Abstract
Objective
Treatment of seizures varies by region with no standard emergency treatment protocol. Febrile status epilepticus (FSE) is often a child’s first seizure; therefore, families are rarely educated about emergency treatment.
Methods
From 2002 to 2010, 199 subjects, age 1 month to 6 years, were recruited as part of a prospective, multicenter study of consequences of FSE. FSE was defined as a febrile seizure or series of seizures lasting >30 minutes. The patients’ charts were reviewed. No standardized treatment protocol was implemented for this observational study.
Results
179 received at least one antiepileptic drug (AED) to terminate FSE and more than one AED was required in 140 patients (70%). Median time from the seizure onset to first AED by EMS or ED was 30 minutes. Mean seizure duration was 81 minutes for subjects given medication prior to ED and 95 minutes for those who did not (p=0.1). Median time from the first dose of AED to end of seizure was 38 minutes. Initial dose of lorazepam or diazepam was suboptimal in 32 of 166 patients (19%). Ninety-five (48%) subjects received respiratory support by EMS or ED. Median seizure duration for respiratory support group was 83 minutes; for non-respiratory support group was 58 minutes (p-value < 0.001). Reducing the time from seizure onset to AED initiation was significantly related to shorter seizure duration.
Significance
FSE rarely stops spontaneously, is fairly resistant to medications and even with treatment persists for a significant period of time. The total seizure duration is composed of two separate factors; the time from seizure onset to AED initiation, and the time from first AED to seizure termination. Earlier onset of treatment results in shorter total seizure duration. A standard pre-hospital treatment protocol should be used nationwide and education of EMS responders is necessary.
Keywords: Seizure, Pediatric, Pre-hospital
INTRODUCTION
Status epilepticus (SE) is the most common neurological life threatening emergency in childhood.1,2 Seizures account for about 1% of ED visits and about 3% of pre-hospital transports.3 Up to 10% of children with febrile seizures develop febrile status epilepticus (FSE) which accounts for 25% of all childhood SE and over two thirds of SE in the second year of life.4–9 Previous studies have not evaluated treatment of prolonged febrile seizures in the community. FEBSTAT did not have an established treatment protocol, but it allowed evaluation of the different treatment paradigms of multiple US regions. There is no standardized treatment protocol for prolonged seizures that all emergency medical services follow. Prolonged seizures of any type are associated with an increased risk of complications, and the time from seizure onset to treatment is critical.10 Longer seizure duration increases potential risk of short and long term morbidity. Indeed prior studies have shown that early treatment of SE by EMS leads to improved outcomes.10–12
The FEBSTAT study is a prospective study of the consequences of FSE.13,14 Subjects were recruited after the episode of FSE so EMS and ED management were done at the discretion of the local clinicians, and varied. We report the acute management of the 199 children who were enrolled in FEBSTAT. The relationship between treatment delay, total seizure duration and associated morbidity are analyzed in this observational study.
METHODS
The FEBSTAT study enrolled 199 subjects age four months to six years of age who presented with FSE between May of 2003 and March 2010 at five centers. The detailed methodologies of the study as well as the inclusion and exclusion criteria have been previously published.13,14 A febrile seizure was defined in accordance with the ILAE criteria as a seizure occurring in the context of a fever (temperature >38.4°C, 101.0°F) in a child with no prior history of afebrile seizures and no other acute neurological insult (meningitis, trauma or severe electrolyte imbalance).15 SE was defined as seizure lasting ≥ 30 minutes or a series of seizures without full recovery in between lasting ≥ 30 minutes.16,17
The five sites in FEBSTAT are Montefiore and Jacobi Medical Centers in the Bronx, Virginia Commonwealth University Hospital in Richmond, Lurie Children’s Hospital in Chicago, Duke University Medical Center and Eastern Virginia Medical School in Norfolk. The International Epilepsy Consortium at Virginia Commonwealth University is the Data Coordinating Center and the Epidemiology/Biostatistics Core is based at Columbia University.
Seizure duration and semiology were classified by clinical features in accordance with the International League Against Epilepsy (ILAE) seizure classification.18 Three clinicians (JMP, DRN, SS) reviewed the medical record, which consisted of the ED records and the ambulance call sheets, if available, the structured interview, and the comments from the local study team. They determined seizure duration, focality and other features, and then reached consensus with excellent interrater reliability as previously described.13,14
For this report, we reviewed information on the pre-hospital and ED management of the children, including the medications administered, EMS management, seizure recognition and response, respiratory support (includes bag-valve mask, positive pressure ventilation and intubation), laboratory data, imaging, length of hospitalization, duration of AED treatment and disposition. The seizure times and duration used were from the consensus reading by the central phenomenology core.
