Abstract
We have analyzed the relationship between CD4+CD8− (CD4SP) thymocyte deletion and CD4SPFoxp3+ Treg formation using mice in which signals transmitted by the TCR have been attenuated by mutation of one of three tyrosine residues (Y145) of the adapter protein Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76). In mice containing polyclonal TCR repertoires, the Y145 mutation caused increased frequencies of CD4SPFoxp3+ thymocytes. CD4SP thymocytes expressing TCR Vβ-chains that are subjected to deletion by endogenous retroviral superantigens were also present at increased frequencies in the context of the Y145 mutation, particularly amongst CD4SPFoxp3+ thymocytes. In transgenic mice in which CD4SP thymocytes expressing an autoreactive TCR undergo both deletion and Treg formation in response to a defined self-peptide, the Y145 mutation abrogated deletion of autoreactive CD4SP thymocytes. Notably, Foxp3+ Treg formation still occurred, albeit with a reduced efficiency, and the Y145 mutation was also associated with decreased Nur77 expression by the autoreactive CD4SP thymocytes. These studies provide evidence that the strength of TCR signal can play a direct role in directing the extent of both thymocyte deletion and Treg differentiation, and suggest that distinct TCR signaling thresholds and/or pathways can promote CD4SP thymocyte deletion versus Treg formation.
Keywords: Immune regulation, Tolerance, Thymic selection
Introduction
Seminal studies demonstrated that positive selection promotes the survival of thymocytes whose TCR has an intrinsic capacity to react with self-peptide-MHC complexes, while those that are strongly reactive in many cases undergo deletion through induction of apoptosis [1–3]. This “avidity” model of thymocyte development can readily explain how an immune system that generates a TCR repertoire by random gene rearrangement can become capable of responding to foreign peptides presented by the host’s MHC molecules while eliminating thymocytes whose TCRs might react with self-antigens that are encountered in the periphery [4]. However, understanding the role that TCR reactivity plays in guiding thymocyte development became more complicated with the discovery that a subset of thymocytes that can react with self-antigens avoid deletion and instead adopt a regulatory phenotype (initially characterized by the expression of CD25, and subsequently by the transcription factor Foxp3) [5, 6]. These Tregs play an obligate role in preventing a severe autoaggressive lymphoproliferative disease that spontaneously develops if these cells are absent [5, 6]. How signals transmitted by the TCR in response to a diverse universe of self-peptides instruct thymocytes to undergo these alternative and/or inter-related fates is not yet understood.
The initial evidence that TCR reactivity with self-peptides can direct thymocytes to develop into Tregs came from transgenic mice expressing MHC class II-restricted TCRs and co-expressing their cognate peptide as self-antigens [7–10]. A notable feature of these studies was that Treg formation was found to be induced by peptides which were defined as “agonists” because of their ability to induce peripheral T cells to elaborate diverse effector functions when present at relatively low concentrations [11]. By contrast, “partial agonists” (typically variants of the agonist peptide) typically require higher concentrations to induce T cell activation and might activate only a subset of the effector pathways that are induced by an agonist counterpart [11]. The notion that Treg formation is induced by self-peptides with which a TCR has a strong intrinsic reactivity seemed counterintuitive in light of the avidity model of thymocyte development (in which high reactivity with self-peptides induces deletion). However, recent studies using a Nur77 reporter mouse have supported the conclusion that thymocytes that develop into Tregs perceive stronger TCR signals than conventional CD4+ T cells [12]. In addition, more recent findings in transgenic mice showed that agonist self-peptides might typically induce thymocyte deletion, but that this process can be incomplete, and Treg formation occurs among a subset of thymocytes that evades deletion [13]. Another study showed that a partial agonist self-peptide can induce thymocyte deletion in the absence of Treg formation [14]. Whether Treg formation can occur in the absence of appreciable deletion has been less well characterized.
To analyze how TCR engagement can affect the ability of autoreactive thymocytes to undergo deletion and/or develop into Foxp3+ Tregs in response to self-peptides, we have examined mutant mice in which one of three tyrosine phosphorylation sites on the SLP76 adapter molecule (designated Y145) has been abolished in order to attenuate the signal transmitted by the TCR. We found that all three of the major pathways that can shape CD4SP thymocyte development (i.e. positive selection, deletion, and Foxp3+ Treg induction) were affected by this mutation, and that effects of the Y145 mutation could be observed at the level of polyclonal CD4SP thymocyte development. However, it can be difficult to interpret the effects of TCR signaling mutation on thymocyte development in mice with polyclonal T cell repertories, because changes in the sensitivity of the TCR that are caused by the mutation could be expected to lead to compensatory changes in the affinity/avidity of the TCRs that are used by thymocytes to direct selection along different developmental pathways. We therefore also examined the effects of the Y145 mutation on the development of CD4SP thymocytes expressing a TCR with a defined specificity for a nominal self-peptide. Comparing the effects of the Y145 mutation on thymocyte development in these different settings allowed us to interpret the effects of the Y145 mutation in the context of the avidity model of thymocyte development, and conclude that the strength of the TCR signal can play a direct role in directing the extent of both autoreactive thymocyte deletion and Treg differentiation.
