Abstract
Studies show 30-47% of persons with heart failure (HF) have concomitant diabetes mellitus (DM). Self-care for persons with both of these chronic conditions is conflicting, complex and often inadequate. This pilot study tested an integrated self-care program for its effects on HF and DM knowledge, self-care efficacy, self-care behaviors and Quality of Life (QOL). Hospitalized HF-DM participants (n=71) were randomized to usual care or intervention using a 1:2 allocation and followed at 30 and 90 days after intervention. Intervention was an integrated education and counseling program focused on HF-DM self-care. Variables included demographic and clinical data, knowledge about HF and DM, HF and DM specific self- efficacy, standard HF and DM QOL scales, and HF and DM self-care behaviors. Analysis included descriptive statistics, multilevel longitudinal models for group and time effects, post-hoc testing and effect size calculations. Sidak adjustments were used to control for Type 1 error inflation. The integrated HF-DM self-care intervention conferred effects on improved HF knowledge (30 days, p=.05), HF self-care maintenance (30 and 90 days, p<.001), self-care management (90 days, p=.05), DM self efficacy (30 days, p=.03; 90 days, p=.004), general diet (30 days, p=.05), HF physical QOL (p=.04) and emotional QOL scores (p=.05) at 90 days within the intervention group. UC also reported increased total and physical QOL. Greater percentages of participants in the intervention group improved self reported exercise between 0-30 days (p=.005 and moderate effect size ES=.47), and foot care between 0-90 days (p=.03, small ES=.36). No group differences or improvements in DM specific QOL were observed. An integrated HF-DM self-care intervention was effective in improving essential components of self-care and had sustained (90 day) effects on selected self-care behaviors. Future studies testing HF-DM integrated self-care interventions in larger samples, with longer follow-up, and on other outcomes such as hospitalization and clinical markers are warranted.
Keywords: Heart Failure, Diabetes, Intervention, Self-care
Introduction
Heart failure (HF) is a serious epidemic affecting over 5.7 million persons in the United States (Roger et al., 2012). The societal and patient burden of HF is enormous, partially due to the high rate of rehospitalizations, reported to be 30% by 90 days (Butler & Kalogeropoulos, 2012) and 45% within 6 months (Ross et al., 2010). A striking 40-60% of rehospitalizations are believed to be preventable by greater provider attention to standards of care and better patient self-care (Heart Failure Society of America, 2010). An estimated 25.6 million adults have diabetes, and the national Medicare claims reflect that the annual incidence in the U.S. increased 23% between the years of 1995 and 2004 with prevalence increasing by 62% (Centers for Disease Control and Prevention, 2011; Sloan, Bethel, Ruiz, Shea, & Feinglos, 2008). Nearly one-third to one-half of people with HF have concomitant DM (Adams et al., 2005; Greenberg et al., 2007; Masoudi & Inzucchi, 2007; Sarma et al., 2013). There is a 40% to 80% increased risk of mortality among HF patients with DM, and a reported 1.6 fold increase in the relative risk for rehospitalization over those without DM (Bobbio et al., 2003; De Groote et al., 2004; Domanski et al., 2003; Dries, Sweitzer, Drazner, Stevenson, & Gersh, 2001; From et al., 2006; Gorelik et al., 2005). This is due to both worsening HF and higher burden of other co-morbidities, such as ischemic heart disease. Therefore, patients with concomitant HF and DM represent an increasing population with greater adverse outcomes, prompting us to intervene in this high-risk group.
HF-DM patients are confronted with intense, and often conflicting, expected self-care. Self-care behaviors for HF patients include following a low sodium diet, taking HF medications, performing and interpreting daily weights, physical activity, and monitoring symptoms of dyspnea, fatigue and edema (Heart Failure Society of America, 2010). DM patients are taught to manage a diabetic diet, take DM medications, monitor blood glucose, perform daily physical activity, and monitor symptoms of hypoglycemia and foot problems (Kerr et al., 2007). HF patients with the comorbidity of DM are at greatest risk of being readmitted due to fluid overload, inadequate glycemic control and other problems that might be preventable with better self-monitoring, self-care, and problem-solving. In addition to knowledge about self-care, self-efficacy has been associated with greater self-care behaviors in HF and DM (Cha et al., 2012; Dickson, Buck, & Riegel, 2013; Wu et al., 2013). Little evidence exists to guide the integration of HF and DM patient teaching or support patients’ understanding of their co-morbid self-care, such as how to follow a low sodium, diabetic diet, managing HF and DM medications and interactions, self monitoring of HF-DM symptoms, or which provider to contact for changes. The presence of HF may actually lead to less DM self-care prioritization and ability (Kerr et al., 2007; Piette & Kerr, 2006). Thus, tested processes and tools for co-morbidity self-management and care do not exist and are important to improve the quality of care and outcomes. The purpose of this pilot study (IMPROVE HF-DM) was to develop and test an integrated self-care education and counseling program for its short term effects on self-care antecedents and behaviors, and to examine the feasibility of such an integrated intervention. The hypothesis tested was that the HF-DM patients randomized to the intervention group would experience greater improvements in HF and DM specific knowledge, self-care efficacy, self-care behaviors and QOL.
Methods
Theoretical Framework
This study was designed around an integrated theoretical framework developed to guide HF self-care studies (Dunbar, Clark, Quinn, Gary, & Kaslow, 2008). The framework synthesizes concepts from patient self management theories and adult patient education concepts for learning and retention. The theoretical relationships of the variables are organized around antecedents and outcomes of self-care behaviors. Antecedents are individual and family factors and include sociodemographic, clinical, knowledge and skills, and behavioral factors. Within behavioral factors, self efficacy is noted as a powerful indicator of self-care performance and predictor of heart failure hospitalization (Riegel, Lee, Albert, Lennie, Chung, Song, Bentley, Heo, Worrall-Carter, Moser, 2011; Sarkar, Ali, Whooley, 2009). The interventions designed for this study targeted the selected antecedents of self-care behaviors of knowledge and self-efficacy through an integrated comorbidity self-care education and counseling intervention. Short term reinforcement, follow up and support were also designed to foster HF and DM knowledge and self efficacy. The framework suggests that improved self-care and simultaneous adherence to multiple self-care behaviors leads to improved outcomes in terms of health status and quality of life and lower resource utilization (Dunbar, Clark, Quinn, Gary, & Kaslow, 2008; Marti, Georgiopoulou, Giamouzis, Cole, Deka, Tang, Dunbar, Smith, Kalogeropoulos, Butler, 2013). We examined the primary effects of the intervention by indicators of HF and DM specific knowledge, self-efficacy, self-care or self-management behaviors and perceived QOL. The intervention also incorporated the chronic care model’s concepts of self-management support (patient education) and systems design (follow up care) (Wagner, 1998).
Design, Setting and Sample
Participants were recruited during an inpatient HF exacerbation at one of three participating hospitals. All were large tertiary care facilities with HF outpatient clinics. Inclusion criteria and exclusion criteria are presented in Table 1. Research nurses screened patients for eligibility through chart reviews, patient interviews, and assessments of depressive symptoms (Patient Health Questionnaire, PHQ-9) (Kroenke et al., 2001). HF-DM patients who were experiencing their first hospitalization for either disease were also excluded. All study procedures were approved by the Institutional Review Board of Emory University and each participating institution, and all participants provided written informed consent.
Table 1.
Participant inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria | ||
|---|---|---|---|
| • | Admitting diagnosis of HF with LVSD | • | Hemodynamically significant angina pectoris |
| • | Concomitant DM Type II treated with oral agents |
• | Renal failure |
| • | Aged 21-80 years | • | HF secondary to untreated medical condition |
| • | Planned discharge from hospital to home setting |
• | Planned cardiac surgery |
| • | English fluency | • | Impaired cognition due to neurological comorbidity |
| • | Without cognitive impairment | • | Psychiatric diagnosis |
| • | Uncorrected visual or hearing problem | ||
| • | Insulin therapy | ||
| • | Depressive symptoms (PHQ-9 > 10) | ||
| • | Evaluation for transplant or ventricular assist device |
||
LVSD, Left Ventricular Systolic Dysfunction
Demographic and clinical variables were collected from each patient and the medical record, and consisted of age, gender, marital status, education, ethnicity, left ventricular ejection fraction (LVEF), NYHA classification, body mass index (BMI), perceived health rating, and discharge medications. These variables, considered antecedent factors influencing self-care, were used to fully describe the sample and to compare treatment groups.
