Abstract
BACKGROUND
Horizontal gaze palsy and progressive scoliosis (HGPPS) is caused by mutations in the ROBO3 gene, which plays a role in axonal guidance during brain development. HGPPS is characterized by the congenital absence of conjugate lateral eye movements with preserved vertical gaze and progressive scoliosis, as well as dysgenesis of brainstem structures and ipsilateral projection of the pyramidal tract.
PATIENT
A 4-year-11-month-old girl presented with psychomotor retardation and autistic traits. Magnetic resonance imaging revealed hypoplasia and malformation of the ventral portion of the pons and medulla oblongata. Diffusion tensor imaging revealed the absence of decussation of the bilateral pyramidal tracts. These findings were similar to the typical findings for HGPPS. However, restriction of horizontal eye movement was minimal, and bilateral polymicrogyria were also noted in the occipitotemporal cortex in the present patient. These findings have not been previously reported in patients with HGPPS. No mutations in the ROBO3, SLIT1, SLIT2, NTN1, SEMA3A and SEMA3F genes were identified.
CONCLUSION
This patient may have a disorder caused by an unidentified factor, other than a mutation in the genes analyzed, involved in corticogenesis, axonal guidance, and brainstem morphogenesis.
Keywords: pontine malformation, brainstem hypoplasia, polymicrogyria, axonal guidance, decussation of the pyramidal tract, horizontal gaze palsy and progressive scoliosis
Introduction
Horizontal gaze palsy and progressive scoliosis (HGPPS), a rare autosomal recessive condition, is characterized by congenital absence of conjugate lateral eye movements with preserved vertical gaze and progressive scoliosis.1,2 Patients generally have a modest delay in motor milestones, whereas intellectual impairment is absent or mild.3 This disorder exhibits a characteristic appearance on magnetic resonance imaging (MRI), consisting of anterior flattening of the hindbrain and an anterior midline cleft in the medulla that can extend into the pons, resulting in a butterfly-like appearance.2,4 Specifically, sensory and motor pathways do not decussate into the contralateral sides at the levels of the spinal cord and the medulla oblongata in HGPPS. This lack of decussation can be demonstrated by diffusion tensor imaging (DTI)5 and by ipsilateral somatosensory evoked potentials or magnetic evoked potentials.2,3,5 The causative gene for this disorder, ROBO3, plays a role in axonal guidance during brain development.2
Here we report the case of a 4-year-old girl who demonstrated brainstem malformation with evidence of abnormal decussation of the pyramidal tract. These findings were compatible with a diagnosis of HGPPS. However, she does not harbor a detectable mutation in ROBO3, and she exhibited severe psychomotor developmental delay. In addition, bilateral polymicrogyria of the occipitotemporal cortex were observed on MRI. These findings suggest that this patient developed a syndrome that is related to, but distinct from HGPPS.
Case report
A 4-year-11-month-old Japanese girl was born uneventfully at full term to nonconsanguineous parents. She had no siblings. She gained the ability to smile socially at 4 months, to control her head at 6 months, to gaze at objects and to sit unsupported at 12 months, and to stand with support at 1 year and 7 months of age. She didn’t have swallowing or chewing difficulties. Brain MRI revealed brainstem malformation, and she was admitted to our hospital at 2 years and 6 months of age for further investigation. On examination, the patient presented with a body height of 82.5 cm (standard deviation [SD]: −1.6), a body weight of 10.0 kg (−1.6 SD), and a head circumference of 47.2 cm (−0.4 SD). No somatic dysmorphisms, including scoliosis, were noted. Her developmental quotient was assessed as 19. She could not utter meaningful words and displayed no social gestures. Stereotyped movements consisting of waving her hand in front of her face were often observed. In addition to these autistic traits, ocular abduction was limited to half of a normal excursion, while adduction and vertical eye movements were complete. She had normal hearing and no other clinical signs of cranial nerve involvement. Muscle tonus and deep tendon reflexes were normal, and pyramidal signs were negative. She showed no muscle weakness or ataxia.
