Abstract
A better understanding of the antigen presentation pathways that lead to CD8+ T cell recognition of HIV epitopes in vivo is needed to achieve better immune control of HIV replication. Here, we show that cross-presentation of very small amounts of HIV proteins from apoptotic infected CD4+ T lymphocytes by dendritic cells to CD8+ T cells is much more efficient than other known HIV presentation pathways, i.e., direct presentation of infectious virus or cross-presentation of defective virus. Unexpectedly, dendritic cells also take up actively antigens into endosomes from live infected CD4+ T lymphocytes and cross-present them as efficiently as antigens derived from apoptotic infected cells. Moreover, live infected CD4+ T cells costimulate cross-presenting dendritic cells in the process. Therefore, dendritic cells can present very small amounts of viral proteins from infected T cells either after apoptosis, which is frequent during HIV infection, or not. Thus, if HIV expression is transiently induced while costimulation is enhanced (for instance after IL-2 and IFNα immune therapy), this HIV antigen presentation pathway could be exploited to eradicate latently infected reservoirs, which are poorly recognized by patients' immune systems.
The prognosis of HIV infection has been greatly improved by highly active antiretroviral treatment. Efficient CD8+ T cell responses are crucial for the development of a protective response against HIV (1). Specific CD8+ T cells are detected during HIV primary infection, but their responses and phenotypes are altered compared with those found in other primary viral infections that are better controlled by the immune system (2, 3). Moreover, latently infected cells constitute viral reservoirs that are inaccessible to highly active antiretroviral treatment and do not reach the antigen expression threshold to stimulate directly HIV-specific CD8+ T cells (4). To obtain better replication control, it would be important to obtain a potent and specific recognition of viral reservoirs by CD8+ T cells.
HIV-specific CD8+ T lymphocytes recognize viral peptides associated with MHC class I molecules on the surface of infected cells. They lyse these cells and produce IFNγ and other antiviral molecules. To proliferate and differentiate into effector cells, naive CD8+ T lymphocytes require antigen presentation by dendritic cells (DC) (5). DC are infrequently infected by the virus as compared with CD4+ T lymphocytes (6). Productive infection may therefore not be the only source of antigen for DC to induce HIV-specific CD8+ T cell responses.
An attractive potential source of HIV antigens in vivo may be the apoptotic infected CD4+ T lymphocytes typically induced by the infection (7). Apoptotic cells are targeted to specific receptors on macrophages and DC, which phagocytose them (8). DC have developed specific cross-presentation pathways that allow MHC class I-restricted presentation of the antigens contained in these apoptotic cells to CD8+ T lymphocytes (9, 10). DC from HIV+ patients can activate autologous CD4+ and CD8+ lymphocytes after coculture with infected apoptotic cells (11, 12). An alternative source of HIV antigens for DC may be defective viral particles, which can fuse with the plasma membrane and be cross-presented without viral replication (13). The relative importance of these mechanisms for HIV presentation has never been evaluated.
We have compared these mechanisms on a quantitative basis to assess those that would be relevant in vivo in infected patients. We found that apoptotic infected CD4+ T cell cross-presentation by DC is much more efficient to present HIV antigens to specific CD8+ T cell lines than direct infection or presentation of defective virus particles or proteins. Surprisingly, we found that DC cross-present equally well HIV antigens from live or apoptotic infected CD4+ T lymphocytes, after active antigen acquisition. A good knowledge of the relative importance of these HIV presentation mechanisms in DC is crucial to stimulate them appropriately in infected patients, in order to help the immune system and control HIV replication.
Materials and Methods
Cell Culture. H9 and 8E5 cells were maintained in complete RPMI medium 1640 supplemented with 10% FCS. HIV protein expression was induced in 8E5 cells by phytohemagglutinin (PHA) (Murex Diagnostics, Chatillon, France) and phorbol 12-myristate 13-acetate (Sigma) for 5 days (14). Primary CD4+ T blasts were obtained from healthy donor peripheral blood mononuclear cells (PBMC; Etablissement Français du Sang, Pitié Salpêtrière, Paris, according to ethical guidelines) after 3 days incubation in 1 μg/ml PHA and 10 units/ml IL-2 (Roche), then positive CD4 immunomagnetic selection (Miltenyi Biotec, Paris). HIV-specific CD8+ T cell lines were generated by using PBMC of HIV+ individuals from cohort studies with the approval of Cochin Hospital's ethics committee as described (15). DC were differentiated from elutriated monocytes from HLA-typed healthy donors for 5–7 days in granulocyte–macrophage colony-stimulating factor (GM-CSF; Schering–Plough) and IL-4 (PeproTech, London) (15). DC loading was performed in H-2000 medium (Stemcell Technologies, Neylan, France) with GM-CSF and IL-4.
Viruses, Peptides, and Antibodies. HIV-1lai and an azidothymidine (AZT)-sensitive WT HIV-1 isolate from an antiretroviral naive patient were used (16). Replication and sensitivity to 200 μM AZT were checked by using CD4+ T cell blasts alone or cocultured with DC, or CD4+ T cell-loaded DC cocultured with CD8+ T cells.
HIV-1 Nef73–82 (QVPLRPMTYK, HLA-A3-restricted), RT476–484 (ILKEPVHGV, HLA-A2), Gag77–85 (SLYNTVATL, HLA-A2), Gag20–28 (RLRPGGKKK, HLA-A3), and human T-lymphotrophic virus-1 Tax11–19 (LLFGYPVYV, HLA-A2) were from Neosystem (Strasbourg, France). The following mAbs were used: anti-MHC class I W6/32 ascitis (1:100), anti-CD11c (pure or phycoerythrin-labeled) and anti-CD3-FITC (Becton Dickinson), and GaMIg-Cy5 (Caltag, South San Francisco, CA).