Participants were randomized into the study groups of usual care (UC) or usual care plus intervention (I). The randomization procedure was generated using a random sorting algorithm with a maximum allowable deviation of 10% (PASS 2008, NCSS LLC, Kaysville, Utah) for a final ratio of 1 UC to 2 Intervention. This allowed for greater efficiency in testing the intervention in a larger sample than if a 1:1 randomization had been used. Data were collected at baseline and at 30 and 90 days after the intervention. The time frames were selected for their importance to usual follow up care and to improve clinical feasibility and relevance of the study. Additionally, measurement of variables were timed to coincide with the expected intervention effects. All variables were measured at baseline, and Ouality of Life was measured again at 90 days as an overall outcome; the interim measurement point of 30 days was used for tracking short term change closer to the intervention for those variables targeted by the intervention - knowledge, self efficacy, and self-care behaviors and the 90 day measurement point was to examine for sustained change.
Variables and Instruments
Knowledge
The Atlanta HF Knowledge Test (AHFKT) was used to measure HF self-care knowledge (Reilly et al., 2009). The thirty item multiple-choice instrument was developed to measure aspects of heart failure understanding, medications, dietary sodium, fluid restriction and symptom recognition. Reliability was demonstrated in a study of HF patients by Cronbach’s alpha of .84 (Reilly et al., 2009), and this same study demonstrated construct validity. Knowledge of diabetes was tested by the Michigan Diabetes Knowledge Test (MDKT), which consists of a 14-item measurement of general diabetes knowledge. The test has adequate reported reliability, with a Cronbach’s alpha of .71 (Fitzgerald et al., 1998). Both measures are scored according to the percent of questions answered correctly.
Self-Efficacy
The self-care confidence scale of the Self-Care in Heart Failure Index Version 6.2 (SCHFI) (Riegel, Carlson, Moser, et al, 2004; Riegel, Lee, Dickson, Carlson, 2009) was used to assess self-efficacy in heart failure and is comprised of six items reflecting confidence in recognizing symptoms and taking HF actions. The responses range from 1=“Not Confident” to 4=“Extremely Confident”, and higher scores indicate more confidence in managing HF (Riegel et al., 2004). Internal reliability consistency is acceptable for the scale (alpha= .82), (Riegel et al., 2009). The Perceived Diabetes Self-Management Scale (PDSMS) is an eight-item instrument designed to test diabetes self-efficacy. The responses for the PDSMS items range from 1=“Strongly Disagree” to 5=“Strongly Agree”. The total score can range from 8 to 40, with higher scores indicating more confidence in managing diabetes; an acceptable Cronbach’s alpha was reported at .83 (Wallston, Rothman, & Cherrington, 2007).
Self-care Behaviors
Two scales of the SCHFI V6.2 (self-care maintenance, Cronbach’s alpha = .60 and self-care management, Cronbach’s alpha= .70) were used to assess self-care in heart failure. The self-care maintenance scale is comprised of 10 items that examine the frequency of performance of adherence and symptom monitoring behaviors and include activities such as daily weighing, following a low sodium diet, performing physical activity, and taking medications. The responses range from 1=“Never or Rarely” to 4=“Always or Daily”. The alpha in the self-care maintenance scale is acceptable in that the scale reflects disparate activities. The self-care management scale assesses the HF patient’s ability to recognize and respond appropriately to symptoms, including interpreting symptoms and contacting the provider, and the 6 items are scored only if symptoms are reported. Higher scores on all SCHFI scales reflect higher self-care within the domain of the scale. (Riegel et al., 2004, 2009) The Summary of Diabetes Self-Care Activities (SDSCA) scale has a core set of 11 items used to assess 5 diabetes self-management behaviors of diet, exercise, smoking, foot checks, and blood glucose testing. A series of seven studies (Toobert, Hampson, & Glasgow, 2000) has demonstrated adequate internal (.70) and retest reliability, with sensitivity to change. Patients report how many days in the previous week they have engaged in a certain activity, and scores are calculated for each of the 5 areas.
Quality of Life
The Minnesota Living with Heart Failure Questionnaire (MLHFQ) has 21-items which are rated on the extent to which various physical and emotional symptoms of HF have prevented them from living as they wanted in the past month (Rector et al., 1995). High scores indicate poorer quality of life. Total, physical and emotional subscale scores can be determined. Construct, convergent and discriminant validity of the MLHFQ has been established, with Cronbach’s alpha of .94 for the total scale and test-retest reliability was high after a 7- to 21-day period (weighted kappa reliability coefficients .84) (Bennet et al., 2002; Rector et al., 1995). A 5-point change on the total score demonstrates a clinically significant difference as validated by Middel et al. (2001) against the Hospital and Anxiety Depression Scale (HADS) and the Short Form Health Survey (SF-36). For DM specific QOL, the ADDQoL was used which consists of 19 items that measure physical functioning, symptoms, psychological well-being, social well-being, role activities, and personal constructs (Wee, Tan, Goh, & Li, 2006; Woodcock et al., 2004); it has been found to be sensitive to the changes in DM treatment and complications of diabetes (Bradley & Speight, 2002; Speight & Bradley, 2000). A 5-point scale measures the impact of diabetes by asking patients how particular aspects of their life would be if they did not have diabetes. The importance of these aspects on their life is rated on a 4-point scale. The two ratings are multiplied and summed for a final impact score that ranges from −9 to +9, where more negative scores indicate more negative impact of diabetes (Sundaram et al., 2007; Wee et al., 2006). Strong reliability (Cronbach’s alpha=0.94) and validity have been reported (Wee et al., 2006).
Intervention and procedures
After consenting to participate, a packet of questionnaires was completed with assistance by the research nurse. Participants received usual care or usual care plus the integrated intervention involving HF-DM education and self-management support. Usual Care was provided by the participants’ HF and DM health care providers, and two brochures, one each on overall HF (Heart Failure Society of America) and DM (American Diabetic Association) self-care, were given to the participants to assure equivalent access to information across enrolling sites. The I group received usual care from their providers and the same brochures, plus an educational and self-management intervention delivered initially by research nurses trained and retrained periodically to meet criteria reflecting their ability to deliver the protocol equivalently. Two 30-45 minute individual education/counseling sessions prior to discharge from the hospital were provided at the patient’s bedside. The intervention was developed to address the themes of self-care dilemmas identified through prior focus groups with HF-DM patients, and the intervention approach as well as materials were reviewed by experts and HF-DM patients, and refined. The intervention nurse uses a flip chart and a script for the educational session with the purpose of increasing knowledge and skills related to diet, medication taking, symptom monitoring, physical activity, and recognition of the interaction between self-management strategies for HF and DM. Participants were given an intervention resource notebook (Healthy Living with Heart Failure and Diabetes) developed for this project with all presented information in written form and additional materials to which they could refer in the home setting. These included integrated dietary sodium, fat, and carbohydrate content charts of common foods and fast foods, quick nutritional reference guides for appropriate snacks, restaurant dining tips, sample meal plans appropriate for low sodium and low carbohydrate goals, recipes, tailored medication informational sheets, an integrated monitoring diary/log for recording daily weight, HF-DM symptom and symptom management information, and schedules for medications, foot and eye care. Smoking cessation referrals were made for those who reported smoking or tobacco use. All materials were printed in large type and prepared at a 4th grade reading level with ample graphics and attention to culturally and regionally relevant information, particularly in dietary information. The discharge education intervention incorporated the individual patient’s medication and follow up care reconciliation.
Additional follow-up education and counseling for integrated self-care was provided with a 15 minute telephone call at approximately 48-72 hours after discharge during which verification of the medication regimen, filling of prescriptions, and daily self monitoring were emphasized. During a clinic visit 2-4 weeks after discharge, the research nurse assessed for difficulty in performing self-care behaviors of diet, physical activity, symptom and self monitoring, and provided reinforcing information and guidance, once again guided by a protocol and script. For intervention fidelity monitoring, research nurses performing the intervention recorded their activities at baseline, telephone calls and follow up visits, to document subjects’ receipt of the notebooks, time required for the intervention, and to document specific questions or issues raised by the participant. Participants completed a brief intervention evaluation form at the end of their participation in the study.
Around 30 and 90 days following the enrollment date, the participants were mailed the same questionnaire packet as completed at baseline and were provided with a stamped return addressed envelope. The exception was that QOL was only measured at baseline and 90 days. The research nurse met the participants on their return follow-up visit to the HF clinic at the study time frames if assistance was needed to collect or complete the packet.
