128] |
| mPEG-PCL based nanoparticles |
SZ: around 80 nm ZP: around −6.5 mV EE: 91% LC: 19% |
Rat C6 glioma cells; dose: 2–32 µM of free or nano-resveratrol; duration: 48 hours |
↑ Sustained release ↓ Cell viability ↑Cellular uptake of resveratrol |
Malignant glioma therapy |
2009 [127] |
EE: encapsulation efficiency; LC: loading capacity; mPEG-PCL: methoxy polyethylene glycol)- poly(caprolactone); NP: nanoparticle; NLC: nanostructured lipid carriers; PCL: poly-caprolactone; PEG: polyethyleneglycol; PI: polydispersity index; SLN: solid lipid nanoparticle; SZ: size; ZP: Z-potential; ↑, increase; ↓, decrease.
Like EGCG and quercetin, circulating resveratrol is rapidly metabolized, and consequently, blood concentrations of free resveratrol are at low mM levels [34]. Different from free resveratrol, nanoencapsulated resveratrol exhibited a sustained release pattern [126–128]. Less than 10% of resveratrol was released from NLCs or SLNs after incubating them at 25°C and 37°C for four hours[126]. Frozza R et al. encapsulated resveratrol into lipid-core nanoparticles and gave male Wistar rats 5 mg/kg body weight of either free or nano-resveratrol daily for 14 days via oral administration [129]. Compared to free resveratrol, nano-resveratrol significantly increased the rat tissue (kidney, brain and liver, but blood was not measured) resveratrol concentrations by more than twofold [129]. In addition, resveratrol loaded lipid-core nanoparticles had higher gastrointestinal safety than free resveratrol [129](Table 3).
Resveratrol has antioxidant, anti-inflammatory, anticarcinogenic properties[34,130,131]. Compared to free resveratrol and void nanoparticles, nano-resveratrol can decrease reactive oxygen species production and increase antioxidant capacity in cell culture and research animal model [45,128,132]. Lee CW et al. orally administered control (no resveratrol), free resveratrol and nano-resveratrol to rats with CCl4-induced hepatotoxicity. They found that nano-resveratrol doubled the beneficial effect of free resveratrol on reducing hepatocyte death, decreasing oxidative stress, lowering inflammatory cytokine production [132]. Shao J et al. demonstrated that nano-resveratrol doubled the inhibitory effect of free resveratrol on the viability of rat C6-glioma cells in vitro[127]. Guo et al. administered saline, free resveratrol and resveratrol loaded bovine serum albumin nanoparticles to the implanted ovarian tumor-bearing mice via intra peritoneal injection once per week for four weeks[133]. The free and nano-resveratrol concentrations were 50, 100, and 200 mg/kg body weight. Compared to free resveratrol, resveratrol loaded bovine serum albumin nanoparticles increased resveratrol concentrations in ovary by 1.8 fold. Both free and nano-resveratrol decreased ovarian tumor weight in a dose dependent manner. The inhibition rates of tumor growth by free and nano-resveratrol at 200 mg/kg body weight were 46% and 62%, respectively. None of the treatments changed animal body weight [133]. In addition, nano-resveratrol has a potential in improving resveratrol’s preventive effect on Alzheimer’s disease [128,129](Table 3).
3.4. Curcumin
Curcumin is a hydrophobic curcuminoid present in the Indian spice turmeric extracted from the herb Curcuma long a[134]. Curcumin has been shown to have several beneficial effects including anti-inflammatory, antiangigenic, and antitumorigenic properties and may potentially help in preventing or even treating some chronic diseases, such as cancer, diabetes, and cardiovascular disease[134]. Despites these advantages, curcumin has poor aqueous solubility, low bioavailability, and is quickly metabolized by hepatic enzymes in humans and research animals [135]. Many biocompatible and biodegradable nanoparticles have been developed to overcome these limitations.
SLNs, nanoemulsions, and PLGA nanoparticles have successfully improved the aqueous solubility and chemical stability of curcumin[136–140]. Nano-curcumin exhibits a sustained release pattern [136,137,141,142]. The bioavailability of curcumin is low. After oral administration of 1 g/kg body weight and 2 g/kg body weight of curcumin to rats, the peak blood concentrations detected at hour 0.8 were1.4 µM and 3.7 µM, respectively [35,143]. In human, consumption of less than 4 g of curcumin resulted in either undetectable or extremely low (less than 1.0 µM) serum curcumin concentrations[144]. If humans consume 4–12 g of curcumin, the peak blood curcumin concentrations are increased, but less than 4.0 µM [35,144–146]. After encapsulating curcumin into SLNs, nanoemulsions and PLGA nanoparticles, the oral bioavailability can be enhanced more than two-fold [62,137,141,142,147,148]. After intravenous administration of nano-curcumin, blood curcumin concentrations and its circulation time are also significantly increased [139,149–151](Table 4).
Table 4.