Cross-Presentation. H9 or 8E5 cells were irradiated (160 or 40 mJ/cm2 at 312 nm, respectively), cultured for 6 h, and then cocultured with DC for 16 h. AZT (200 μM; Sigma) was added when specified. Primary CD4+ T cell blasts were irradiated at 20 mJ/cm2 24 h after infection (11), cultured for 6 h, and then cocultured overnignt with DC in AZT. After 1 h of culture, 1 μg/ml lipopolysaccharide (LPS) (Escherichia coli, Calbiochem) was added if required. Thereafter, DC were extensively washed, then used as antigen-presenting cells (20,000 per well) in a 6-h IFNγ enzyme-linked immunospot assay (15) using as effectors CD8+ T cell lines or PBMC. CD4+ T cells were depleted or CD8+ T cells were enriched by using anti-CD4 beads or CD8 T cell isolation kit II, respectively (Miltenyi Biotec). Two or more effector:DC ratios were systematically tested in triplicate. DC class I haplotype was chosen according to CD8+ cell line restriction. In some experiments, apoptotic cells were purified or excluded by using annexin-V-conjugated magnetic microbeads (Miltenyi Biotec), which were then separated by using 10 mM EDTA-PBS. Transwell plates were from Costar. N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-FMK) (Z-VAD, 10 μM, Sigma) was added to T cells for 6 h. HIV p24 was quantified in viral stocks or infected cell lysates by ELISA (Innogenetics).
DC Loading. T cells were labeled with PKH67 or PKH26 (Sigma) before irradiation. At the end of the DC/apoptotic cell coculture, annexin-V-APC (Bender MedSystems, Vienna) and CD11c labeling was performed. In competition assays, DC were incubated with 10 mg/ml mannan, 500 μg/ml polyG or 10 μg/ml RGD (Arg-Gly-Asp) (all from Sigma) for 40 min at 4°C before coculture with PKH67-labeled live or UV-irradiated H9-HIV cells. After 1 h, cells were washed and stained with anti-CD11c-phycoerythrin in presence of 5 mM EDTA. Cells were then fixed with 1% paraformaldehyde and analyzed by using a FACScalibur flow cytometer and cellquest software (Beckton Dickinson). At least 10,000 viable DC were acquired. For microscopy purposes, cells were settled onto slides in mounting medium (DAKO) and coverslipped after paraformaldehyde fixation. In some experiments, transferrin-Alexa488 (10 μg/ml) or acetylated low density lipoprotein (AcLDL)-Alexa488 (10 μg/ml, Molecular Probes) were added during incubation to define the early and the late endosomal/lysosomal compartment, respectively. In situ Cell Death Detection Kit, TMR red was from Roche. Samples were acquired by using a TCS SP2 inverted confocal microscope (Leica Microsystems).
Results
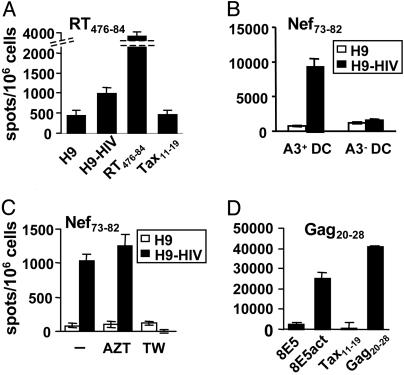
DC Loaded with HIV-Infected Apoptotic T Cells Stimulate HIV-Specific CD8+ Lines. To test cross-presentation of HIV antigens present in apoptotic T lymphocytes, the CD4+ T lymphoma cell line H9 chronically infected by HIV-1lai was UV-irradiated. Apoptotic cells were isolated by using annexin-V immunomagnetic separation and incubated with monocyte-derived DC from noninfected donors as described in Materials and Methods. As shown in Fig. 1A, an HLA-A2-restricted HIV-1 reverse transcriptase (RT)-specific T cell line recognized the epitope on the surface of DC. Other CD8+ T cell lines specific for the HLA-A2- and HLA-A3-restricted Nef- and Gag-derived epitopes were also stimulated after presentation by DC loaded with apoptotic H9-HIV cells (Fig. 1 B–D). This recognition was restricted by MHC class I molecules because it was blocked by an anti-class I mAb, and only CD8+ T cells secreted IFNγ, as assessed by intracellular cytokine flow cytometry (not shown). In addition, recognition depended on DC haplotype (Fig. 1B), excluding direct viral presentation by apoptotic H9-HIV cells.
Fig. 1.
DC presentation of MHC class I molecule-restricted viral epitopes from HIV-infected apoptotic CD4+ T cells. (A) DC were incubated with apoptotic cells purified from irradiated H9 or H9-HIV cells at an H9:DC ratio of 3:1, then with LPS, and tested by using a CD8+ T cell line specific for RT476–84 epitope at a DC:effector ratio of 1:3. As control, DC were incubated with 1 μM peptide. (B) The test was carried out by using DC expressing or not the class I MHC restriction molecule HLA-A3. (C) DC:H9 coculture was performed in the presence or not of AZT or in transwells (TW). (D) DC were cultured with irradiated 8E5 cells expressing viral proteins after activation (8E5act) in the absence of LPS and tested as before. Data are representative of at least three experiments each, except for B and C (two experiments).