Results
SLP76 mutation alters thymocyte selection in polyclonal BALB/c.Y145 mice
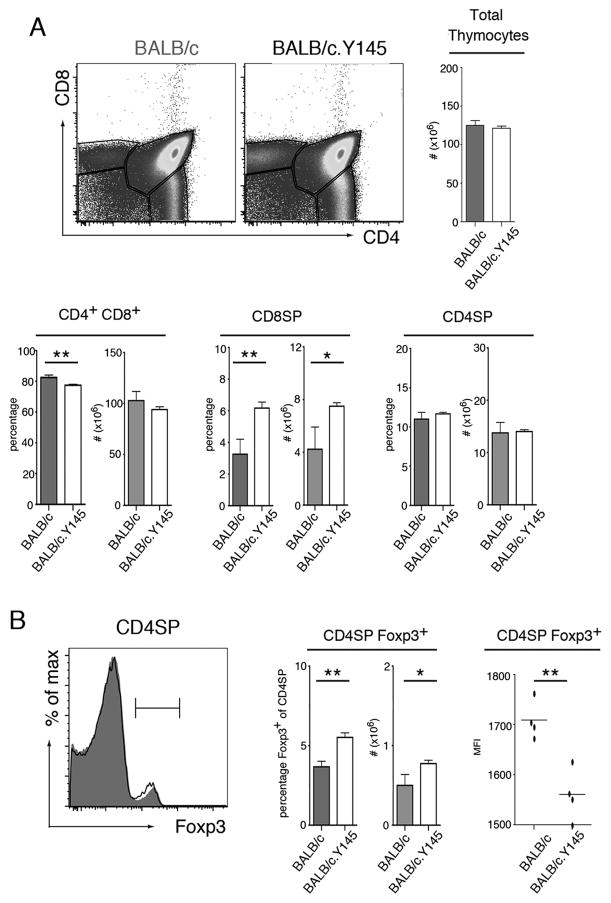
Y145 mice are homozygous for a “knock-in” mutation in which tyrosine 145 of the signaling adaptor molecule SLP76 (SH2 domain-containing leukocyte protein of 76 kDa) has been replaced with phenylalanine [15]. Previous studies showed that the Y145 mutation leads to decreased Ca2+ flux and diminished MAP-kinase mediated signaling in response to TCR stimulation via anti-CD3 crosslinking [15, 16]. As an initial step toward understanding how this mutation influences selection of thymocytes expressing an I-Ed restricted TCR, we generated BALB/c.Y145 mice. Similar to the effects of the Y145 mutation in thymocytes expressing polyclonal TCRs in a MHC H-2b background [15], there were increased frequencies of CD8SP thymocytes in BALB/c.Y145 mice relative to BALB/c mice with wild-type SLP76 alleles. Notably, while the number of CD4SP thymocytes did not significantly differ, both the percentages and number of CD4SPFoxp3+ thymocytes was higher in BALB/c.Y145 mice than in BALB/c mice (Fig. 1B). Although their frequency was increased, the CD4SPFoxp3+ thymocytes in BALB/c.Y145 mice expressed slightly lower levels of Foxp3 (Fig. 1B). Thus introduction of the SLP-76 mutation led to alterations in CD8+ and CD4SPFoxp3 thymocyte formation in BALB/c mice containing a polyclonal T cell repertoire.
Figure 1. SLP-76 mutation alters thymocyte selection and causes increased frequencies of CD4SPFoxp3+ thymocytes in polyclonal BALB/c.Y145 mice.
(A) Dot plots show staining for CD4 and CD8 of thymocytes from BALB/c and BALB/c.Y145 mice. Bar graphs indicate mean numbers or percentages of indicated populations ± SD (filled bars, BALB/c mice; open bars, BALB/c.Y145 mice). (B) Histograms show Foxp3 straining by CD4SP thymocytes from BALB/c (grey shading) and BALB/c.Y145 (line) mice. Bar graph indicates mean numbers or percentages of CD4SP thymocytes that are Foxp3+ ± SD (filled bars, BALB/c mice; open bars, BALB/c.Y145 mice). Scatter plots show MFI with mean indicated for Foxp3 expression by CD4SPFoxp3+ thymocytes from BALB/c and BALB/c.Y145 mice. *p<0.05, **p<0.01. Data are representative of at least 4 independent determinations.