The characteristics, functions and application of curcumin nanoparticles
| NP type | NP characteristics |
Experiment model/dose/route | Functions | Application | Year reference |
|---|---|---|---|---|---|
| PLGA NPs PLGA-PEG NPs |
SZ: around 152 nm EE: over 70% |
Male adult Wistar; dose: 50 mg/kg of free or nano-curcumin; route: oral administration. |
↑ Oral bioavailability | Bioavailability | 2013 [62] |
| SLNs | SZ: 190 nm ZP: –20.7 mV EE: 75% LC: 28% |
Balb/c mice; dose: 400 mg/kg of free or nano-curcumin; route: intraperitoneal injection. |
↑ Curcumin concentrations in lungs ↓ Inflammatory response in lung |
Asthma therapy |
2012 [153] |
| TPGS NPs | SZ: 210.2 nm | Kunming mice; dose: 250 mg/kg of free or nano-curcumin; route: oral gavage. |
↑ Oral bioavailability | Bioavailability | 2012 [147] |
| PLGA-APgp (conjugate anti-P- glycoprotein ) NPs |
SZ: around 130 nm ZP: −23.1 to − 40.3 mV EE: 60% –99% |
Multidrug resistant (KB-V1) and drug sensitive (KB–3– 1) cervical carcinoma cells; dose: 5 –30 µM of free or nano- curcumin. |
↑ Targeting and binding affinity to cancer cells; ↑ Cellular uptake ↓ Cancer cell viability |
Cancer | 2012 [155] |
| Emulsions | SZ: 218 nm | Caco-2 cells; dose: 20 µg/mL of free or nano-curcumin to measure the transportation of curcum in Female CD-1 mice; dose: 240 mg/kg of free or nano-curcumin; route: oral gavage. |
↑ Permeation rate across Caco-2 cells ↑Oral bioavailability |
Bioavailability | 2012 [148] |
| PLGA NPs | SZ: 80–100nm EE: 31% LC: 15% |
Human neuroblastoma SK-N-SH cells; dose: 0.035 to 0.1 µM of free or nano- curcumin. |
↓ Reactive oxygen species accumulation ↑ Neurons against oxidative damage |
Alzheimer’s disease |
2012 [152] |
| PLGA NPs | SZ: 120–190 nm EE: 74–90% |
HeLa cells; dose: 5–25 µM of free or nano-curcumin. |
↑ Aqueous solubility ↑ Sustained release ↑ Cellular uptake ↑ Anticancer efficacy |
Cancer | 2012 [136] |
| Liposomes | N/A | Insulin resistant ob/ob mice; route: intraperitoneal injection of free or nano-curcumin. |
↓Fasting blood glucose an insulin levels and HOMA-IR ↓ Inflammatory response ↑ Peripheral insulin sensitivity |
Type 2 diabetes |
2011 [154] |
| PLGA NPs | SZ: <200 nm EE: 92% LC: 5.8% |
Male Sprague-Dawley rats; dose and route: 10 mg/kg body weight by intravenous injection or 100 mg/kg body weight by oral administration. |
↑ Stability ↑ Sustained release ↑ Oral bioavailability |
Bioavailability | 2011 [137] |
| PLGA and PEG-PLGA micelles |
SZ: 26.29 nm ZP: −0.71 mV mV EE: 70 ± 0.34%. LC: 6.4 ± 0.02% |
Kunming mice; dose: 10 mg/kg body weight of free or nano-curcumin; route: tail vein injection. |
↑ Bioavailability ↓ Curcumin uptake by liver and spleen ↑ Curcumin uptake by lung and brain |
Bioavailability | 2011 [158] |
| PLGA NPs | SZ: 163 nm ZP: −12.5 mV PI: 0.05 EE: 47% |
Male Sprague-Dawley rats; dose: 25 mg/kg body weight of free or nano- curcumin; route: intravenous injection. |
↑ Intravenous bioavailability ↑ Curcumin concentrations in brain |
Brain disease | 2011 [149] |
| SLNs | SZ: 134 nm EE: 82% Total drug content: 94% |
Wistar male rats; dose: 50 mg/kg body weight of free or nano-curcumin; route: oral administration. |
↑ Oral bioavailability | Bioavailability | 2010 [159] |
| Human serum albumin NPs |
SZ: 130–150 nm ZP: −23 mV LC: 7.2% |
Human colon cancer cells (HCT116); dose: 0–60 µM of free or nano- curcumin. Balb/c nu/nu mice were inoculated with HCT116 cells; dose: 10 or 20 mg/kg body weight of free or nano-curcumin; route: intravenous injection every other days; duration: 10 days. |
↑ Aqueous solubility ↓ Viability of HCT116 cells ↓ Colon tumor size, but no effect on body weight |
Cancer | 2011 [138] |
| SLNs | SZ: around 135nm EE: 84% |
Male Wistar rats; dose: 1–50 mg /kg body weight of nano-curcumin, or 50mg/kg body weight of free curcumin; route: oral administration. |
↑ Stability ↑ Sustained release ↑ Oral bioavailability |
Bioavailability | 2011 [141] |
| PLGA NPs | SZ: 76.2 – 560.4nm ZP: −0.56mV- 0.06mV EE: 49.56 ± 4.52% – 89.53 ± 3.26% |
A2780CP cells & MDA-MB-231 cells; dose: 0–40µM of free or nano- curcumin. |
↑ Cur stability ↑ cellular drug uptake |
Cancer | 2010 [140] |
| Nanoemulsi ons |
SZ: around 192 nm ZP: −32 mV EE: around 90 % |
Cancer cells (PANC-1, MIA PaCa-2, K562, MCF 7, A549, and HCT-116); dose: 0–40 µM of free or nano- curcumin. BALB/c mice; dose: 30mg/kg body weight of free or nano-curcumin; route: tail vein injection. |
↑ Aqueous solubility ↑ Cellular uptake ↓ Viability of cancer cells ↑ Intravenous bioavailability |
Cancer | 2010 [139] |
| PLGA NPs | SZ: 80.9 nm EE: 97.5% |
KBM-5 cancer cells; dose: 0–25 µM of free or nano-curcumin. Balb/c mice; dose: 2.5 mg/kg body weight of free or nano-curcumin; route: intravenous injection. |
↑ Cellular uptake ↓ NF-kB activation ↑ Intravenous bioavailability |
Cancer | 2010 [150] |
| PLGA NPs | SZ: 264 nm EE: 76.9% LC: 15% |
Male Sprague Dawley rats; dose: 250 mg/kg body weight of free curcumin, or 100 mg/kg body weight of nano- curcumin; route: oral gavage. |