Data Analysis
All data were reviewed for data entry errors, potential outliers, missing data as well as any differences between groups on demographic and clinical variables using t-test and chi-square tests. Fewer participants in the UC group were African American (AA) (41.2%) compared to the I Group (68.2%, p=.05) (Table 2). Thus race was included as a covariate in the models. Distributions were also assessed for deviations from normality. No differences were noted in missing data between groups over time. However, due to a minor amount of intermittent missing items in the MLWHF (5 items of less) substitutions were made using the average response across items within a given subscale within each individual with missing items. SCHFI exercise, blood glucose and foot care were significantly skewed and were dichotomized (SCHFI exercise = 0 versus >0; SCHFI Blood Glucose and Foot care <7 versus =7). Multilevel mixed (MLM) longitudinal models (SPSS Statistics, version 20, copyright IBM Corporation, 2011 [SPSS v.20] MIXED) were used for testing group, time and group-by-time effects after adjusting for covariates for outcomes measured at all 3 time points (AHFKT, MDKT, three SCHFI scales, PDSMS, SDSCA General and Specific Diet). In addition to age, NYHA class, gender, education, ejection fraction, and BMI were also considered as potential covariates. Only those with p<.05 were included as covariates in the models. For the dichotomized SDSCA exercise, blood glucose and foot care scales, multilevel mixed longitudinal models for a binomial response function with a logit link function (e.g. logistic regression) was used (SPSS v.20 GENLINMIXED) to test for time effect after adjusting for age within each group and included planned contrasts for time using baseline as the reference. Repeated measures analysis of covariance (RM-ANCOVA) (SPSS v.20 GLM) was used for the quality of life outcomes measured at only 2 time points (baseline and 90 days for MLWHF total, physical and emotional scores and ADDQOL) after adjusting for age. All planned post hoc pairwise tests were performed using Sidak alpha adjustment, which is slightly more powerful than Bonferroni (DeMuth, 2006). All effect sizes (ES) for significant post hoc comparisons are noted throughout and were calculated as the ratio of the mean difference divided by the standard deviation which is Cohen’s d (d=0.30 is considered a small ES, d=0.5 a moderate ES and d=0.8 a large ES), and for the multiliel logistic regression models, Cohen’s d was calculated as a function of the test statistic for the corresponding effect’s test (Cohen, 1988). To test the internal consistency reliability of the instruments in this sample, Cronbach’s alpha was determined and is reported in the relevant table describing results for the appropriate instrument. The Cronbach’s alpha before and after substitutions for MLWHF were the same on this instrument (reported in Table 9).
Table 2.
Demographic and Clinical Variables
| All (n=61) |
Usual Care (n=17) |
Intervention (n=44) |
Group Differences (p-value) |
|
|---|---|---|---|---|
| Age years (mean (SD)) | 59.7 (10.6) | 63.8 (9.5) | 58.2 (10.7) | .064* |
| Gender (% male) | 67.2 % | 70.6 % | 65.9 % | .727** |
| Race (% AA) | 60.7 % | 41.2 % | 68.2 % | .053** |
| Married (% married) | 45.9 % | 29.4 % | 52.3 % | .108** |
| Education (% hs or less) | 36.7 % | 29.4% | 39.5% | .463** |
| NYHA Class II (%) | 31.7 % | 17.6 % | 37.2 % | .319** |
| Class III (%) | 56.7 % | 70.6 % | 51.2 % | |
| Class IV (%) | 11.7 % | 11.8 % | 11.6 % | |
| LVEF % (mean (SD)) | 32.7 (14.9) | 29.1 (12.3) | 34.1 (15.8) | .394c |
| BMI (mean (SD)) | 37.0 (8.8) | 36.2 (9.6) | 37.3 (8.5) | .688* |
| Health rating (% poor) | 44.8 % | 46.7 % | 44.2 % | .868** |
| HgA1c (mean (SD)) | 8.1 (1.8) | 8.3 (2.1) | 8.0 (1.7) | .581c |
| Prior MI (% yes) | 53.3 % | 50.0 % | 54.5 % | .755** |
| PVD (% yes) | 11.9 % | 11.8 % | 11.9 % | 1.00d |
| Renal Disease (% yes) | 31.1 % | 29.4 % | 31.8 % | .856** |
Abbreviations: SD, Standard Deviation; AA, African American; HS, High School; NYHA, New York Heart Association; LVEF, Left Ventricular Ejection Fraction; BM, Body Mass Index; MI, Myocardial Infarction; PVD, Peripheral Vascular Disease.
Student’s t-test
Chi-square test
Mann Whitney test
Fisher’s Exact Test (2-sided)
Table 9.
HF and Diabetes QOL Unadjusted Means and SDs
| Measure | Baseline | 90d | ||
|---|---|---|---|---|
|
| ||||
| n | Mean (SD) | n | Mean (SD) | |
| MLWHF (Cα=0.93) | 57 | 63.9 (23.4) | 46 | 47.1 (25.9) |
|
| ||||
| Usual Care | 16 | 61.3 (20.5) | 11 | 41.8 (19.7) |
| Intervention | 41 | 64.9 (24.6) | 35 | 48.8 (27.5) |
|
| ||||
| MLWHF Physical | 57 | 27.3 (9.9) | 46 | 20.9 (12.0) |
|
| ||||
| Usual Care | 16 | 26.9 (8.0) | 11 | 19.0 (10.7) |
| Intervention | 41 | 27.5 (10.6) | 35 | 21.6 (12.4) |
|
| ||||
| MLWHF Emotional | 57 | 12.0 (7.7) | 45 | 9.5 (7.7) |
|
| ||||
| Usual Care | 16 | 10.5 (6.9) | 11 | 8.8 (7.3) |
| Intervention | 41 | 12.6 (8.1) | 34 | 9.8 (7.9) |
|
| ||||
| ADDQOL (Cα=0.92) | 56 | −3.61 (2.3) | 47 | −3.24 (2.6) |
|
| ||||
| Usual Care | 16 | −2.85 (1.8) | 12 | −2.04 (1.9) |
| Intervention | 40 | −3.91 (2.5) | 35 | −3.66 (2.7) |
Abbreviations: SD, standard deviation; Cα, Cronbach’s alpha reliability.
Results
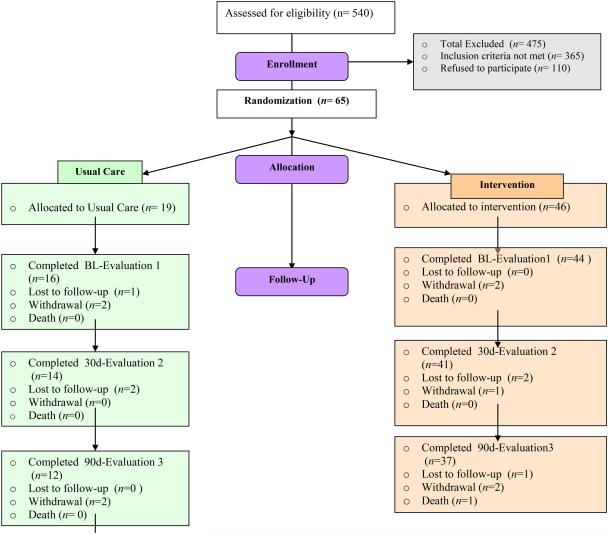
A total of 540 individuals were assessed for eligibility, and 65 participants were enrolled, with 19 individuals allocated to the UC group with 16 completing baseline and 46 allocated to I group with 44 completing baseline (Figure 1). Of the 475 excluded from the study, 110 people declined to participate, and 365 did not meet inclusion criteria or were not enrolled for reasons such as living too far from the research center. Those declining to participate gave reasons of feeling too uncertain about their future, too busy, feeling too sick, and in rare cases, already knowing the information. Throughout the course of the study, six individuals were lost to follow-up, nine participants withdrew, and two participants were lost due to death for an overall 25% attrition rate (16/65) (37% for UC (7/19) and 20% (9/46) for I, no significant differences by group: X2(1)=2.19, p=.14).
Figure 1.
IMPROVE HF-DM Consort Chart. BL, baseline.
In spite of the randomization procedures, comparison of the demographic and clinical variables of the UC and I groups (Table 2) revealed a significant group difference in the proportion of African Americans with the I group having a greater percentage than the UC group. There were more men and a lower education level in participants from one clinical site, however this did not affect randomized group composition. There was also a borderline but not significant group difference in age (UC slightly older than I) and ejection fraction (LVEF) with participants in UC having slightly lower values than I. There were no group differences in any other variables.
The mean age of participants was 59.7 years old, and while the majority were male, the characteristics reveal good representation overall of women (32.8%) and African Americans (60.7%). Forty-six percent were married with most were fairly well educated with around a third having a high school or less level of education. The majority were NYHA class III with mean LVEF of 32.7%. The overall body mass index was in the obese range (mean 37), and 44.8% considered their health to be poor. Regarding their DM status and glycemic control, all were Type II on oral agents as specified in the inclusion criteria, and the average HgA1c level was 8.1%. The onset of DM preceded the onset of HF in 64.9% of participants by a mean of 5.8 ± 8.9 years.