SLP76 mutation alters the representation of TCR β-chain variable region gene segments among CD4SP thymocytes
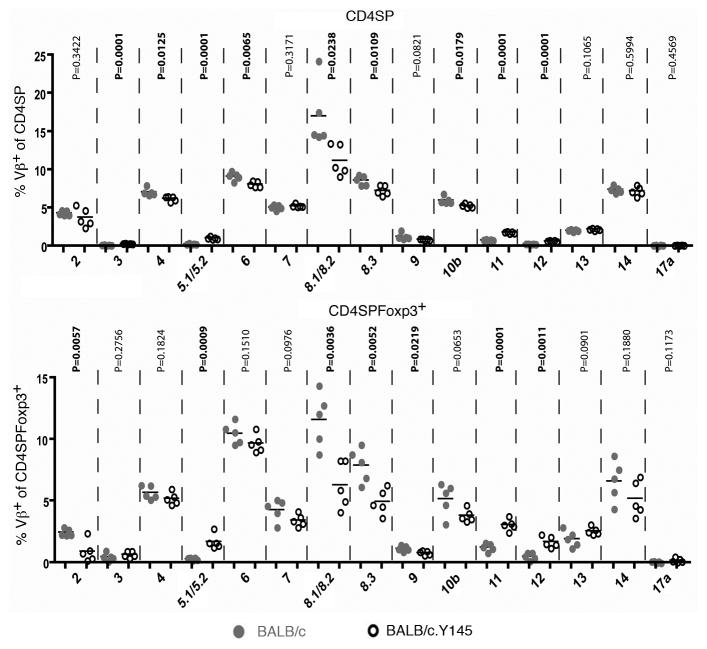
We also analyzed whether the Y145 mutation has any effects on the representation of different Vβ gene segments in CD4SP thymocytes from BALB/c versus BALB/c.Y145 mice. Indeed, there were significant decreases in the representation of specific gene segments (e.g. Vβ6, Vβ8.1/8.2 and Vβ8.3) in both CD4SP and CD4SPFoxp3+ thymocytes from BALB/c.Y145 mice compared to WT BALB/c mice (Fig. 2). Conversely, the frequencies of some gene segments (e.g. Vβ3, Vβ5.1/5.2, Vβ11 and Vβ12) were significantly increased among CD4SP thymocytes from BALB/c.Y145 mice (Fig. 2). Importantly, the TCR Vβ chains that were present at increased frequencies in BALB/c.Y145 mice confer specificity for the Mlsf group of mouse mammary tumor virus-encoded superantigens that are carried by BALB/c mice and, in association with I-Ed, can mediate deletion of CD4SP thymocytes [17]. Moreover, while the representation of these Mlsf-reactive TCR Vβ genes increased from mean frequencies of 1.5% to 3.7% among CD4SP thymocytes from BALB/c.Y145 mice, the increase was even more pronounced among CD4SP Foxp3+ thymocytes, where the mean frequencies increased from 2.1% to 6.5% in BALB/c.Y145 mice versus BALB/c mice. Thus, the Y145 mutation impaired the deletion of thymocytes bearing autoreactive TCR Vβ gene segments, and CD4SPFoxp3+ thymocytes were enriched relative to CD4SP thymocytes for the presence of these autoreactive TCR Vβ gene segments in BALB/c.Y145 mice.
Figure 2. SLP-76 mutation alters the representation of TCR Vβ gene segments in CD4SP thymocytes from BALB/c.Y145 mice.
Scatter plots show the percentages of CD4SP thymocytes (upper panel) and of CD4SPFoxp3+ thymocytes (lower panel) that express the indicated TCR Vβ gene segments. Data from individual mice are shown (grey circles, BALB/c mice; open circles, BALB/c.Y145 mice) (n=5) along with means for each group and the probabilities that each pairing has the same mean.
SLP76 mutation leads to decreased positive selection of thymocytes expressing the 6.5 TCR
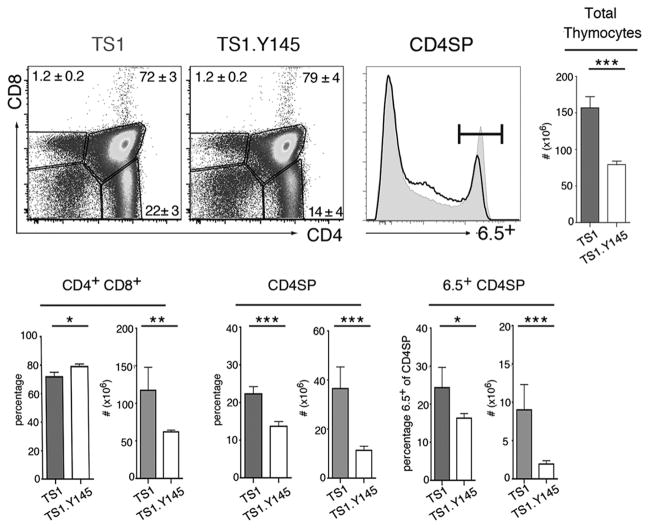
To further examine how the Y145 mutation can affect thymocyte development, we made use of TS1 mice, which express a transgenic Vα4/Vβ8.2 TCR that can be identified by the anti-clonotypic mAb 6.5 and recognizes the site 1 (S1) determinant of influenza virus PR8 HA in the context of I-Ed [18]. TS1 mice were mated with Y145 mice to produce TS1.Y145 mice, and we first examined whether the Y145 mutation exerts any effects on positive selection of thymocytes expressing the 6.5 TCR. Indeed, total thymic cellularity was significantly reduced in TS1.Y145 mice relative to TS1 mice, and while the percentages of CD4+CD8+ thymocytes increased, their numbers were reduced in line with the lower overall thymic cellularity of TS1.Y145 mice (Fig. 3). In addition, both the percentages and numbers of thymocytes that were CD4SP were reduced, and of the CD4SP thymocytes, the percentages and numbers that were 6.5+ were diminished in TS1.Y145 mice relative to TS1 mice. Thus, the introduction of the Y145 mutation in TS1.Y145 mice decreased the efficiency of positive selection of 6.5+CD4SP thymocytes relative to TS1 mice expressing wild-type SLP-76 alleles.
