Abstract
Background
Accelerated phase CML (CML-AP) most frequently represents a progression state in CML. However, some patients present with AP features at the time of diagnosis. There is limited information on the outcome of these patients when receiving tyrosine kinase inhibitors (TKI) as initial therapy.
Methods
We analyzed the outcome of 51 consecutive patients with CML who presented with features of AP at the time of diagnosis, including blasts ≥15% (n=6), basophils ≥20%, (n=22), platelets <100×109/L (n=3), cytogenetic clonal evolution (n=17), or more than 1 feature (n=3). Patients received initial therapy with imatinib (n=30), dasatinib (n=5) or nilotinib (n=16).
Results
The rate of complete cytogenetic response (CCyR) for patients treated with imatinib was 80%, and with dasatinib or nilotinib was 90%. Major molecular response (MMR, BCR-ABL/ABL ≤0.1%, by International Scale [IS]) was achieved in 69% including complete molecular responses (MR4.5, BCR-ABL/ABL ≤0.0032% IS) in 49%. MMR rates for patients treated with imatinib were 63%, and with second generation TKI (2GTKIs) 76%. Overall survival at 36 months was 87% with imatinib and 95% with 2GTKI’s.
Conclusion
TKIs should be considered standard initial therapy for patients with AP at the time of diagnosis.
Keywords: tyrosine kinase inhibitors (TKI), accelerated phase CML (CML-AP), complete cytogenetic response (CCyR), major molecular response (MMR), second generation TKI (2GTKIs)
INTRODUCTION
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasm characterized by the BCR-ABL fusion gene.1 This fusion gene produces the constitutively activated tyrosine kinase BCR-ABL, the therapeutic target of BCR-ABL tyrosine kinase inhibitors (TKIs).2 The disease usually evolves in a tri-phasic clinical course with an initial chronic phase (CP), followed by an intermediate accelerated phase (AP) and a frequently terminal blast-phase (BP).3
Associated with cytogenetic instability, progressive impairment of myeloid cell differentiation, and eventually blast phase progression, accelerated phase CML (CML-AP) has an aggressive clinical course, historically associated with a median survival of only 6–18 months.1,4,5 Approximately 5–10% of patients with CML present with AP features at the time of diagnosis.4
Imatinib, dasatinib, and nilotinib are standard initial TKI therapies for patients with CML in CP. Most studies exploring the use of TKIs for CML-AP have included patients progressing to AP after failing previous therapies.1 Little is known about the outcomes of patients with CML-AP features at the time of presentation receiving initial therapy with imatinib6 and there is no published data on nilotinib and dasatinib as initial therapy for de novo CML-AP.7–11 The aim of this study was to describe the efficacy of imatinib, dasatinib, and nilotinib as initial therapy for patients with de novo CML-AP.
MATERIALS AND METHODS
Study Group
From September 1999 through May 2011, 51 adult patients (age ≥18 years) with a confirmed diagnosis of CML-AP were treated with TKIs as initial therapy on consecutive or parallel clinical trials and were included in this analysis. Patients with any of the following features of CML-AP were eligible: blasts ≥15% in peripheral blood (PB) or bone marrow (BM), blasts + promyelocytes ≥30% (PB or BM), basophils ≥20% (PB or BM), platelets <100×109/L unrelated to therapy, and/or cytogenetic clonal evolution.4 The presence of any clonal abnormality other than a single Ph, was classified as cytogenetic clonal evolution.12,13
Other inclusion criteria included ECOG performance status 0–2, and acceptable end organ function including total bilirubin <1.5 x upper limit of normal (ULN), SGPT <2.5 xULN, creatinine <1.5 xULN). For women of childbearing potential, a negative pregnancy test was required for inclusion. Except for hydroxyurea, patients could not have received more than minimal therapy, defined as <1 month of prior interferon-alpha (with or without cytarabine) and/or imatinib (for patients receiving nilotonib or dasatinib).
Written informed consent was obtained from all patients, according to institutional guidelines. The protocols were approved by the MDACC Institutional Review Board and were executed in adherence to the Declaration of Helsinki.
