Abstract
It has been firmly established that opening and closing the eyes strongly modulate the electro- and magnetoencephalography (EEG and MEG) signals acquired during wakeful rest. Certain features of the resting EEG are altered in chronic alcoholics and their offspring, and have been proposed as biomarkers for alcoholism. Spontaneous brain oscillations are also affected by pharmacological manipulations, but the spectral and spatial characteristics of these changes are not clear. This study examined effects of the eyes-open (EO) and eyes-closed (EC) resting paradigm and alcohol challenge on the spatial profile of spontaneous MEG and EEG oscillations. Whole-head MEG and scalp EEG signals were acquired simultaneously from healthy social drinkers (n = 17) who participated in both alcohol (0.6 g/kg ethanol for men, 0.55 g/kg for women) and placebo conditions in a counterbalanced design. Power of the signal was calculated with Fast Fourier Transform and was decomposed into its constituent theta (4–7 Hz), alpha (8–12 Hz), and beta (15–20 Hz) frequency bands. High-resolution structural MRI images were additionally obtained from all participants and used to constrain distributed minimum norm inverse source power estimates. The spatial estimates of the main generator nodes were in agreement with studies using a combined fMRI-EEG approach. Alpha band oscillations dominated the spectral profile and their source was estimated to the medial parieto-occipital area. Power in theta and beta bands was weaker overall and their sources were estimated to a more focal medial prefrontal area. EO and EC manipulation most strongly modulated power in the alpha band, but a wide-band power increase was observed during the EC condition. Alcohol intoxication increased alpha power, particularly during the EC condition. Application of this methodology to cohorts of chronic alcoholics or individuals at risk could potentially provide insight into the neural basis of oscillatory differences that may be predictive of the vulnerability to alcoholism.
Keywords: theta, alpha, beta, eyes-open, eyes-closed, alcohol
Introduction
Spontaneous brain oscillations during wakeful rest have been investigated extensively with EEG and MEG (Buzsaki, 2006; Hari & Salmelin, 1997; Nunez & Srinivasan, 2006). In addition to providing insight into the basic organizational principles of cortical dynamics, such studies are highly relevant to clinical applications in patient populations with wide-ranging neurological abnormalities and communicative abilities (Schomer & Lopes da Silva, 2011). The frequency spectrum is canonically divided into bands including theta (4–7 Hz), alpha (8–12 Hz), and beta (15–20 Hz), with functional attributions differing across the spectrum (Nunez & Srinivasan, 2006). Alpha rhythm is the most prominent oscillatory feature during resting with eyes closed. It is modulated by opening or closing the eyes and altering attentional states and it has been studied during both passive resting and active task manipulations (Nunez, Wingeier, & Silberstein, 2001; Pfurtscheller & Lopes da Silva, 1999). However, it is not clear to what degree these effects are widely distributed in the cortex, especially in the light of the evidence of different generating networks and functional profiles of alpha oscillations (Lopes da Silva, 2011). Most human studies have used EEG methodology, but its poor spatial resolution precludes reliable spatial estimates. In recent years there has been an upsurge of fMRI studies using very low frequency blood oxygen level dependent (BOLD) signal fluctuations to explore functionally correlated brain networks (Deco, Jirsa, & McIntosh, 2011; Fox & Raichle, 2007). Observations that interconnected brain regions show increased activity levels during the resting state have led some to propose that the default mode network (DMN) subserves a baseline mode of brain function (Gusnard & Raichle, 2001; Raichle et al., 2001). However, the neurophysiological underpinnings of the DMN and the ways in which it relates to brain oscillatory activity remain unclear. In an effort to combine advantages of both methods and investigate EEG generating networks, some studies have employed concurrent recordings of the EEG and the BOLD signal and examined correlations between the BOLD and power fluctuations in different EEG frequency bands (Ullsperger & Debener, 2010). The most reliable finding is the negative correlation between the occipital alpha activity and the BOLD activity in the visual cortex (Becker, Reinacher, Freyer, Villringer, & Ritter, 2011; Bridwell, Wu, Eichele, & Calhoun, 2013; Feige et al., 2005; Goldman, Stern, Engel, & Cohen, 2002; Laufs et al., 2006; Mantini, Perrucci, Del Gratta, Romani, & Corbetta, 2007; Mo, Liu, Huang, & Ding, 2013; Moosmann et al., 2003; Olbrich et al., 2009). However, combining fMRI data with EEG data reveals intrinsic ambiguities in spatio-spectral mapping. As a consequence, studies differ in the number of extracted alpha components and vary in their spatial estimates of other bands such as theta or beta (Ben-Simon, Podlipsky, Arieli, Zhdanov, & Hendler, 2008; de Munck, Goncalves, Mammoliti, Heethaar, & Lopes da Silva, 2009; Mantini et al., 2007; Olbrich et al., 2009). These inconsistencies may be related to the relative temporal insensitivity of the BOLD signal and dependence on the assumptions of a particular analysis model. Even though the BOLD signal is an excellent mapping tool, its temporal resolution is limited by the fact that it reflects neural changes only indirectly via neurovascular coupling (Buxton, 2002). Furthermore, the BOLD signal’s direct dependence on the hemodynamic response renders it highly sensitive to vasoactive influences during pharmacological manipulations (Iannetti & Wise, 2007). During alcohol challenge studies, intoxication may affect cerebral blood flow and confound the neural activity with vascular changes (Rickenbacher, Greve, Azma, Pfeuffer, & Marinkovic, 2011). And yet, gaining a better understanding of the neural basis of alcohol’s effects on brain function is of great importance. Consequently, in the context of pharmacological manipulations, there is a great advantage in relying on methods that are free of vascular confounds such as EEG and MEG. Even though these two methods reflect the same postsynaptic currents directly, they are sensitive to different biophysical properties of the signal and therefore provide complementary information (Hämäläinen, Hari, Ilmoniemi, Knuutila, & Lounasmaa, 1993). Given its superior temporal resolution, the combined MEG/EEG approach provides accurate insight into oscillatory dynamics of the signal. Furthermore, reasonable spatial estimates can be obtained when physiologically appropriate solution constraints are applied (Dale & Halgren, 2001; Dale & Sereno, 1993).
