Abstract
Purpose
In mouse models of prostate cancer, increased epinephrine levels accelerated tumor growth via the beta2-adrenoreceptor/PKA signaling pathway. It is unknown, however, whether men experience increased epinephrine levels sufficient to activate the beta2-adrenoreceptor/ PKA pathway in the prostate gland. We measured epinephrine levels in blood samples collected immediately prior to prostate biopsies and measured phosphorylation of S133CREB (PKA site), S112BAD, T202/Y204ERK, and S473 Akt in prostate biopsy tissue samples.
Methods
Tissue samples and 3 ml of blood were obtained from men (n=20) recruited from the patients scheduled for prostate biopsies. Epinephrine levels were measured by ELISA. Proteins were extracted from biopsied tissue and protein phosphorylation was measured by Western blotting with phospho-specific antibodies. Pearson and Spearman rank correlations were analyzed to assess relationships among blood epinephrine levels and phosphorylation of CREB, BAD, AKT, and ERK.
Results
Epinephrine levels above 1 nM were detected in 5 of 20 patients. A strong positive correlation was observed between increased epinephrine levels and CREB phosphorylation. In contrast, no correlation was observed between epinephrine levels and phosphorylation of ERK, BAD, or of AKT.
Conclusion
Our results suggest that increased blood epinephrine levels activate the beta2-adrenoreceptor/PKA signaling pathway in human prostate glands. These results will inform future studies to examine whether beta2-selective blockers can inhibit activation of the epinephrine/ADRB2/PKA pathway in prostate tumors of men with increased epinephrine levels, and explore the use of beta2-selective blockers as adjuvant therapy for prostate cancer.
Keywords: Prostate cancer, apoptosis, emotional stress, CREB, BAD, ERK, AKT
Introduction
Chronic stress and depression have been connected recently with human cancer progression and mortality [1,2]. Prostate cancer patients reportedly experience increased levels of stress and anxiety [3]. Yet, analysis of relationships between stress levels and prostate cancer susceptibility and progression in humans show conflicting results [4–6], unlike the strong support for this association in animal models. When epinephrine increases in response to stress, it inhibits apoptosis in prostate cancer and stimulates tumor growth via the beta2-adrenoreceptor/ cAMP-dependent protein kinase (ADRB2/PKA) signaling pathway in mice [7]. Furthermore, activation of ADRB2 was connected with accelerated prostate cancer cell migration and metastases in animal models of prostate cancer [8]. Beta-blockers, which act as epinephrine antagonists and inhibit activation of the ADRB2/PKA signaling pathway, completely reversed the effects of stress and the effects of injected epinephrine or norepinephrine on the prostate [8].
Analyses of data from the Quebec population-based prescription database linked to the provincial cancer registry showed that beta-blockers taken for 4 years reduced the chance of being diagnosed with prostate cancer by 18% (odds ratio 0.86% (95% CI 0.77–0.96)) [9]. However, some studies have not replicated these findings [10, 11]. Still, two studies based on Norwegian Cancer Registry and US FDA Adverse Event Reporting System reported decreased mortality among patients scheduled for androgen deprivation therapy [12] and among patients with male reproductive neoplasms [13] who took beta blockers.
Taken together, these results from animal models and epidemiologic studies imply that signaling via the ADRB2 pathway could be a primary mediator of the effects of stress on prostate cancer development. Still, it is not known whether elevated epinephrine leads to activation of ADRB2/PKA signaling or of other signaling pathways in the prostate gland in men.
To assess signaling pathways activated by epinephrine, we examined phosphorylation of CREB (cAMP response element-binding protein), an established substrate of PKA [14], and ERK and Akt phosphorylation in prostate biopsies, and measured epinephrine levels in blood collected immediately prior to prostate biopsies. A significant positive correlation has been observed between blood epinephrine levels and intensity of CREB phosphorylation in prostate glands.
Our results suggest that increased blood epinephrine leads to activation of the ADRB2/PKA signaling pathway in human prostate glands, which has been connected with inhibition of apoptosis and androgen independence in prostate cancer models [15–17]. Identifying prostate cancer patients with increased epinephrine levels will allow assessments of the contribution of ADRB2/PKA signaling to prostate cancer progression, and future studies to test the use of ADRB2-selective antagonists as adjuvant therapy for these patients.