Patient Evaluation
Patients were followed with complete blood counts every 1–2 weeks for the first 2–3 months, and then every 4–6 weeks. Bone marrow aspirations were performed at least every 3 months for the first 12 months, then every 6–12 months. Cytogenetic responses were also evaluated on those specimens. Response criteria for CML-AP have been previously described.14 Briefly, a complete hematologic response (CHR) was characterized by the following: resolution of signs and symptoms of CML, normalization of the blast percentage in the peripheral blood and bone marrow (≤5% marrow blasts); leukocytes <10 × 109/L; normal peripheral blood differential (with no peripheral blasts, promyelocytes, or myelocytes); and platelet counts <450 × 109/L. If thrombocytopenia (<100 × 109/L) was present before treatment, then normalization of platelet counts to >100 × 109/L was required for a CHR. Patients with a normal platelet count prior to starting therapy, who developed thrombocytopenia <100 × 109/L as a consequence of TKIs could be considered to have achieved CHR if they had all the other features of CHR.14
Patients were evaluated for cytogenetic response by conventional cytogenetic analysis in 20 metaphases.1 Cytogenetic responses were classified as minor (mCyR) if the percentage of Philadelphia chromosome (Ph)-positive metaphases was 36–95%, partial (PCyR) if 5 to 35%, and complete (CCyR) if 0%. A major cytogenetic response (MCyR) included a complete and partial cytogenetic response (i.e., ≤35% Ph+ metaphases).
BCR-ABL transcripts were identified with real-time quantitative RT-PCR (Q-PCR) analysis on peripheral blood and/or bone marrow aspirate.1 A major molecular response (MMR) was defined as BCR-ABL/ABL transcripts ≤0.1% as assessed on the international scale (IS). A MR4.5 was defined as BCR-ABL/ABL ≤0.0032% (IS).15
Statistical Considerations
Categorical variables were analyzed by chi-square and Fisher’s exact tests. Survival probabilities were estimated by the Kaplan-Meier method. The log-rank test was used for comparing survival estimates. Event free survival (EFS) was measured from the date of start of therapy to the occurrence of an event. The following occurrences constituted an event: loss of CHR, loss of MCyR, transformation, and death (on study). Transformation free survival (TFS) was calculated from the date treatment was started to the date of transformation to blast phase or death while on study. Overall survival (OS) was measured from the date treatment started to the date of death at any time from any cause, or last follow-up. We also analyzed failure free survival (FFS) where failure included an event (as defined above) or loss of CCyR, toxicity, or discontinuation for any reason.
RESULTS
A total of 51 patients with the following features of AP at the time of diagnosis were included: blasts ≥15% (n=6), basophils ≥20% (n=22), platelets <100×109/L (n=3), cytogenetic clonal evolution (n=17), or more than 1 feature (n=3). The median age was 46 years (range: 22 to 81 years); 55% were males (Table 1). Patient characteristics were similar in all treatment groups. Thirty (59%) patients received initial therapy with imatinib and 21 (41%) with a 2GTKI (16 received nilotinib and 5 dasatinib). Among the 30 patients treated with imatinib, 5 received an initial dose of 400 mg/day, 21 received 600 mg/day, and 4 >600/day (800 mg/day, n=3; 1000 mg/day, n=1). The starting dose of nilotinib was 800 mg daily (400 mg bid) and of dasatinib 100 mg daily (or 50 mg bid). The median time to initiate TKI therapy was 1 month from diagnosis (range 0–10 months).
Table 1.