Figure 3. SLP-76 mutation alters positive selection of thymocytes expressing the 6.5 TCR.
Dot plots show staining for CD4 and CD8 of thymocytes from TS1 and TS1.Y145 mice. Histograms show 6.5 expression by CD4SP thymocytes from TS1 (gray shading) and TS1.Y145 (line) mice. Bar graphs indicate mean numbers or percentages of indicated populations ± SD (filled bars, TS1 mice; open bars, TS1.Y145 mice). *p<0.05, **p<0.01, ***p<0.001. Data are representative of at least 5 independent determinations.
SLP-76 mutation decreases the sensitivity of the 6.5 TCR to cognate peptide
To examine how the Y145 mutation affects the sensitivity of the 6.5 TCR to stimulation by cognate peptide, we isolated CD4+ T cells from the LNs of TS1 and TS1.Y145 mice and compared their abilities to upregulate CD25 and CD69 at early time points following stimulation with S1 peptide. Consistent with a response to an agonist peptide, the 6.5+CD4+ T cells from TS1 mice upregulated both CD25 and CD69 in response to sub-micromolar concentrations of S1, while those from TS1.Y145 mice required 2- to 4-fold higher concentrations of S1 peptide to achieve comparable CD25 or CD69 up-regulation (Fig. 4). Thus, the Y145 mutation causes attenuated TCR signaling in 6.5+CD4+ T cells responding to the S1 peptide.
Figure 4. SLP-76 mutation decreases the sensitivity of CD4+ T cells to cognate peptide.
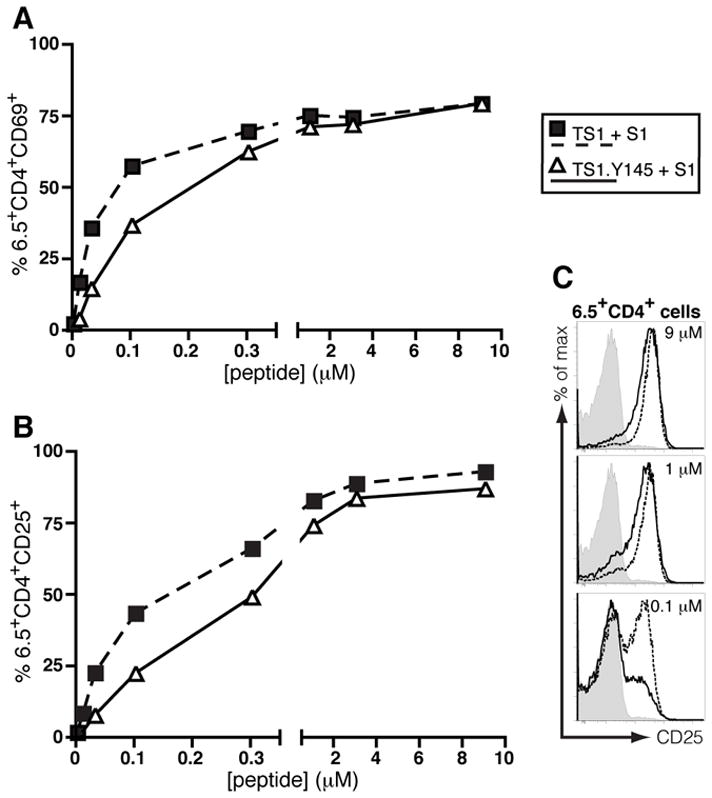
6.5+CD4+ cells were FACS-purified from the spleens and LNs of TS1 and TS1.Y145F mice and co-cultured with BALB/c splenocytes pulsed with graded doses of S1 peptide. Sixteen hours later the cells were stained for CD69 (A) and CD25 (B) and the percentage of 6.5+CD4+ cells that expressed each marker was determined by flow cytometry. (C) Histograms showing staining of 6.5+CD4+ T cells from TS1 mice (dashed line) and TS1.Y145 mice (solid line) stimulated with S1 peptide, or from TS1 mice without peptide (filled). Data are representative of two independent determinations.