Materials and Methods
Study participants
Eligible participants for this study were men scheduled for prostate biopsy, ages 18 to 80 years, with a life expectancy greater than 3 months. Potential participants had to be able to understand and sign a written informed consent (in English). Exclusion criteria included any patients with (1) chronically elevated levels of epinephrine due to pheochromocytoma; (2) serious cognitive problems likely to impair their ability to give informed consent (i.e. diagnosis of mental retardation, dementia, or other serious neurocognitive conditions); (3) active psychosis or prominent suicidal ideation at enrollment screening; or (4) inability to have blood samples drawn via standard venipuncture procedures. The investigation was done in accordance with the Declaration of Helsinki, including obtaining written informed consent from all participants. The study was approved by the Institutional Review Board at Wake Forest University Health Sciences.
Blood sample collection
Men scheduled for prostate biopsies were recruited from the Department of Urology at Wake Forest Baptist Health. After signing written informed consent and within one hour prior to prostate biopsy, 3 ml of blood was drawn into a heparinized vial. Blood was stored on ice and within 1 hour was centrifuged at 1000g for 10 minutes to pellet cells. Then, sodium metabisulfite was added to plasma to a final concentration of 4 mM, and plasma was snap frozen and stored at −80°C. 10–12 needle biopsies of average size 1 × 15 mm were collected by Dr. Hemal from each patient, and one core was snap frozen in liquid nitrogen and stored at −80°C prior to analysis. The remaining prostate biopsy tissue was used to prepare paraffin-embedded sections for pathologic evaluation.
Epinephrine measurements
Plasma epinephrine concentrations were measured by ELISA, using commercially available assays (BA-0100 from Labor Diagnostika) purchased through Rocky Mountain Diagnostics. Epinephrine was first extracted using a cis-diol-specific affinity gel, acetylated to N-acyladrenaline, and then derivatized enzymatically to N-acylmetanephrine. Acylated adrenaline from the standards, controls, and samples then compete for a fixed number of antiserum binding sites later detected by ELISA (lower detection limit is 0.05nM), as described by the manufacturer.
Western blot analysis
Prostate biopsy samples were homogenized using a glass dounce tissue homogenizer in a cold lysis buffer (20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5% Na+deoxycholate, 1% Nonidet P-40, and 1 mM DTT, pH 7.4), containing protease inhibitors (10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 1 mM benzamidine, and 1 mM PMSF) and phosphatase inhibitors (1 mM NaVO4, 50 mM β-glycerophosphate, 40 mM p-nitrophenylphosphate, 40 mM NaF, and 1 μg/mL microcystin). All reagents were purchased from Sigma-Aldrich. Prostate biopsy tissue lysates were separated by polyacryamide gel electrophoresis and transferred to nitrocellulose membranes for analysis with appropriate antibodies. The nitrocellulose membranes were incubated overnight at 4°C with primary antibodies against human pCREB, CREB, pBAD, BAD, pAKT, AKT, pERK, ERK (from Cell Signaling Technology), using β-actin (from Sigma-Aldrich) as a control, followed by 1 hr incubation at room temperature in fluorescently labeled secondary antibodies. Western blots were analyzed with the Odyssey CLx Infrared Imaging System (Li-Cor Biosciences), according to the manufacturer’s instructions. Protein bands were quantified using ImageJ software (National Institutes of Health).
Statistical analyses
Statistical analysis was conducted by Dr. D’Agostino using SAS version 9.2. This study was designed to have 80% power to detect a correlation of 0.60 with 20 patients enrolled assuming a 2-sided test. With 13 patients (number with phosphorylation data available), we had 80% power to detect a correlation of 0.71. Correlations were estimated between blood epinephrine levels and BAD, CREB, AKT, and ERK phosphorylation on the subsample of patients with these measures (n=13). Four sets of correlations were calculated; the first two were Pearson correlations on all available patients, which were then repeated after removing one patient with an extremely high level of blood epinephrine (Patient 14). The second two were Spearman rank correlations on all available patients, which were then repeated after removing one patient with an extremely high level of blood epinephrine (Patient 14). These four methods were used to provide confirmatory results for any correlations seen (or not seen). The Pearson correlations are model-based and assume that the variables have somewhat normal distributions, whereas the Spearman rank correlation is a non-parametric approach that estimates the correlation of the ranks of the measures. A p-value ≤0.05 was considered statistically significant, and all tests were performed using 2-sided tests.