Patient Characteristics
| Total Patients | 51 |
| Median Age (range) | 46 [22–81] |
| Median Months to start TKI | 1 [0–10] |
| Male (%) | 28 (55) |
| AP Features | No (%) |
| Increased Basophils | 22 (43) |
| Increased Blasts | 6 (12) |
| Clonal Evolution (CE) | 17 (33) |
| Decreased Platelets | 3 (6) |
| More than 1 factor | 3 (6) |
TKI, Tyrosine Kinase Inhibitor; AP, Accelerated Phase
Responses by treatment cohort, time to response, and median follow-up by treatment cohort are shown in Table 2. After a median follow-up of 65 months (range 3–144), a CHR was achieved in 49 patients (96%) with a median time to CHR of 1 month (0–12 months). A cytogenetic response was achieved by 44 (86%) patients, including PCyR in 1 (2%) and CCyR in 43 (84%). The rate of CCyR for patients treated with imatinib was 80% (n=24), and with 2GTKIs 90% (n=19). The median time to CCyR was 3 months (range 2–44 months). A MMR was achieved in 35 patients (69%), and MR4.5 in 25 (49%). The median time to MMR was 9.6 months (0–44 months) (12 months with imatinib and 6 months with 2GTKI, respectively). MMR rates for patients treated with imatinib were 63%, and with 2GTKIs 76%. Response by time on therapy is shown in Table 3 by intention to treat analysis (ITT). By ITT, the 12-month rate of CCyR was 48% with imatinib and 88% with 2GTKIs, and the 12-month MMR rate was 38% for imatinib and 71% for 2GTKIs.
Table 2.
Outcome after treatment with TKI.
| Responses | No. (%), or Median [range] | ||
|---|---|---|---|
| All (%) | Imatinib (%) | 2GTKI(%) | |
| N | 51 | 30 | 21 |
| CHR | 49 (96) | 29 (97) | 20 (95) |
| Cytogenetic | |||
| mCyR | 1 (2) | 1 (3) | 0 (0) |
| PCyR | 1 (2) | 1 (3) | 0 (0) |
| CCyR | 43 (84) | 24 (80) | 19 (90) |
| MCyR | 44 (86) | 25 (83) | 19 (90) |
| Molecular | |||
| MMR | 35 (69) | 19 (63) | 16 (76) |
| MR4.5 | 25 (49) | 15 (50) | 10 (63) |
| Follow-up (months) | 65 [3–144] | 113 [48–144] | 28 [3–73] |
| Months to CHR | 1 [0–12] | 1 [0–12] | 1 [0–3] |
| Months to MMR | 10 [0–44] | 12 [3–44] | 6 [0–24] |
| Months to CCyR | 3 [2–44] | 6 [2–44] | 3 [2–6] |
TKI, Tyrosine kinase Inhibitor; 2GTKI, Second Generation Tyrosine Kinase Inhibitor; CHR, Complete Hematologic Response; mCyR, Minor Cytogenetic Response; PCyR, Partial Cytogenetic Response; CCyR, Complete Cytogenetic Response; MCyR, Major Cytogenetic Response; MMR, Major Molecular Response; MR4.5 defined as BCR-ABL/ABL ≤0.0032%
Table 3.
Response by Time on Therapy by ITT
| n/N (%) | |||
|---|---|---|---|
| 6 month | 12 month | 18 month | |
| ITT Rate CCyR | |||
| Overall | 36/50 (72) | 29/46 (63) | 26/44 (59) |
| Imatinib | 19/30 (63) | 14/29 (48) | 14/28 (50) |
| 2GTKI | 17/20 (85) | 15/17 (88) | 12/16 (75) |
| ITT Rate MMR | |||
| Overall | 15/50 (30) | 23/46 (50) | 22/44 (50) |
| Imatinib | 3/30 (10) | 11/29 (38) | 13/28 (46) |
| 2GTKI | 12/20 (60) | 12/17 (71) | 9/16 (56) |
- Patients lost to follow-up or who did not reach the given time point for analysis were excluded from the ITT denominator
- For the evaluable population only, response rates are higher. For example at 18 months, CCyR is 81% for all patients (n=32), 82% for imatinib (n=17) and 80 for 2GTKI (n=15). Corresponding rates for MMR are 81% (n=27), 100% (n=13) and 64% (n=14), respectively.
Results on an intention-to-treat (ITT) are shown below Table 3. Generally CCyR and MMR response rates were higher among evaluable cases. For example, for imatinib-treated patients evaluable for molecular responses at 6, 12, and 18 months, the respective MMR rates were 100% (3/3), 85% (11/13), and 100 % (13/13). Among evaluable cases, MMR rates appear lower with 2GTKI compared to imatinib due to a shorter median follow-up of 28 months versus 113 months with imatinib. When adjusted by time, responses are higher with 2GTKI. For example, at 18 months, rates of CCyR are 50% with imatinib and 75% with 2GTKI, and for MMR 46% and 56%, respectively.