Monocyte-derived DC do not constitute a preferential target for HIV, and only minor amounts of viral RNA are produced when they are infected in vitro (17, 18). Nevertheless, in our experimental system, DC were incubated overnight with HIV-producing cells; they could be infected and generate sufficient amounts of HIV epitopes to be presented to CD8+ HIV-specific lines. To exclude direct viral presentation, DC and apoptotic cells were cocultured in the presence of AZT to block replication (19). DC presented HIV antigens in the absence of productive viral particle infection (Fig. 1C). Nonproductive infection of DC by defective viral particles can also yield antigen presentation to an HIV-specific CD8+ clone (13). To investigate this potential mechanism, apoptotic cells were incubated in the upper compartment of a 0.45-μm transwell plate, allowing virion and soluble protein passage onto DC in the lower compartment, as measured by Bradford and Gag p24 ELISA tests, but preventing DC/apoptotic cell contact. In these conditions no specific response was detected (Fig. 1C), indicating that a contact or high proximity between DC and apoptotic cells was required for antigen uptake. These results suggested that viral infection, defective virion, or soluble antigen uptake are poorly efficient viral antigen acquisition mechanisms for MHC class I-restricted presentation by DC, compared with internalization of apoptotic debris. To study cross-presentation in the absence of infective virions, we used 8E5 cells, which are infected with an RT-defective HIV-1lai variant and cannot produce infective viral particles, but produce low amounts of viral proteins after activation. The mean amount of p24 protein was 27 ± 36 ng of p24 per million cells for H9-HIV and 2 ± 1.5 pg of p24 per million cells for activated 8E5 cells. Activated, irradiated 8E5 cells cocultured with DC induced efficient antigen presentation (Fig. 1D). These data show that DC actually cross-present MHC class I-restricted HIV antigens from apoptotic infected T cell lines to specific CD8+ T lymphocytes, in the absence of direct infection or of viral particles, even when low amounts of viral proteins are available.
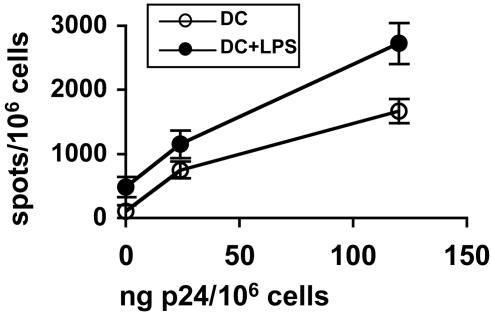
Cross-Presentation of HIV Antigens from Apoptotic Primary CD4+ Lymphocytes Infected with a Wild-Type Virus Isolate. To investigate whether cross-presentation of chronically infected CD4+ lymphoid lines after apoptosis was transposable to primary T lymphocytes, CD4+ T cell blasts from an HLA-class I-mismatched donor were infected with different amounts of an AZT-sensitive primary HIV-1 isolate, irradiated, and incubated with DC before testing recognition by an HIV-specific CD8 T cell line. After overnight incubation of T cells or DC with 120 pg of p24 per 106 cells, p24 was detected in T cell lysates (28 ± 15 pg per million cells) but not in DC, where p24 production could be detected only after longer culture times, indicating poor infectability of DC compared with T cells (20). Nevertheless, to avoid any direct presentation by DC, DC-CD4+ T lymphocyte cocultures were performed with AZT. As seen in Fig. 2, loaded DC presented the Nef73–82 epitope to a specific CD8+ line proportionally to the amount of virus used to infect the CD4+ T blasts. Presentation was obtained even in the absence of LPS stimulation during DC-T cell coculture, although at a lower level than that in the presence of LPS. Indeed, CD4+ T cell blasts induced DC maturation (Fig. 6, which is published as supporting information on the PNAS web site). Therefore, DC cross-present HIV antigens from apoptotic primary CD4+ cells infected with WT HIV.
Fig. 2.
DC cross-present a viral epitope from CD4+ primary lymphocytes infected with a WT HIV isolate. DC were cultured with irradiated CD4+ T cell blasts infected with different amounts of a primary HIV isolate in the presence or absence of LPS and tested by using an anti-Nef73–82 CD8+ line as in Fig. 1. This experiment was carried out in the presence of AZT.
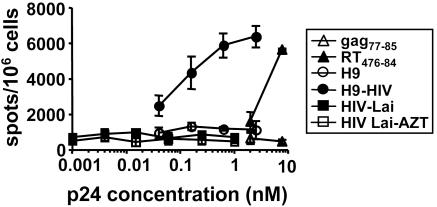
Cross-Presentation of HIV Antigens from Apoptotic T Cells Is More Efficient Than Direct Presentation or Presentation of Nonreplicating Virus. The different mechanisms of HIV antigen uptake and presentation mentioned above were compared quantitatively, by using p24 measurement in viral isolates or in apoptotic infected T cells before incubation with DC. Different amounts of HIV-1lai, replication competent or not (without or with AZT), or apoptotic T cells (with AZT to avoid direct presentation) were incubated overnight with DC. As seen in Fig. 3, low amounts of p24 contained in apoptotic infected cells induced a half-maximal response around 0.1 nM, stronger than an equivalent molar concentration of the synthetic RT476–484 peptide. Concentrations as high as 1 nM of p24 (equivalent to a multiplicity of infection of 200) of free virus, either infectious or inactivated by AZT, were not high enough to induce specific responses. When similar experiments were carried out by using activated 8E5 cells, even lower viral antigen concentrations (10-5 to 10-4 nM) induced CD8 T cell responses, even in the absence of LPS, probably due to more effective DC maturation in the presence of these cells (data not shown). Therefore, cross-presentation of HIV antigens from apoptotic infected CD4+ T cells was much more efficient on a quantitative basis than cross-presentation of defective virus or direct DC infection.