Results
Patient characteristics
We recruited 20 men scheduled for prostate biopsy due to elevated prostate-specific antigen (PSA) to participate in this study; two patients refused to participate. Participants ranged in age from 46 to 77 years, with a mean age of 67.5 (SD = 6.8) years. Participants included 16 Caucasian and 4 African-American men. Four participants were taking beta1-selective beta blockers. Following prostate biopsy, 10 men (50%) were diagnosed with prostate adenocarcinoma (Gleason score 6–9), 2 men were diagnosed with prostate intraepithelial neoplasia (PIN), and 8 men were diagnosed with benign prostate hyperplasia (Table 1).
Table 1.
Pathology Reports, blood epinephrine (Epi) and beta blockers use for Study Participants
| Pt. IDs | Age | Prostate biopsy Histopathology | Blood Epi (nM) | Beta blockers |
|---|---|---|---|---|
| 1 | 68 | Benign Hyperplasia of Prostate & Chronic inflammation | <0.05 | na |
| 2 | 69 | 3 out of 12; GS is 6 to 7 Adenocarcinoma of Prostate |
0.09 | na |
| 3 | 61 | Benign Hyperplasia of Prostate | 1.63 | Atenolol |
| 4 | 72 | Benign Hyperplasia of Prostate | <0.05 | na |
| 5 | 46 | 1 out of 12; GS is 3+3=6 Adenocarcinoma of Prostate |
<0.05 | na |
| 6 | 70 | Benign Hyperplasia of Prostate | <0.05 | Toprol XL |
| 7 | 67 | Benign Hyperplasia of Prostate | 0.23 | na |
| 8 | 68 | 12 out of 12; GS is 3+4=7 Adenocarcinoma of Prostate |
<0.05 | na |
| 9 | 62 | 1 out of 12; GS is 3+4=7 Adenocarcinoma of Prostate |
0.13 | na |
| 10 | 65 | Prostatic intraepithelial neoplasia | 1.05 | na |
| 11 | 58 | 6 out of 12; GS is 6 to 8 Adenocarcinoma of Prostate |
0.35 | na |
| 12 | 68 | 5 out of 5; GS is 3+4=7 Adenocarcinoma of Prostate |
0.28 | Atenolol |
| 13 | 59 | Benign Hyperplasia of Prostate & Chronic inflammation | <0.05 | na |
| 14 | 73 | Prostatic intraepithelial neoplasia | 13.42 | na |
| 15 | 65 | 3 out of 12; GS is 3+4=7 Adenocarcinoma of Prostate |
1.28 | na |
| 16 | 68 | Chronic inflammation | 0.41 | na |
| 17 | 58 | 2 out of 12; GS is 3+4=7 Adenocarcinoma of Prostate |
0.47 | na |
| 18 | 65 | 12 out of 12; GS is 7 to 9 Adenocarcinoma of Prostate |
2.30 | na |
| 19 | 77 | Benign Hyperplasia of Prostate | 0.89 | Metoprolol |
| 20 | 71 | 1 out of 12; GS is 3+3=6 Adenocarcinoma of Prostate |
1.95 | na |
Epinephrine levels and cancer diagnosis
With the assays used, epinephrine could be measured down to 0.05 nM. In this pilot study, we found no significant correlations between diagnoses of adenocarcinoma or inflammation or plasma epinephrine levels (Table 1). An epinephrine level of 1nM was chosen as a cutpoint to stratify patients into “high” and “low” epinephrine groups. We chose this cutpoint based on tissue culture and mouse model studies that showed robust activation of the PKA/BAD signaling pathway when epinephrine levels were above 1nM [7,16].
Epinephrine levels and activation of signaling pathways in prostate biopsies
Protein extracts suitable for analysis by Western blotting were obtained from 13 prostate biopsies and examined with antibodies that recognize pS112BAD, pS133CREB, pS473Akt, pT202/pY204ERK. To control for total protein expression, lysates of prostate biopsies were also probed with antibodies to non-phosphorylated BAD, CREB, AKT, and ERK (Figure 1). Ratios of signal intensity of phosphorylated and corresponding total proteins were calculated to determine the activation status of each signaling molecule tested. Beta-actin was used as a control to equalize loading. Activation levels of each signaling molecule were compared with each other as well as with blood epinephrine levels.
Figure 1. Western blot analysis of prostate biopsies.