There is extensive evidence of the EEG signal’s sensitivity to pharmacological manipulations, including alcohol (Bauer & Bauer, 2011; Porjesz & Begleiter, 1996). Because of the high heritability of individual characteristics of resting EEG (van Beijsterveldt & van Baal, 2002), resting EEG has been used extensively in studies of chronic alcoholics and their offspring, with some of its aspects proposed as biological markers of the predisposition to develop alcoholism (Begleiter & Porjesz, 2006; Porjesz et al., 2005; Rangaswamy & Porjesz, 2008). The present study employed a within-subject factorial design to examine effects of both eyes-open (EO) and eyes-closed (EC) resting states and alcohol challenge vs. placebo on spontaneous oscillations as measured simultaneously by MEG and EEG. One study (Nikulin, Nikulina, Yamashita, Rossi, & Kähkönen, 2005) recorded MEG/EEG and examined the effects of alcohol (0.8 g/kg) compared to placebo during EO and EC. The overall power in the lower alpha (8–10 Hz) was increased, and beta power (17–25 Hz) was decreased by alcohol in the EC condition. The aim of the present study was to extend these findings by estimating cortical sources of the principal frequency bands including theta, alpha, and beta during resting and as a function of intoxication and eyes-open or closed (EOC) states. We employed a distributed, anatomically constrained MEG (aMEG) approach (Dale et al., 2000; Lin et al., 2004). The aMEG combines whole-head high-density MEG and distributed source modeling with high-resolution MRI and cortical reconstruction, which relies on a realistic head shape obtained for each participant (Dale et al., 2000; Dale & Sereno, 1993). EEG signal was acquired simultaneously to provide a direct comparison with previous evidence.
Methods
Participants
Seventeen healthy, non-smoking, right-handed volunteers (9 men, mean [± SD] age = 28.3 ± 5.4 years) completed all sessions of this study. None reported alcohol or drug-related problems, serious head injury, head trauma, seizures, neuropsychiatric, or other medical problems, and none was taking any psychoactive medications at the time of the study. They did not have alcoholism-related symptoms as assessed by the Short Michigan Alcoholism Screening Test (SMAST) (Selzer, Vinokur, & Van Rooijen, 1975) and were negative for family history of alcoholism and drug abuse. Subjects reported light-to-moderate alcohol use in social situations, imbibing 2.3 ± 0.8 drinks 1.7 ± 0.9 times per week. The study’s procedures were approved by the Institutional Review Board of the University of California at San Diego, and written informed consent was obtained from each subject prior to the experiment. Subjects were compensated for their participation and provided with transportation to and from the laboratory.
Experimental Protocol
Each participant took part in four sessions conducted on separate days including the MEG familiarization session, two recording sessions with beverage administration, and an MRI scan. Over the course of a beverage-free introductory session, subjects completed a battery of questionnaires. The participants were queried on their handedness (Oldfield, 1971) and medical history as well as their alcohol drinking habits, including the frequency and quantity of alcohol consumption (modified from Cahalan, Cisin, & Crossley, 1969), the magnitude of their response to alcohol (Self-Rating of the Effects of Alcohol, SRE, Schuckit, Smith, & Tipp, 1997), and the severity of their alcoholism-related symptoms (SMAST, Selzer et al., 1975). In addition, their disinhibitory and novelty-seeking traits were assessed by the Zuckerman Sensation Seeking Scale (Zuckerman, 1971) and Eysenck Impulsiveness and Venturesomeness Scale (Eysenck & Eysenck, 1978). At this time, subjects practiced experimental tasks (to be reported separately) and were familiarized with the experimental setup. They completed a mock recording with the purpose of reducing situation-induced arousal effects in the ensuing experimental sessions (Maltzman & Marinkovic, 1996).
Subsequently, participants took part in both alcohol and placebo sessions in a counterbalanced manner in the within-subject design. Upon arrival to the lab, participants were queried about their compliance with the requirement to refrain from drinking for 48 h and from food for 3 h before each session. They provided urine samples for multi-drug screening and women were additionally tested for pregnancy. All tests were negative. Subjects imbibed either an alcohol beverage (0.60 g/kg for men, 0.55 g/kg for women, presented as a cocktail containing vodka [Grey Goose] as 20% v/v in orange juice), or a placebo beverage (the same volume of orange juice with the glass rim swabbed with vodka). Throughout the session, the subjects’ breath alcohol concentration (BrAC) was monitored with a breathalyzer (Draeger, Inc.), except during scanning due to electronic interference with the MEG device. At that time, a Q.E.D. Saliva Alcohol Test (OraSure Technologies, Inc.) was used to measure BrAC. Participants rated their moods and feelings with the Biphasic Alcohol Effects Scale (BAES) (Martin, Earleywine, Musty, Perrine, & Swift, 1993) at baseline, and on the ascending and descending limbs of their BrAC. The resting state measurement was started about 1 h after the beverage was administered. At this time, the average BrAC level was 0.060 ± 0.01%, indicating that the signal acquisition took place at or near the peak of the blood alcohol curve. At the end of the session, subjects rated the alcohol content of the beverage, their perceived level of intoxication, and their feelings of nausea and dizziness on 1–5 Likert scales. High-resolution structural MR images were obtained from all participants in a separate session.
Data Acquisition
Structural MRI
Structural images were acquired with a 1.5 T General Electric EXCITE HG whole-body scanner. The acquisition protocol included a conventional 3-plane localizer, calibration scan, and 2 high-resolution T1-weighted IR-FSPGR scans (TR = 8.5 s, TE = 3.75 ms, TI = 500 ms, flip angle = 10 degrees, FOV = 240, 166 sagittal slices, 1.2 mm slice thickness, in-plane resolution 0.94 × 0.94 mm). Each person’s cortical surface was reconstructed from these structural images with FreeSurfer, https://surfer.nmr.mgh.harvard.edu/ (Dale, Fischl, & Sereno, 1999) (Fischl, Sereno, & Dale, 1999). Inner skull surface was used for a boundary element model of the volume conductor in the forward calculations. Each subject’s gray-white matter surface was morphed into average space after aligning their cortical sulcal-gyral patterns (Fischl, Sereno, Tootell, & Dale, 1999). The solution space was approximated by ~5000 free-rotating dipoles spaced ~7 mm apart.
MEG
A whole-head Elekta Neuromag Vectorview system housed in an IMEDCO-AG magnetically shielded room was used to acquire MEG data. A continuous signal was recorded from 204 channels (102 pairs of planar gradiometers) at a 1000 Hz sampling rate and with minimal filtering (0.1 to 200 Hz). Four head-position indicator coils were affixed to the scalp. Their positions, along with those of the nasion, bilateral pre-auricular points, and numerous random head points were digitized with a 3Space Isotrak II system for later precise co-registration with MR images. Subjects were seated comfortably in the scanner and were in a state of relaxed but alert wakefulness. The resting state data were collected during two 2-min intervals as the subjects focused on a fixation cross (eyes open, EO), or kept their eyes closed (EC).