↑ Sustained release ↑ Oral bioavailability |
Bioavailability | 2009 [142] |
EE: encapsulation efficiency; LC: loading capacity; mPEG-PA: methoxy poly(ethylene glycol)-Palmitate; NP: nanoparticle; PEG: polyethyleneglycol; PLGA: poly (D,L-lactide-co-glycolide); PVA: Poly(vinyl alcohol); SLN: Solid lipid nanoparticle; SZ: size; TPGS: D-α-tocopheryl polyethylene glycol 1000 succinate
Nanoencapsulation increases curcumin bioactivities[138–140,150,152–154]. Researchers have investigated the anticancer activities of nanoencapsulated curcumin in a variety of cancer cells including HCT116, A2780CP, MDA-MB-231, KBM-5, PANC-1, MIA PaCa-2, K562, MCF 7, A549 cells[138–140,150]. Nano-curcumin significantly enhanced the inhibitory effect of curcumin on cancer cell viability, which is accompanied by an increase in curcumin uptake by cancer cells[136,138–140]. Importantly, the void nanoparticles including void nanoemulsions, PLGA nanoparticles and human serum albumin nanoparticles did not change viability of those cancer cells, indicating the safety of those void nanoparticles [136,139,140]. Punfa W et al. conjugated anti-P-glycoprotein (P-gp) to the surface of PLGA nanoparticles [155]. They found that targeting antibodies significantly increased the binding and targeting specificity of nanoparticles to cancer cells and further enhanced the uptake of nanoparticles by cancer cells [155]. Kim TH et al. inoculated HCT116 or MIA PaCa2 cells to Balb/c nu/nu male mice[138]. After inoculation for five days, they intravenously administered 10 mg/kg body weight of free or nanoencapsulated curcumin every other day for ten days[138]. Compared to control (saline), free and nanoencapsulated curcumin decreased the tumor volume by 20% and 45%, respectively. Importantly, they did not change body weight [138]. Curcumin-PLGA nanoparticles can decrease reactive oxygen species accumulation in neurons, improve neurons against oxidative damage in vitro, and increase curcumin accumulation in rat brain, which indicates its potential application in preventing Alzheimer’s disease and other neuron degenerative diseases [149,152].
Wang W et al. gave 400 mg/kg body weight of free curcumin or nano-curcumin (curcumin-SLNs) to Balb/c mice via intra peritoneal injection [153]. They found that nano-curcumin dramatically increased curcumin concentrations in lung and doubled inhibitory effect of free curcumin on inflammatory responses in lung, which implies its application in asthma therapy [153]. In addition, curcumin liposomes can decrease inflammatory response and reverse insulin resistance in an animal model [154]. After intra peritoneal injection of curcumin-liposomes to insulin resistance (ob/ob) mice, blood fasting glucose and insulin levels and homeostasis model assessment-estimated insulin resistance index (HOMA-IR) were significantly decreased, inflammatory responses were reduced, and peripheral insulin sensitivity was improved [154](Table 4). Curcumin nanoparticle research is growing. When we used key words”curcumin nanoparticle” to search on Pub Med, we found 10, 19, 48, 79, 109 articles in 2008, 2009, 2010, 2011 and 2012, respectively.
4. Challenges and limitations
Nanomedicine is a very promising field especially when applied for disease prevention and/or treatment using phytochemicals and other dietary supplements. However, this is a very new field and is still in its infancy, thus presenting many technical and translational challenges and limitations.
The major challenge is potential toxicity of nanoparticles. Many components in nanoparticles, such as nucleic acids, antibody fragments, peptides and proteins, can function as antigens, resulting in increased immunotoxicity [160]. Moreover, if the encapsulation efficacy and loading capacity of nanoparticles are low, people would be consuming or receiving large amounts of nanocarriers containing surfactants and co-surfactants or emulsifiers, which may cause adverse effects. While clinical trials can be used to assess the short-term toxicity of nanoparticles, the possibility of long-term toxicity due to chronic exposure and accumulation needs to be carefully addressed. Currently, there are no good in vivo models, guidelines, and standardized safety test variables established to determine toxicity and adverse effects of nanoparticles. Incorporation of target ligands on the surface of nanoparticles can increase targeted delivery of encapsulated phytochemicals to targeted abnormal cells, which can be used to decrease toxicity and adverse effects [161]. However, many physical and biological barriers exist between nanoparticles and abnormal cells such as cancer cells [162]. These barriers include the blood vessel wall, blood-brain barrier, extra cellular matrix, interstitial fluid pressure gradients, among many others [163]. It is necessary for nanoparticles to overcome all these barriers before acting on the targeted cells.