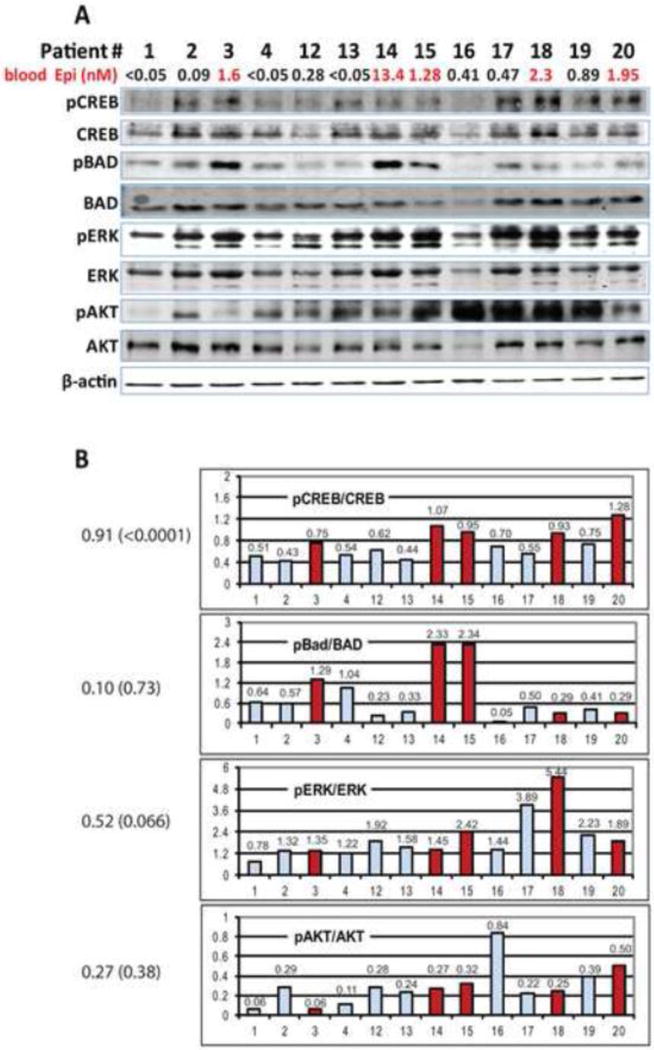
A) Proteins extracted from prostate biopsies were analyzed with antibodies that recognize pS133CREB and total CREB, pS112BAD and total BAD, pT202/pY204ERK and total ERK, pS473Akt and total Akt. Beta-actin was used as a control. Fluorescently labeled secondary antibodies were used to detect and quantify signal intensities.
B) Quantitation of pS133CREB, pS112BAD, pT202/pY204ERK and pS473Akt phosphorylation are presented as ratios of signal intensities of phosphorylated protein and total protein (values are shown above the bars). Samples of patients with epinephrine levels over 1nM are shown in dark (red). Values of Spearman correlations between epinephrine levels and CREB, BAD, ERK and AKT phosphorylation are shown on the right [correlation (p value)].
We found a statistically significant positive correlation between blood epinephrine levels and both BAD phosphorylation (r=0.6; p=0.024) and CREB phosphorylation (r=0.55; p=0.05) using a Pearson correlation coefficient. Analysis using Spearman’s rank correlation coefficient found a highly significant correlation between blood epinephrine levels and CREB phosphorylation (r=0.9; p<0.0001), but not BAD phosphorylation (p =0.73). When we repeated these correlation analyses removing patient 14 as an outlier, the Pearson correlation between blood epinephrine levels and BAD phosphorylation became non-significant (r=0.16, p=0.62) and the Pearson correlation between blood epinephrine levels and CREB phosphorylation became highly significant (r=0.86; p=0.0003). Thus, the positive correlation between blood epinephrine and CREB phosphorylation was not driven by the single patient with extremely high epinephrine levels. No other significant correlations were detected between other tested parameters (Table 2).
Table 2.
Comparison of plasma epinephrine levels and phosphorylation intensity of pS112BAD, pS133CREB, pT202/pY204ERK and pS473Akt using Pearson and Spearman correlation tests.
| Pearson Correlations (Correlation (p-value)) |
Spearman Correlations (Correlation (p-value)) |
|
|---|---|---|
| Variable | Blood Epinephrine | Blood Epinephrine |
| pBAD/BAD | 0.62 (0.02) | 0.10 (0.73) |
| pCREB/CREB | 0.55 (0.05) | 0.91 (<0.0001) |
| pERK/ERK | −0.02 (0.94) | 0.52 (0.066) |
| pAKT/AKT | −0.01 (0.97) | 0.27 (0.38) |
Four of 20 patients (Patients 3, 6, 12, 19), regularly took beta1-selective beta blockers. Nonetheless, CREB phosphorylation was found in Patients 3 and 19.