EEG
The EEG signal was recorded simultaneously with the MEG to verify concordance with previous studies using the EOC paradigm. Electrodes were placed at the Fz, Cz, Pz, C3, and C4 sites. The reference electrode was affixed to the tip of the nose and the ground to the underside of each subject’s forearm. A bipolarly referred electro-oculogram (EOG) was also recorded to assist in data quality control. Electrode impedance was kept well below 5 kΩ in all sessions. Sixteen subjects yielded good quality EEG data sets across both beverages, and these data were analyzed in the sensor space using the same method applied to the MEG, as described below.
Data Processing and Analysis
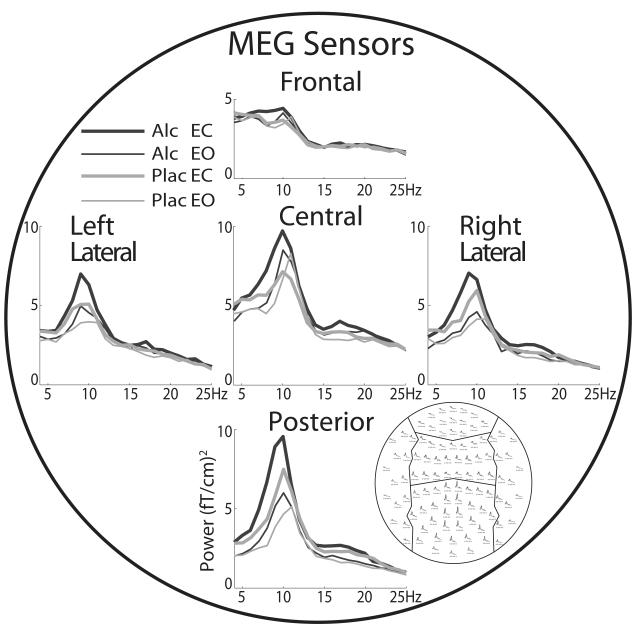
The MEG and EEG data were analyzed with custom-made MATLAB routines in addition to publicly available algorithms including FieldTrip (Oostenveld, Fries, Maris, & Schoffelen, 2011) and MNE (http://martinos.org/mne/). Continuous data were downsampled to 500 Hz and low-pass filtered at 100 Hz. Noisy and flat channels were removed, and the data were epoched into 4-sec periods of clean data as determined by careful visual inspection. The complex power spectrum of each epoch was then computed using a multitapered Fast Fourier Transform with Hanning tapers (Oostenveld et al., 2011) and averaged across trials. The number of epochs was equated across both beverage and EOC conditions in order to eliminate potential bias. Group averages of sensor space spectral power profiles for the factors of beverage and EOC for the MEG and EEG are shown in Fig. 1 and Fig. 4, respectively.
Fig. 1.
Sensor-space group average profiles of spectral power acquired with MEG gradiometers positioned over the frontal, central, posterior, and left and right lateral areas of the head. Average power spectra for the four conditions resulting from the within-subject factorial design incorporating the factors of eyes-open (EO) and eyes-closed (EC) and beverage (alcohol [Alc] and placebo [Plac]) are superimposed.
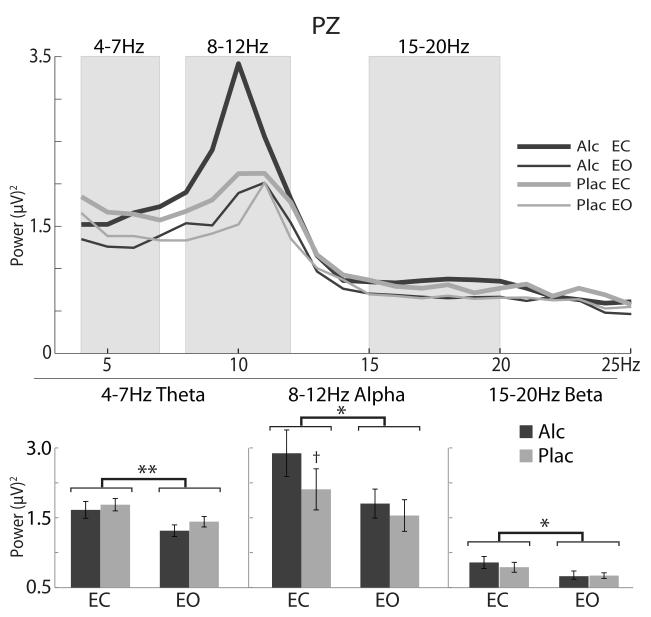
Fig. 4.
Grand average EEG spectral distribution plots are superimposed for each eyes-closed (EC), eyes-open (EO), and beverage condition for the signal recorded at Pz. Opening of the eyes resulted in a wideband decrease in alpha power. Alcohol intoxication marginally increased alpha power only under the EC condition. +p < 0.05; *p < 0.01; **p < 0.001.
MEG source estimates were calculated in 1 Hz increments for the frequency range spanning 1–50 Hz. Source power was estimated using the spectral dynamic statistical parametric mapping approach (Lin et al., 2004) in conjunction with applying anatomically constrained minimum norm estimation (Dale et al., 2000; Kovacevic et al., 2012; Marinkovic, Rosen, Cox, & Kovacevic, 2012). Empty room data were pooled across sessions and used to generate the noise covariance matrix, thereby avoiding any bias in the inverse solution against spontaneous brain oscillations. The source-space solution was noise-sensitivity normalized with an identity matrix. For each subject and each frequency, a map of spectral source power was calculated across epochs. Group and frequency band average source estimate maps for theta (4–7 Hz), alpha (8–12 Hz), and beta (15–25 Hz) are shown in Fig. 2. Region-of-interest (ROI) analysis was used to examine the statistical significance of the observed effects and to probe for interactions between EOC and beverage factors for each frequency band. ROIs were selected based on the overall group average across all subjects, EOC, and beverage conditions. They comprised dipole locations with most notable source power in each frequency band. These ROIs were applied to all subjects with an automatic spherical morphing procedure (Fischl, Sereno, Tootell, et al., 1999) in a manner blind to individual activations. Estimates of spectral source power were averaged across the cortical dipoles within each ROI for each subject, EOC condition, and beverage condition within the three frequency bands (Fig. 2). Repeated-measures ANOVAs were carried out for MEG ROIs and EEG channels with beverage and EOC as within-subject factors for each frequency band (SPSS, 2001).
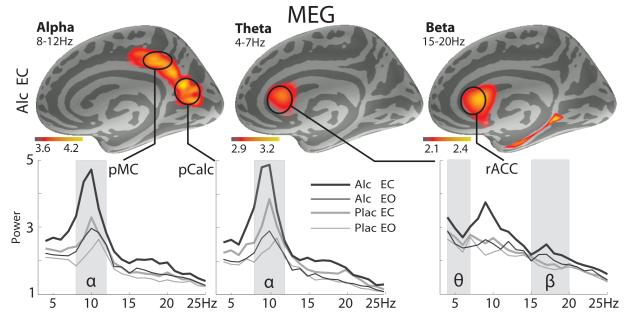
Fig. 2.