Another relevant issue worth noting is the administration route of nanoparticles. In general, oral administration is the most practical and acceptable route for long-term administration of phytochemicals and dietary supplements. However, little is known about the absorption and metabolism of nanoparticles in the gastrointestinal tract, thus limited data exists about bioavailability of nanocarriers and their tissue specific pharmacokinetics [164]. Biocompatible and biodegradable nanocarriers, such as lipid nanoparticles, can be digested or degraded in the gastrointestinal tract. Even though the phytochemical encapsulated nanoparticles can be absorbed, the structure, characteristics and pharmacokinetics of nanoparticles may be changed after the digestion or degradation of nanocarriers.
Finally, the cost of applying this nanotechnology is another major limitation. Indeed, the synthesis of nanoparticles, especially multifunctional nanoparticles (e.g. incorporation of both preventive and therapeutic phytochemicals in nanoparticles), is an expensive and complicated process, which requires special ingredients, certain instruments and optimal conditions[160, 165]. Lowering the cost/benefit ratio is critical in the application of nanotechnology in nutrition research.
5. Conclusions
Many biocompatible and biodegradable nanoparticles are currently available for encapsulating bioactive compounds including phytochemicals. Each nanoparticle has its own advantages, disadvantages, and characteristics. We have demonstrated that nanoparticles can overcome some limitations in using phytochemicals for health promotion and prevention, and enhance their bioactivities. Given the wide use of dietary supplements (most of which are phytochemicals), and potential toxicity and safety concerns with some of these supplements, nanotechnology is a promising tool for limiting the dosage while increasing bioavailability and bioactivities. Even though nanotechnology offers promising approaches in nutraceutical applications, additional innovative research is needed to address the cost-effectiveness and long-term safety of those nanoparticles.
Acknowledgement
This project was supported by Grant Number R15AT007013 from the National Center for Complementary & Alternative Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Institutes of Health. Additional support was provided by Texas Tech University, College of Human Sciences, Lubbock, TX. The authors would like to thank Caraline Trotter for her thoughtful critical review of the manuscript.
Funding sources: The project described was supported by Grant Number R15AT007013 from the National Center for Complementary & Alternative Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishiyama N. Nanomedicine: nanocarriers shape up for long life. Nat Nanotechnol. 2007;2:203–204. doi: 10.1038/nnano.2007.88. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles: in medicine therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 3.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional: nanoparticles cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangham AD, Horne RW. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J Mol Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 6.Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16:307–321. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 7.Bangham AD, Horne RW, Glauert AM, Dingle JT, Lucy JA. Action of saponin on biological cell membranes. Nature. 1962;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- 8.Langer R. New methods of drug delivery. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 9.Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18:309–327. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- 10.Newman GC, Huang C. Structural studies on phophatidylcholine-cholesterol mixed vesicles. Biochemistry. 1975;14:3363–3370. doi: 10.1021/bi00686a012. [DOI] [PubMed] [Google Scholar]
- 11.Harrison M, Tomlinson D, Stewart S. Liposomal-entrapped doxorubicin: an active agent in AIDS-related Kaposi's sarcoma. J Clin Oncol. 1995;13:914–920. doi: 10.1200/JCO.1995.13.4.914. [DOI] [PubMed] [Google Scholar]
- 12.Mozafari MR, Pardakhty A, Azarmi S, Jazayeri JA, Nokhodchi A, Omri A. Role of nanocarrier systems in cancer nanotherapy. J Liposome Res. 2009;19:310–321. doi: 10.3109/08982100902913204. [DOI] [PubMed] [Google Scholar]
- 13.Abreu AS, Castanheira EM, Queiroz MJ, Ferreira PM, Vale-Silva LA, Pinto E. Nanoliposomes for encapsulation and delivery of the potential antitumoral methyl 6-methoxy-3-(4-methoxyphenyl)-1H-indole-2-carboxylate. Nanoscale Res Lett. 2011;6:482. doi: 10.1186/1556-276X-6-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozafari MR. Nanoliposomes: preparation and analysis. Methods Mol Biol. 2010;605:29–50. doi: 10.1007/978-1-60327-360-2_2. [DOI] [PubMed] [Google Scholar]
- 15.Shoji Y, Nakashima H. Nutraceutics and delivery systems. J Drug Target. 2004;12:385–391. doi: 10.1080/10611860400003817. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Zhang X, Huang X, Wang X, Liao G, Chen Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int J Nanomedicine. 2013;8:1285–1292. doi: 10.2147/IJN.S41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang S, Gao D, Zhao T, Zhou J, Zhao X. An evaluation of the anti-tumor efficacy of oleanolic acid-loaded PEGylated liposomes. Nanotechnology. 2013;24:235102. doi: 10.1088/0957-4484/24/23/235102. [DOI] [PubMed] [Google Scholar]
- 18.Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta. 1991;1070:187–192. doi: 10.1016/0005-2736(91)90162-2. [DOI] [PubMed] [Google Scholar]