Antiepileptic drugs (AEDs)
AEDs reported to be administered were lorazepam, diazepam, fosphenytoin, phenytoin, phenobarbital, midazolam, levetiracetam and propofol. Other medication that were given but were excluded from this review were vecuronium, fentanyl, succinylcholine, ketamine, etomidate and rocuronium, as these medications were used for rapid sequence intubation and not for seizure termination.
The earliest dose of AED was defined as the time of the first AED after seizure initiation. When the exact time of the first dose was not documented a mid-range was estimated based on available information. This was common for EMS drug administration times and, in this case, the beginning of the range was the time EMS arrived at the child’s home and the end of the range as the time that the seizure terminated before ED arrival or the time of ED arrival.
AEDs given first in the ED were not assigned a range of administration time, except when the documentation indicated that an AED was given between two doses of medication for which the times were documented.
Dosing of diazepam and lorazepam
Appropriate dosing of diazepam was considered to be > 0.3 mg/kg when rectally administered and > 0.1 mg/kg for IV, IO or IM administration.19 The appropriate dose of lorazepam was considered to be > 0.05 mg/kg for IV, IO or IM administration.20 Only children with complete information about the dose were included in this part of the analysis.
Seizure recognition
Seizures were considered recognized by EMS if documentation of the active seizure was present in the EMS record. Similarly, seizure recognition was considered present if active seizures were recorded in the ED record.
Statistical Analysis
The statistical analyses were conducted with SAS v.9.2. Frequencies and medians were used to describe the data. All 199 children had valid seizure duration data. They were all included in the analysis of comparison the median seizure duration among different groups. Seizure durations for children who received an AED prior to ED arrival and those who did not were not normally distributed. Therefore, the Wilcoxon rank-sum was used to test for the medians.
Since the seizure duration was not normally distributed, the log transformed linear regression were conducted when analyze the relationship between seizure duration and time from seizure onset to the first AED. In order to find out the relationship between the seizure duration after the first AED and the time from seizure onset to the first AED, both methods, non-parametric regression and log transformed linear regression were performed.
RESULTS
FEBSTAT enrolled 199 children. Of these, 179 (90%) received at least one AED administered by the family, EMS or the ED to terminate FSE. The number of AEDs given by the family, EMS and ED per subject ranged from 0 to 5 medications with a median of 2 in those who required medication. FSE stopped spontaneously without need for AED administration in only 20 children (10%). Of the 179 children who received medications, 140 (78%) required more than one AED before the FSE stopped.
Administration of AEDs
The first AED was given by the family in two cases (1%), EMS in 73 cases (41%) and in the ED in 104 cases (58%). There were 58 children (32%) who were given medication both by EMS and the ED, and 41 children (23%) were treated with multiple doses of benzodiazepines in the ED in addition to EMS doses. Two families administered rectal diazepam prior to EMS arrival, and these were given to children with a prior history of febrile seizures, but not FSE. The only medication administered by families was rectal diazepam, which terminated FSE for both children. The only medications given by EMS were diazepam, lorazepam and midazolam. The treatment of children by EMS varied by region (Table 1).
Table 1.
Treatment with antiepileptic drugs by emergency medical services (EMS) in 199 children with febrile status epilepticus.
| Site | Number treated by EMS/Number recruited (%) |
|---|---|
| Virginia Commonwealth University | 14/37 (38%) |
| Montefiore and Jacobi | 10/55 (18%) |
| Duke University Medical Center | 9/25 (36%) |
| Lurie Children’s Hospital | 23/45 (51%) |
| Eastern Virginia Medical School | 17/37 (46%) |
Among children given an AED prior to ED arrival the median seizure duration was 68 minutes, interquartile range (IQR) 45. The median was 72 minutes (IQR 70) for children who received their first dose of medication after arrival to the ED (p = 0.1).
Activation of EMS
Of the 199 children, 46 (23%) did not activate EMS. Six of these children (3%) were in the ED when the seizure began, having presented with fever or complaints related to illness, and 40 (97%) were brought in by family. Of the 153 children transported by EMS, 148 children (97%) continued to have seizures at the time of EMS arrival.