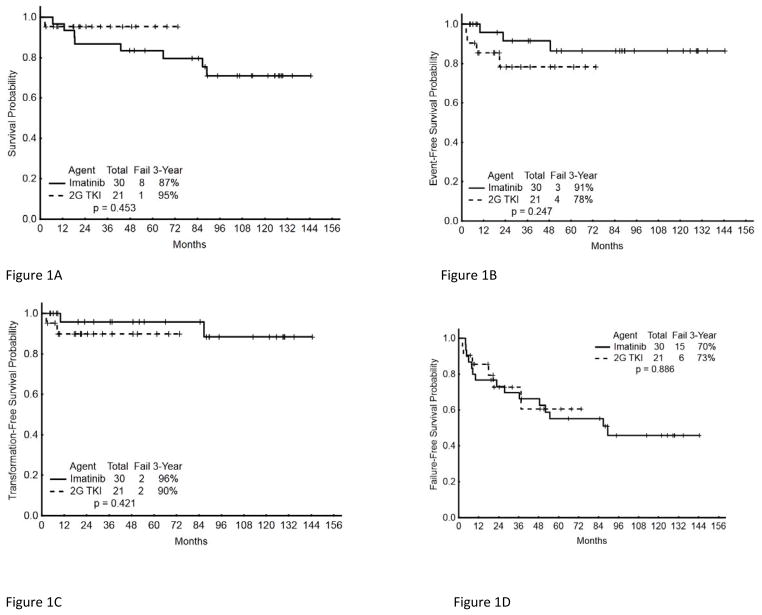
Patients with clonal evolution as the only criterion for CML-AP had a CCyR rate of 94% (16/17) compared to 79% (27/34) for those with other criteria (p=0.174). Survival probability by drug for EFS, TFS, OS, and FFS are shown in Figures 1A–1D. The projected 36-month EFS for all patients was 86% (imatinib 91%; 2GTKI 78%) (Table 4). Three (6%) patients have progressed to BP, including one treated with imatinib and two treated with 2GTKI. This resulted in a projected 36-month TFS of 93%: 96% with imatinib and 90% with 2GTKIs (Table 4). Overall, 9 patients have died at any time during follow-up (8 in the imatinib cohort and 1 in the 2GTKI cohort). The projected overall survival at 36 months was 89% (87% for imatinib and 95% for 2GTKI).
Figure 1. Long-term outcome of patients with CML AP according to initial therapy.
(A) Overall Survival, (B) Event Free Survival, (C) Transformation Free Survival, and (D) Failure Free Survival.
Table 4.
Long Term Outcome
| Outcome | Percentage at 36 months | ||||
|---|---|---|---|---|---|
| EFS | TFS | FFS | OS | ||
| Overall | 86 | 93 | 71 | 89 | |
| Imatinib | 91 | 96 | 70 | 87 | |
| 2GTKI | 78 | 90 | 73 | 95 | |
| 12-months | CCyR | 96 | 100 | 88 | 100 |
| No CCyR | 75 | 100 | 75 | 100 | |
| 18-months | MMR | 100 | 100 | 90 | 100 |
| No MMR | 75 | 100 | 75 | 100 | |
CCyR, Complete Cytogenetic Response; MMR, Major Molecular Response; 2GTKI, Second Generation tyrosine kinase inhibitor; EFS, Event Free Survival; TFS, Transformation Free Survival; FFS, Failure Free Survival; OS, Overall Survival
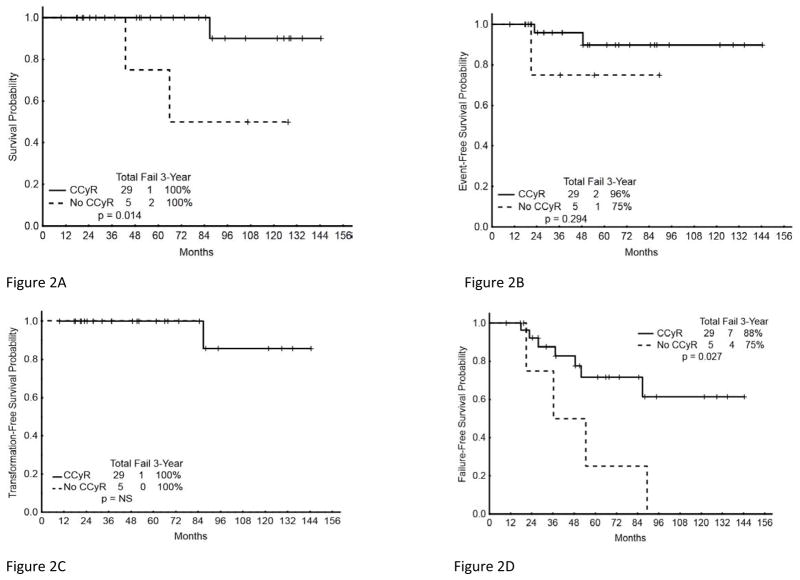
The 36-month TFS probability for patients with or without CCyR at 12-months was 100%. Of note however, as noted earlier, all 3 patients that transformed to AP did so before 12 months. Similarly, the TFS rate was 100% for those with or without a MMR at 18 months (Table 4). Only 4 patients had a 12-month CCyR but no 18-month MMR, and none transformed to blast phase. OS, EFS, TFS, and FFS by CCyR at 12 months are shown in Figure 2A–2D.