Group and frequency average maps of source estimates for theta (θ), alpha (α), and beta (β) frequency bands are shown in the upper panel. The lower panel shows group average spectral distribution plots of the source power estimates averaged across cortical dipoles within each region of interest (ROI), shown in arbitrary units. Estimates for the eyes-closed (EC) and eyes-open (EO) for alcohol (Alc) and placebo (Plac) conditions are superimposed. The ROIs comprise dipole locations with the most notable group average source power in each frequency band, including posteromedial cortex (pMC) and pericalcarine cortex (pCalc) for alpha bands, and rostral anterior cingulate cortex (rACC) for theta and beta bands, as marked with vertical gray overlays.
Results
Mood ratings and post-experiment questionnaire
Self-ratings obtained with the BAES questionnaire (Martin et al., 1993) and post-experiment Likert-scale ratings violated the assumption of normality and were analyzed with a non-parametric related-samples Friedman test (SPSS, 2001).
The BAES ratings obtained on the ascending and descending BrAC phases were analyzed relative to baseline for the effects of beverage and phase. Overall, participants reported feeling more sedated (χ2 = 7.1, p < 0.01) and less stimulated (χ2 = 4.8, p < 0.05) at the end of the experimental session. They reported feeling significantly more stimulated (χ2 = 4.8, p < 0.05) and “high” (χ2 = 11.3, p < 0.001) under alcohol as compared to placebo.
As a part of the post-experiment questionnaire, participants rated the beverage content on the Likert scale ranging from 1 (definitely not alcohol) to 5 (definitely alcohol). They discerned the content with ratings at 4.7 ± 0.5 under alcohol and 1.7 ± 0.8 under placebo (χ2 = 17.0, p < 0.001). On the scale ranging from 1 (not at all) to 5 (very much), participants reported feeling moderately intoxicated under alcohol (2.6 ± 0.8) and not at all under placebo (1.1 ± 0.2) (χ2 = 15.0, p < 0.001). Whereas alcohol intoxication did not increase the feeling of nausea, participants reported being slightly, but significantly more dizzy under alcohol (1.8 ± 0.6) than under placebo (1.2 ± 0.7) (χ2 = 8.3, p < 0.01).
MEG Spectral Power Source Estimates
Group average maps of theta (4–7 Hz), alpha (8–12 Hz), and beta (15–20 Hz) source power estimates are shown in Fig. 2. Alpha power was estimated to the posteromedial cortex (pMC) and the pericalcarine cortex (pCalc) bilaterally, whereas theta and beta power were primarily estimated to the bilateral rostral anterior cingulate cortex (rACC). Spectral distribution plots for these ROIs are shown in the lower panel of Fig. 2. As there were no hemispheric differences, source estimates were averaged across symmetrical ROIs and used for statistical comparisons.
EOC Effects
Group averages of spectral power in sensor space are shown in Fig. 1 and spatial estimates of the principal generators are shown in Fig. 2. Resting theta power was estimated to rACC and was reduced significantly in the EO condition as shown with the main effect of EOC, F(1,16) = 9.7, p < 0.01 (Fig. 3). Alpha power was estimated to the posteromedial cortex (pMC) and pericalcarine area (pCalc). As expected, opening of the eyes decreased alpha power significantly in both ROIs, F(1,16) = 21.9, p < 0.001 and F(1,16) = 22.1, p < 0.001. Beta power source was estimated primarily to the rACC. It was marginally reduced by opening of the eyes, F(1,16) = 6.3, p < 0.05.
Fig. 3.
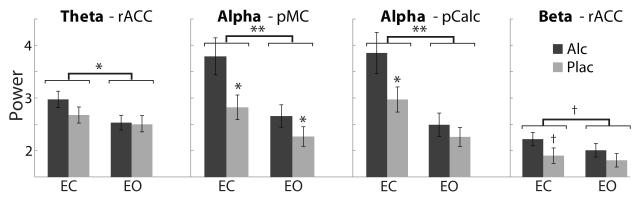
Effects of the factors of EC, EO, and beverage on source MEG estimates for the ROIs relevant for the three frequency bands were evaluated with repeated-measures ANOVAs. A wide-band power decrease was observed during the eyes-open (EO) condition. The effects of alcohol intoxication were primarily reflected in increased alpha power during the eyes-closed (EC) condition. +p < 0.05; *p < 0.01; **p < 0.001.
Alcohol Effects
Alcohol intoxication did not affect power in the theta frequency band. The strongest effects of alcohol intoxication were visible in the alpha frequency band as alcohol intoxication increased alpha power in both pMC [F(1,16) = 14.5, p < 0.01] and pCalc [F(1,16) = 9.9, p < 0.01]. However, this effect was particularly apparent during EC. As shown in Fig. 3, there was a significant interaction between the factors of beverage and EOC in the pCalc area, F(2,32) = 8.9, p < 0.01, due to the alcohol-induced alpha power increase under the EC condition only, F(1,16) = 11.0, p < 0.01. Beta power was marginally increased by alcohol in rACC only under the EC condition, F(1,16) = 5.9, p < 0.05. Similarly, alcohol increased beta power in the pMC and pCalc under EC, F(1,16) = 11.8, p < 0.01, and F(1,16) = 8.6, p < 0.01, respectively.
EEG
Grand average spectral distributions for each EOC and beverage condition as recorded at Pz are shown in Fig. 4. Repeated-measures ANOVA was performed on the band average power in each of the three frequency bands. Power under the EC condition was consistently greater than under the EO condition across all three bands with F(1,15) = 25.9, p < 0.001 (theta), F(1,15) = 9.5, p < 0.01 (alpha), and F(1,15) = 12.6, p < 0.01 (beta). Alcohol had no effect on power in the theta and beta bands, but it marginally increased alpha band power only under the EC condition, F(1,15) = 4.6, p < 0.05.
Discussion
The current study utilized aMEG to investigate the spectral and spatial characteristics of resting state networks as a function of alcohol intoxication and in the presence or absence of visual sensory input. Power of the acquired signal was decomposed into its constituent theta, alpha, and beta frequency bands. Consistent with other evidence, oscillations in the alpha range (8–12 Hz) were dominant and maximal over the posterior scalp, and their source was estimated to a broad medial parieto-occipital area. Power in theta and beta bands was weaker overall and their sources were estimated to originate from a more focal medial frontal area. Opening and closing the eyes most strongly modulated power in the alpha band, but a wide-band power increase was observed during the EC condition. Although the effect of alcohol intoxication was weaker overall, it increased alpha power particularly during the EC condition.