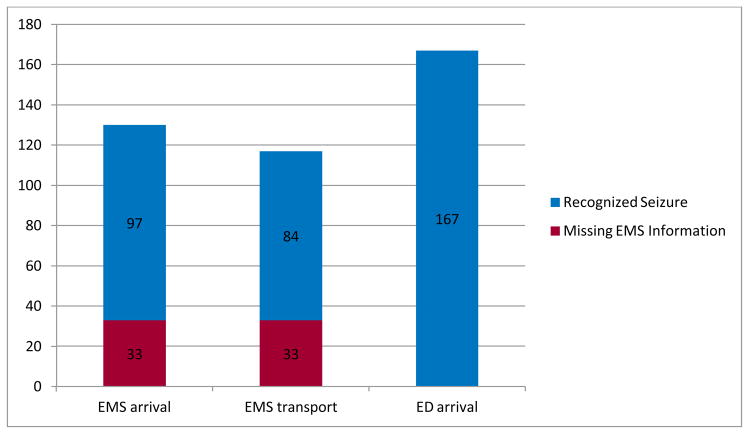
Seizure recognition (Figure 1)
Figure 1.
Recognition of seizure by emergency medical services (EMS) and emergency department (ED) personnel.
Of the 148 children who were actively seizing on EMS arrival, EMS did not recognize seizures in 18 children on arrival (12%). In this group active seizures on EMS arrival were recognized by the parents, but EMS documented that the event was not a seizure or that the child was post-ictal. At the time of EMS arrival to the home, seizures were recognized in 97 children (63%). EMS recognized clinical seizures during transport in 84 children (57%), but not 31 (20%). Thirty-three children (22%) were missing EMS forms.
Recognition of active seizures by the ED staff was superior to EMS. Including children for whom EMS was not activated, 169 children (85%) were still having a seizure on arrival to the ED. ED providers recognized seizures on arrival in 167 children (99%). The two that were not recognized were documented to be post-ictal with a description of active seizure in the records.
Respiratory Support (Table 3)
Table 3.
Respiratory support in children with febrile status epilepticus.
| Median Seizure Duration (minutes) | Mean number of AEDs | Mean number of doses | |
|---|---|---|---|
| Respiratory support | 83 | 2.7 | 4.7 |
| No Respiratory Support | 58 | 1.7 | 2.7 |
Ninety-five children (48%) were given respiratory support, defined as bag-valve mask, positive pressure ventilation or intubation. Patients that only received supplementary oxygen via blow-by, nasal cannula and face-mask were excluded from this group because it was unclear if the administration of oxygen was based on protocol or necessity. Of the 95 subjects that received respiratory support, four (4%) were given support by EMS only, 77 (81%) received support only in the ED and 14 (15%) received support by both EMS and ED (Table 2). Eighty-one children (41%) were intubated, all in the ED. Of the 81 subjects that were intubated, 68 (86%) had a known seizure onset time and a known respiratory support start time. The median time from seizure onset to intubation was 56 minutes. Children requiring respiratory support had a median seizure duration of 83 minutes compared to a median of 58 minutes for those that did not require respiratory support (p = 0.0003).
Table 2.
Respiratory support required by children with febrile status epilepticus.
| Respiratory support given by | Number of Children |
|---|---|
| EMS only | 4 |
| ED only | 77 |
| EMS and ED | 14 |
EMS – Emergency Medical Services; ED – Emergency Department
Among the 104 children not requiring respiratory support, AEDs included lorazepam, fosphenytoin/phenytoin, phenobarbital, diazepam and midazolam. The mean number of AEDs given was 1.7 (median 2.0). The mean number of AED doses administered was 2.7 (median 3.0).
Among the 95 children requiring respiratory support, levetiracetam and propofol were used in addition to the AEDs used in the group not requiring respiratory support. The mean number of AEDs used in children requiring respiratory support was 2.7 (median 3.0). The mean number of AED doses was 4.7 (median 4.5). Children given respiratory support received more AEDs than those not requiring such support (p = <0.0001).
Time from seizure onset to administration of the first AED (Table 4)
Table 4.
Timeline of seizure onset to AED administration.
| Median time | |
|---|---|
| Seizure onset to EMS arrival | 12.5 minutes |
| EMS arrival to 1st AED* | 9.8 minutes |
| Seizure onset to ED arrival | 33 minutes |
| ED arrival to 1st AED** | 10 minutes |
Only includes children given AED by EMS.
Only includes children given 1st AED by ED.