Knowledge
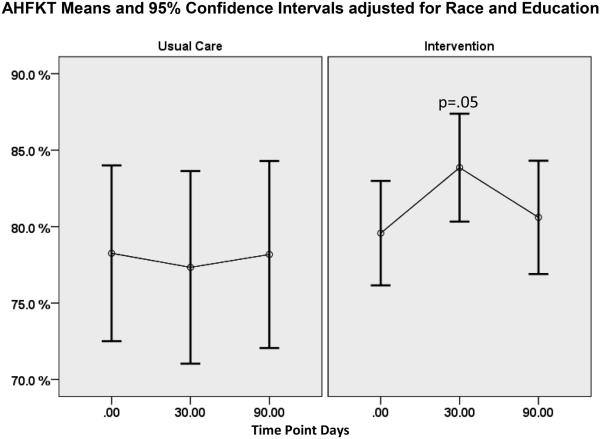
On average, HF knowledge was 78% or higher for both groups baseline (Table 3). Race and education were found to be significant or near significant covariates for assessment of knowledge on both tests (AHFKT race p=0.035, education p=.06); MDKT race p=0.001, education p=.002) where AA scored significantly lower than White participants, and participants with higher formal education had higher HF or DM knowledge scores. For the I group, AHFKT scores improved on average by 4.3 percentage points from baseline to 30 days (Table 4, p=.05, small ES=0.38), although this improvement was mostly lost by 90 days (Figure 2). Diabetes knowledge scores averaged 58% or higher and tended to be lower than HF knowledge scores overall (Table 3). The MLM longitudinal model for diabetes knowledge did not result in significant change (Table 4). Cronbach’s alpha obtained n this study for both instruments were acceptable as noted in Table 3.
Table 3.
Knowledge of HF and Diabetes (Unadjusted Means and SDs)
| Measure | Baseline | 30d | 90d | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| AHFKT (Cα=0.83) | 60 | 78.8 (12.0) | 52 | 81.5 (13.4) | 48 | 79.0 (14.7) |
|
| ||||||
| Usual Care | 16 | 81.0 (12.9) | 12 | 80.8 (8.9) | 13 | 81.0 (15.1) |
| Intervention | 44 | 78.0 (11.6) | 40 | 81.7 (14.6) | 35 | 78.2 (14.7) |
|
| ||||||
| MDKT (Cα=0.74) | 60 | 58.6 (19.9) | 51 | 59.7 (16.5) | 48 | 61.1 (18.9) |
|
| ||||||
| Usual Care | 16 | 64.6 (20.9) | 12 | 62.8 (16.7) | 13 | 69.2 (16.2) |
| Intervention | 44 | 56.4 (19.4) | 39 | 58.8 (16.5) | 35 | 58.1 (19.1) |
Abbreviations: AHFKT, Atlanta Heart Failure Knowledge Test; MDKT, Michigan Diabetes Knowledge Test; SD, standard deviation; Cα, Cronbach’s alpha reliability.
Table 4.
Multilevel Longitudinal Models and Planned Contrast Tests for Knowledge
| Outcome Measure Model Effect |
F(df1,df2) | p-value | Planned Pairwise Post Hoc Contrasts (Sidak adjusted p-value < .10) |
|---|---|---|---|
| AHFKT | |||
|
| |||
| Race | F(1,54.954)=8.939 | .004 | |
| Education | F(1,55.420)=3.711 | .06 | |
| Group | F(1,55.704)=1.383 | .25 | |
| Time | F(2,100.648)=0.448 | .64 | |
| Group-x-Time | F(2,100.629)=1.108 | .33 | |
| Time within Usual Care | F(2,101.068)=0.050 | .95 | |
| Time within Intervention | F(2,99.195)=3.077 | .05 | BL – 30 days (p=.05) Δ=4.28 (SD 11.13) ES 0.38 |
|
| |||
| MDKT | |||
|
| |||
| Race | F(1,56.217)=12.575 | .001 | |
| Education | F(1,56.406)=10.276 | .002 | |
| Group | F(1,56.622)=0.024 | .88 | |
| Time | F(2,101.715)=1.037 | .36 | |
| Group-x-Time | F(2,101.732)=0.603 | .55 | |
| Time within Usual Care | F(2,102.119)=1.026 | .36 | |
| Time within Intervention | F(2,100.356)=0.240 | .79 | |
Abbreviations: BL baseline; Δ, mean difference; SD; standard deviation; ES, effect size
Figure 2.
AHFKT Test Means and 95% Confidence Intervals Adjusted for Race and Education.
Self-Efficacy
The SCHFI-Confidence scores were not significantly different between the groups at baseline (Table 5). After adjusting for race (which was a significant covariate, p=.03, with lower scores obtained in AA versus White participants), no effects (group, time, group x time) were significant for SCHFI confidence scores (Table 6). Age (p=.01) and LVEF (p<.001) were significant covariates for the PDSMS score where older participants and those with lower LVEF had higher PDSMS scores. For both groups, PDSMS scores increased from baseline with a significant time effect (p<.001) in the MLM longitudinal model after adjusting for age, LVEF, and race (Table 6). Post hoc tests revealed significant PDSMS improvements from baseline to 30 days for the I group (p=.03, small to moderate ES=0.42) and from baseline to 90 days for both UC (p=.05, large ES=0.73) and I (p=.004, moderate to large ES=0.61).
Table 5.
Heart Failure and Diabetes Self-Efficacy (Unadjusted Means and SDs)
| Measure | Baseline | 30 Days | 90 Days | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| SCHFI–Confidence (Cα=0.85) |
56 | 70.5 (19.9) | 48 | 75.4 (17.1) | 45 | 75.2 (16.5) |
|
| ||||||
| Usual Care | 16 | 67.8 (19.2) | 11 | 73.3 (16.5) | 13 | 71.9 (20.5) |
| Intervention | 40 | 71.6 (20.3) | 37 | 76.0 (17.5) | 32 | 76.6 (14.7) |
|
| ||||||
| PDSMS (Cα=0.77) | 54 | 27.3 (6.2) | 50 | 29.8 (5.5) | 42 | 31.5 (5.7) |
|
| ||||||
| Usual Care | 12 | 27.4 (5.9) | 12 | 31.0 (5.9) | 11 | 32.7 (4.7) |
| Intervention | 42 | 27.2 (6.3) | 38 | 29.4 (5.4) | 31 | 31.0 (5.9) |
Abbreviations: SD, standard deviation; Cα, Cronbach’s alpha reliability
Table 6.
Multilevel Longitudinal Models and Planned Contrast Tests for Self-Efficacy
| Outcome Measure (Model Effect) |
F(df1,df2) | p-value | Planned Pairwise Post Hoc Contrasts (Sidak adjusted p-value < .10) |
|---|---|---|---|
| SCHFI – confidence | |||
|
| |||
| Race | F(1,57.297)=5.261 | .03 | |
| Group | F(1,58.169)=1.748 | .19 | |
| Time | F(2,95.071)=2.705 | .07 | |
| Group-x-Time | F(2,94.995)=0.038 | .96 | |
| Time within Usual Care | F(2,94.996)=1.025 | .33 | |
| Time within Intervention | F(2,94.898)=2.207 | .11 | |
|
| |||
| PDSMS | |||
|
| |||
| Age | F(1,51.739)=6.368 | .02 | |
| Ejection Fraction | F(1,53.706)=13.931 | <.001 | |
| Race | F(1,53.318)=0.281 | .60 | |
| Group | F(1,54.823)=0.378 | .54 | |
| Time | F(2,93.707)=7.548 | .001 | |
| Group-x-Time | F(2,93.609)=0.106 | .90 | |
| Time within Usual Care | F(2,94.904)=3.062 | .05 | BL – 90 days (p=.05) Δ=4.50 (SD 6.19) ES 0.73 |
| Time within Intervention | F(2,89.995)=6.367 | .003 | BL – 30 days (p=.03); BL – 90 days (p=.004) Δ=2.55 (SD 5.99) ES 0.42; Δ=3.52 (SD 5.75) ES 0.61 |
Abbreviations: BL, baseline; Δ, mean difference; SD, standard deviation; ES, effect size
Self-Care Behaviors
Self-care maintenance and management scores were not significantly different between the groups at baseline (Table 7). After adjusting for race and education, the time effect for SCHFI-Maintenance was significant (p<.001, Table 8) with significant improvements in the I group from baseline to 30 days (p<.001, very large ES=1.08) and from baseline to 90 days (p<.001, large ES=0.93). Usual Care group also showed a increase from baseline to 30 days but this was not sustained at 90 days. Self-care management scores were calculated only on a subset of participants who answered yes to item 11 “In the past month, have you had trouble breathing or ankle swelling?” After adjusting for race and LVEF, there was a significant improvement in SCHFI-Management scores for the I group from baseline to 90 days (p=.05, moderate ES=0.56) (Table 8).
Table 7.