Figure 2. Long-term outcome by cytogenetic response at 12 months of patients with CML AP treated with TKI as initial therapy.
(A) Overall Survival, (B) Event Free Survival, (C) Transformation Free Survival, and (D) Failure Free Survival.
Patient Status
At last follow-up 30 of the 51 (59%) patients remain on therapy with their initial TKI. One patient experienced treatment failure off protocol. Because this patient came off protocol only because imatinib became commercially available, and was followed off protocol for several years, the failure was counted for FFS calculations. Eight patients discontinued therapy because of treatment failure including transformation to blast phase (n=3) and resistance to therapy (n=5). Among the three patients who transformed to blast phase, the time intervals from start of therapy to transformation were 2, 8 and 10 months; all 3 had increased blasts ≥ 20% in peripheral blood or bone marrow at baseline. Disease resistance included cytogenetic clonal evolution, loss of cytogenetic response, and minimal or no cytogenetic response. Among five patients with treatment failure who had ABL sequencing performed, all had one or more of the following ABL mutations at the time of treatment failure: Y253H (n=3), E255K (n=2), T3151 (n=2). One patient who eventually transformed to BP had all 3 mutations. The rest of the patients discontinued therapy due to other reasons (n=12) e.g. (noncompliance, patient choice, difficulties obtaining drug), and toxicity (n=1; diarrhea).
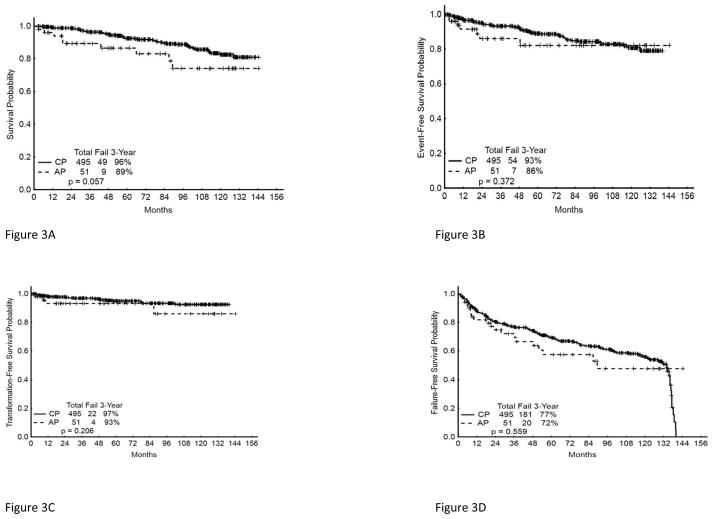
To put these results into context, we compared the outcome of this cohort of patients with de novo CML-AP to a cohort of patients with newly diagnosed chronic phase CML (CML-CP) treated on clinical trials at our institution during the same time period. CML-AP and CML-CP patients were compared in terms of rates of CCyR, MMR, EFS, TFS, and OS. The CML-AP and CML-CP cohorts had similar overall rates of CCyR [84 %(AP); 88% (CP)], MMR [69%(AP); 82%(CP)], 36-month EFS [86%(AP) and 93% (CP) and 36-month OS16 [89%(AP) and 96%(CP)] (Figures 3A–D).