Most of the 199 children were given more than one medication. Among the 189 children that were given AEDs, ten (5%) received AED for prophylaxis after the FSE had been terminated. The AEDs given for prophylaxis were all given by the ED after FSE was terminated, and included lorazepam, diazepam, midazolam, fosphenytoin/phenytoin and phenobarbital. Of the 179 children requiring an AED to terminate their FSE, 161 (90%) had a recorded administration time in the records. Most children experienced a significant delay initiating treatment, even though the seizure was recognized. The median time from the seizure onset to the first dose of AED by EMS or ED was 30 minutes (IQR=35; range=1–175). The median time from seizure onset to EMS arrival was 12.5 minutes (mean=20; IQR=18; range=0–95), with EMS median response time of 6 minutes (activation to arrival) (mean=8; IQR=6; range 0–33). The median time from EMS arrival to first AED, in children treated by EMS, was 10 minutes (mean=10; IQR=7.5; range=0–28). The median time from seizure onset to arrival in the ED was 33 minutes (mean=38; IQR=29; range=0–239 minutes). The median time from seizure onset to first AED, in children first treated in the ED, was 10 minutes (mean=19; IQR=23; range=0–107). In the 161 children with recorded times, the median time from the first dose of AED to end of seizure was 38 minutes (IQR=52).
There are two components; the time from seizure onset to AED initiation, and the time from first AED to seizure termination. The relationship between seizure duration and the time from seizure onset to the first AED was analyzed. Since the dependent variable, seizure duration, was not normally distributed, the simple linear regression was conducted with both variables log transformed. The results showed a significant positive linear relationship between these two log transformed variables. Three patients’ seizure durations were considered as potential outliers. With the three outliers excluded, the results still showed a significant positive linear relationship between log transformed seizure duration and log transformed time from seizure onset to the first AED. The estimated parameter is 0.28, which means a one unit (2.72 minute) increase in log transformed seizure onset to the first AED is associated with a 0.28 unit (1.32 minute) increase in log transformed seizure duration.
A non-parametric regression and a log transformed linear regression were performed with the time from seizure onset to the first AED as the explanatory variable and the seizure duration after the first AED as the dependent variable. There was not a statistically significant relationship found in this cohort between earlier initial AED treatment and a decrease in seizure duration. Although a longer seizure duration is implied when treatment is delayed because the seizures are usually not stopping spontaneously.
Dosing for diazepam and lorazepam
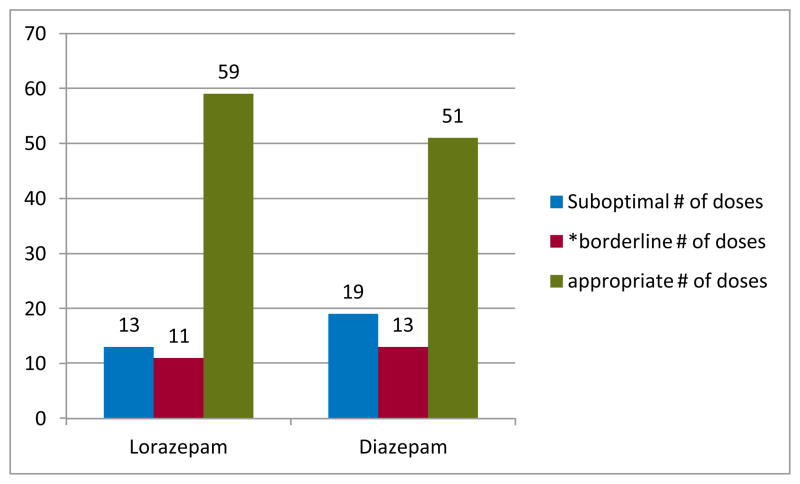
Of the 179 children who received AEDs, 166 (93%) received either lorazepam (N=83; 46%) or diazepam (N=83; 46%) as their first AED. Of these, 20 (11%) did not have dosage or route recorded. Among the 146 children (88%) with complete information, 32 (22%) had suboptimal dosing (Figure 2) including 19 with diazepam and 13 with lorazepam. There was another subgroup of 24 children (14%) that were given an appropriate dose of medication, but at the lower end of the range (Figure 2) including 13 with diazepam and 11 with lorazepam.
Figure 2.
Dosing for diazepam and lorazepam.
*Borderline dosing of diazepam was considered to be = 0.3 mg/kg for a rectally administered dose and = 0.1 mg/kg for IV, IO or IM administration. The borderline dose of lorazepam was considered to be = 0.05 mg/kg for IV, IO or IM administration.