HF and Diabetes Self-care (Unadjusted Means and SDs or Frequencies and Percents)
| Measure | Baseline | 30 Days | 90 Days | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Mean (SD) | n | Mean (SD) |
n | Mean (SD) | |
| SCHFI–Maintenance (Cα=0.77) | 53 | 67.1 (19.4) | 44 | 82.9 (12.5) |
39 | 79.1 (14.9) |
|
| ||||||
| Usual Care | 15 | 67.8 (23.7) | 9 | 83.0 (18.4) |
11 | 76.1 (18.0) |
| Intervention | 38 | 66.8 (17.9) | 35 | 82.8 (10.9) |
28 | 80.3 (13.6) |
|
| ||||||
| SCHFI–Management* (Cα=0.73) | 53 | 68.4 (23.7) | 29 | 76.7 (18.4) |
26 | 76.9 (19.9) |
|
| ||||||
| Usual Care | 15 | 65.3 (23.1) | 4 | 80.0 (4.1) | 6 | 72.5 (17.0) |
| Intervention | 38 | 69.6 (24.1) | 25 | 76.2 (19.8) |
20 | 78.3 (20.9) |
|
| ||||||
| SDSCA–General Diet (Cα=0.80) | 57 | 4.94 (1.9) | 50 | 5.76 (1.2) | 48 | 5.22 (1.5) |
|
| ||||||
| Usual Care | 14 | 5.07 (1.3) | 12 | 6.17 (1.0) | 13 | 4.73 (1.9) |
| Intervention | 43 | 4.90 (2.1) | 38 | 5.63 (1.2) | 35 | 5.40 (1.3) |
|
| ||||||
| SDSCA-Specific Diet (Cα=0.06) | 59 | 4.41 (1.6) | 50 | 4.84 (1.4) | 48 | 4.74 (1.5) |
|
| ||||||
| Usual Care | 15 | 4.43 (1.4) | 12 | 5.13 (1.0) | 13 | 4.35 (1.4) |
| Intervention | 44 | 4.40 (1.7) | 38 | 4.75 (1.5) | 35 | 4.89 (1.5) |
|
| ||||||
| count/n | (%) | count/n | (%) | count/n | (%) | |
|
| ||||||
| SDSCA–Exercise [%>0]†
(Cα=0.78) |
33/58 | (56.9 %) | 42/51 | (82.4 %) | 35/46 | (76.1 %) |
|
| ||||||
| Usual Care | 9/14 | (64.3 %) | 9/12 | (75.0 %) | 10/13 | (76.9 %) |
| Intervention | 24/44 | (54.5 %) | 33/39 | (84.6 %) | 25/33 | (75.8 %) |
|
| ||||||
| SDSCA–Blood Glucose [%=7]†
(Cα=0.87) |
34/58 | (58.6 %) | 30/51 | (58.8 %) | 23/46 | (50.0 %) |
|
| ||||||
| Usual Care | 7/15 T | (46.7 %) | 9/12 | (75.0 %) | 6/13 | (46.2 %) |
| Intervention | 27/43 | (62.8 %) | 21/39 | (53.8 %) | 17/33 | (51.5 %) |
|
| ||||||
| SDSCA–Foot Care [%=7]†
(Cα=0.53) |
21/59 | (35.6 %) | 21/51 | (41.2 %) | 27/46 | (58.7 %) |
|
| ||||||
| Usual Care | 5/15 | (33.3 %) | 4/12 | (33.3 %) | 6/13 | (46.2 %) |
| Intervention | 16/44 | (36.4 %) | 17/39 | (43.6 %) | 21/33 | (63.6 %) |
Abbreviations: SD, standard deviation; Cα, Cronbach’s alpha reliability
SCHFI-Management scores calculated only on subjects who answered yes to item 11, “In the past month, have you had trouble breathing or ankle swelling?”
SDSCA – Exercise, -Blood Glucose and –Foot Care were dichotomized since the underlying distributions were highly skewed with the majority of the scores either zero and non-zero for exercise and <7 or =7 for Blood Glucose and Foot Care.
Table 8.
Multilevel Longitudinal Models and Planned Contrast Tests for Self-Care
| Outcome Measure Model Effect |
F(df1,df2) | p-value | Planned Pairwise Post Hoc Contrasts (Sidak adjusted p-value < .10) |
|---|---|---|---|
| SCHFI–Maintenance | |||
|
| |||
| Race | F(1,56.568)=8.997 | .004 | |
| Education | F(1,58.340)=3.744 | .058 | |
| Group | F(1,58.730)=2.353 | .13 | |
| Time | F(2,85.952)=16.991 | <.001 | |
| Group-x-Time | F(2,86.182)=0.542 | .58 | |
| Time within Usual Care | F(2,86.460)=3.910 | .02 | BL – 30 days (p=.05); BL – 90 days (p=.09) Δ=11.23 (SD 13.69) ES 0.82; Δ=9.43 (SD 14.31) ES 0.66 |
| Time within Intervention | F(2,84.527)=22.642 | <.001 | BL – 30 days (p<.001); BL – 90 days (p<.001) Δ=16.11 (SD 14.95) ES 1.08; Δ=13.52 (SD 14.52) ES 0.93 |
|
| |||
| SCHFI–Management | |||
|
| |||
| Race | F(1,51.309)=2.347 | .13 | |
| Ejection Fraction | F(1,48.272)=8.927 | .004 | |
| Group | F(1,65.692)=3.349 | .07 | |
| Time | F(2,68.000)=2.318 | .11 | |
| Group-x-Time | F(2,67.939)=0.677 | .51 | |
| Time within Usual Care | F(2,68.713)=0.874 | .42 | |
| Time within Intervention | F(2,64.353)=3.152 | .05 | BL – 90 days (p=.05) Δ=11.22 (SD 20.12) ES 0.56 |
|
| |||
| SDSCA – general diet | |||
|
| |||
| Race | F(1,46.569)=0.144 | .71 | |
| Group | F(1,48.431)=0.020 | .89 | |
| Time | F(2,94.843)=5.046 | .09 | |
| Group-x-Time | F(2,94.794)=1.346 | .27 | |
| Time within Usual Care | F(2,96.252)=3.285 | .04 | 30 days – 90 days (p=.05) Δ=1.34 (SD 1.98) ES 0.67 |
| Time within Intervention | F(2,90.599)=2.947 | .05 | BL – 30 days (p=.05) Δ=0.73 (SD 1.86) ES 0.39 |
|
| |||
| SDSCA – specific diet | |||
|
| |||
| Race | F(1,57.937)=1.484 | .23 | |
| Group | F(1,59.768)=0.099 | .75 | |
| Time | F(2,107.552)=1.893 | .15 | |
| Group-x-Time | F(2,107.539)=1.132 | .33 | |
| Time within Usual Care | F(2,108.478)=1.516 | .22 | |
| Time within Intervention | F(2,104.798)=1.421 | .25 | |
|
| |||
| SDSCA–Exercise > 0† |
[planned time contrasts using baseline as
reference] |
||
|
| |||
| Race | F(1,148)=2.105 | .15 | |
| Group | F(1,148)=0.127 | .72 | |
| Time | F(2,148)=2.522 | .08 | |
| Group-x-Time | F(2,148)=0.436 | .65 | |
| Usual Care: BL-30d | F(1,148)=0.449 | .51 | |
| Usual Care: BL-90d | F(1,148)=0.605 | .44 | |
| Intervention: BL-30d | F(1,148)=8.319 | .005 | T=−2.884 (df=148), ES=0.47 |
| Intervention: BL-90d | F(1,148)=3.557 | .06 | |
|
| |||
| SDSCA– Blood Glucose = 7† |
[planned time contrasts using baseline as
reference] |
||
|
| |||
| Race | F(1,148)=2.350 | .13 | |
| Group | F(1,148)=0.059 | .81 | |
| Time | F(2,148)=1.234 | .29 | |
| Group-x-Time | F(2,148)=1.726 | .18 | |
| Usual Care: BL-30d | F(1,148)=2.800 | .10 | |
| Usual Care: BL-90d | F(1,148)=0.019 | .89 | |
| Intervention: BL-30d | F(1,148)=0.655 | .42 | |
| Intervention: BL-90d | F(1,148)=1.561 | .21 | |
|
| |||
| SDSCA–Foot Care = 7† |
[planned time contrasts using baseline as
reference] |
||
|
| |||
| Race | F(1,149)=0.161 | .69 | |
| Group | F(1,149)=0.328 | .57 | |
| Time | F(2,149)=2.109 | .13 | |
| Group-x-Time | F(2,149)=0.078 | .93 | |
| Usual Care: BL-30d | F(1,149)=0.000 | .99 | |
| Usual Care: BL-90d | F(1,149)=0.875 | .35 | |
| Intervention: BL-30d | F(1,149)=0.381 | .54 | |
| Intervention: BL-90d | F(1,149)=4.768 | .03 | t=−2.184 (df=149), ES=0.36 |
Abbreviations: BL, baseline; Δ, mean difference; SD, standard deviation; ES, effect size
SDSCA-Exercise>0, SDSCA-Blood Glucose=7 and SDSCA-Foot Care=7 were analyzed using generalized multilevel longitudinal models for a Binomial distribution with a Logit link function using planned contrasts to test for changes from baseline to 30days and baseline to 90 days for each group.