Alpha oscillations are most prominent during periods of wakeful rest, especially when the eyes are closed, precluding any visual sensory stimulation. Animal experiments indicate that the alpha oscillations are mainly generated in the visual cortex and are propagated by intracortical connections, with different laminar organization underlying specific alpha rhythm functional profiles (Bollimunta, Chen, Schroeder, & Ding, 2008; Lopes da Silva, 2011). Alpha rhythm emerges from an active interaction with the visual thalamic nuclei within a thalamo-cortical re-entrant network (Chatila, Milleret, Rougeul, & Buser, 1993; Hughes & Crunelli, 2005). The striking alpha suppression upon opening of the eyes is a classical effect (Adrian & Matthews, 1934) which reflects the thalamocortical visual input (Bollimunta, Mo, Schroeder, & Ding, 2011; Hughes & Crunelli, 2005). This visual stimulation could be partially mediated by the RAS, reflecting widespread cortical engagement (Palva & Palva, 2007). Indeed, alpha power has been proposed as a measure of resting-state arousal as it is negatively correlated with skin conductance levels (Barry, Clarke, Johnstone, Magee, & Rushby, 2007). The alpha rhythm is also modulated by cognitive tasks and initially it was conceptualized as an index of “cortical idling” and inactivity (Pfurtscheller, Stancák, & Neuper, 1996). Such a view, however, does not fully account for recent evidence indicating that increased alpha is predictive of errors (Hanslmayr et al., 2007), whereas decreased alpha power is associated with attention (Thut, Nietzel, Brandt, & Pascual-Leone, 2006). Similarly, a MEG study showed that an increase in pre-stimulus alpha power estimated to the parieto-occipital sulcus was associated with attenuated visual discrimination ability (van Dijk, Schoffelen, Oostenveld, & Jensen, 2008). Therefore, it has been proposed that increasing alpha activity reflects active attentional suppression (Rihs, Michel, & Thut, 2007), with attentional capacity fluctuating as a function of a “pulsed inhibition” tied to alpha cycles (Mathewson, Gratton, Fabiani, Beck, & Ro, 2009). On that view, alpha may reflect a modulating gate function influencing visual perception and attention (Jensen & Mazaheri, 2010; Lopes da Silva, 2011). The neurochemical basis of alpha oscillations has been investigated in animal and human imaging studies. In addition to the cholinergic input (Lörincz, Crunelli, & Hughes, 2008), GABA-ergic transmission mediates phasic inhibition and may underlie modulating or gating influences in the thalamo-cortical networks (Lopes da Silva, 2011). Recent MEG studies have examined the effects of GABA on alpha oscillations by administering benzodiazepines that result in enhanced GABA efficacy. Whereas spontaneous alpha oscillations in the visual cortex were augmented in one study (Hall, Barnes, Furlong, Seri, & Hillebrand, 2010), they were reduced by increased GABA levels in another (Ahveninen et al., 2007). These contradictory findings may have resulted from differences in drug type, dosing, nonlinear effects, or experimental and model parameters.
The most prominent effect of beverage in the current study is an increase in alpha power in the posteromedial and visual cortices, particularly during the eyes-closed state, confirming other similar findings (Nikulin et al., 2005). Given that acute alcohol enhances GABA-ergic function (Nevo & Hamon, 1995; Santhakumar, Wallner, & Otis, 2007), it would be expected that alcohol would increase alpha oscillations to the extent that they are mediated by GABA. Indeed, the highest density of GABA-ergic neurons is found in the human primary visual cortex (Zilles, Palomero-Gallagher, & Schleicher, 2004), which is the strongest estimated cortical generator of alpha oscillations. Alpha oscillations are generated by a resonating circuit comprising thalamic nuclei, nucleus reticularis thalami, and cortical neurons (Amzica & Lopes da Silva, 2011). The thalamic cells alternate between depolarizing and hyperpolarizing phases maintaining oscillatory generation (Bollimunta et al., 2011; Hughes & Crunelli, 2005; Steriade, Domich, Oakson, & Deschênes, 1987; Steriade, McCormick, & Sejnowski, 1993). The thalamic alpha generating circuit probably relies on the GABA-ergic intrinsic currents, lh and lt, much as does sleep spindle generation (Destexhe & Sejnowski, 2003; McCormick, 1992). These currents are inactivated when thalamic cells are depolarized during waking, and especially in the presence of visual stimulation in the case of the lateral geniculate nucleus (LGN), resulting in decreased alpha power (Steriade et al., 1993). Hyperpolarization induced by removal of visual stimulation may reactivate the critical currents, which is enhanced by alcohol-induced increased GABA function (Nevo & Hamon, 1995; Santhakumar et al., 2007). Consequently, alpha oscillations are particularly sensitive to the effects of alcohol in the absence of visual stimulation.
In addition to GABA, alcohol is known to affect other neurotransmitter systems. For instance, glutamatergic excitatory effects are suppressed by acute intoxication (Nevo & Hamon, 1995). Thalamocortical input from the lateral geniculate nucleus to the visual cortex is mediated by metabotropic glutamate receptors (Govindaiah, Venkitaramani, Chaki, & Cox, 2012), and the excitability of the visual cortex is inversely proportional to alpha power, whereby opening of the eyes results in increased cortical excitability and suppression of alpha power (Romei, Rihs, Brodbeck, & Thut, 2008). By modulating both ion channels and receptors, alcohol can alter synaptic events at multiple levels of regulation and with multiple neurotransmitters (Vengeliene, Bilbao, Molander, & Spanagel, 2008). Therefore, alcohol-induced increase of alpha power may result from combined influences of increased GABA inhibition and reduced glutamatergic excitation (Fitzgerald & Nestler, 1995; Vengeliene et al., 2008). Recent findings of genetic studies suggest that alcoholism and the vulnerability to alcoholism are associated with the GABRA2 receptor gene which may mediate alterations in brain oscillations resulting from the inhibition/excitation imbalance (Porjesz & Rangaswamy, 2007; Porjesz et al., 2005; Winterer et al., 2003).