- 19.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Momekova D, Rangelov S, Yanev S, Nikolova E, Konstantinov S, Romberg B, Storm G, Lambov N. Long-circulating, pH-sensitive liposomes sterically stabilized by copolymers bearing short blocks of lipid-mimetic units. Eur J Pharm Sci. 2007;32:308–317. doi: 10.1016/j.ejps.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 22.Sharpe M, Easthope SE, Keating GM, Lamb HM. Polyethylene glycol-liposomal doxorubicin: a review of its use in the management of solid and haematological malignancies and AIDS-related Kaposi's sarcoma. Drugs. 2002;62:2089–2126. doi: 10.2165/00003495-200262140-00012. [DOI] [PubMed] [Google Scholar]
- 23.Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multi center trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Liu D. Long-circulating emulsions (oil-in-water) as carriers for lipophilic drugs. Pharm Res. 1995;12:1060–1064. doi: 10.1023/a:1016274801930. [DOI] [PubMed] [Google Scholar]
- 25.Mun S, Decker EA, McClements DJ. Influence of droplet characteristics on the formation of oil-in-water emulsions stabilized by surfactant-chitosan layers. Langmuir. 2005;21:6228–6234. doi: 10.1021/la050502w. [DOI] [PubMed] [Google Scholar]
- 26.McClements DJ, Decker EA, Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72:R109–R124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 27.McNiff BL. Clinical use of 10% soybean oil emulsion. Am J Hosp Pharm. 1977;34:1080–1086. [PubMed] [Google Scholar]
- 28.Press M, Kikuchi H, Shimoyama T, Thompson GR. Diagnosis and treatment of essential fatty acid deficiency in man. Br Med J. 1974;2:247–250. doi: 10.1136/bmj.2.5913.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Anton N, Vandamme TF. Nano-emulsions micro-emulsions: clarifications of the critical differences. Pharm Res. 2011;28:978–985. doi: 10.1007/s11095-010-0309-1. [DOI] [PubMed] [Google Scholar]
- 31.Pinnamaneni S, Das NG, Das SK. Comparison of oil-in-water emulsions manufactured by microfluidization and homogenization. Pharmazie. 2003;58:554–558. [PubMed] [Google Scholar]
- 32.Singh B, Bandopadhyay S, Kapil R, Singh R, Katare O. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit Rev Ther Drug Carrier Syst. 2009;26:427–521. doi: 10.1615/critrevtherdrugcarriersyst.v26.i5.10. [DOI] [PubMed] [Google Scholar]
- 33.Hollman PC, vd Gaag M, Mengelers MJ, van Trijp JM, de Vries JH, Katan MB. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med. 1996;21:703–707. doi: 10.1016/0891-5849(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 34.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 35.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 36.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin Drug Deliv. 2012;9:1305–1317. doi: 10.1517/17425247.2012.719870. [DOI] [PubMed] [Google Scholar]
- 38.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller RH, Maassen S, Weyhers H, Mehnert W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J Drug Target. 1996;4:161–170. doi: 10.3109/10611869609015973. [DOI] [PubMed] [Google Scholar]
- 40.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 41.Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 42.Uner M. Preparation characterization physico-chemical properties of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375–386. [PubMed] [Google Scholar]
- 43.Sanad RA, Abdelmalak NS, Elbayoomy TS, Badawi AA. Formulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs) AAPS PharmSciTech. 2010;11:1684–1694. doi: 10.1208/s12249-010-9553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gokce EH, Korkmaz E, Dellera E, Sandri G, Bonferoni MC, Ozer O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: evaluation of antioxidant potential for dermal applications. Int J Nanomedicine. 2012;7:1841–1850. doi: 10.2147/IJN.S29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aditya NP, Shim M, Lee I, Lee Y, Im MH, Ko S. Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agric Food Chem. 2013;61:1878–1883. doi: 10.1021/jf305143k. [DOI] [PubMed] [Google Scholar]
- 47.Teeranachaideekul V, Muller RH, Junyaprasert VB. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)--effects of formulation parameters on physicochemical stability. Int J Pharm. 2007;340:198–206. doi: 10.1016/j.ijpharm.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 48.Jenning V, Lippacher A, Gohla SH. Medium scale production of solid lipid nanoparticles (SLN) by high pressure homogenization. J Microencapsul. 2002;19:1–10. doi: 10.1080/713817583. [DOI] [PubMed] [Google Scholar]
- 49.Puglia C, Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin Drug Deliv. 2012;9:429–441. doi: 10.1517/17425247.2012.666967. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system recent advances in drug delivery. J Drug Target. 2012;20:813–830. doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 51.Bargoni A, Cavalli R, Caputo O, Fundaro A, Gasco MR, Zara GP. Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm Res. 1998;15:745–750. doi: 10.1023/a:1011975120776. [DOI] [PubMed] [Google Scholar]
- 52.Cavalli R, Bargoni A, Podio V, Muntoni E, Zara GP, Gasco MR. Duodenal administration of solid lipid nanoparticles loaded with different percentages of tobramycin. J Pharm Sci. 2003;92:1085–1094. doi: 10.1002/jps.10368. [DOI] [PubMed] [Google Scholar]
- 53.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 54.Lim SB, Banerjee A, Onyuksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Control Release. 2012;163:34–45. doi: 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 56.Gong J, Chen M, Zheng Y, Wang S, Wang Y. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159:312–323. doi: 10.1016/j.jconrel.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 57.Kwon GS, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Physical entrapment of adriamycin in AB block copolymer micelles. Pharm Res. 1995;12:192–195. doi: 10.1023/a:1016266523505. [DOI] [PubMed] [Google Scholar]