Fig. 3.
Efficiency of cross-presentation of HIV antigens from apoptotic cells compared with free virus. DC were cultured with different amounts and sources of HIV epitope: synthetic peptides, UV-irradiated, apoptotic H9 and H9-HIV cells in the presence of AZT, or HIV-1-Lai with or without AZT; then LPS was added. After coculture, DC were tested by using an anti-RT476–84 CD8+ line. Equivalent Gag p24 values were calculated after titration of viruses or lysed cells. For synthetic peptides, molar concentrations are represented. Data are representative of two experiments.
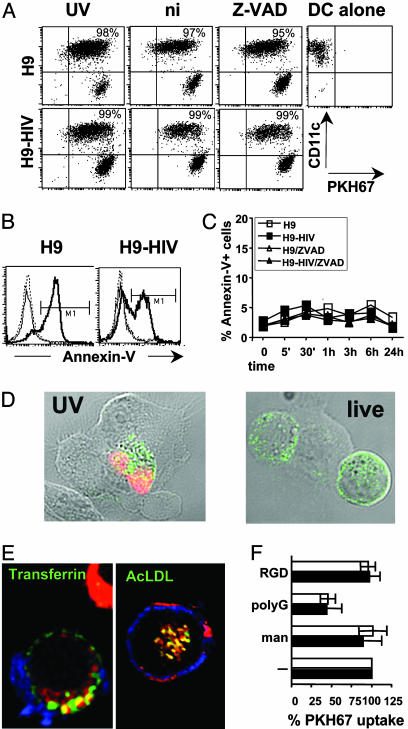
DC Acquire and Cross-Present Antigens from Live HIV-Infected T Cells. To test the dependence on apoptotic death of HIV cross-presentation, infected and non-infected H9 cells were labeled with the PKH67 cell membrane dye and cocultured with DC after being UV-irradiated or not. As shown in Fig. 4A, most of the DC (70–96% depending on the experiment) acquired PKH67 labeling, and neither the percentage of PKH67+ DC nor the fluorescence intensity was decreased if target cells were not previously irradiated. At 4°C, PKH67 acquisition was decreased to 19–24% of weakly positive DC, indicating that DC actively acquired material from live as well as apoptotic T cells (not shown). HIV-stimulated DC might have induced apoptosis in T lymphocytes, then phagocytosed the resulting debris (21). However, treatment of the cocultures with the pan-caspase inhibitor Z-VAD (22) did not reduce labeled material uptake (Fig. 4A). Apoptosis may also occur independently of caspases, but, in this experimental setting with low DC:H9 cell ratios, DC did not induce detectable apoptosis in nonirradiated cells anytime (Fig. 4 B and C; see also Fig. 4D, live). When live H9-HIV cells were cocultured with DC in the presence of Z-VAD, intimate DC-T contact and no T cell apoptosis were observed by confocal microscopy (Fig. 4D). All these data show that apoptosis induction is not required for antigen transfer. Material transfer from T lymphocytes into DC was visualized (Fig. 7, which is published as supporting information on the PNAS web site): PKH67-labeled membrane patches and intracellular vesicles were found in DC, and CD3 surface-labeling stained not only T lymphocytes, but also patches on DC. Similar patterns were obtained when H9-HIV cells were labeled with carboxyfluorescein diacetatesuccinimidyl ester (data not shown), a dye that labels cytoplasmic proteins, indicating that transfer is not restricted to plasma membrane-associated material. Equivalent results were obtained by using live HIVlai-infected primary CD4+ T cell blasts: PKH67-labeled material was found in 26 ± 6% of DC at 20 min and 50 ± 14% at 2 h. Clear vesicular aspects were evidenced in half of the cells that had incorporated PKH67-stained material. A majority of PKH-positive vesicles colocalized with transferrin (64 ± 5%) or AcLDL (75 ± 7%, Fig. 4E). Therefore, live cell material was effectively internalized into DC and undergoes classical trafficking from early (transferrin-positive) to late endosomes (positive for AcLDL).
Fig. 4.
Uptake of cell material from HIV-infected live cells by DC. (A) CD11c and PKH67 labeling of DC gated using forward scatter (FSC) and side scatter (SSC) criteria (note: some H9 cells appear in this gate as CD11c-negative events) after overnight coculture with PKH67-labeled H9 cells at an H9:DC ratio of 3:1. Dot plots show DC that have acquired H9-derived material as CD11c+ PHK67+ events, and their percentages are noted. UV, UV-irradiated; ni, nonirradiated; Z-VAD, nonirradiated Z-VAD-treated H9 cells. (B) Annexin-V staining of H9 cells gated using FSC and SSC criteria and CD11c-, PKH67+ labeling after overnight incubation alone (dotted lines), or with DC (thick line, UV-irradiated; thin line, nonirradiated H9 cells). (C) Annexin-V staining of H9 cells after different incubation times with DC. (D) Microscopy visualization of DC interaction with H9-HIV cells. Transmission light and confocal fluorescence overlay. DC were cultured for 15 min with UV-irradiated (UV) or nonirradiated (live) H9-HIV cells, treated with Z-VAD. They were fixed, permeabilized, and labeled for CD3 (green) and TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) reaction (red) to detect apoptotic cells. Intimate membrane interactions were found between DC (unlabeled) and H9-HIV cells (green). (E) Material from live, infected, PKH26-labeled (red) CD4+ T cell blasts is internalized and colocalizes (in yellow) with transferrin or AcLDL after 2 h of incubation with DC. (F) Competition of live (filled) or UV-irradiated (open) H9-HIV-associated (positive for CD11c, in blue) PKH67 uptake by DC using several adhesion molecule ligands. man, mannan. Mean values and SDs of at least three independent experiments are represented. DC were 60–95% PKH67-positive in absence of competitors. Data are representative of at least three experiments, except for C (two experiments).