Attenuated TCR signaling alters autoreactive thymocyte deletion and Foxp3 induction
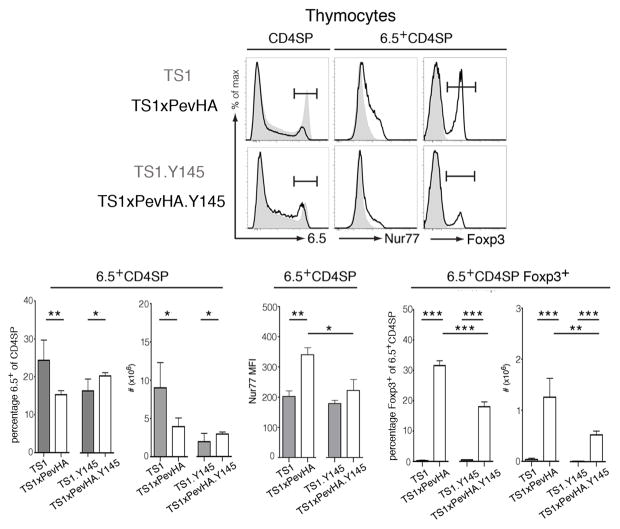
We have previously shown that 6.5+CD4SP thymocytes undergo a combination of deletion and CD25+Foxp3+ Treg formation in response to the S1 self-peptide when TS1 mice are mated with PevHA mice that express HA as a self-antigen, to produce TS1xPevHA mice [13, 19]. Consistent with these previous studies, TS1xPevHA mice contained significantly fewer 6.5+CD4SP thymocytes than TS1 mice (Fig. 5). Moreover, Nur77 levels, as determined by the MFI, were significantly higher on 6.5+CD4SP thymocytes from TS1xPevHA mice than from TS1 mice (Fig. 5). These increased levels of Nur77 are consistent with studies showing that Nur77 expression by thymocytes correlates with strength of TCR signaling [12]; in this case, increased Nur77 levels reflect the ability of the 6.5 TCR to react with the S1 self-peptide. Increased Nur77 levels in the context of decreased numbers of 6.5+CD4SP thymocytes are also consistent with studies showing that Nur77 upregulation is associated with thymocyte deletion [20, 21]. Among the 6.5+CD4SP thymocytes that persisted in TS1xPevHA mice despite thymocyte deletion, approximately one third expressed Foxp3 (Fig. 5). This occurrence reflects increased Foxp3+ Treg formation driven by interactions with the S1 peptide, since 6.5+CD4SPFoxp3+ thymocytes were present in significantly higher numbers in TS1xPevHA mice than in TS1 mice (Fig. 5).
Figure 5. Attenuated TCR signaling differentially affects autoreactive thymocyte deletion and Treg formation.
Representative histograms show staining for 6.5 by CD4SP thymocytes, and of Nur77 and Foxp3 by 6.5+CD4SP thymocytes of indicated strains. Shaded histograms are for TS1 and TS1.Y145 mice, lines are for TS1xPevHA and TS1xPevHA.Y145 mice as indicated. Bar graphs indicate mean numbers or percentages of indicated populations ± SD (filled bars, TS1xPevHA mice; open bars, TS1xPevHA.Y145 mice). *p<0.05, **p<0.01, ***p<0.001. Data are representative of at least 5 independent determinations.
To examine the effects of SLP-76 mutation on these processes, we compared 6.5+CD4SP thymocyte development in TS1.Y145 and TS1xPevHA.Y145 mice to mice expressing wild-type SLP-76 alleles. Notably, the presence of the S1 self-peptide in TS1xPevHA.Y145 mice led to increases in the both the percentage and number of 6.5+CD4SP thymocytes relative to TS1.Y145 mice, sharply contrasting the deletion of 6.5+CD4SP thymocytes that was induced by S1 peptide in TS1xPevHA mice with wild-type SLP-76 alleles (Fig. 5). Consistent with the failure to observe 6.5+CD4SP thymocyte deletion, the Nur77 MFI was significantly lower in 6.5+CD4SP thymocytes from TS1xPevHA.Y145 mice than from TS1xPevHA mice, and did not differ significantly from TS1.Y145 mice (Fig. 5). While there was no evidence that interactions with the S1 peptide induced deletion of 6.5+CD4SP thymocytes in TS1xPevHA.Y145 mice, these interactions could still induce Treg formation, since the numbers of 6.5+CD4SPFoxp3+ thymocytes were significantly higher in TS1xPevHA.Y145 mice than in TS1.Y145 mice (Fig. 5). The Y145 mutation did, however, reduce the efficiency of Foxp3 induction because the numbers of 6.5+CD4SP thymocytes that became Foxp3+ were significantly lower in TS1xPevHA.Y145 mice than in TS1xPevHA mice (Fig. 5). Similar differences in Foxp3 expression were found in peripheral CD4+ T cells from TS1xPevHA.Y145 mice and TS1xPevHA mice (Supporting Information Fig. 1). Thus, the Y145 mutation abrogated 6.5+CD4SP thymocyte deletion in TS1xPevHA.Y145 mice, but Foxp3+ Treg formation still occurred, albeit with reduced efficiency relative to mice containing wild-type SLP-76 alleles.