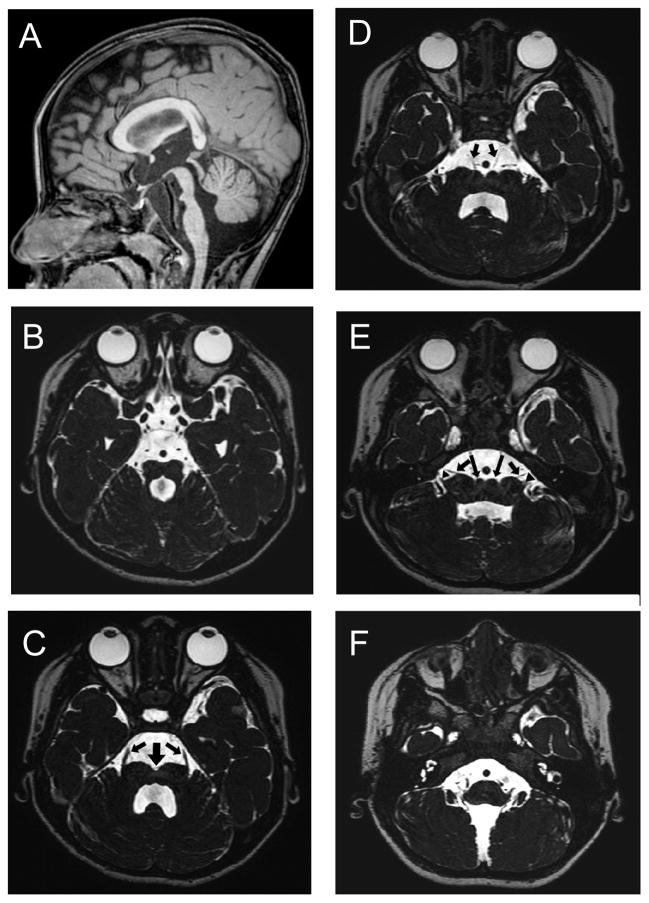
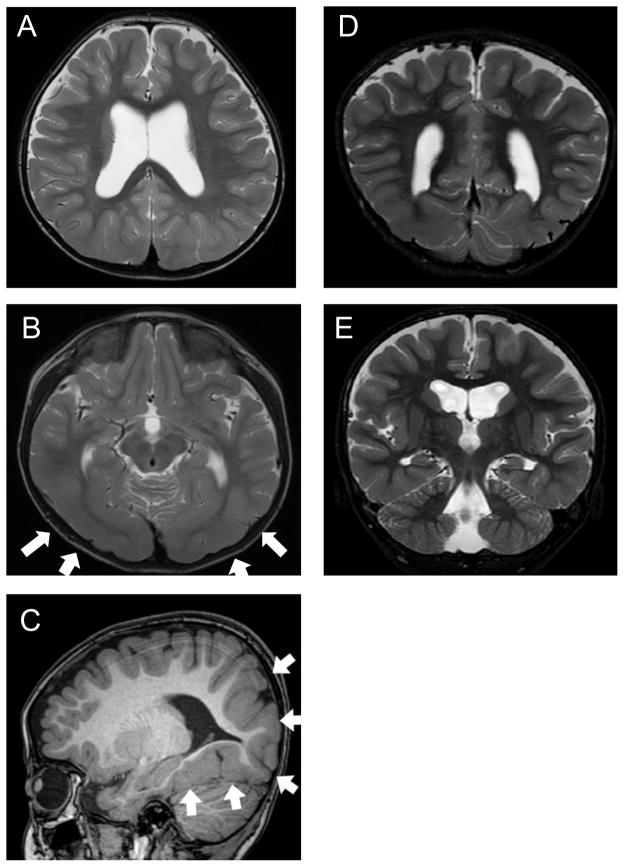
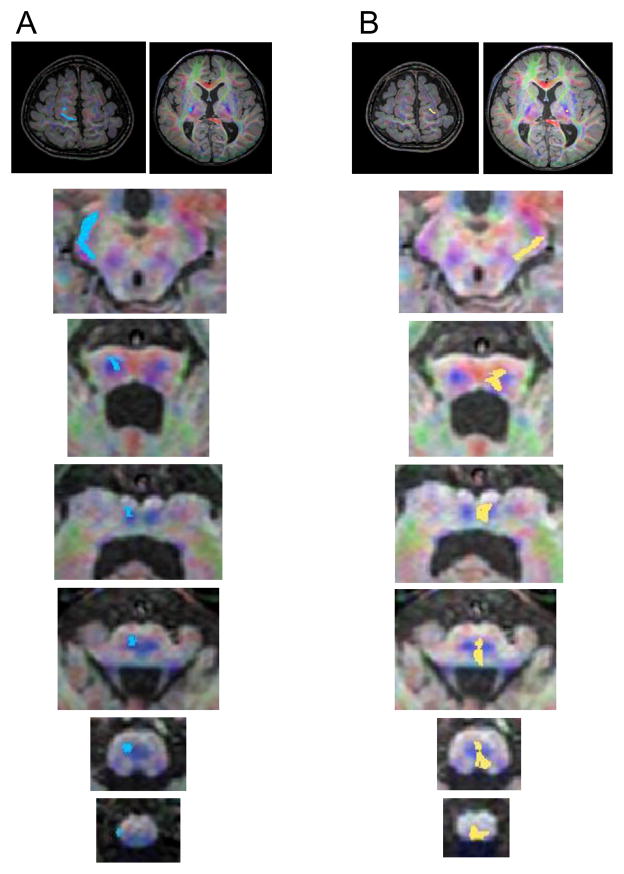
No abnormalities were found on general blood and urine analyses and on electroencephalography. MRI revealed pontine hypoplasia (Fig. 1) with a ventral slit at the midline of the pons (thick arrow in Fig. 1C) and small colliculi on both sides of the slit (long arrows in Fig. 1E). The prominences of the inferior olivary nuclei and bilateral pyramids were absent at the ventral medulla oblongata (Fig. 1F). There were no findings of a dorsal bump at the levels of upper pons, such as reported in pontine cap dysplasia (PTCD).6 Constructive interference in steady-state imaging revealed laterally displaced entry of the trigeminal nerve roots at the ventral pons (Fig. 1C) and unusually widely spaced entry points of the facial and vestibulocochlear nerves at the junction of the pons and medulla oblongata (Fig. 1E). The bilateral abducens nerves were identified along with their origins at the ventral colliculi of the pons (Fig. 1D). In addition, thickening of the cerebral cortex and reduced volume of the white matter were noted in the bilateral temporal and occipital lobes (Fig. 2A to 2D; arrows in B and C) accompanied by findings suggestive of subcortical heterotopia (arrows in Fig. 2D). The insular cortex also appeared to be dysmorphic (Fig. 2E). DTI revealed that the pyramidal tract, originating from both sides of the motor cortex, descended into the ipsilateral spinal cord without decussation (Fig. 3). Somatosensory evoked potentials demonstrated decussated responses with delayed latencies of the cortical components on both sides.
Figure 1.
Brainstem structures visualized on magnetic resonance imaging (MRI) of the patient at 2.5 years of age (A: T1-weighted sagittal section; B to F: constructive interference in steady-state sequences). A: The brainstem tegmentum appears hypoplastic at the level of the isthmus, and the protrusion of the ventral pons is attenuated. B: A molar tooth-like appearance is noted at the