Other AEDs used initially to stop FSE were midazolam (N=6; 3%), fosphenytoin (N=2; 1%) and phenobarbital (N=1; 0.5%). No medication was given to 20 children (10%) and 4 (2%) had an unknown medication status because no times were recorded, the sequence could not be determined or documentation of treatment was unavailable.
DISCUSSION
We have previously reported that the great majority of children with FSE required abortive medication to terminate their seizure.13,14 This analysis which examined the details of treatment found that not only did most require abortive medication, but the majority required administration of more than one AED to terminate their seizure. This study demonstrated FSE initial AED treatment was significantly delayed. Majority of children do not respond to initial AED and require multiple AEDs to terminate FSE. This indicates that these seizures can be refractory to treatment. When initial treatment is significantly delayed the entire treatment paradigm shifts, which prolongs total seizure duration. EMS does not have a standard treatment protocol. This analysis demonstrated the median time from seizure onset to arrival in the ED was 33 minutes, which supports the importance of pre-hospital treatment. The total seizure duration is composed of two separate factors; the time from seizure onset to AED initiation, and the time from first AED to seizure termination. We found that reducing the time from seizure onset to AED initiation was significantly related to shorter seizure duration.
Previous reports from the FEBSTAT cohort noted that FSE is not recognized by the ED in approximately one third of cases. This study evaluated recognition of seizure, not FSE, and found that EMS often recognized seizures on arrival and during transport, and the ED recognized almost all seizures on arrival. Although seizures are being recognized, the treatment remains delayed. This supports the conclusion that recognition of seizure activity by the ED is not the cause of the delay, but possibly the lack of recognition of SE affects treatment delay. While the current definition of SE (30 minutes) may theoretically contribute to delayed recognition, it is unlikely to have been a major factor, as treatment initiation was based on whether the child was recognized to be convulsing and whether the local EMS protocol allowed the administration of AEDs prior to ED arrival. The long median time from seizure onset to ED arrival (> 30 minutes) implies that if EMS has not treated the child prior to ED arrival the child would have been likely to have met the 30 minute definition of SE by ED arrival.
The treatment of available by EMS varies by region, and, despite the accumulated data to support uniform practice, EMS still cannot administer AEDs in many jurisdictions. The delay in treatment when EMS cannot provide AEDs correlates with a longer seizure duration. We also found a suboptimal dose of the first AED in 19% of the children given lorazepam or diazepam.
While studies have shown that short-term morbidity and mortality of FSE is low, there is evidence that even a single episode of SE may interfere with normal psychomotor development in infants and children without underlying structural abnormalities or delays.21–23 FSE is also associated with an increased risk of developing epilepsy.6,24
Our finding that the great majority of patients required AEDs to stop the seizures indicates that FSE rarely stops spontaneously. Once a seizure lasts for more than 5 minutes it is likely it will continue for some time.7,25 As seizure duration lengthens, spontaneous resolution becomes more difficult and this may particularly be true of febrile seizures.7,13,26,27
Delays in treatment of FSE have been observed in previous studies.10,16,26 A review of the Virginia Commonwealth University SE database, which includes all SE and both pediatrics and adults, found that less than half of SE patients received their first dose of an AED within 30 minutes of seizure onset.28 We observed a median of 30 minutes from FSE onset to AED treatment, an unacceptable lag to treatment. Multiple studies have recommended treatment after 5 minutes of seizing though with somewhat different rationales.16,25,26 The current accepted treatment parameters have not been changed since the initial enrollment in FEBSTAT. Thus, a change in treatment paradigm would not be a contributing factor to the significant treatment delay found in this study. The need for respiratory support was common and more frequent in children with a longer duration of seizure.
The observed tendency of prolonged seizures to continue, sometimes even if treated, may explain the protracted seizure duration of the patients in this study. Other studies that have shown pre-hospital administration of medication is associated with SE of shorter duration.11 Only 20% of the children had a history of febrile seizures, and 7% had a history of possible or definite FSE.14 Thus, most families did not have medication available for administration prior to EMS arrival. Pre-hospital treatment of prolonged seizures reduces the duration of the seizure, but it is often not utilized in part because there are regional differences in medications that can be administered.29 EMS care of the children participating in FEBSTAT varied considerably. Each EMS squad had different rules and regulations governing treatment paradigms. Some volunteer squads were not allowed to administer AEDs and others were only able to administer treatment when given a direct order from a local ED physician. Additionally, each region had multiple EMS squads.