SLP-76 mutation impairs Foxp3−CD25+ Treg precursor cell formation
To determine whether the Y145 mutation causes a decrease in Treg precursor formation, we analyzed CD4SPCD25+Foxp3− thymocytes, which have previously been identified as a precursor population that is enriched among immature heat stable antigen (HSA)highCD4SP thymocytes and that has received a TCR signal and can upregulate Foxp3 and complete Treg differentiation in response to IL-2 or other common γ-chain cytokines [22]. Consistent with this, ~32% of the mature HSAlow6.5+CD4SP thymocytes in TS1xPevHA mice co-expressed CD25 and Foxp3, and ~9.1% of the immature HSAhigh6.5+CD4SP thymocytes expressed the CD25+Foxp3− Treg precursor phenotype (Fig. 6). By contrast, <1% of the HSAhigh6.5+CD4SP thymocytes in TS1 mice expressed the CD25+Foxp3− Treg precursor phenotype, and we again found few or no HSAhigh6.5+CD4SPCD25+Foxp3+ thymocytes in this lineage. In the case of TS1xPevHA.Y145 mice, the frequency of mature HSAlow6.5+CD4SPCD25+Foxp3+ thymocytes was approximately one third of that found in TS1xPevHA mice (~9.3 versus ~32%), resembling what was seen in the preceding studies (see Fig. 5). Notably, the percentage of immature HSAhigh6.5+CD4SP thymocytes in TS1xPevHA.Y145 mice that expressed the CD25+Foxp3− Treg precursor phenotype was also reduced to approximately one third of that found in TS1xPevHA mice (2.4 versus 9.1 percent, respectively). Thus, the reduced formation of 6.5+CD4SPFoxp3+ thymocytes that is caused by the Y145 mutation in TS1xPevHA.Y145 mice is closely associated with, and likely a consequence of, decreased formation of 6.5+CD4SPCD25+Foxp3− Treg precursor cells in response to the S1 self-peptide.
Figure 6. SLP-76 mutation attenuates Foxp3−CD25+ Treg precursor formation.
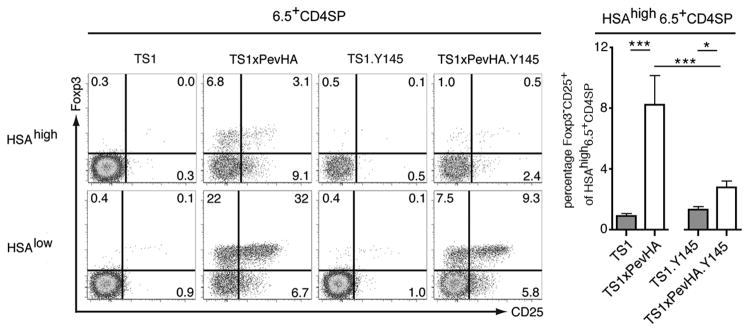
Histograms show CD25 and Foxp3 staining by immature (HSAhigh) and mature (HSAlow) 6.5+CD4SP thymocytes from indicated strains. Numbers indicate percentages in indicated gates from representative mouse. Bar graphs show the mean percentages ± SD of HSAhigh6.5+CD4SP cells that are CD25+Foxp3− in the indicated strains (filled bars, TS1 and TS1xPevHA mice; open bars, TS1.Y145 and TS1xPevHA.Y145 mice as indicated). *p<0.05, ***p<0.001. Data are representative of at least 5 independent determinations.
Discussion
The studies here have shown that alterations of TCR signaling caused by elimination of one of three critical tyrosine phosphorylation sites on the SLP76 adapter molecule can affect multiple aspects of thymocyte development (i.e. positive selection, deletion, and Foxp3+ Treg induction). With respect to positive selection, there were significant decreases in the frequencies of CD4SP thymocytes using several different Vβ gene segment, including Vβ8.1/8.2 gene segments, in BALB/c.Y145 mice relative to BALB/c mice. Since other TCR Vβ gene segments were present at similar frequencies in these two backgrounds, these observations suggest that the Y145 mutation does not cause a global decrease in the overall efficiency of positive selection of CD4SP thymocytes. It is notable then that the frequencies of CD4SP thymocytes expressing the 6.5 TCR (which uses a Vβ8.2 gene segment) were also significantly reduced in TS1, Y145 mice relative to TS1 mice with wild-type SLP76 alleles. This is consistent with the conclusion that the Y145 mutation can affect the efficiency of positive selection of CD4SP thymocytes expressing (among others) the Vβ8.2 gene segment, and suggests that the Y145 mutation may reduce the sensitivity of the TCRs expressed by a subset of TCRs (identified at least in part by Vβ gene segment usage) such that they are below a signaling threshold necessary for positive selection.