Several receptors have been implicated in apoptotic or live cell material uptake by DC (23–25). Both live and apoptotic cell-associated material uptake were inhibited by polyG and fucoidan, and not by mannan, Arg-Gly-Asp (RGD), or Arg-Gly-Asp-Ser (RGDS) (Fig. 4F and not shown), indicating uptake mediated by scavenger receptors and not mannose receptors or α integrins.
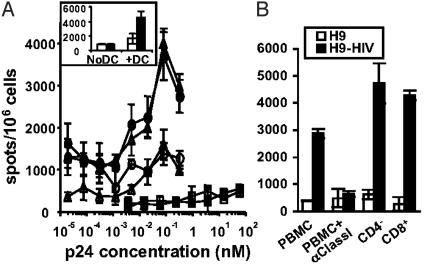
To determine whether this transfer from live cells led to HIV antigen presentation, DC were cultured overnight with irradiated or live Z-VAD-treated H9-HIV cells. Amazingly, the HIV-specific CD8+ cell line recognized live infected H9 cells with a dose–response curve entirely superimposed to that of apoptotic cells (Fig. 5A). Similar results were obtained with Z-VAD untreated cells (data not shown). Live H9-HIV cells were not recognized in the absence of DC (Fig. 5A Inset). When DC-T contact was blocked by using a 0.45-μm transwell, cross-presentation was prevented (Fig. 8A, which is published as supporting information on the PNAS web site), despite passage of particles with a size similar to that of 100- to 430-nm beads (Fig. 8B). Consistently with previous results, a three-log excess of free virus over the maximal viral protein concentration in infected cells was unable to induce a detectable CD8+ T cell stimulation (Fig. 5A). These data indicate that HIV antigen can be transferred from both apoptotic and live cells to DC, and that both mechanisms are equally efficient for MHC class I-restricted presentation. Moreover, cross-presented HIV antigens from live infected cells were recognized by circulating CD8+ T cells isolated from HIV-infected patients (Fig. 5B), adding more in vivo relevance to these results.
Fig. 5.
Cross-presentation of HIV antigens from live infected cells. (A) DC were tested as in Fig. 3 after coculture with UV-irradiated (circles) or live, Z-VAD-treated (triangles) H9 (empty symbols) or H9-HIV (filled symbols) cells in the presence of AZT, by using an anti-RT476–84 CD8+ line (DC:T ratio 1:1), all in the presence of LPS. DC were also incubated with free viral particles in the presence (open squares) or absence (filled squares) of AZT as in Fig. 3. (Inset) Live H9 (open bars) and H9-HIV (filled bars) cells were incubated or not with DC in the presence of Z-VAD and tested as before. (B) Recognition of DC cocultured with Z-VAD-treated H9-HIV cells by circulating lymphocytes from an HIV+ patient (DC:T ratio 1:5) in the presence of LPS and AZT. PBMC were incubated with an anti-class I ascitis, CD4-depleted or CD8-enriched. Data are representative of two experiments.
Discussion
The data presented here show that antigens from not only apoptotic, but also live, infected CD4+ T cells can be presented by DC much more efficiently than live virus or replication-deficient viral particles by using quantitative tests. This cross-presentation was restricted by DC MHC class I molecules, occurred in the absence of viral replication or viral particles, and required contact between DC and infected T cells. Antigen transfer from nonirradiated CD4+ lymphocytes to DC and subsequent cross-presentation occurred from live T cells and not from contaminating apoptotic cells, either present as a minor population in the culture or secondarily induced by HIV-exposed DC. Indeed, it was not prevented by a pan-caspase inhibitor because in these conditions apoptosis was not induced by DC anytime during coculture, and the dose–response curves obtained with irradiated and nonirradiated CD4+-infected cells were superimposable. Therefore, we demonstrate cross-presentation of infectious antigens from live antigen-donor cells as in an elegant model using OVA-expressing recombinant vaccinia viruses (26). We questioned the mechanism of antigen acquisition from live infected cells and the potential relevance of these different presentation mechanisms for HIV infection.
Different mechanisms for antigen exchange between T lymphocytes and DC are possible: exosome (27) or microparticle transfer (28), or antigen uptake from whole cells (29, 30). Antigen cross-presentation was blocked by a 0.45-nm membrane, despite demonstrated passage of particles with sizes compatible with that of exosomes (50–90 nm), or microvesicles generated by H9-HIV cells (50–500 nm) (31). If these particles were the major source of HIV antigens for DC cross-presentation, then their transfer would not need close DC:T cell contact, as those found by microscopy. The nibbling mechanism, implying cell-associated material exchange, has been described for antigen transfer from DC, macrophages, B cells, and activated T lymphocytes to monkey DC, allowing cross-presentation of a tumor antigen to CD8+ lymphocytes (30). Here, capture of both live and apoptotic cell material seemed dependent on scavenger receptors, and not on mannose-binding lectins or integrins. Redundant pathways difficult to inhibit by a single antibody may be involved. This process is distinct from the reverse acquisition of membrane material from antigen-presenting cells by T cells, which occurs after immunological synapse formation, only during cognate interaction between the T cell receptor and epitope-loaded MHC molecules (32, 33). Interaction between DC and T lymphocytes has already been reported in the absence of cognate antigen or relevant MHC expression in DC (34). This interaction, characterized as an antigen-independent synapse, induces signaling in T lymphocytes, as well as in DC (35) that may stimulate nibbling. HIV antigens may be recruited at the site of the synapse, as occurs in interactions between HIV-infected DC and T cell lines (36).