Access to EMS as well as the treatment offered by EMS varies by location, and it is accepted that it is not always feasible to activate and await EMS arrival during an emergency. However, it is somewhat surprising and discouraging that in some jurisdictions very few children with ongoing seizures were getting treatment by EMS during the time period studied, which is fairly recent. When access is available patients have the opportunity for earlier recognition and treatment of FSE. The treatment of FSE should be as prompt and aggressive as treatment of SE caused by other etiologies. Interestingly, a recent study of prolonged febrile seizures including FSE in Israel reports much shorter time to treatment as the children received aggressive benzodiazepine treatment in the ambulance and this was associated with shorter seizure durations than those in FEBSTAT.30 This highlights the importance of early aggressive pre-hospital treatment of prolonged seizures. Our results that show a consistent median time from first AED administration to end of seizure and therefore a longer total seizure duration when first AED is delayed, are consistent with these findings and highlight the need for earlier aggressive therapy of prolonged febrile seizures.
The administration of rescue medication to children with prolonged seizures is poorly covered in current treatment paradigms.31 Management of children with prolonged febrile seizures need to begins prior to hospital arrival, but most published guidelines are limited to hospital settings.31 There is evidence that more than two doses of a benzodiazepine can increase the need for respiratory support, and physicians who disregard pre-hospital treatment may give more doses of benzodiazepines than those who do take pre-hospital treatment into account.32 Our study also finds that there are an increased number of doses of AEDs given to children needing respiratory support. It is unclear if this is primarily due to the multiple doses of benzodiazepines leading to respiratory depression or the effect of prolonged seizure per se which has been shown in adults to lead to respiratory depression more often than benzodiazepine administration.33 Similarly, we found that the ED does not always consider the treatment given by EMS, resulting in children receiving several doses of benzodiazepines in the ED after EMS had administered multiple doses. A previously published study of SE found that only one in every six children with SE admitted to the pediatric intensive care unit was appropriately treated for SE using current SE treatment guidelines.32 This emphasizes the importance of pre-hospital and ED protocols for FSE treatment, and a protocol education for EMS and ED personnel.
Paradoxically, FSE is often not treated because of a concern that medication may cause the need for respiratory support. However we found that the need for respiratory support was more frequent in subjects with more prolonged seizures and in those with an increased number of AED doses. Other studies have shown that treatment of seizures with benzodiazepines does not increase the rate of pre-hospital or ED intubation in children or adults.11,33 The increased number of doses in the respiratory support group may not be an indicator that the medications cause respiratory distress, but rather that an adequate dose of a medication is delayed and the seizure leads to respiratory depression.
This analysis has some limitations. The FEBSTAT children were enrolled in a prospective study, but they were recruited after the episode was over and were not done according to a standard protocol, EMS and ED treatment protocols varied widely. However, the results clearly confirm that FSE is a neurological emergency requiring prompt recognition and treatment. The findings of this study stress the need for a uniform EMS protocol. More aggressive treatment with adequate doses of effective medication is needed to stop FSE and prevent associated morbidity, including short-term consequences of intubation and long-term consequences of hippocampal injury and long-term consequences of hippocampal injury.34 Our findings have implications for the future treatment of FSE and for the role of EMS and the ED in the acute setting.
Acknowledgments
Supported by grant NINDS R01 NS 43209 (PI S. Shinnar, M.D., Ph.D.). This research received support from award number P60MD002256 from the National Institute on Minority Health and Health Disparities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities or the National Institutes of Health.
APPENDIX. FEBSTAT study team
Montefiore Medical Center and Jacobi Medical Center, Albert Einstein College of Medicine, Bronx, NY: Shlomo Shinnar M.D. Ph.D. (PI), Jennifer Ayala B.A., Jacqueline Bello M.D., Mootoo Chunasamy R.T. M.S., Patricia Clements, Ronda L. Facchini Ph.D., James Hannigan R.T., George Lantos M.D., Ann Mancini M.A., David Masur Ph.D., Solomon L. Moshé M.D., Ruth C. Shinnar R.N. M.S.N., Maryana Sigalova M.A., Yoshimi Sogawa, M.D., EricaWeiss M.A.
Lurie Children’s Hospital, Northwestern University Feinberg School of Medicine, Chicago, IL: Douglas Nordli M.D. (PI), John Curran M.D., Leon G. Epstein M.D., Aaliyah Hamidullah M.S., Andrew Kim M.D., Julie Renaldi Ph.D.