Figure 3. Long-term outcome of patients with CML CP or AP receiving TKI as initial therapy.
(A) Overall Survival, (B) Event Free Survival, (C) Transformation Free Survival, (D) Failure Free Survival.
DISCUSSION
Imatinib was the first TKI approved in 2001 for patients with CML-CP at doses of 400 mg daily and for CML-AP and blast phase (CML-BP) at doses of 600 mg daily.17–20 The pivotal IRIS trial demonstrated the superiority of imatinib over interferon-alpha (IFN) plus cytarabine in CML-CP.21 With an 8-year follow-up, the estimated event-free survival (EFS) was 81%, freedom from progression to CML-AP or CML-BP was 92%, and the overall survival (OS) was 85%. When only CML-related deaths were considered, the OS reached 93%.22
Information on the use of TKI’s for CML-AP has been extensively described for patients who have failed prior therapy and/or have progressed from CML-CP.1,9,23 The use of TKIs as initial therapy for de novo CML-AP has been scarce and mostly limited to imatinib.6 Use of second generation TKI’s as front-line therapy for de novo CML-AP has not yet been reported, and no TKI is currently approved for use as initial therapy on patients with presenting features of CML-AP. TKIs are known to be effective therapy for CML-AP patients treated after progression from CML-CP. In this setting imatinib was initially reported to induce a CCyR in 28% with projected 1-year EFS (event-free survival) of 59% and overall survival of 74%.24 Second generation TKIs have also been explored in the setting of CML-AP after resistance or intolerance to imatinib therapy. With dasatinib, CCyR was achieved in 32% and MCyR in 39%. This resulted in a 1- year progression free survival (PFS) of 66% and overall survival of 82%.23 Nilotinib was also effective in a similar setting with a CCyR rate of 21%, MMR of 11% (in evaluable patients), a 2-year PFS of 33%, and overall survival of 70%.25
The clinical benefit of TKI therapy in CML-AP compared to therapies used before the availability of TKI was analyzed by Kantarjian et al. Imatinib therapy administered to 176 patients in this single-institution experience induced a CHR in 82% of patients compared to less than 50% for patients treated with other modalities (historical control) (n=213). The population of CML-AP patients was heterogeneous in terms of duration of CML prior to initiation of imatinib and the number of patients with de novo CML-AP was not specified; however, 112 patients had CML for less than 12 months prior to imatinib. The rest had CML for 12–35 months or ≥36 months. The CCyR-rate for imatinib-treated patients was 43% compared to 0% to 6% for the historical control group. This resulted in an improved 4-year survival rate of 53% with imatinib compared to 42% with interferon-alpha and 0% to 21% with other therapies.1 The use of TKIs has resulted in improved survival compared to that achieved with historical therapies.7
In this series we report an excellent outcome with the use of TKI as initial therapy for patients who present with CML-AP features at the time of diagnosis. The rate of CCyR was 84% and MMR 69%. Recently, Rea et al reported their experience with imatinib as initial therapy for patients with de novo CML-AP. In that series a total of 42 patients were treated, all with imatinib. The rate of CCyR was 60% and MCyR 74 %, with a projected 2-year failure free survival of 54.2% and OS of 87.2%. Importantly, in our report there is a suggestion that therapy with second generation TKIs resulted in an improved outcome with rates of CCyR of 90% (compared to 80% with imatinib). Likewise, the overall MMR rate was higher with 2GTKI at 76% vs. 63% with imatinib. This however did not translate into improved EFS, FFS or TFS.
As shown in Figure 1A–1D, while the imatinib-treated cohort was not inferior to the 2GTKI cohort with respect to EFS, TFS, and FFS, the 2GTKI cohort did appear to show a non-statistically significant trend for a superior OS. While one nilotinib-treated patient died with blast phase, 2/8 deaths in imatinib-treated patients were due to CML, while the rest died of comorbidities or unknown causes. The survival advantage with 2GTKI then might be related to longer follow-up with the corresponding greater time at risk for deaths from other causes. Thus, although 2GTKI provide a trend for better response rate, due to the small cohort size, we cannot specifically conclude that 2GTKI should be recommended over imatinib, however we can conclude that TKIs should be the standard of care for all patients with de novo CML-AP.