The different MHC class I-restricted presentation pathways that have been described for HIV antigens in DC, i.e., classical presentation after direct infection and cross-presentation after defective virus entry (13) or after phagocytosis of infected apoptotic CD4+ T lymphocytes (11, 12), had never been compared quantitatively. Our experiments attempted to reproduce in vivo conditions by using CD8+ T cell lines with a relative low avidity or even CD8+ cells purified from HIV+ patient PBMC. We also validated the results obtained with chronically infected immortalized CD4+ T cells using primary CD4+ T lymphocytes together with a primary viral isolate. The role of apoptosis in enhancing cross-presentation has up to now been extensively compared with that of necrosis (10, 37), but not quantitatively with that of live cell-associated antigen transfer. The equivalent efficacy of apoptotic or live cell cross-presentation in the present study may be coincidental because the two pathways presumably use different and redundant receptors to internalize antigens into DC (23, 24).
We show that apoptotic or live cell cross-presentation needs very low amounts of HIV proteins to reach the threshold for efficient CD8+ T cell stimulation. Conversely, HIV epitope presentation after direct infection of DC was not detectable, even with high amounts of replicative virus. Efficient presentation after DC infection by live or defective virus probably needs recognition by high-avidity CD8+ T clones (13) whereas it was not found in another study using PBMC from patients (11). Therefore, in vivo, cross-presentation should allow recognition of cells expressing very low amounts of viral proteins by naive or average-avidity memory CD8+ T lymphocytes.
Cross-presentation can induce immunity or tolerance, depending on the environment and the activation state of the DC. In the absence of CD40-CD40L costimulation, it leads to tolerance or even suppression of immune responses (10, 38, 39). A striking correlation was shown between the frequency of cytotoxic T lymphocytes specific for vinculin, an antigen overexpressed in apoptotic cells, and the proportion of peripheral apoptotic CD40L+ T cells in HIV-infected patients, implying that vinculin is cross-presented by DC from CD40L+ T cells (22). In the present study, the apparent lack of requirement for DC maturation stimuli may be related to the use of HIV-specific CD8+ T cell lines, which need less costimulation than PBMC. It may also be related to the HIV- and apoptosis-independent induction of DC maturation by primary CD4+ T cell blasts. In our hands, the proportion of CD83+ (mature) cells (Fig. 6) and CD40 expression (not shown) increased when DC were incubated with primary CD4+ T cell blasts, whether these blasts were apoptotic or not, independently of HIV infection. In former studies, when PBMC from HIV-infected patients and not T cell lines were used, soluble CD40L or CD4+ T cell help or LPS were indeed required (11). From all these data, and because HIV infects predominantly activated lymphocytes (40), it is likely that, in vivo, live and apoptotic HIV-infected T lymphocytes can supply antigens and costimulation signals for MHC class I-restricted presentation by DC and thus immunostimulation. On the other hand, they may induce tolerance, depending on HIV infection stage, because, in HIV patients with low CD4 counts, triggering of CD40L on T cells is impaired (41). Finally, the outcome of live infected cell cross-presentation might be stimulation or tolerance and needs to be further explored.
In vivo, the infection route was shown to influence the nature of the pathway used for MHC class I-restricted presentation (42). This pathway may depend on the cell types encountered by the antigens. DC are required for CD8+ lymphocyte cross-priming in vivo, even after infection with bacteria infecting macrophages (5). Cross priming after HSV-1 s.c. infection occurs rapidly in lymph nodes in the absence of local virus, suggesting rapid and efficient antigen uptake and presentation by DC (43). This activity requires viral protein synthesis, indicating that DC capture antigens from productively infected cells, but the model did not discriminate whether donor-infected cells were alive or apoptotic. Cross-presentation of live cell-associated antigens might allow the development of an early immune response at the first stages of HIV infection, before apoptosis is massively induced. Moreover, HIV antigen acquisition from live cells by DC could be an efficient mechanism to induce recognition of very low amounts of viral proteins and destruction of latent viral reservoirs. This mechanism could probably be enhanced by using either granulocyte colony-stimulating factor to promote limited viral production in resting T lymphocytes and macrophages (44, 45), or IL-2 to promote viral production in T cells and restore T cell help (46, 47), combined with IFNα to enhance cross-presentation, and CD4+ T helper 1 and CD8+ T cell effector functions (48, 49), during highly active antiretroviral treatment-structured interruptions. Thus, eradication of reservoirs might be obtained.
Supplementary Material
Acknowledgments
We acknowledge R. Thomas, A. Trautmann, and R. Cheynier for critically reading the manuscript, S. Wain Hobson for interesting discussion, A. Benmerah for transferrin and valuable advice, A. C. Ripoche for technical help, A. Jobard and the Cochin Institute confocal platform for teaching confocal microscopy, M. Lichtner and M. Andreoni (Sapienza University, Rome) for WT virus, and P. Langlade (PasteurInstitute, Paris) for W6/32 ascitis. This work was supported by grants from Agence Nationale de Recherche sur le SIDA (ANRS), Ensemble Contre le SIDA (ECS), and fellowships from Institut National de la Santé et de la Recherche Médicale (to C.M.), the European Community (QLK2-2000-52160, to C.M.), Ministère de la Recherche et de la Technologie (to W.C.), and ECS (J.-F.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AZT, azidothymidine; DC, dendritic cell(s); PBMC, peripheral blood mononuclear cells; LPS, lipopolysaccharide; Z-VAD, N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone; RT, reverse transcriptase; Ac-LDL, acetylated low density lipoprotein.