With respect to thymocyte deletion, there were significant increases in the representation of CD4SP thymocytes expressing Vβ gene segments that confer specificity for endogenous Mlsf superantigens in BALB/c.Y145 mice relative to BALB/c mice, resembling a previous demonstration that the Y145 mutation impairs deletion of Mls-reactive thymocytes in an H-2b background [15]. Similarly, both the percentages and numbers of 6.5+CD4SP thymocytes were increased in TS1xPevHA.Y145 mice relative to TS1.Y145 mice, contrasting the significant deletion of 6.5+CD4SP thymocytes that occurred in response to the S1 self-peptide in TS1xPevHA mice containing wild-type SLP-76 alleles. Notably, the impaired deletion of 6.5+CD4SP thymocytes in TS1xPevHA.Y145 mice was associated with a significant decrease in the level of Nur77. Strong TCR signals have previously been found to be associated with both upregulation of Nur77 and thymocyte deletion [12]. The studies here provide additional evidence linking these processes, since decreasing the levels Nur77 through Y145 mutation was associated with reduced thymocyte deletion. It is also noteworthy that deletion of 6.5+CD4SP thymocytes was abrogated despite a relatively small effect of the Y145 mutation on the ability of mature CD4+ T cells to become activated in response to the cognate S1 peptide, suggesting that phosphorylation of amino acid Y145 of SLP-76 plays an important role in directing thymocyte deletion in response to self-peptides.
The Y145 mutation exerted more complex effects on the formation of Foxp3+ CD4SP thymocytes in the different experimental systems. In polyclonal BALB/c.Y145 mice, there was an increased representation of CD4SPFoxp3+ thymocytes relative to BALB/c mice, resembling studies in mice expressing a TCR CD3 ζ-chain in which six ITAMs had been mutated to prevent phosphorylation, and which were found to contain increased frequencies of Foxp3+ Tregs [23]. However, in this previous study, the effects of the mutated allele on the selection of a TCR with a defined specificity were not examined. We found that the Y145 mutation led to a decreased efficiency of Foxp3+ Treg formation that was evident at the level of Treg precursor formation in TS1xPevHA.Y145 mice. How can these apparently opposing observations be reconciled? We have previously provided evidence in the TS1xHA system that Foxp3+ Tregs arise from thymocytes that have evaded deletion by self-antigens, and we propose that the increased representation of Tregs in BALB/c.Y145 mice is a reflection of the impaired negative selection of autoreactive thymocytes. Indeed, the CD4SPFoxp3+ thymocytes in BALB/c.Y145 mice were enriched for Mlsf-reactive TCR Vβ chains. Thus, despite the reduced efficiency of Treg precursor formation at the level of thymocytes expressing an individual autoreactive TCR specificity that was observed TS1xPevHA.Y145 mice, the Y145 mutation deletion led to overall increases in the numbers of autoreactive thymocytes that could evade deletion and enter the Treg differentiation pathway in polyclonal BALB/c.Y145 mice. The observation that the CD4SPFoxp3+ thymocytes that developed in BALB/c.Y145 mice expressed on average slightly lower levels of Foxp3 than were found in mice with wild-type SLP-76 alleles may also be a reflection of reduced TCR signaling caused by the Y145 mutation. Alternatively, these lower levels of Foxp3 could be a reflection of competition for limiting resource(s) that was caused by an increase in the numbers of autoreactive thymocytes entering the Treg pathway [24, 25].
The adapter protein SLP-76 occupies a relatively TCR-proximal position in the diverse signal transduction pathways that can be triggered when thymocytes and T cells interact with selecting and/or activating ligands. The effects of the Y145 mutation on thymocyte development observed here can be readily interpreted in the context of an overall reduction in the strength of signal that is transmitted following TCR engagement, since the mutation also reduced the upregulation of molecules associated with TCR stimulation of both 6.5+CD4SP thymocytes (i.e. Nur77) and peripheral 6.5+CD4+ T cells (i.e. CD69 and CD25) that had been stimulated with the S1 peptide in either in vivo or in vitro settings. However, it is also possible that some of the effects observed here reflect selectivity of this mutation for particular signaling pathways and/or the selective reliance on particular signals for different thymocyte fates during selection. Since positive selection is dependent upon both Ca2+ and Erk signals [26–28], the impaired positive selection observed in TS1.Y145 mice is consistent with previous work demonstrating poor activation of these pathways in thymocytes expressing the Y145F mutation [15]. Similarly, it might be predicted that signals thought to be important for deletion, such as activation of p38 [29, 30], are defective in the presence of the Y145F mutation, although this has not been directly examined. While defining the signals required for Treg development is still an area of active investigation, several upstream modulators of NF-κB have been shown to have a more selective effect on Treg development than on conventional T cells [31]. Thus, it is possible that the activity of such effectors is relatively maintained in the context of the Y145F mutation. Nonetheless, the association between decreased Nur77 expression and impaired Treg precursor formation that was observed in TS1xPevHA.Y145 mice strongly suggests that the strength of signal transmitted by the TCR can play a direct role in directing Treg differentiation. The data further suggest that either the signaling thresholds, or the signaling pathways that are involved in deletion and Treg formation can be at least partially non-overlapping, because 6.5+CD4SPFoxp3+ thymocyte formation could occur in TS1xPevHA.Y145 mice (albeit with reduced efficiency), even though there was no evidence that thymocyte deletion had occurred. In this regard, it is noteworthy that we previously found evidence for an obverse phenotype (i.e. significant deletion of 6.5+CD4SP thymocytes in the absence of Foxp3+ Treg cell formation) when TS1 mice were mated with a lineage of HA transgenic mice expressing a self-peptide with which the 6.5 TCR is only weakly reactive [14]. It will be of interest in future experiments to determine how differences in TCR signaling might shape thymocyte commitment to the Treg lineage through an ability to induce distinct epigenetic changes in Foxp3 and/or other Treg lineage-associated genes [32].