Columbia University School of Medicine, New York, NY: Dale C. Hesdorffer Ph.D. (PI), Emilia Bagiella Ph.D., Stephen Chan M.D., Veronica J. Hinton Ph.D., Claire Litherland, Yuxin Zhang M.S.
Duke University Medical Center, Durham, NC: Darrell Lewis M.D. (PI), Melanie Bonner Ph.D., William Gallentine DO, James MacFall Ph.D., James Provenzale M.D., Elizabeth Rende R.N. M.S.N. C.P.N.P., James Voyvodic, Ph.D., Allen Song Ph.D., Yuan Xu B.S.
Eastern Virginia Medical School, Norfolk, VA: L. Matthew Frank M.D. (PI), Joanne Andy R.T., Terrie Conklin R.N., Susan Grasso M.D., Diane James R-EEG T, David Kushner M.D., Susan Landers R.T., Virginia Van de Water Ph.D.
Virginia Commonwealth University, Richmond, VA: John M. Pellock M.D. (PI), Tanya Bazemore REEG-T, James Culbert Ph.D., Kathryn O’Hara R.N., Syndi Seinfeld D.O., Jean Snow RT-R.
International Epilepsy Consortium at Virginia Commonwealth University, Richmond VA: Shumei Sun Ph.D., Brian J Bush MSMIT, Sreedevi Chandrasekaran, Lori L. Davis, John M. Pellock M.D., Christiane Rogers, Cynthia Shier Sabo M.S., Helen Wang.
Collaborators: Joan Conry M.D., Children’s National Medical Center, Washington DC –Safety.
Monitor: Tracy Glauser M.D., Cincinnati Children’s Hospital, Cincinnati Ohio – Genomics substudy. Jeffrey L. Noebels M.D. Ph.D., Baylor College of Medicine, Houston, Texas –Genetics substudy.
Footnotes
Presented in part at the 40th Annual Child Neurology Society meeting (2011), 65th Annual American Epilepsy Society meeting (2011) and 64th Annual American Academy of Neurology meeting (2012).
DISCLOSURES
None of the authors has any relevant conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with the guidelines of the Committee on Publication Ethics (COPE) guidelines for ethical publication (http://publicationethics.org/).
References
- 1.DeLorenzo RJ, Hauser WA, Towne AR. A prospective, population based epidemiological study of status epilepticus in Richmond, VA. Neurology. 1996;46:1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 2.Chin RF, Neville BG, Peckham C, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368:222–229. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- 3.Martindale JL, Goldstein JN, Pallin DJ. Emergency department seizure epidemiology. Emerg Med Clin North Am. 2011;29:15–27. doi: 10.1016/j.emc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295:1029–1033. doi: 10.1056/NEJM197611042951901. [DOI] [PubMed] [Google Scholar]
- 5.Verity CM, Ross EM, Golding J. Outcome of childhood status epilepticus and lengthy febrile convulsions: finding of national cohort study. BMJ. 1993;307:225–228. doi: 10.1136/bmj.307.6898.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg AT, Shinnar S. Unprovoked seizures in children with febrile seizures: short-term outcome. Neurology. 1996;47:562–568. doi: 10.1212/wnl.47.2.562. [DOI] [PubMed] [Google Scholar]
- 7.Hesdorffer DC, Benn EK, Bagiella E, et al. Distribution of febrile seizure duration and associations with development. Ann Neurol. 2011;70:93–100. doi: 10.1002/ana.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maytal J, Shinnar S, Moshe SL, et al. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83:323–331. [PubMed] [Google Scholar]
- 9.Shinnar S, Pellock JM, Moshe SL, et al. In whom does status epilepticus occur: Age-related differences in children. Epilepsia. 1997;38:907–914. doi: 10.1111/j.1528-1157.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 10.Pellock JM, Marmarou A, DeLorenzo R. Time to treatment in prolonged seizure episodes. Epilepsy Behav. 2004;5:192–196. doi: 10.1016/j.yebeh.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Alldredge BK, Wall DB, Ferriero DM. Effect of Prehospital Treatment on the Outcome of Status Epilepticus in Children. Pediatr Neurol. 1995;12:213–216. doi: 10.1016/0887-8994(95)00044-g. [DOI] [PubMed] [Google Scholar]
- 12.Dreifuss FE, Rosman NP, Cloyd JC, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338:1869–1875. doi: 10.1056/NEJM199806253382602. [DOI] [PubMed] [Google Scholar]