- 58.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27:2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennet D, Marimuthu M, Kim S, An J. Dual drug-loaded nanoparticles on self-integrated scaffold for controlled delivery. Int J Nanomedicine. 2012;7:3399–3419. doi: 10.2147/IJN.S32800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakraborty S, Stalin S, Das N, Choudhury ST, Ghosh S, Swarnakar S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials. 2012;33:2991–3001. doi: 10.1016/j.biomaterials.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 62.Khalil NM, do Nascimento TC, Casa DM, Dalmolin LF, de Mattos AC, Hoss I, Romano MA, Mainardes RM. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 63.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 64.Kumari A, Yadav SK, Pakade YB, Singh B, Yadav SC. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf B Biointerfaces. 2010;80:184–192. doi: 10.1016/j.colsurfb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 67.Peng SF, Tseng MT, Ho YC, Wei MC, Liao ZX, Sung HW. Mechanisms of cellular uptake and intracellular trafficking with chitosan/DNA/poly(gamma-glutamic acid) complexes as a gene delivery vector. Biomaterials. 32:239–248. doi: 10.1016/j.biomaterials.2010.08.081. [DOI] [PubMed] [Google Scholar]
- 68.Chiu YL, Ho YC, Chen YM, Peng SF, Ke CJ, Chen KJ, Mi FL, Sung HW. The characteristics, cellular uptake and intracellular trafficking of nanoparticles made of hydrophobically-modified chitosan. J Control Release. 146:152–159. doi: 10.1016/j.jconrel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 69.Müller RH, Rühl D, Runge SA. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. International Journal of Pharmaceutics. 1996:115–121. [Google Scholar]
- 70.Olbrich C, Muller RH. Enzymatic degradation of SLN-effect of surfactant and surfactant mixtures. Int J Pharm. 1999;180:31–39. doi: 10.1016/s0378-5173(98)00404-9. [DOI] [PubMed] [Google Scholar]
- 71.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm Res. 2002;19:875–880. doi: 10.1023/a:1016121319668. [DOI] [PubMed] [Google Scholar]
- 72.Urbinati G, Marsaud V, Renoir JM. Anticancer drugs in liposomal nanodevices: a target delivery for a targeted therapy. Curr Top Med Chem. 2012;12:1693–1712. doi: 10.2174/156802612803531423. [DOI] [PubMed] [Google Scholar]
- 73.Wang S, Noh SK, Koo SI. Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats. J Nutr. 2006;136:2791–2796. doi: 10.1093/jn/136.11.2791. [DOI] [PubMed] [Google Scholar]
- 74.Wang S, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A(2) and intestinal absorption of lipids in ovariectomized rats. J Nutr Biochem. 2006;17:492–498. doi: 10.1016/j.jnutbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Basu A, Lucas EA. Mechanisms and effects of green tea on cardiovascular health. Nutr Rev. 2007;65:361–375. doi: 10.1301/nr.2007.aug.361-375. [DOI] [PubMed] [Google Scholar]
- 76.Chyu KY, Babbidge SM, Zhao X, Dandillaya R, Rietveld AG, Yano J, Dimayuga P, Cercek B, Shah PK. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;109:2448–2453. doi: 10.1161/01.CIR.0000128034.70732.C2. [DOI] [PubMed] [Google Scholar]
- 77.Proniuk S, Liederer BM, Blanchard J. Preformulation study of epigallocatechin gallate, a promising antioxidant for topical skin cancer prevention. J Pharm Sci. 2002;91:111–116. doi: 10.1002/jps.10009. [DOI] [PubMed] [Google Scholar]
- 78.de Pace RC, Liu X, Sun M, Nie S, Zhang J, Cai Q, Gao W, Pan X, Fan Z, Wang S. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J Liposome Res. 2013;23:187–196. doi: 10.3109/08982104.2013.788023. [DOI] [PubMed] [Google Scholar]
- 79.Barras A, Mezzetti A, Richard A, Lazzaroni S, Roux S, Melnyk P, et al. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int J Pharm. 2009;379:270–277. doi: 10.1016/j.ijpharm.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 80.Hu B, Ting Y, Zeng X, Huang Q. Bioactive Peptides/Chitosan Nanoparticles Enhance Cellular Antioxidant Activity of (−)-Epigallocatechin-3- gallate. J Agric Food Chem. 2013;61:875–881. doi: 10.1021/jf304821k. [DOI] [PubMed] [Google Scholar]
- 81.Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, et al. Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem. 2011;54:1321–1332. doi: 10.1021/jm1013715. [DOI] [PubMed] [Google Scholar]
- 82.Siddiqui IA, Adhami VM, Ahmad N, AhmadMukhtar H. Nanochemoprevention: sustained release of bioactive food components for cancer prevention. Nutr Cancer. 2010;62:883–890. doi: 10.1080/01635581.2010.509537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 84.Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr. 2001;131:1731–1737. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- 85.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 86.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 87.Hung PF, Wu BT, Chen HC, Chen YH, Chen CL, Wu MH, Liu HC, Lee MJ, Kao YH. Antimitogenic effect of green tea (−)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the ERK and Cdk2 pathways. Am J Physiol Cell Physiol. 2005;288:C1094–C1108. doi: 10.1152/ajpcell.00569.2004. [DOI] [PubMed] [Google Scholar]
- 88.Kim H, Hiraishi A, Tsuchiya K, Sakamoto K. (−) Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology. 2010;62:245–255. doi: 10.1007/s10616-010-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur J Pharm Sci (pharmacokinetics) 2011;44:422–426. doi: 10.1016/j.ejps.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Hu B, Ting Y, Yang X, Tang W, Zeng X, Huang Q. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem Commun (Camb) 2012;48:2421–2423. doi: 10.1039/c2cc17295j. [DOI] [PubMed] [Google Scholar]
- 91.Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur J Pharm Sci. 2010;41:219–225. doi: 10.1016/j.ejps.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Werle M, Takeuchi H. Chitosan-aprotinin coated liposomes for oral peptide delivery: Development, characterisation and in vivo evaluation. Int J Pharm. 2009;370:26–32. doi: 10.1016/j.ijpharm.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Werle M, Takeuchi H, Bernkop-Schnurch A. Modified chitosans for oral drug delivery. J Pharm Sci. 2009;98:1643–1656. doi: 10.1002/jps.21550. [DOI] [PubMed] [Google Scholar]