The results we report here for patients with features of accelerated phase at the time of diagnosis are very similar to those that have been reported among patients with CML-CP receiving initial therapy with TKIs. We analyzed patients treated at our institution with TKI during the same treatment period as those in CML-AP reported here and found similar rates of CCyR [84 %(AP); 88% (CP)], MMR [69%(AP); 82%(CP)], 36-month EFS [86%(AP) and 93% (CP) and 36-month OS16 [89%(AP) and 96%(CP)]
Allogeneic stem-cell transplant is a frequent consideration for patients with CML-AP. However, the results with SCT in CML-AP are not as favorable as when SCT is performed in first CP. In the International Bone Marrow Transplant Registry (IBMTR) analysis of patients with CML in second chronic phase/CML-AP who received myeloablative marrow (n = 192) or peripheral blood allo-SCT (n = 251) between 2000 and 2009, the estimated 5-year survival rates were approximately 36 and 38% respectively.16 Similarly the European Group for Blood and Marrow Transplantation (EBMT) reported 444 patients with CML-AP receiving allo-SCT between 1980 and 1990 and noted a 5-year survival probability of 29%. 26 Results with SCT may be superior to those with TKI in the setting of transformation to CML-AP. Jiang et al. compared imatinib therapy (n=87) to allo-SCT (n=45) in a non-randomized prospective analysis. In that analysis allo-SCT in high-risk and intermediate-risk CML-AP patients yielded a significant survival advantage over imatinib treatment. Conversely, in low-risk patients, the outcomes of the 2 therapies were similar.27 Results of SCT for patients with CML AP at the time of presentation have not been reported. However, our observations, together with that of Rea et al, suggest that when CML-AP occurs at the time of diagnosis, treatment considerations might be different than when AP evolves after progression from CP. The excellent response rate with TKI with a projected 36-month overall survival of 90% suggest that patients with de novo CML-AP may be properly treated with TKI as initial therapy and may not need SCT. However, in our series, all three patients who transformed to BP had blasts ≥ 20% in peripheral blood or bone marrow at baseline. This suggests that patients with such elevated baseline blasts may be considered for early allogeneic stem cell transplantation. Also, patients with no adequate response at 12 months have an inferior outcome and may be considered for alternative therapies. It is also possible that earlier time points (3 or 6 month response) may already identify patients destined to fail, similar to what has been reported in CML-CP. 28.
The majority of the study cohort had a diagnosis of AP based on isolated clonal evolution (CE) or basophilia. The percentage of patients with isolated clonal evolution cases in our study of de novo CML-AP (33%) is similar to what we have reported on a larger study of CML-AP1 (mostly on patients progressing from CML-CP) in which isolated clonal evolution accounted for 36%; conversely, compared to that earlier study in which basophilia accounted for 12%,18 our study had a larger percentage of cases with basophilia (43%) as the sole criteria for CML-AP. It is very likely that different clinical presentations of CML-AP have diverse biologic backgrounds that may respond differently to diverse treatment interventions. It would be important to understand these biologic differences and to incorporate this knowledge into a more biologically relevant disease classification for CML.
Compared to patients with other features of CML-AP, patients with clonal evolution as the sole criteria for CML-AP are known to have favorable outcomes with TKIs29 as well as in the pre-imatinib era when treated with interferon and low-dose cytarabine.30,31 Similarly, our study also demonstrated that patients with clonal evolution as the only criterion for CML-AP had a more favorable CCyR rate of 94% (16/17) compared to those with other criteria, 79% (27/34).
CONCLUSION
In conclusion, patients who present with features of CML-AP at the time of diagnosis have an excellent outcome with frontline therapy with TKIs, particularly with second generation TKI. The outcome in this setting mirrors what has been reported for patients with CML-CP receiving TKI at the time of diagnosis. Thus, patients who present with features of CML-AP at the time of diagnosis can be offered therapy with TKI and do not need a SCT. Longer follow-up and larger, prospective studies are needed to confirm this observation. If confirmed, this finding would bring into question the current criteria used to classify patients into chronic and accelerated phase based only on morphologic criteria.