- 13.Shinnar S, Hesdorffer DC, Nordli DR, Jr, et al. Phenomenology of Prolonged Febrile Seizures: Results of the FEBSTAT study. Neurology. 2008;71:170–176. doi: 10.1212/01.wnl.0000310774.01185.97. [DOI] [PubMed] [Google Scholar]
- 14.Hesdorffer DC, Shinnar S, Lewis DV, et al. Design and Phenomenology of the FEBSTAT Study. Epilepsia. 2012;53:1471–1480. doi: 10.1111/j.1528-1167.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Commission on Epidemiology and Prognosis, International League Against Epilepsy. . Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 16.Dodson WE, DeLorenzo RJ, Pedley TA, et al. The treatment of convulsive status epilepticus: recommendations of the Epilepsy Foundation of America’s working group on status epilepticus. JAMA. 1993;270:854–859. [PubMed] [Google Scholar]
- 17.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52:2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 18.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 19.Meberg A, Langslet A, Bredesen JE, et al. Plasma concentration of diazepam and N-desmethyldiazepam in children after a single rectal or intramuscular dose of diazepam. Eur J Clin Pharmacol. 1978;14:273–276. doi: 10.1007/BF00560461. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain JM, Capparelli EV, Brown KM, et al. Pharmacokinetics of intravenous lorazepam in pediatric patients with and without status epilepticus. J Pediatr. 2012;160:667–672. doi: 10.1016/j.jpeds.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinnar S, Pellock JM, Berg AT, et al. Short-Term Outcomes of Children with Febrile Status Epilepticus. Epilepsia. 2001;42:47–53. doi: 10.1046/j.1528-1157.2001.10000.x. [DOI] [PubMed] [Google Scholar]
- 22.Roy H, Lippe S, Lussier F, et al. Developmental outcome after a single episode of status epilepticus. Epilepsy Behav. 2011;21:430–436. doi: 10.1016/j.yebeh.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 23.van Esch A, Ramlal IR, Van Steensel-Moll HA, et al. Outcome after febrile status epilepticus. Dev Med Child Neurol. 1996;38:19–24. doi: 10.1111/j.1469-8749.1996.tb15028.x. [DOI] [PubMed] [Google Scholar]
- 24.Annegers JF, Hauser WA, Elveback LR, et al. The risk of epilepsy following febrile convulsions. Neurology. 1979;29:297–303. doi: 10.1212/wnl.29.3.297. [DOI] [PubMed] [Google Scholar]
- 25.Shinnar S, Berg AT, Moshe SL, et al. How long do new onset seizures in children last? Ann Neurol. 2001;49:659–664. [PubMed] [Google Scholar]
- 26.Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–122. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 27.Besli GE, Saltik S, Erguven M, et al. Status Epilepticus in Children: Causes, clinical features and short-term outcome. Pediatrics International. 2010;52:749–753. doi: 10.1111/j.1442-200X.2010.03164.x. [DOI] [PubMed] [Google Scholar]
- 28.Pellock JM. Treatment considerations: Traditional anti-epileptic drugs. Epilepsy Behav. 2002;3:S18–S23. doi: 10.1016/s1525-5050(02)00538-3. [DOI] [PubMed] [Google Scholar]
- 29.Chin RF, Neville BG, Peckham C, et al. Treatment of community-onset, childhood convulsive status epilepticus: A prospective population based study. Lancet Neurol. 2008;7:696–703. doi: 10.1016/S1474-4422(08)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassan H, Barzilay M, Shinnar S, et al. Prolonged febrile seizures: clinical characteristics and acute management. Epilepsia. 2013;54:1092–1098. doi: 10.1111/epi.12164. [DOI] [PubMed] [Google Scholar]
- 31.Wait S, Lagae L, Arzimanoglou A, et al. The administration of rescue medication to children with prolonged acute convulsive seizures in the community: What happens in practice? Eur J Paediatr Neurol. 2013;17:14–23. doi: 10.1016/j.ejpn.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Chin R, Verhulst L, Neville B, et al. Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004;75:1584–1588. doi: 10.1136/jnnp.2003.032797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 34.Shinnar S, Bello JA, Chan S, et al. MRI abnormalities following Febrile Status Epilepticus in Children: The FEBSTAT Study. Neurology. 2012;79:871–877. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]