Discussion
There has been substantial interest in determining whether stress contributes to accelerated prostate cancer progression and reduced therapeutic sensitivity [18]. Although there is little doubt that effects of stress on prostate tumors are mediated by changes in endocrine (or paracrine) parameters, only a few studies have used biochemical analyses to follow changes of stress hormones in cancer patients. Thus, Schatzl and colleagues detected increased cortisol levels in 75 prostate cancer patients, and another report examined epinephrine levels in 50 cancer patients, including 3 prostate cancer patients [19,20]. Unfortunately, in the latter study individual levels of epinephrine for cancer patients were not reported.
Our recent data [7], together with this report, provide a first survey of epinephrine levels in prostate cancer patients and in men scheduled for prostate biopsy. We found a highly significant correlation between epinephrine concentrations over 1 nM and phosphorylation of S133CREB, a well-established PKA substrate.
Increased CREB phosphorylation in patients with elevated blood epinephrine levels was observed regardless of whether the diagnosis was benign prostate hyperplasia, prostate intraepithelial neoplasia, or prostate adenocarcinoma. This observation supports clinical relevance of our recent experiments in tissue culture models and mouse models of prostate cancer, which showed that epinephrine at levels over 1nM activate the ADRB2/PKA signaling pathway, inhibit apoptosis, and promote tumor growth [7,16]. In contrast, no significant correlations were found between epinephrine levels and activation of the ERK or PI3K/AKT signaling pathways. We also did not observe loss of CREB phosphorylation in patients with increased epinephrine levels who took beta1-selective beta blockers (atenolol in Patient 3 or metoprolol in Patient 19). This result is consistent with the earlier reports that epinephrine-induced activation of PKA/CREB pathway in prostate cells depends on beta2-adrenoreceptors [7,16,21].
Despite the significant correlation we found between epinephrine and CREB phosphorylation in prostate biopsies, a causal relationship remains to be established. Comparing CREB phosphorylation in repeated prostate biopsies from patients with higher epinephrine levels before and after beta-blockers are taken, would directly test the role of epinephrine/ADRB2 signaling in induction of CREB phosphorylation. Such a comparison could also shed light on whether doses of beta blockers that target ADRB2, prescribed to treat hypertension and anxiety (e.g. propranolol), are sufficient to inhibit epinephrine-induced CREB phosphorylation.
Analysis of downstream targets of the ADRB2/PKA pathway could potentially allow more precise selection of patients who will respond to beta-blockers. Thus, experiments in tissue culture and mouse models have identified BAD as a downstream target of antiapoptotic ADRB2/PKA signaling in prostate cancer [7,16]. Earlier studies in prostate cancer cells showed that BAD could be regulated by several redundant mechanisms, including PKA, PI3K, and Ras signaling [22]. If BAD is indeed a critical target of ADRB2/PKA in prostate cancer, then prostate tumors with active signaling pathways that phosphorylate BAD independently from ADRB2/PKA are unlikely to respond to sole inhibition of ADRB2 signaling. In such cases, beta-blockers should be administered with inhibitors of other signaling pathways, and BAD phosphorylation should be monitored to confirm efficacy of inhibitors.
Conclusion
In this pilot study, we found an association between increased blood epinephrine levels and activation of the PKA/CREB pathway in prostate glands. This result suggests that increased epinephrine levels may contribute to prostate cancer progression and therapy resistance, as has been shown in mouse models of prostate cancer [7,8]. Results of this pilot study provide a foundation for future studies that will examine whether beta2-selective blockers will inhibit activation of the epinephrine/ADRB2/PKA pathway in prostate tumors of men with increased epinephrine levels, and will inform future studies exploring the use of beta2-selective blockers as adjuvant therapy in men with prostate cancer.
Acknowledgments
The authors are grateful to Karen Klein for critical reading and for helpful suggestions; to Denise Young for helping with patients recruitment and sample handling; and to Megan J. Whelan, Lisa Dixon, and Claire Kimbrough for IRB protocol assistance. Project described was supported by Award Number R01CA118329 from the National Cancer Institute to George Kulik. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of interest statement.
Authors declare no conflict of interest that pertains to this publication.