The SDSCA-General and -Specific Diet scores were very similar between usual care and intervention groups at baseline (Table 7). After adjusting for race, there were significant increases in SDSCA-General Diet scores for the I group from baseline to 30 days (p=.05, small to moderate ES =.39) and significant decreases for UC from 30 to 90 days (p=.05). Group, time, or group x time effects were not significant for SDSCA Specific Diet scores after adjusting for race (Table 8).
The dichotomized SDSCA-Exercise scores split participants into those that scored 0 versus >0, and generalized MLM longitudinal models were run for a binomial response function with a logit link function (e.g. logistic regression) to test the group, time, and group x time effects after adjusting for race (Table 8). Sixty-four percent of UC group had SDSCA-Exercise scores >0 at baseline which increased to 75% at 30 days and 77% at 90 days, however these changes were not significant (Table 8). Fifty-five percent of I group had SDSCA- Exercise scores >0 at baseline which was not significantly different from UC at baseline. However, by 30 days 85% of the I group had SDSCA-Exercise scores >0 (p=.005, moderate ES=.0.47, Table 8) and 76% trended toward still having scores >0 at 90 days (p=.06). For SDSCA-Blood Glucose, fewer usual care participants (46.7%) had scores =7 at baseline than intervention participants (62.8%) but this was not significant. For UC, by 30 days, 75% had SDSCA-Blood Glucose scores =7 but this dropped back to 46.2% by 90 days. For I group, SDSCA-Blood Glucose scores =7 dropped slightly from 62.8% at baseline to 53.8% at 30 days and 51.5% at 90 days. However, these changes were not significant over time after adjusting for race (Table 8). Both UC and I had only about one-third of the participants with SDSCA-Foot Care scores =7 at baseline. Although both groups increased the percentage of participants with SDSCA-Foot Care scores =7 by 90 days, the change in UC was not significant whereas I increased to 43.6% at 30 days and 63.6% at 90 days (p=.03, small ES=0.36) after adjusting for race (Table 8).
The SDSCA has a self-report measure of smoking, and a yes to whether they have smoked any cigarettes in the past 7 days prompts for a report of how many cigarettes they smoke on an average day. At baseline, three participants acknowledged smoking in the past week, with all smoking three cigarettes per day. All were in the I group, and all subsequently answered no to this question at 30 and 90 days. There were too few participants who smoked to track the effect on this behavior, however the self-report data revealed some positive trends.
Quality of Life
Descriptive statistics for HF failure QOL (MLWHF total, physical and emotional subscales) and diabetes QOL (ADDQOL) at the baseline and 90-day time points for both groups are provided in Table 9. Acceptable Cronbach’s alphas were obtained as reported in Table 9. Significant improvements from baseline were seen for overall heart failure quality of life (MLWHF total) for both UC (p=.03, large ES=0.69) and I (p=.001, moderate to large ES=0.65) after adjusting for race and age (Table 10). This improvement was also seen for both the I group for the MLWHF physical subscale (p=.01, ES=0.47) and UC group (p=.01, ES=.77) after adjusting for race. There was a small improvement in MLWHF Emotional subscale scores the I group (p=.05, small ES = .35). No significant changes were seen for ADDQOL scores after adjusting for race.
Table 10.
Repeated Measures Models for QOL
| Outcome Measure Model Effect |
F(df1,df2) | p-value |
|---|---|---|
| MLWHF total | ||
|
| ||
| Race | F(1,41)=0.932 | .34 |
| Race-x-Time | F(1,41)=0.082 | .78 |
| Age | F(1,41)=5.680 | .02 |
| Age-x-Time | F(1,41)=0.001 | .98 |
| Group | F(1,41)=0.882 | .35 |
| Time | F(1,41)=15.380 | <.001 |
| Group-x-Time | F(1,41)=0.033 | .86 |
| Time within Usual Care | F(1,41)=5.302 | .03 [Δ=18.94 (SD 27.27) ES 0.69] |
| Time within Intervention | F(1,41)=14.269 | .001 [Δ=17.19 (SD 26.53) ES 0.65] |
|
| ||
| MLWHF physical | ||
|
| ||
| Race | F(1,42)=0.670 | .42 |
| Race-x-Time | F(1,42)=0.964 | .33 |
| Group | F(1,42)=0.622 | .44 |
| Time | F(1,42)=12.999 | .001 |
| Group-x-Time | F(1,42)=0.793 | .38 |
| Time within Usual Care | F(1,42)=6.468 | .02 [Δ=9.83 (SD 12.82) ES 0.77] |
| Time within Intervention | F(1,42)=7.375 | .01 [Δ=5.82 (SD 12.50) ES 0.47] |
|
| ||
| MLWHF emotional | ||
|
| ||
| Race | F(1,41)=0.035 | .85 |
| Race-x-Time | F(1,41)=0.054 | .82 |
| Group | F(1,41)=0.643 | .427 |
| Time | F(1,41)=1.873 | .18 |
| Group-x-Time | F(1,41)=0.373 | .55 |
| Time within Usual Care | F(1,41)=0.169 | .68 |
| Time within Intervention | F(1,41)=4.020 | .05 [Δ=2.68 (SD 7.66) ES 0.35] |
|
| ||
| ADDQOL | ||
|
| ||
| Race | F(1,42)=4.864 | .03 |
| Race-x-Time | F(1,42)=0.443 | .51 |
| Group | F(1,42)=1.842 | .18 |
| Time | F(1,42)=2.739 | .11 |
| Group-x-Time | F(1,42)=0.138 | .71 |
| Time within Usual Care | F(1,42)=1.330 | .26 |
| Time within Intervention | F(1,42)=1.485 | .23 |
Abbreviations: SD, standard deviation; Δ, mean difference; ES, effect size
Participants in the intervention group rated the intervention as very positive overall, and expressed that they had not received integrated information in prior patient education sessions. In particular, information viewed as new and useful related to the integrated diet, role of exercise in improving outcomes in both HF and DM, impact of ankle edema from HF on diabetic feet and importance of foot care, and how to interpret overall symptoms of HF and DM and their interaction.
Discussion and Conclusions
The data revealed that the integrated HF-DM self-care intervention conferred effects on HF knowledge at 30 days, improved HF self-care maintenance at 30 days and sustained to 90 days, improved self-care management at 90 days, improved DM but not HF self efficacy at 30 and 90 days, improved self-reported general diet and exercise at 30 days, and improved foot care by 90 days. These results indicate that important self-care antecedents of knowledge and self-efficacy as well as actual self-care behaviors are modifiable in this HF-DM population with intervention, and the change in self-care management reflects the important ability to take action upon detection of HF symptoms. It also suggests that an integrated co-morbidity approach to self-care education such as focusing on behaviors important to both conditions such as exercise, diet and foot care may be effective. Specific DM diet behavior was moderately high at baseline and may have required longer or a larger sample to affect a change. Although within the intervention group, an improvement in physical and small improvement in emotional HF QOL were observed, the UC group also improved overall and physical QOL, and no overall between group differences in overall HF or DM specific QOL were observed. This may be due to the attention received from being in a study or that more time is needed to observe the impact of improved self-care on symptoms and perceived QOL. Importantly, the trend for increased perceived physical QOl in the intervention group corresponds with the trend for increased self-reported exercise for that group.
As with other studies (Mercier, Âeladeau, & Tempier, 1998; Netuveli & Blane, 2008), older participants reported better QOL as measured by the MLWHF scale at each time point. At baseline, participants in both groups reported overall poor perceived quality of life related to both HF and DM, thus any improvement was deemed important. Because groups were different in race, it was included in the models but was not significant in comparing group change on QOL.
Participants demonstrated moderate self-care knowledge. Although the onset of DM preceded HF in most participants, both UC and I groups performed better on the assessment of HF knowledge (AHFKT) than on the assessment of DM knowledge (MDKT). All participants in this study were recruited from a HF exacerbation requiring a hospital stay. Thus, HF education should have been occurring for both groups during the hospital stay whereas DM education may have occurred in the more distant past. Regardless, the study reflects the need to develop strategies for retention of knowledge concerning both conditions. The integrated comorbidity HF-DM intervention was useful for improving HF knowledge, and while knowledge alone is not sufficient for behavior change, it is an imperative factor for self-care (Clark, AM, et al 2009). We only measured HF- and DM-specific knowledge, and the acquisition of integrated HF-DM knowledge and clinically meaningful change would be important for future studies.