- 94.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 96.You JO, Liu YC, Peng CA. Efficient gene transfection using chitosan-alginate core-shell nanoparticles. Int J Nanomedicine. 2006;1:173–180. doi: 10.2147/nano.2006.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer's disease. Int J Pharm. 2010;389:207–212. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–1716. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–740. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- 100.Kumari A, Yadav SK, Pakade YB, Singh B, Yadav SC. Development of biodegradable nanoparticles for delivery of quercetin. Colloids and Surfaces B-Biointerfaces. 2010;80:184–192. doi: 10.1016/j.colsurfb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 101.Yu YB, Miyashiro H, Nakamura N, Hattori M, Park JC. Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch Pharm Res. 2007;30:820–826. doi: 10.1007/BF02978831. [DOI] [PubMed] [Google Scholar]
- 102.Stewart LK, Soileau JL, Ribnicky D, Wang ZQ, Raskin I, Poulev A, Majewski M, Cefalu WT, Gettys TW. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 2008;57:S39–S46. doi: 10.1016/j.metabol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumari A, Kumar V, Yadav SK. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS One. 2012;7:e41230. doi: 10.1371/journal.pone.0041230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Srinivas K, King JW, Howard LR, Monrad JK. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. Journal of Food Engineering. 2010;100:208–218. [Google Scholar]
- 105.Tan BJ, Liu Y, Chang KL, Lim BK, Chiu GN. Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int J Nanomedicine. 2012;7:651–661. doi: 10.2147/IJN.S26538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumari A, Kumar V, Yadav SK. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: a green approach. PLoS One. 2012;7:e41230. doi: 10.1371/journal.pone.0041230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song X, Zhao Y, Hou S, Xu F, Zhao R, He J, Chen Q. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm. 2008;69:445–453. doi: 10.1016/j.ejpb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 108.Bose S, Michniak-Kohn B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur J Pharm Sci. 2012;48:442–452. doi: 10.1016/j.ejps.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133:238–244. doi: 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998;128:593–597. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- 111.Hong YJ, Mitchell AE. Metabolic profiling of flavonol metabolites in human urine by liquid chromatography and tandem mass spectrometry. J Agric Food Chem. 2004;52:6794–6801. doi: 10.1021/jf040274w. [DOI] [PubMed] [Google Scholar]
- 112.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 113.Li W, Yi S, Wang Z, Chen S, Xin S, Xie J, Zhao C. Self-nanoemulsifying drug delivery system of persimmon leaf extract: Optimization and bioavailability studies. Int J Pharm. 2011;420:161–171. doi: 10.1016/j.ijpharm.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 114.Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 115.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 116.Adebamowo CA, Cho E, Sampson L, Katan MB, Spiegelman D, Willett WC, Holmes MD. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int J Cancer. 2005;114:628–633. doi: 10.1002/ijc.20741. [DOI] [PubMed] [Google Scholar]
- 117.Nothlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166:924–931. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 118.Wang G, Wang JJ, Yang GY, Du SM, Zeng N, Li DS, Ye F. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int J Nanomedicine. 2012;7:271–280. doi: 10.2147/IJN.S26935. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63:342–351. doi: 10.1111/j.2042-7158.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 120.Ghosh D, Ghosh S, Sarkar S, Ghosh A, Das N, Das Saha K, Mandal AK. Quercetin in vesicular delivery systems: evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem Biol Interact. 2010;186:61–71. doi: 10.1016/j.cbi.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 121.Chen-yu G, Chun-fen Y, Qi-lu L, Qi T, Yan-wei X, Wei-na L, Guang-xi Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int J Pharm. 2012;430:292–298. doi: 10.1016/j.ijpharm.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 122.Orallo F. Comparative studies of the antioxidant effects of cis- and trans-resveratrol. Curr Med Chem. 2006;13:87–98. [PubMed] [Google Scholar]
- 123.Sanna V, Roggio AM, Siliani S, Piccinini M, Marceddu S, Mariani A, Sechi M. Development of novel cationic chitosan-and anionic alginate-coated poly(D,L-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int J Nanomedicine. 2012;7:5501–5516. doi: 10.2147/IJN.S36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Detoni CB, Souto GD, da Silva AL, Pohlmann AR, Guterres SS. Photostability and skin penetration of different E-resveratrol-loaded supramolecular structures. Photochem Photobiol. 2012;88:913–921. doi: 10.1111/j.1751-1097.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- 125.Teskac K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 2010;390:61–69. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 126.Neves AR, Lúcio M, Martins S, Lima JL, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomedicine. 2013;8:177–187. doi: 10.2147/IJN.S37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shao J, Li X, Lu X, Jiang C, Hu Y, Li Q, Fu Z. Enhanced growth inhibition effect of resveratrol incorporated into biodegradable nanoparticles against glioma cells is mediated by the induction of intracellular reactive oxygen species levels. Colloids Surf B Biointerfaces. 2009;72:40–47. doi: 10.1016/j.colsurfb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Lu X, Ji C, Xu H, Li X, Ding H, Ye M, Guo X. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int J Pharm. 2009;375:89–96. doi: 10.1016/j.ijpharm.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 129.Frozza RL, Bernardi A, Paese K, Hoppe JB, da Silva T, Battastini AM, Salbego C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J Biomed Nanotechnol. 2010;6:694–703. doi: 10.1166/jbn.2010.1161. [DOI] [PubMed] [Google Scholar]