Reference List
- 1.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 2.Armaiz-Pena GN, Lutgendorf SK, Cole SW, Sood AK. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traeger L, Penedo FJ, Gonzalez JS, Dahn JR, Lechner SC, Schneiderman N, Antoni MH. Illness perceptions and emotional well-being in men treated for localized prostate cancer. J Psychosom Res. 2009;67:389–397. doi: 10.1016/j.jpsychores.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen NR, Kristensen TS, Zhang ZF, Strandberg-Larsen K, Schnohr P, Gronbaek M. Sociodemographic status, stress, and risk of prostate cancer. A prospective cohort study. Ann Epidemiol. 2007;17:498–502. doi: 10.1016/j.annepidem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe C, Davey SG, Macleod J, Hart C. The role of self-reported stress in the development of breast cancer and prostate cancer: a prospective cohort study of employed males and females with 30 years of follow-up. Eur J Cancer. 2007;43:1060–1065. doi: 10.1016/j.ejca.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma: a structured review of the literature. Cancer. 2005;104:467–478. doi: 10.1002/cncr.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R, Jr, Danial N, Datta SR, Kulik G. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123:874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palm D, Lang K, Niggemann B, Drell TL, Masur K, Zaenker KS, Entschladen F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 9.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 10.Friedman GD, Udaltsova N, Habel LA. Norepinephrine antagonists and cancer risk. Int J Cancer. 2011;128:737–738. doi: 10.1002/ijc.25351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate. 2013;73:250–260. doi: 10.1002/pros.22564. [DOI] [PubMed] [Google Scholar]
- 13.Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, Han LY, Mangala LS, Villares GJ, Vivas-Mejia P, Rodriguez-Aguayo C, Nagaraja AS, Gharpure KM, Wu Z, English RD, Soman KV, Shazhad MM, Zigler M, Deavers MT, Zien A, Soldatos TG, Jackson DB, Wiktorowicz JE, Torres-Lugo M, Young T, De Geest K, Gallick GE, Bar-Eli M, Lopez-Berestein G, Cole SW, Lopez GE, Lutgendorf SK, Sood AK. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. doi: 10.1038/ncomms2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 15.Kasbohm EA, Guo R, Yowell CW, Bagchi G, Kelly P, Arora P, Casey PJ, Daaka Y. Androgen receptor activation by G(s) signaling in prostate cancer cells. J Biol Chem. 2005;280:11583–11589. doi: 10.1074/jbc.M414423200. [DOI] [PubMed] [Google Scholar]
- 16.Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A, Carson JP, Weber MJ, Register TC, Chen YQ, Penn RB, Kulik G. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem. 2007;282:14094–14100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- 17.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 18.Ullrich PM, Carson MR, Lutgendorf SK, Williams RD. Cancer fear and mood disturbance after radical prostatectomy: consequences of biochemical evidence of recurrence. J Urol. 2003;169:1449–1452. doi: 10.1097/01.ju.0000053243.87457.60. [DOI] [PubMed] [Google Scholar]
- 19.Schatzl G, Reiter WJ, Thurridl T, Waldmuller J, Roden M, Soregi S, Madersbacher S. Endocrine patterns in patients with benign and malignant prostatic diseases. Prostate. 2000;44:219–224. doi: 10.1002/1097-0045(20000801)44:3<219::aid-pros6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Lechin F, van der DB, Vitelli-Florez G, Lechin-Baez S, Azocar J, Cabrera A, Lechin A, Jara H, Lechin M, Gomez F. Psychoneuroendocrinological and immunological parameters in cancer patients: involvement of stress and depression. Psychoneuroendocrinology. 1990;15:435–451. doi: 10.1016/0306-4530(90)90067-j. [DOI] [PubMed] [Google Scholar]
- 21.Nagmani R, Pasco DS, Salas RD, Feller DR. Evaluation of beta-adrenergic receptor subtypes in the human prostate cancer cell line-LNCaP. Biochem Pharmacol. 2003;65:1489–1494. doi: 10.1016/s0006-2952(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 22.Sastry KS, Smith AJ, Karpova Y, Datta SR, Kulik G. Diverse antiapoptotic signaling pathways activated by vasoactive intestinal polypeptide, epidermal growth factor, and phosphatidylinositol 3-kinase in prostate cancer cells converge on BAD. J Biol Chem. 2006;281:20891–20901. doi: 10.1074/jbc.M602928200. [DOI] [PubMed] [Google Scholar]