isthmus, composed by parallel orientation of superior cerebellar peduncles and an enlarged forth ventricle. B to E: A cleft (thick arrow) is seen at the midline along the entire length of the ventral pons. Small colliculi are noted on both sides of this cleft (long arrows in E). C: Entry of the trigeminal nerve roots (arrows) is dislocated to the most lateral end of the ventral pons. D: The abducens nerves (arrows) originate at the colliculli lateral to the midline cleft. E: The facial (arrows) and vestibulocochlear (arrowheads) nerves, which normally leave the brainstem in parallel and closely apposed to each other, originate separately.
Figure 2.
Supratentorial structures on MRI (A, B, D and E: T2-weighted images; C: T1-weighted image). The cerebral cortex is thick in the posterior areas, including the bilateral temporal and occipital lobes (white arrows in B and C). The multi-convoluted cortical surface (C) and irregular gray-white matter boundary (B, C) are compatible with the findings in polymicrogyria. A myelinated layer is noted regionally within the thick cortex, which is suggestive of subcortical heterotopia (arrows in E). In D, hippocampal formation appears somewhat plump, but is not obviously dysplastic.
Figure 3.
Diffusion tensor images of the bilateral pyramidal tracts. The tract from the right precentral gyrus is seen in blue (A), and that from the left precentral gyrus is seen in yellow (B). The levels of the central sulcus, the basal ganglia, the midbrain, the rostral and caudal pons, the rostral and caudal medulla oblongata, and the medullospinal transition are shown from top to bottom. Note that these pathways descend through the ipsilateral brainstem, and there is no evidence of decussation. Anomalous Sylvian fissures can be observed in the images at the level of central sulcus.
Chromosomal microarray testing7 did not reveal chromosomal deletions. Genetic analysis of ROBO3 and other genes involved in axonal guidance, including SLIT1, SLIT2, NTN1, SEMA3A and SEMA3F, revealed no pathological mutations in their exons.