Materials and methods
Mice
TS1 and PevHA mice [13, 14, 18, 19] are in the BALB/c background and were intermated to produce TS1xPevHA mice. SLP-76.Y145 mice expressing the SLP-76Y145F allele [15] were bred to homozygosity for the H-2d haplotype and then backcrossed onto the BALB/c background for at least 5 generations prior to intermating to produce TS1.Y145 and TS1xPevHA.Y145 mice (homozygous for the Y145 mutation). All experiments were performed according to protocols approved by The Wistar Institutional Animal Care and Use Committee.
Flow cytometry
Antibodies were obtained from BD or eBioscience. Data were collected on a BD LSRII and analyzed using FlowJo..
In vitro T cell culture
6.5+CD4+ LN cells were FACS-purified and co-cultured with BALB/c splenocytes and graded doses of S1 (SFERFEIFPKE) peptide. After 16 h cells were stained for CD25 and CD69, and analyzed by flow cytometry.
Statistical methods
Unpaired two-tailed Student t-tests were performed to compare data sets within an experimental group. ANOVA with Tukey post-tests were performed when data was compared across multiple experimental groups.
Supplementary Material
Acknowledgments
We thank V. Garcia, O. Perng, F. Bedoya, the Wistar Flow Cytometry Core and the Wistar Animal Facility staff for their assistance. This research was funded by the NIH grant AI59166 (A.J.C), by NCI core grant P30 CA10815, and by the Commonwealth of Pennsylvania.
Abbreviations
- SLP-76
Src homology 2 domain-containing leukocyte protein of 76 kDa
- S1
site 1
- SP
single positive
- HSA
heat stable antigen
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 2.Sebzda E, Wallace VA, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 3.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 4.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 7.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 8.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 9.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 11.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 12.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picca CC, Oh S, Panarey L, Aitken M, Basehoar A, Caton AJ. Thymocyte deletion can bias Treg formation toward low-abundance self-peptide. Eur J Immunol. 2009;39:3301–3306. doi: 10.1002/eji.200939709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cozzo Picca C, Simons DM, Oh S, Aitken M, Perng OA, Mergenthaler C, Kropf E, Erikson J, Caton AJ. CD4CD25Foxp3 regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion. Proc Natl Acad Sci USA. 2011;108:14890–14895. doi: 10.1073/pnas.1103810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan MS, Sadler J, Austin JE, Finkelstein LD, Singer AL, Schwartzberg PL, Koretzky GA. Functional hierarchy of the N-terminal tyrosines of SLP-76. J Immunol. 2006;176:2430–2438. doi: 10.4049/jimmunol.176.4.2430. [DOI] [PubMed] [Google Scholar]
- 17.Herman A, Kappler JW, Marrack P, Pullen AM. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 18.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerman MA, Larkin J, 3rd, Cozzo C, Jordan MS, Caton AJ. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J Immunol. 2004;173:236–244. doi: 10.4049/jimmunol.173.1.236. [DOI] [PubMed] [Google Scholar]
- 20.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205:1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T, Cheng J, Yang P, Wang Z, Liu C, Su X, Bluethmann H, Mountz JD. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J Exp Med. 1996;183:1879–1892. doi: 10.1084/jem.183.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lio CWJ, Hsieh CS. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang S, Song KD, Lesourne R, Lee J, Pinkhasov J, Li L, El-Khoury D, Love PE. Reduced TCR signaling potential impairs negative selection but does not result in autoimmune disease. J Exp Med. 2012;209:1781–1795. doi: 10.1084/jem.20120058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bautista JL, Lio CWJ, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 27.Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 28.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565–574. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 30.Gong Q, Cheng AM, Akk AM, Alberola-Ila J, Gong G, Pawson T, Chan AC. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 31.Oh H, Ghosh S. NF-kB: roles and regulation in different CD4(+) T-cell subsets. Immunol Rev. 2013;252:41–51. doi: 10.1111/imr.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.