In a fully crossed design, the present study manipulated the level of visual input (EOC state) and acute alcohol intoxication. The strongest effect was attenuation of wideband power upon eye opening, with the most prominent effects on alpha oscillations (Osipova, Hermes, & Jensen, 2008; Romei, Brodbeck, et al., 2008). Traditionally, the EO-induced alpha suppression, also termed “alpha block” (Niedermeyer, 1999), has been interpreted as a replacement by higher frequencies which is indeed suggested by visual inspection of the raw EEG signal. However, numerical comparisons indicate a decrease in wideband power upon opening the eyes, albeit the decrease in the beta band is usually smaller than in the alpha band (Barry et al., 2007; Heister et al., 2013; Ishii et al., 2013). Overall, effects of the pharmacological manipulation with moderate alcohol intoxication were weaker than the EOC effects, with alcohol increasing power in alpha and beta bands, especially under the eyes-closed condition. Our observation of alcohol-induced alpha increase is consistent with other evidence obtained with EEG (Kaplan, Hesselbrock, O’Connor, & DePalma, 1988; Lansbergen, Dumont, van Gerven, Buitelaar, & Verkes, 2011; Lukas, Mendelson, Kouri, Bolduc, & Amass, 1990). Furthermore, the increase in alpha power during alcohol challenge is higher in the offspring of alcoholics (Cohen, Porjesz, & Begleiter, 1993; Ehlers & Schuckit, 1991). Studies not administering beverages have reported alpha power reduction in chronic alcoholics (Saletu-Zyhlarz et al., 2004) as well as in the offspring of alcoholics (Finn & Justus, 1999). Dispositional factors such as impulsivity could further interact with alcohol effects. In the current study, the occipital alpha during EC was negatively correlated with behavioral disinhibition traits such as venturesomeness, r = −0.57, p < 0.01 (Eysenck & Eysenck, 1978), and adventure seeking, r = −0.59, p < 0.01 (Zuckerman, 1971). Negative association was observed across both beverage conditions, suggesting that more impulsive individuals have lower alpha power overall. In the context of GABA-ergic mediation of alpha rhythm, this finding is consistent with reports of a negative correlation between impulsivity and GABA concentration (Boy et al., 2011), and it provides insight into neurophysiological underpinnings of dispositional differences. It is well known that the impulsivity trait is reliably associated with alcohol use and dependence (Aragues, Jurado, Quinto, & Rubio, 2011; Sher & Trull, 1994). Indeed, inability to maintain inhibitory control over drinking is considered fundamental to hazardous drinking and abuse (Field, Wiers, Christiansen, Fillmore, & Verster, 2010; Lyvers, 2000). As a premorbid trait, impulsivity may predispose individuals to a spectrum of conduct disorders including alcohol dependence (Begleiter & Porjesz, 2006; Nigg et al., 2006), and evidence suggests that the same genetic pathways may mediate both addiction and impulsivity (Bevilacqua et al., 2010; Goldman, Oroszi, & Ducci, 2005). All these lines of evidence converge with the idea that GABA-mediated alpha is attenuated in individuals who are more impulsive and more likely to develop alcohol dependence. Resting EEG is stable and highly heritable (Smit, Posthuma, Boomsma, & Geus, 2005; van Beijsterveldt & van Baal, 2002). Additionally, the heritability index increases under acute intoxication (Sorbel, Morzorati, O’Connor, Li, & Christian, 1996). Therefore, studies investigating acute alcohol effects on brain oscillations, together with studies on chronic alcoholics and at-risk populations, can provide valuable contributions to a better understanding of alcohol neurotoxicity and genetic predisposition toward alcoholism.
In the present study, alcohol intoxication exerted its effects primarily on the alpha band but it also increased power in the beta band without affecting the theta band. An increase in alcohol-induced beta power during resting has been reported in chronic alcoholics (Coutin-Churchman, Moreno, Añez, & Vergara, 2006; Rangaswamy et al., 2002). Indirect support for those observations is provided by a positive association between beta power and drinking rate. In this study, beta power correlated with the typical drinking rate under alcohol, r = 0.62, p < 0.005, but not under placebo, r = 0.15, ns, suggesting that the beta power is associated with the amount of alcohol that is regularly consumed. Since it has also been observed in the offspring of alcoholics, beta increase has been proposed as a predictive endophenotype for the vulnerability to alcoholism (Rangaswamy et al., 2004).
Our results corroborate the effects of alcohol on the alpha band reported by Nikulin and colleagues (2005), but contradict them in the beta band. However, we fully concur with their conclusion that the MEG signal is more sensitive than EEG to the effects of alcohol during wakeful rest, because in the present study alcohol only marginally increased EEG alpha during the EC condition. As an extension of those findings, our study employed the aMEG method which combines distributed source modeling and MRI-based cortical reconstruction. This multimodal approach made it possible to estimate the spatial context from which the sensitivities to EOC and alcohol intoxication arise. Previous MEG studies of spontaneous oscillations relied on dipole models to estimate the source of alpha power. In agreement with our results, they estimated sources in the posterior medial cortex (Ciulla, Takeda, & Endo, 1999; Hari & Salmelin, 1997; Manshanden, De Munck, Simon, & Lopes da Silva, 2002; Osipova et al., 2008; Williamson & Kaufman, 1989). Our study has additionally estimated principal theta and beta generators to rACC. More recently, numerous studies have employed concurrent recordings of EEG and BOLD to investigate the spatial distribution and oscillatory characteristics of resting state networks. Extensive literature indicates that a network of regions is active during the resting state that is associated with self-referential thoughts, memory, and mind wandering (Gusnard, Akbudak, Shulman, & Raichle, 2001; Mason et al., 2007). Very low frequency fluctuations of the BOLD signal (< 0.1 Hz) are synchronized among the principal regions of the DMN, including posteromedial and medial prefrontal cortices as well as parietal regions (Buckner, Andrews-Hanna, & Schacter, 2008). The EEG signal is analyzed as power fluctuations, making it possible to obtain correlations with low frequency BOLD signal (Mantini et al., 2007). Despite these significant methodological differences in the analysis of the temporally sensitive EEG signal, our spatial estimates are consistent with studies showing that the main activation foci are in anterior and posterior medial prefrontal, and pericalcarine cortices across alpha, theta, and beta bands (Bridwell et al., 2013; Mantini et al., 2007; Olbrich et al., 2009; Scheeringa et al., 2008). These observations lend strong support to the inverse estimates provided by the aMEG method employed in the current study. The main advantage of the MEG signal is that it directly reflects postsynaptic current flow in the dendrites of cortical neurons and can thus be used to examine momentary changes in neural activity due to fluctuating state of arousal, cognitive demands, or pharmacological effects (Cohen & Halgren, 2009; Hämäläinen et al., 1993; Lewine & Orrison, 1995). Several studies have reported a negative correlation between the BOLD signal and alpha power during resting (Feige et al., 2005; Goldman et al., 2002; Laufs et al., 2006; Moosmann et al., 2003). Simultaneous acquisition of the near infrared spectroscopy (NIRS) signal along with the EEG and fMRI reveals that the increased alpha in the posterior cortex is associated with metabolic deactivation (Moosmann et al., 2003), which is compatible with the idea of decreased neural activity (Pfurtscheller et al., 1996).