Discussion
A defect in the guidance of hindbrain neurons and axons across the midline is thought to exist in HGPPS. A similar pathogenesis may be responsible for the malformations in the present patient. The guidance of hindbrain neurons is orchestrated by ROBO3, which acts as a receptor for Slit.2 Slit acts as a major regulator of the midline crossing of leading axons and migrating neurons in the developing nervous system.8 In particular, an essential function of ROBO3 is to allow midline decussation of the migrating neurons of the pontine and inferior olivary nuclei originating from the lateral recess of the fourth ventricle. These neurons migrate circumferentially beneath the pia mater to the ventral brainstem and further migrate beyond the midline to the contralateral side. The midline of the pons appears less permissive in ROBO3 mutant mice,9 resulting in defects of the pontine nuclei and commissural hindbrain projections.2,8,10 Although the exact role of the SLIT/ROBO pathway in pyramidal tract decussation and formation of the abducens nucleus is unclear, it is presumed to be essential for the normal development of these structures.4 Scoliosis is also believed to be caused by defects in the development of extrapyramidal projections.2
Apart from HGPPS, several disorders that present with defects in axonal guidance have been identified.11 PTCD has some features in common with HGPPS and the phenotype of the present patient, including pontine hypoplasia with narrow isthmus, as well as defective decussation of corticospinal tract and pontine transverse fibers.6,11 However, it is questionable whether the present case can be diagnosed as PTCD, because some of the telltale symptoms of PTCD were not present in this patient, such as vaulted pontine tegmentum on MRI, as well as impaired swallowing and facial nerve palsy. Joubert syndrome and other congenital cranial dysinnervation disorders could be excluded, based on the clinical and MRI features. Since there have been no reports on cortical malformation in diseases that involve defective axonal guidance, the condition of this patient may represent a novel disorder in this category, as well as in the category of hereditary bilateral polymocrogyria.12–14
The present patient did not have a mutation in ROBO3 or its ligand SLIT genes, nor did she have a mutation in factors in the netrin/DCC and semaphorin/neuropilin signaling pathways that are involved in axonal guidance. Presumably, there may be unidentified mutations in a gene other than these candidates that are implicated in pyramidal tract decussation in the developing brainstem. In particular, the findings of this case suggest that there may be factors that play roles similar to the SLIT/ROBO pathway in the development of brainstem structures, but may play a different role in the development of the cerebral cortex. Thus, this case report expands the category of HGPPS-like diseases and implies the presence of genetic defects in gene other than ROBO3, which is expressed in both the developing cerebral cortex and the brainstem and controls neuronal migration and axonal guidance. Alternatively, defects in angiogenesis might explain the brainstem anomalies accompanying bilateral polymicrogyria.
References
- 1.Dretakis EK, Kondoyannis PN. Congenital scoliosis associated with encephalopathy in five children of two families. J Bone Joint Surg Am. 1974;56(8):1747–1750. [PubMed] [Google Scholar]
- 2.Jen JC, Chan WM, Bosley TM, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304(5676):1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosley TM, Salih MA, Jen JC, et al. Neurologic features of horizontal gaze palsy and progressive scoliosis with mutations in ROBO3. Neurology. 2005;64(7):1196–1203. doi: 10.1212/01.WNL.0000156349.01765.2B. [DOI] [PubMed] [Google Scholar]
- 4.Rossi A, Catala M, Biancheri R, Di Comite R, Tortori-Donati P. MR imaging of brain-stem hypoplasia in horizontal gaze palsy with progressive scoliosis. AJNR Am J Neuroradiol. 2004;25(6):1046–1048. [PMC free article] [PubMed] [Google Scholar]
- 5.Sicotte NL, Salamon G, Shattuck DW, et al. Diffusion tensor MRI shows abnormal brainstem crossing fibers associated with ROBO3 mutations. Neurology. 2006;67(3):519–521. doi: 10.1212/01.wnl.0000227960.38262.0c. [DOI] [PubMed] [Google Scholar]
- 6.Barth PG, Majoie CB, Caan MWA, et al. Pontine tegmental cap dysplasia:a novel brain malformation with a defect in axonal guidance. Brain. 2007;130(Pt9):2258–2266. doi: 10.1093/brain/awm188. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Shimoima K, Nishizawa T, Matsuo M, Ito M, Imai K. Clinical manifestations of the deletion Down syndromcritical region including DYRK1A nd KCNJ6. Am J Med Genet A. 2011;155A(1):113–119. doi: 10.1002/ajmg.a.33735. [DOI] [PubMed] [Google Scholar]
- 8.Wong K, Park HT, Wu JY, Rao Y. Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev. 2002;12(5):583–591. doi: 10.1016/s0959-437x(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 9.Sabatier C, Plump AS, Le Ma, et al. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117(2):157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- 10.Marillat V, Sabatier C, Failli V, et al. The Slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron. 2004;43(1):69–79. doi: 10.1016/j.neuron.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Engle EC. Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol. 2010;2:a001784. doi: 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkovich AJ, Hevner R, Guerrini R. Syndromes of bilateral symmetrical polymicrogyria. AJNR Am J Neuroradiol. 1999;20:1814–1821. [PMC free article] [PubMed] [Google Scholar]
- 13.Barkovich AJ. Current concepts of polymicrogyria. Neuroradiology. 2010;52:479–487. doi: 10.1007/s00234-009-0644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leventer RJ, Jansen A, Pilz DT, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133:1415–1427. doi: 10.1093/brain/awq078. [DOI] [PMC free article] [PubMed] [Google Scholar]