- 130.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 132.Lee CW, Yen FL, Huang HW, Wu TH, Ko HH, Tzeng WS, Lin CC. Resveratrol nanoparticle system improves dissolution properties and enhances the hepatoprotective effect of resveratrol through antioxidant and anti-inflammatory pathways. J Agric Food Chem. 2012;60:4662–4671. doi: 10.1021/jf2050137. [DOI] [PubMed] [Google Scholar]
- 133.Guo L, Peng Y, Yao J, Sui L, Gu A, Wang J. Anticancer activity and molecular mechanism of resveratrol-bovine serum albumin nanoparticles on subcutaneously implanted human primary ovarian carcinoma cells in nude mice. Cancer Biother Radiopharm. 2010;25:471–477. doi: 10.1089/cbr.2009.0724. [DOI] [PubMed] [Google Scholar]
- 134.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 135.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425:44–52. doi: 10.1016/j.ijpharm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 137.Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, Wang H, Zhou Q, Yu S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59:9280–9289. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]
- 138.Kim TH, Jiang HH, Youn YS, Park CW, Tak KK, Lee S, Kim H, Jon S, Chen X, Lee KC. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403:285–291. doi: 10.1016/j.ijpharm.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 139.Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597–6611. doi: 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 140.Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 141.Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2011;55:495–503. doi: 10.1002/mnfr.201000310. [DOI] [PubMed] [Google Scholar]
- 142.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 143.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 144.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 145.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 146.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao Y, Wang C, Sun M, Wang X, Yu A, Li A, Zhai G. In vivo evaluation of curcumin loaded nanosuspensions by oral administration. J Biomed Nanotechnol. 2012;8:659–668. doi: 10.1166/jbn.2012.1425. [DOI] [PubMed] [Google Scholar]
- 148.Yu H, Huang Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J Agric Food Chem. 2012;60:5373–5379. doi: 10.1021/jf300609p. [DOI] [PubMed] [Google Scholar]
- 149.Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin, its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416:331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 150.Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79:330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Gao Y, Li Z, Sun M, Li H, Guo C, Cui J, Li A, Cao F, Xi Y, Lou H, Zhai G. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm. 2010;36:1225–1234. doi: 10.3109/03639041003695139. [DOI] [PubMed] [Google Scholar]
- 152.Doggui S, Sahni JK, Arseneault M, Dao L, Ramassamy C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J Alzheimers Dis. 2012;30:377–392. doi: 10.3233/JAD-2012-112141. [DOI] [PubMed] [Google Scholar]
- 153.Wang W, Zhu R, Xie Q, Li A, Xiao Y, Li K, Liu H, Cui D, Chen Y, Wang S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine. 2012;7:3667–3677. doi: 10.2147/IJN.S30428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yekollu SK, Thomas R, O'Sullivan B. Targeting curcusomes to inflammatory dendritic cells inhibits NF-kappaB and improves insulin resistance in obese mice. Diabetes. 2011;60:2928–2938. doi: 10.2337/db11-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Punfa W, Yodkeeree S, Pitchakarn P, Ampasavate C, Limtrakul P. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin. 2012;33:823–831. doi: 10.1038/aps.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rocha S, Generalov R, Pereira MoC, Peres I, Juzenas P, Coelho MA. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine (Lond) 2011;6:79–87. doi: 10.2217/nnm.10.101. [DOI] [PubMed] [Google Scholar]
- 157.Weiss-Angeli V, Poletto FS, de Marco SL, Salvador M, da Silveira NP, Guterres SS, &Pohlmann AR. Sustained antioxidant activity of quercetin-loaded lipid-core nanocapsules. J NanosciNanotechnol. 2012;12:2874–2880. doi: 10.1166/jnn.2012.5770. [DOI] [PubMed] [Google Scholar]
- 158.Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, Zhai G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci. 2011;354:116–123. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 159.Kakkar V, Singh S, Singla D, Sahwney S, Chauhan AS, Singh G, Kaur IP. Pharmacokinetic applicability of a validated liquid chromatography tandem mass spectroscopy method for orally administered curcumin loaded solid lipid nanoparticles to rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3427–3431. doi: 10.1016/j.jchromb.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 160.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm. 2011;8:2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 162.Wei A, Mehtala JG, Patri AK. Challenges and opportunities in the advancement of nanomedicines. J Control Release. 2012;164:236–246. doi: 10.1016/j.jconrel.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev ClinOncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Srinivas PR, Philbert M, Vu TQ, Huang Q, Kokini JL, Saltos E, Chen H, Peterson CM, Friedl KE, McDade-Ngutter C, Hubbard V, Starke-Reed P, Miller N, Betz JM, Dwyer J, Milner J, Ross SA. Nanotechnology research: applications in nutritional sciences. J Nutr. 2010;140:119–124. doi: 10.3945/jn.109.115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]