References
- 1.Letvin, N. L. & Walker, B. D. (2003) Nat. Med. 9, 861-866. [DOI] [PubMed] [Google Scholar]
- 2.Wilson, J. D., Ogg, G. S., Allen, R. L., Davis, C., Shaunak, S., Downie, J., Dyer, W., Workman, C., Sullivan, S., McMichael, A. J. & Rowland-Jones, S. L. (2000) AIDS 14, 225-233. [DOI] [PubMed] [Google Scholar]
- 3.Dalod, M., Dupuis, M., Deschemin, J.-C., Goujard, C., Deveau, C., Meyer, L., Ngo, N., Rouzioux, C., Guillet, J.-G., Delfraissy, J.-F., et al. (1999) J. Clin. Invest. 104, 1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankson, J. N., Persaud, D. & Siliciano, R. F. (2002) Annu. Rev. Med. 53, 557-593. [DOI] [PubMed] [Google Scholar]
- 5.Jung, S., Unutmaz, D., Wong, P., Sano, G., De los Santos, K., Sparwasser, T., Wu, S., Vuthoori, S., Ko, K., Zavala, F., et al. (2002) Immunity 17, 211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIlroy, D., Autran, B., Cheynier, R., Wain-Hobson, S., Clauvel, J. P., Oksenhendler, E., Debre, P. & Hosmalin, A. (1995) J. Virol. 69, 4737-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badley, A. D., Pilon, A. A., Landay, A. & Lynch, D. H. (2000) Blood 96, 2951-2964. [PubMed] [Google Scholar]
- 8.Henson, P. M., Bratton, D. L. & Fadok, V. A. (2001) Nat. Rev. Mol. Cell Biol. 2, 627-633. [DOI] [PubMed] [Google Scholar]
- 9.Albert, M. L., Sauter, B. & Bhardwaj, N. (1998) Nature 392, 86-89. [DOI] [PubMed] [Google Scholar]
- 10.Heath, W. R. & Carbone, F. R. (2001) Annu. Rev. Immunol. 19, 47-64. [DOI] [PubMed] [Google Scholar]
- 11.Zhao, X. Q., Huang, X. L., Gupta, P., Borowski, L., Fan, Z., Watkins, S. C., Thomas, E. K. & Rinaldo, C. R., Jr. (2002) J. Virol. 76, 3007-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson, M., Fonteneau, J. F., Lirvall, M., Haslett, P., Lifson, J. D. & Bhardwaj, N. (2002) AIDS 16, 1319-1329. [DOI] [PubMed] [Google Scholar]
- 13.Buseyne, F., Le Gall, S., Boccaccio, C., Abastado, J. P., Lifson, J. D., Arthur, L. O., Riviere, Y., Heard, J. M. & Schwartz, O. (2001) Nat. Med. 7, 344-349. [DOI] [PubMed] [Google Scholar]
- 14.Wang, D., de la Fuente, C., Deng, L., Wang, L., Zilberman, I., Eadie, C., Healey, M., Stein, D., Denny, T., Harrison, L. E., et al. (2001) J. Virol. 75, 7266-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrieu, M., Loing, E., Desoutter, J. F., Connan, F., Choppin, J., Gras-Masse, H., Hanau, D., Dautry-Varsat, A., Guillet, J. G. & Hosmalin, A. (2000) Eur. J. Immunol. 30, 3256-3265. [DOI] [PubMed] [Google Scholar]
- 16.Nicastri, E., Sarmati, L., d'Ettorre, G., Parisi, S. G., Palmisano, L., Galluzzo, C., Montano, M., Uccella, I., Amici, R., Gatti, F., et al. (2003) J. Clin. Microbiol. 41, 3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakri, Y., Schiffer, C., Zennou, V., Charneau, P., Kahn, E., Benjouad, A., Gluckman, J. C. & Canque, B. (2001) J. Immunol. 166, 3780-3788. [DOI] [PubMed] [Google Scholar]
- 18.Cameron, P. U., Lowe, M. G., Crowe, S. M., O'Doherty, U., Pope, M., Gezelter, S. & Steinman, R. M. (1994) J. Leukocyte Biol. 56, 257-265. [DOI] [PubMed] [Google Scholar]
- 19.Tsunetsugu-Yokota, Y., Akagawa, K., Kimoto, H., Suzuki, K., Iwasaki, M., Yasuda, S., Hausser, G., Hultgren, C., Meyerhans, A. & Takemori, T. (1995) J. Virol. 69, 4544-4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granelli-Piperno, A., Delgado, E., Finkel, V., Paxton, W. & Steinman, R. M. (1998) J. Virol. 72, 2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtner, M., Maranon, C., Vidalain, P. O., Azocar, O., Hanau, D., Lebon, P., Burgard, M., Rouzioux, C., Vullo, V., Yahita, H., et al. (2004) AIDS Res. Hum. Retroviruses 20, 175-182. [DOI] [PubMed] [Google Scholar]
- 22.Propato, A., Cutrona, G., Francavilla, V., Ulivi, M., Schiaffella, E., Landt, O., Dunbar, R., Cerundolo, V., Ferrarini, M. & Barnaba, V. (2001) Nat. Med. 7, 807-813. [DOI] [PubMed] [Google Scholar]
- 23.Harshyne, L. A., Zimmer, M. I., Watkins, S. C. & Barratt-Boyes, S. M. (2003) J. Immunol. 170, 2302-2309. [DOI] [PubMed] [Google Scholar]