In summary, our results substantiate previous evidence of the strong modulatory influence of eyes-open/eyes-closed states on wideband spectral characteristics and are consistent with reports of alcohol-induced increases in alpha power. Our study extends previous evidence by providing spatial estimates of the main generator nodes for the alpha (posteriomedial and pericalcarine cortex), theta, and beta bands (medial prefrontal cortex), which are in agreement with fMRI-BOLD studies. When applied to chronic alcoholics or individuals at risk, this methodology could provide insight into the neural basis of oscillatory differences that may be predictive of the vulnerability to alcoholism.
Acknowledgments
This work was supported by funds from the National Institutes of Health (R01-AA016624). The authors thank Jason Sherfey and Eric Halgren for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian ED, Matthews BHC. The Berger rhythm: potential changes from the occipital lobes in man. Brain: A Journal of Neurology. 1934;57:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Ahveninen J, Lin FH, Kivisaari R, Autti T, Hämäläinen M, Stufflebeam S, et al. MRI-constrained spectral imaging of benzodiazepine modulation of spontaneous neuromagnetic activity in human cortex. NeuroImage. 2007;35:577–582. doi: 10.1016/j.neuroimage.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Amzica F, Lopes da Silva FH. Cellular substrates of brain rhythms. In: Schomer D, Lopes da Silva FH, editors. Niedermeyer’s Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. pp. 33–63. [Google Scholar]
- Aragues M, Jurado R, Quinto R, Rubio G. Laboratory paradigms of impulsivity and alcohol dependence: a review. European Addiction Research. 2011;17:64–71. doi: 10.1159/000321345. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clinical Neurophysiology. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Bauer G, Bauer R, Schomer D. EEG, drug effects, and central nervous system poisoning. In: Lopes da Silva FH, editor. Niedermeyer’s Electroncephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. pp. 901–922. [Google Scholar]
- Becker R, Reinacher M, Freyer F, Villringer A, Ritter P. How ongoing neuronal oscillations account for evoked fMRI variability. The Journal of Neuroscience. 2011;31:11016–11027. doi: 10.1523/JNEUROSCI.0210-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. International Journal of Psychophysiology. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Ben-Simon E, Podlipsky I, Arieli A, Zhdanov A, Hendler T. Never resting brain: simultaneous representation of two alpha related processes in humans. PLoS One. 2008;3:e3984. doi: 10.1371/journal.pone.0003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. The Journal of Neuroscience. 2008;28:9976–9988. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic α oscillations. The Journal of Neuroscience. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, et al. Dorsolateral prefrontal γ-aminobutyric acid in men predicts individual differences in rash impulsivity. Biological Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridwell DA, Wu L, Eichele T, Calhoun VD. The spatiospectral characterization of brain networks: fusing concurrent EEG spectra and fMRI maps. NeuroImage. 2013;69:101–111. doi: 10.1016/j.neuroimage.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging. Cambridge University Press; New York, NY: 2002. [Google Scholar]
- Buzsaki G. Rhythms of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. Monograph #6. Rutgers Center of Alcohol Studies; New Brunswick, NJ: 1969. [Google Scholar]
- Chatila M, Milleret C, Rougeul A, Buser P. Alpha rhythm in the cat thalamus. Comptes rendus de l’Académie des sciences. Série III, Sciences de la vie. 1993;316:51–58. [PubMed] [Google Scholar]
- Ciulla C, Takeda T, Endo H. MEG characterization of spontaneous alpha rhythm in the human brain. Brain Topography. 1999;11:211–222. doi: 10.1023/a:1022233828999. [DOI] [PubMed] [Google Scholar]
- Cohen D, Halgren E. Magnetoencephalography. In: Squire LR, editor. Encyclopedia of Neuroscience. Academic Press; Oxford: 2009. pp. 615–622. [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H. The effects of ethanol on EEG activity in males at risk for alcoholism. Electroencephalography and Clinical Neurophysiology. 1993;86:368–376. doi: 10.1016/0013-4694(93)90132-f. [DOI] [PubMed] [Google Scholar]
- Coutin-Churchman P, Moreno R, Añez Y, Vergara F. Clinical correlates of quantitative EEG alterations in alcoholic patients. Clinical Neurophysiology. 2006;117:740–751. doi: 10.1016/j.clinph.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Current Opinion in Neurobiology. 2001;11:202–208. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- de Munck JC, Goncalves SI, Mammoliti R, Heethaar RM, Lopes da Silva FH. Interactions between different EEG frequency bands and their effect on alpha-fMRI correlations. NeuroImage. 2009;47:69–76. doi: 10.1016/j.neuroimage.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature Reviews. Neuroscience. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Sejnowski TJ. Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiological Reviews. 2003;83:1401–1453. doi: 10.1152/physrev.00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. Evaluation of EEG alpha activity in sons of alcoholics. Neuropsychopharmacology. 1991;4:199–205. [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ. Impulsiveness and venturesomeness: their position in a dimensional system of personality description. Psychological Reports. 1978;43:1247–1255. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. Journal of Neurophysiology. 2005;93:2864–2872. doi: 10.1152/jn.00721.2004. [DOI] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A. Reduced EEG alpha power in the male and female offspring of alcoholics. Alcoholism: Clinical and Experimental Research. 1999;23:256–262. [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Nestler EJ. Molecular and cellular adaptations in signal transduction pathways following ethanol exposure. Clinical Neuroscience. 1995;3:165–173. [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nature Reviews. Genetics. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Jr., Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Venkitaramani DV, Chaki S, Cox CL. Spatially distinct actions of metabotropic glutamate receptor activation in dorsal lateral geniculate nucleus. Journal of Neurophysiology. 2012;107:1157–1163. doi: 10.1152/jn.00401.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews. Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hall SD, Barnes GR, Furlong PL, Seri S, Hillebrand A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Human Brain Mapping. 2010;31:581–594. doi: 10.1002/hbm.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography - theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics. 1993;65:413–497. [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml KH. Prestimulus oscillations predict visual perception performance between and within subjects. NeuroImage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends in Neurosciences. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Heister D, Diwakar M, Nichols S, Robb A, Angeles AM, Tal O, et al. Resting-state neuronal oscillatory correlates of working memory performance. PLoS One. 2013;8:e66820. doi: 10.1371/journal.pone.0066820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magnetic Resonance Imaging. 2007;25:978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Ishii R, Canuet L, Aoki Y, Ikeda S, Hata M, Takahashi H, et al. Frequency diversity of posterior oscillatory activity in human revealed by spatial filtered MEG. Journal of Integrative Neuroscience. 2013;12:343–353. doi: 10.1142/S0219635213500209. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Frontiers in Human Neuroscience. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RF, Hesselbrock VM, O’Connor S, DePalma N. Behavioral and EEG responses to alcohol in nonalcoholic men with a family history of alcoholism. Progress in Neuro-psychopharmacology & Biological Psychiatry. 1988;12:873–885. doi: 10.1016/0278-5846(88)90083-8. [DOI] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K. Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One. 2012;7:e43957. doi: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen MM, Dumont GJ, van Gerven JM, Buitelaar JK, Verkes RJ. Acute effects of MDMA (3,4-methylenedioxymethamphetamine) on EEG oscillations: alone and in combination with ethanol or THC (delta-9-tetrahydrocannabinol) Psychopharmacology (Berl) 2011;213:745–756. doi: 10.1007/s00213-010-2031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, et al. Where the BOLD signal goes when alpha EEG leaves. NeuroImage. 2006;31:1408–1418. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Orrison WW. Magnetoencephalography and magnetic source imaging. In: Orrison WW, Lewine JD, Sanders JA, Hartshorne MF, editors. Functional Brain Imaging. St. Louis; Mosby: 1995. pp. 369–418. [Google Scholar]