The SCHFI-Confidence scale and the PDSMS reflected low to moderate levels of both HF and DM self-efficacy. HF self-efficacy was not improved through the intervention which was surprising and may reflect the impact of confronting lack of knowledge with HF self-care. African American participants did report lower HF and DM self-efficacy than White participants which has implications for design of culturally relevant self-care interventions. Although the intervention group improved DM self-efficacy at 30 and was sustained to 90 days as reflected by changes on the PSMDS, an increase was also observed in the UC group. This may have been due to unknown changes in clinical practice or the fact that the study refocused attention on their DM care since most had had DM for a longer period. The relationship of age to DM self-efficacy but not to HF self-efficacy scores possibly reflects the longer time living with DM versus HF in this sample.
Improving self-care behaviors, even at a modest level has the potential to improve important outcomes in HF and DM. The intervention increased overall HF self-care maintenance activities at the early (30 days) and sustained (90 days) time points, and improved symptoms interpretation and self-care by 90 days. Additionally the intervention improved self-reported exercise across time, efforts to improve general diet, and improved foot care. The addition of the reinforcing education and counseling after discharge from the hospital and in the early follow up visit were important to sustaining the change. Importantly, at baseline, both groups demonstrated low to moderate HF and DM self-care activities with some reporting zero days of participation in exercise or glucose monitoring. There is an opportunity to make a difference in this population through continued development and strengthening of the integrated self-care intervention
The study has several limitations, including the small sample size, numerous analyses, and the total attrition rate which was 25%. Attrition rates were consistent with other separate HF and DM studies reported to be 20-28% and 20-33%, respectively (E.Stull & Houghton, 2013; Heisler et al., 2013; Tang, Funnell, Noorulla, Oh, & Brown, 2012). Retention in longitudinal behavior change studies with patients who have HF alone, and HF complicated by a comorbidity is challenging, and although all efforts were exerted to make participation convenient for participants, retention was lower than desired due to progression of HF severity and death. Of the 534 individuals assessed for eligibility, a large portion were excluded primarily for not meeting inclusion criteria for the study (other comorbitidies, age outside the specified range, inadequate renal function, and presence of a neurological or psychological diagnosis preventing participation). Some were also excluded due to living too distant from the enrolling center to return for follow-up, thus the sample may represent a more urban population. The criteria were selected to reduce confounding with the study variables, however, this rigor excluded a large portion of the overall HF-DM population such as those with more severe renal problems. Nevertheless, the sample size was adequate to identify important trends and effect sizes which will be essential to inform future studies. While the sample size of usual care was small, the combined results from the three time points increased the apparent sample size for most models, and allowed calculation of effect sizes as noted throughout. Additionally the sample was diverse which increases the generalizability.
The intervention materials were prepared at a level below the standard 6th grade level used in low health literacy studies (Baker et al, 2011; Rothman et al., 2004), and around one third of participants reported a high school education or less. Education was found to be a significant covariate for assessment of knowledge on both tests, with the higher the formal education, the higher the HF or DM knowledge score. Studies have shown that patients who demonstrate low health literacy are less likely to have a high school education, however substituting education level for health literacy has proven to be an insufficient measure. In fact, health literacy is associated with adverse outcomes, independent of level of education (Peterson et al., 2011). In older individuals, education level does not account for lifelong learning or age-related declines in reading ability (Baker et al., 2007). In persons with heart failure, memory and learning deficits often arise due to decreased cerebral flow. Moreover, 27% - 54% of patients with heart failure have low health literacy (Evangelista et al., 2010). It is likely that low health literacy among some participants affected both HF and DM knowledge. Attention to health literacy in future studies of educational interventions for those with HF and DM is warranted. As more internet and media based formats are developed and made available, it is likely that future studies might test different methods of delivering this integrated co-morbidity patient education at varying health literacy levels.
This pilot study provided insight into the many issues of recruitment, retention as well as positive trends data on the intervention effect, and effect sizes for determining appropriate samples for future work. This study reveals the potential to be gained from teaching integrated self-care in this population with multiple chronic conditions, and with further study, the intervention could be adapted to other patient populations with intensive recommended self-care such as persons with HF and concomitant chronic obstructive pulmonary disease or renal dysfunction. With the current changes in Medicare reimbursement rates for readmissions and with HF having the highest readmission rate, this small nurse-led intervention could be a great benefit to patients. Considering the current health care environment, and the growing numbers of people with both HF and DM, this intervention could be quite translatable into current practice. More research is needed to fine tune the approach to providing integrated comorbidity self-care patient education. Future studies testing HF-DM integrated self-care interventions in larger samples, with longer follow-up, and on other outcomes such as hospitalization and clinical status, are warranted.
Acknowledgement
Funded in part by the National Institutes of Health National Institute of Nursing Research grant number R21NRO11204 (PI-S. Dunbar) and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 (PI-D. Stephens), and the Atlanta Veterans Administration Medical Center. Also effort was funded for B. Butts through the National Institutes of Health National Institute of Nursing Research grant number T32NR012715 (PI-S. Dunbar). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KF, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American Heart Journal. 2005;149(2):209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Baker DW, Wolf MS, Feinglass J, Thompson JA, Gazmararian JA, Huang H. Health literacy and mortality among elderly persons. JAMA. 2007;167(14):1503–1509. doi: 10.1001/archinte.167.14.1503. [DOI] [PubMed] [Google Scholar]
- Baker DW, DeWalt DA, Schillinger D, Hawk V, Ruo B, Bibbins-Domingo K, Weinberger M, Macabasco-O’Connell A, Pignone M. Teach to goal”: theory and design principles of an intervention to improve heart failure self-management skills of patients with low health literacy. J Health Commun. 2011;16(Suppl 3):73–88. doi: 10.1080/10810730.2011.604379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Deer M, Murray MD. Discriminant properties of commonly used quality of life measures in heart failure. Quality of Life Research. 2002;11(4):349–359. doi: 10.1023/a:1015547713061. [DOI] [PubMed] [Google Scholar]
- Bobbio M, Ferrua S, Opasich C, Porcu M, Lucci D, Scherillo M, Tavazzi L, Maggioni AP. Survival and hospitalization in heart failure patients with or without diabetes treated with beta-blockers. Journal of Cardiac Failure. 2003;9(3):192–202. doi: 10.1054/jcaf.2003.31. [DOI] [PubMed] [Google Scholar]
- Bradley C, Speight J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diabetes/Metabolism Research and Reviews. 2002;18(Supplement 3):S64–69. doi: 10.1002/dmrr.279. [DOI] [PubMed] [Google Scholar]
- Butler J, Kalogeropoulos A. Hospital strategies to reduce heart failure readmissions: where is the evidence? Journal of the American College of Cardiology. 2012;60(7):615–617. doi: 10.1016/j.jacc.2012.03.066. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- Cha E, Clark PC, Reilly CM, Higgins M, Lobb M, Smith AL, Dunbar SB. Educational Needs for Improving Self-Care in Heart Failure Patients With Diabetes. Diabetes Educator. 2012 Sep-Oct;38(5):673–84. doi: 10.1177/0145721712450923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- De Groote P, Lamblin N, Mouquet F, Plichon D, McFadden E, Belle E, Bauters C. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. European Heart Journal. 2004;25(8):656–662. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- DeMuth JE. Basic Statistics and Pharmceutical Statistical Applications. 2nd Chapman and Hall/CRC; Boca Raton, FL: 2006. [Google Scholar]
- Dickson VV, Buck H, Riegel B. Multiple comorbid conditions challenge heart failure self-care by decreasing self-efficacy. Nursing Research. 2013;62(1):2–9. doi: 10.1097/NNR.0b013e31827337b3. [DOI] [PubMed] [Google Scholar]