- 24.Albert, M. L., Pearce, S. F., Francisco, L. M., Sauter, B., Roy, P., Silverstein, R. L. & Bhardwaj, N. (1998) J. Exp. Med. 188, 1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubartelli, A., Poggi, A. & Zocchi, M. R. (1997) Eur. J. Immunol. 27, 1893-1900. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez, M. C. & Sigal, L. J. (2002) J. Immunol. 169, 6733-6742. [DOI] [PubMed] [Google Scholar]
- 27.Wolfers, J., Lozier, A., Raposo, G., Regnault, A., Thery, C., Masurier, C., Flament, C., Pouzieux, S., Faure, F., Tursz, T., et al. (2001) Nat. Med. 7, 297-303. [DOI] [PubMed] [Google Scholar]
- 28.Mack, M., Kleinschmidt, A., Bruhl, H., Klier, C., Nelson, P. J., Cihak, J., Plachy, J., Stangassinger, M., Erfle, V. & Schlondorff, D. (2000) Nat. Med. 6, 769-775. [DOI] [PubMed] [Google Scholar]
- 29.Knight, S. C., Iqball, S., Roberts, M. S., Macatonia, S. & Bedford, P. A. (1998) Eur. J. Immunol. 28, 1636-1644. [DOI] [PubMed] [Google Scholar]
- 30.Harshyne, L. A., Watkins, S. C., Gambotto, A. & Barratt-Boyes, S. M. (2001) J. Immunol. 166, 3717-3723. [DOI] [PubMed] [Google Scholar]
- 31.Gluschankof, P., Mondor, I., Gelderblom, H. R. & Sattentau, Q. J. (1997) Virology 230, 125-133. [DOI] [PubMed] [Google Scholar]
- 32.Trambas, C. M. & Griffiths, G. M. (2003) Nat. Immunol. 4, 399-403. [DOI] [PubMed] [Google Scholar]
- 33.Hudrisier, D., Riond, J., Mazarguil, H., Gairin, J. E. & Joly, E. (2001) J. Immunol. 166, 3645-3649. [DOI] [PubMed] [Google Scholar]
- 34.Revy, P., Sospedra, M., Barbour, B. & Trautmann, A. (2001) Nat. Immunol. 2, 925-931. [DOI] [PubMed] [Google Scholar]
- 35.Montes, M., McIlroy, D., Hosmalin, A. & Trautmann, A. (1999) Int. Immunol. 11, 561-568. [DOI] [PubMed] [Google Scholar]
- 36.McDonald, D., Wu, L., Bohks, S. M., KewalRamani, V. N., Unutmaz, D. & Hope, T. J. (2003) Science 300, 1295-1297. [DOI] [PubMed] [Google Scholar]
- 37.Sauter, B., Albert, M. L., Francisco, L., Larsson, M., Somersan, S. & Bhardwaj, N. (2000) J. Exp. Med. 191, 423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. (2003) Annu. Rev. Immunol. 21, 685-711. [DOI] [PubMed] [Google Scholar]
- 39.Martin, E., O'Sullivan, B., Low, P. & Thomas, R. (2003) Immunity 18, 155-167. [DOI] [PubMed] [Google Scholar]
- 40.Scales, D., Ni, H., Shaheen, F., Capodici, J., Cannon, G. & Weissman, D. (2001) J. Immunol. 166, 6437-6443. [DOI] [PubMed] [Google Scholar]
- 41.Vanham, G., Penne, L., Devalck, J., Kestens, L., Colebunders, R., Bosmans, E., Thielemans, K. & Ceuppens, J. L. (1999) Clin. Exp. Immunol. 117, 335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen, X., Wong, S. B., Buck, C. B., Zhang, J. & Siliciano, R. F. (2002) J. Immunol. 169, 4222-4229. [DOI] [PubMed] [Google Scholar]
- 43.Mueller, S. N., Jones, C. M., Smith, C. M., Heath, W. R. & Carbone, F. R. (2002) J. Exp. Med. 195, 651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armstrong, W. S. & Kazanjian, P. (2001) Clin. Infect. Dis. 32, 766-773. [DOI] [PubMed] [Google Scholar]
- 45.Aladdin, H., Ullum, H., Dam Nielsen, S., Espersen, C., Mathiesen, L., Katzenstein, T. L., Gerstoft, J., Skinhoj, P. & Pedersen, B. K. (2000) J. Infect. Dis. 181, 1148-1152. [DOI] [PubMed] [Google Scholar]
- 46.Chun, T. W., Engel, D., Mizell, S. B., Hallahan, C. W., Fischette, M., Park, S., Davey, R. T., Jr., Dybul, M., Kovacs, J. A., Metcalf, J. A., et al. (1999) Nat. Med. 5, 651-655. [DOI] [PubMed] [Google Scholar]
- 47.Levy, Y., Durier, C., Krzysiek, R., Rabian, C., Capitant, C., Lascaux, A. S., Michon, C., Oksenhendler, E., Weiss, L., Gastaut, J. A., et al. (2003) AIDS 17, 343-351. [DOI] [PubMed] [Google Scholar]
- 48.Le Bon, A., Etchart, N., Rossmann, C., Ashton, M., Hou, S., Gewert, D., Borrow, P. & Tough, D. F. (2003) Nat. Immunol. 4, 1009-1015. [DOI] [PubMed] [Google Scholar]
- 49.Emilie, D., Burgard, M., Lascoux-Combe, C., Laughlin, M., Krzysiek, R., Pignon, C., Rudent, A., Molina, J. M., Livrozet, J. M., Souala, F., et al. (2001) AIDS 15, 1435-1437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.