- Lin FH, Witzel T, Hämäläinen MS, Dale AM, Belliveau JW, Stufflebeam SM. Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage. 2004;23:582–595. doi: 10.1016/j.neuroimage.2004.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva FH. Neurocognitive processes and the EEG/MEG. In: Schomer D, Lopes da Silva FH, editors. Niedermeyer’s Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; Philadelphia: 2011. pp. 1083–1112. [Google Scholar]
- Lörincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8–13 Hz) rhythms in sensory thalamic nuclei in vitro. The Journal of Neuroscience. 2008;28:660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Kouri E, Bolduc M, Amass L. Ethanol-induced alterations in EEG alpha activity and apparent source of the auditory P300 evoked response potential. Alcohol. 1990;7:471–477. doi: 10.1016/0741-8329(90)90034-a. [DOI] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Maltzman I, Marinkovic K. Alcohol, alcoholism, and the autonomic nervous system: A critical account. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; New York: 1996. pp. 248–306. [Google Scholar]
- Manshanden I, De Munck JC, Simon NR, Lopes da Silva FH. Source localization of MEG sleep spindles and the relation to sources of alpha band rhythms. Clinical Neurophysiology. 2002;113:1937–1947. doi: 10.1016/s1388-2457(02)00304-8. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rosen BQ, Cox B, Kovacevic S. Event-related theta power during lexical-semantic retrieval and decision conflict is modulated by alcohol intoxication: Anatomically constrained MEG. Frontiers in Psychology. 2012;3:121. doi: 10.3389/fpsyg.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. The Journal of Neuroscience. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Progress in Neurobiology. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Mo J, Liu Y, Huang H, Ding M. Coupling between visual alpha oscillations and default mode activity. NeuroImage. 2013;68:112–118. doi: 10.1016/j.neuroimage.2012.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, et al. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochemistry International. 1995;26:305–336. doi: 10.1016/0197-0186(94)00139-l. discussion 337–342. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva FH, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Lippincott Williams & Wilkins; Baltimore, MD: 1999. pp. 149–173. [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Nikulina AV, Yamashita H, Rossi EM, Kähkönen S. Effects of alcohol on spontaneous neuronal oscillations: a combined magnetoencephalography and electroencephalography study. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2005;29:687–693. doi: 10.1016/j.pnpbp.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: The neurophysics of EEG. Oxford University Press; New York: 2006. [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Human Brain Mapping. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich S, Mulert C, Karch S, Trenner M, Leicht G, Pogarell O, et al. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. NeuroImage. 2009;45:319–332. doi: 10.1016/j.neuroimage.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipova D, Hermes D, Jensen O. Gamma power is phase-locked to posterior alpha activity. PLoS One. 2008;3:e3990. doi: 10.1371/journal.pone.0003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends in Neurosciences. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical Neurophysiology. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A, Jr., Neuper C. Event-related synchronization (ERS) in the alpha band--an electrophysiological correlate of cortical idling: a review. International Journal of Psychophysiology. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. The pharmacology of alcohol and alcohol dependence. Oxford University Press; New York: 1996. pp. 207–247. [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. TheScientificWorldJournal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Research. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, et al. Beta power in the EEG of alcoholics. Biological Psychiatry. 2002;52:831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, et al. Resting EEG in offspring of male alcoholics: beta frequencies. International Journal of Psychophysiology. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Rickenbacher E, Greve DN, Azma S, Pfeuffer J, Marinkovic K. Effects of alcohol intoxication and gender on cerebral perfusion: an arterial spin labeling study. Alcohol. 2011;45:725–737. doi: 10.1016/j.alcohol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. The European Journal of Neuroscience. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cerebral Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Rihs T, Brodbeck V, Thut G. Resting electroencephalogram alpha-power over posterior sites indexes baseline visual cortex excitability. Neuroreport. 2008;19:203–208. doi: 10.1097/WNR.0b013e3282f454c4. [DOI] [PubMed] [Google Scholar]
- Saletu-Zyhlarz GM, Arnold O, Anderer P, Oberndorfer S, Walter H, Lesch OM, et al. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol and Alcoholism. 2004;39:233–240. doi: 10.1093/alcalc/agh041. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41:211–221. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Schomer D, Lopes da Silva FH, editors. Niedermeyer’s Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The Self-Rating of the Effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997;92:979–988. [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Sorbel J, Morzorati S, O’Connor S, Li TK, Christian JC. Alcohol effects on the heritability of EEG spectral power. Alcoholism: Clinical and Experimental Research. 1996;20:1523–1527. doi: 10.1111/j.1530-0277.1996.tb01694.x. [DOI] [PubMed] [Google Scholar]
- SPSS . SPSS for Windows. SPSS Inc.; Chicago, IL: 2001. [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. Journal of Neurophysiology. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. The Journal of Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Debener S. Simultaneous EEG and fMRI: recording, analysis and application. Oxford University Press; New York: 2010. [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biological Psychology. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. The Journal of Neuroscience. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. British Journal of Pharmacology. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson SJ, Kaufman L. Advances in neuromagnetic instrumentation and studies of spontaneous brain activity. Brain Topography. 1989;2:129–139. doi: 10.1007/BF01128850. [DOI] [PubMed] [Google Scholar]
- Winterer G, Enoch MA, White KV, Saylan M, Coppola R, Goldman D. EEG phenotype in alcoholism: increased coherence in the depressive subtype. Acta Psychiatrica Scandinavica. 2003;108:51–60. doi: 10.1034/j.1600-0447.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Schleicher A. Transmitter receptors and functional anatomy of the cerebral cortex. Journal of Anatomy. 2004;205:417–432. doi: 10.1111/j.0021-8782.2004.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Dimensions of sensation seeking. Journal of Consulting and Clinical Psychology. 1971;36:45–52. [Google Scholar]