- Domanski M, Krause-Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, Haffner S, Katz R, Lindedfeld J, Lowes BD, Martin W, McGrew F, Bristow MR. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. Journal of the American College of Cardiology. 2003;42(5):914–922. doi: 10.1016/s0735-1097(03)00856-8. [DOI] [PubMed] [Google Scholar]
- Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. Journal of the American College of Cardiology. 2001;38(2):421–428. doi: 10.1016/s0735-1097(01)01408-5. [DOI] [PubMed] [Google Scholar]
- Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. Journal of Cardiovascular Nursing. 2008;23(3):258–265. doi: 10.1097/01.JCN.0000305093.20012.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista LS, Rasmusson KD, Laramee AS, Barr J, Ammon SE, Dunbar SB, Ziesche S, Patterson JH, Yancy CW. Health literacy and the patient with heart failure - implications for patient care and research: a consensus statement of the Heart Failure Society of America. Journal of Cardiac Failure. 2010;16(1):9–16. doi: 10.1016/j.cardfail.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JT, Funnell MM, Hess GE, Barr PA, Anderson RM, Hiss RG, Davis WK. The reliability and validity of a brief diabetes knowledge test. Diabetes Care. 1998;21(5):706–710. doi: 10.2337/diacare.21.5.706. [DOI] [PubMed] [Google Scholar]
- From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. American Journal of Medicine. 2006;119(7):591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Gorelik O, Almoznino-Sarafian D, Alon I, Shteinshnaider M, Chachashvily S, Tzur I, Modai D, Cohen N. Heart failure in diabetes mellitus: clinical features and prognostic implications. Cardiology. 2005;103(3):161–166. doi: 10.1159/000084587. [DOI] [PubMed] [Google Scholar]
- Greenberg BH, Abraham WT, Albert NM, Chiswell K, Clare R, Stough WG, Gheorghiade M, O’Connor CM, Sun JL, Yancy CW, Young JB, Fonarow GC. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) American Heart Journal. 2007;154277(2):e271–278. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Heart Failure Society of America Executive summary: HFSA 2010 comprehensive heart failure practice guideline. Journal of Cardiac Failure. 2010;16(6):475–539. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Heisler M, Halasyamani L, Cowen ME, Davis MD, Resnicow K, Strawderman RL, Choi H, Mase R, Piette JD. A randomized controlled effectiveness trial of reciprocal peer support in heart failure. Circulation: Heart Failure. 2013;6(2):246–53. doi: 10.1161/CIRCHEARTFAILURE.112.000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, Piette JD. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients' treatment priorities and self-management? Journal of General Internal Medicine. 2007;22(12):1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti CN, Georgiopoulou VV, Giamouzis G, Cole RT, Deka A, Tang WH, Dunbar SB, Smith AL, Kalogeropoulos AP, Butler J. Patient-Reported Selective Adherence to Heart Failure Self-Care Recommendations: A Prospective Cohort Study: The Atlanta Cardiomyopathy Consortium. Congest Heart Fail. 2013;19(1):1751–7133. doi: 10.1111/j.1751-7133.2012.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoudi FA, Inzucchi SE. Diabetes mellitus and heart failure: epidemiology, mechanisms, and pharmacotherapy. American Journal of Cardiology. 2007;19(99(4A)):113B–132B. doi: 10.1016/j.amjcard.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Mercier CÂ, Âeladeau NP, Tempier R. Age, gender and quality of life. Community Mental Health Journal. 1998;34(5):487–500. doi: 10.1023/a:1018790429573. [DOI] [PubMed] [Google Scholar]
- Middel B, Jongste M. d., Sonderen E. v., Niemeijer MG, Crijns H, van den Heuvel W. Psychometric properties of the Minnesota Living with Heart Failure Questionnaire (MLHF-Q) Clinical Rehabilitation. 2001;15(5):489–500. doi: 10.1191/026921501680425216. [DOI] [PubMed] [Google Scholar]
- Netuveli G, Blane D. Quality of life in older ages. British Medical Bulletin. 2008;85(1):113–126. doi: 10.1093/bmb/ldn003. [DOI] [PubMed] [Google Scholar]
- Peterson PN, Shetterly SM, Clarke CL, Bekelman DB, Chan PS, Allen LA, Matlock DD, Magid DJ, Masoudi FA. Health literacy and outcomes among patients with heart failure. JAMA Internal Medicine. 2011;305(16):1695–1701. doi: 10.1001/jama.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald K, Keeler CA, Silver MA. Use of the Living WIth Heart Failure quiestionnaire to ascertain patients’ perspectives on improvement in quality of life versus risk of drug-induced death. Journal of Cardiac Failure. 1995;1(3):201–206. doi: 10.1016/1071-9164(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Reilly CM, Higgins M, Smith A, Gary RA, Robinson J, Clark PC, McCarty F, Dunbar SB. Development, psychometric testing, and revision of the Atlanta Heart Failure Knowledge Test. Journal of Cardiovascular Nursing. 2009;24(6):500–509. doi: 10.1097/JCN.0b013e3181aff0b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel B, Carlson B, Moser DK, M MS, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. Journal of Cardiac Failure. 2004;10(4):350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Riegel B, Lee CS, Dickson VV, Carlson B. An Update on the Self-Care of Heart Failure Index. Journal of Cardiovascular Nursing. 2009;24(6):485–497. doi: 10.1097/JCN.0b013e3181b4baa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel B, Lee CS, Albert N, Lennie T, Chung M, Song EK, Bentley B, Heo S, Worrall-Carter L, Moser DK. From novice to expert: confidence and activity status determine heart failure self-care performance. Nursing Research. 2011;60(2):132–138. doi: 10.1097/NNR.0b013e31820978ec. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Shifan D, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics - 2012 Update. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JS, Chen J, Lin Z, Bueno H. c., Curtis JP, Keenan PS, Normand S-L, Schreiner G, Spertus JA, Vida’n MT, Wang Y, Wang Y, Krumholz HM. Recent national trends in readmission rates after heart failure hospitalization. Circulation: Heart Failure. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RL, DeWalt DA, Malone R, Bryant B, Shintani A, Crigler B, Weinberger M, Pignone M. Influence of patient literacy on the effectiveness of a primary care-based diabetes disease management program. JAMA. 2004;292(14):1711–1716. doi: 10.1001/jama.292.14.1711. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Ali S, Whooley MA. Self-efficacy as a marker of cardiac function and predictor of heart failure hospitalization and mortality in patients with stable coronary heart disease: findings from the Heart and Soul Study. Healthy Psychology. 2009;28(2):166–173. doi: 10.1037/a0013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma S, Mentz RJ, Kwasny MJ, Fought AJ, Huffman M, Subacius H, Nodari S, Konstam M, Swedberg K, Maggioni AP, Zannad F, Bonow RO, Gheorghiade M. Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings fromthe EVEREST trial. European Journal of Heart Failure. 2013 Feb;15(2):194–202. doi: 10.1093/eurjhf/hfs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan FA, Bethel MA, Ruiz D, Jr., Shea AM, Feinglos MN. The growing burden of diabetes mellitus in the US elderly population. Archives of Internal Medicine. 2008;168(2):192–199. doi: 10.1001/archinternmed.2007.35. [DOI] [PubMed] [Google Scholar]
- Speight J, Bradley C. ADDQoL indicates negative impact of diabetes on quality of life despite high levels of satisfaction with treatment. Diabetologia. 2000;43(Supplement 1):A225. [Google Scholar]
- Stull DE, Houghton K. Identifying differential responders and their characteristics in clinical trials: Innovative methods for analyzing longitudinal data. Value in Health. 2013;16:164–176. doi: 10.1016/j.jval.2012.08.2215. [DOI] [PubMed] [Google Scholar]
- Sundaram M, Kavookjian J, Patrick JH, Miller L-A, Madhavan SS, Scott VG. Quality of life, health status and clinical outcomes in Type 2 diabetes patients. Quality of Life Research. 2007;16(2):165–177. doi: 10.1007/s11136-006-9105-0. [DOI] [PubMed] [Google Scholar]
- Tang TS, Funnell MM, Noorulla S, Oh M, Brown MB. Sustaining short-term improvements over the long-term: Results from a 2-year diabetes self-management support (DSMS) intervention. Diabetes Research and Clinical Practice. 2012;95(1):85–92. doi: 10.1016/j.diabres.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Effective Clinical Practice. 1998;1(1):2–4. [PubMed] [Google Scholar]
- Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS) Journal of Behavioral Medicine. 2007;30(5):395–401. doi: 10.1007/s10865-007-9110-y. [DOI] [PubMed] [Google Scholar]
- Wee H-L, Tan C-E, Goh S-Y, Li S-C. Usefulness of the Audit of Diabetes-Dependent Quality-of-Life (ADDQoL) questionnaire in patients with diabetes in a multiethnic Asian country. Pharmacoeconomics. 2006;24(7):673–682. doi: 10.2165/00019053-200624070-00006. [DOI] [PubMed] [Google Scholar]
- Woodcock A, Bradley C, Plowright R, Fftyche T, Kennedy-Martin T, Hirsch A. The influence of diabetic retinopathy on quality of life interviews to guide the design of a condition-specific, indivualized questionnaire: the RetDQoL. Patient Education and Counseling. 2004;53(3):365–383. doi: 10.1016/j.pec.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Wu SF, Huang YC, Lee MC, Wang TJ, Tung HH, Wu MP. Self-efficacy, self-care behavior, anxiety, and depression in Taiwanese with type 2 diabetes: A cross-sectional survey [Epub ahead of print] Nursing and Health Sciences. 2013 doi: 10.1111/nhs.12022. doi: 10.1111/nhs.12022. [DOI] [PubMed] [Google Scholar]