Supplementary Material
CLINICAL PRACTICE POINTS.
Patients with CML who present with features of accelerated phase (CML-AP) at initial diagnosis have excellent outcomes.
Second generation tyrosine kinase inhibitors (2GTKI) are associated with particularly favorable outcomes in de novo CML-AP.
TKIs should be considered standard initial therapy for patients with AP at the time of diagnosis.
Acknowledgments
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672, and Award Number P01 CA049639 from the National Cancer Institute. The authors would like to acknowledge Mark Brandt for his assistance with graphics.
Appendix
Authorship Contributions
Maro Ohanian: collected and analyzed the data, writing and final approval of the manuscript
Hagop Kantarjian: analysis of the data, contributed patients, reviewed and approved manuscript
Alfonso Quintas-Cardama: contributed patients, reviewed and approved manuscript
Elias Jabbour: contributed patients, reviewed and approved manuscript
Lynne Abruzzo: performed cytogenetic analysis, reviewed and approved manuscript
Srdan Verstovsek: contributed patients, reviewed and approved manuscript
Gautam Borthakur: contributed patients, reviewed and approved manuscript
Farhad Ravandi-Kashani: contributed patients, reviewed and approved manuscript
Guillermo Garcia-Manero: contributed patients, reviewed and approved manuscript
Richard Champlin: contributed patients, reviewed and approved manuscript
Sherry Pierce: analyzed the data, reviewed and approved manuscript
Mona Lisa Alattar: collected data, reviewed and approved manuscript
Long Xuan Trinh: collected data, reviewed and approved manuscript
Raja Luthra: preformed molecular analysis, reviewed and approved manuscript
Alessandra Ferrajoli: contributed patients, reviewed and approved manuscript
Tapan Kadia: contributed patients, reviewed and approved manuscript
Susan O’Brien: contributed patients, reviewed and approved manuscript
Jorge Cortes: designed the research, analyzed the data, treated patients, writing and final approval the manuscript
Conflicts of Interest Disclosures
Maro Ohanian: None
Hagop Kantarjian: Research grants from Novartis, BMS, Ariad, Pfizer
Alfonso Quintas-Cardama: honoraria from BMS and Novartis
Elias Jabbour: Consultancy honoraria from BMS, Novartis, Pfizer, and Ariad
Lynne Abruzzo: none
Srdan Verstovsek: none
Gautam Borthakur: Advisory board Novartis
Farhad Ravandi-Kashani: honoraria from BMS, Novartis, Pfizer
Guillermo Garcia-Manero
Richard Champlin: none
Sherry Pierce: none
Mona Lisa Alattar: none
Long Xuan Trinh: none
Raja Luthra: none
Alessandra Ferrajoli
Tapan Kadia: none
Susan O’Brien: none
Jorge Cortes: Research support from BMS, Novartis, Ariad, Chemgenex, and Pfizer. Consultancy: Pfizer, Ariad Teva
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kantarjian H, Talpaz M, O’Brien S, et al. Survival benefit with imatinib mesylate therapy in patients with accelerated-phase chronic myelogenous leukemia--comparison with historic experience. Cancer. 2005;103(10):2099–2108. doi: 10.1002/cncr.21032. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. The New England journal of medicine. 2001;344(14):1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 3.Cortes J. Natural history and staging of chronic myelogenous leukemia. Hematology/oncology clinics of North America. 2004;18(3):569–584. viii. doi: 10.1016/j.hoc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Dixon D, Keating MJ, et al. Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer. 1988;61(7):1441–1446. doi: 10.1002/1097-0142(19880401)61:7<1441::aid-cncr2820610727>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Cortes J, Kantarjian H. Advanced-phase chronic myeloid leukemia. Seminars in hematology. 2003;40(1):79–86. doi: 10.1053/shem.2003.50005. [DOI] [PubMed] [Google Scholar]
- 6.Rea D, Etienne G, Nicolini F, et al. First-line imatinib mesylate in patients with newly diagnosed accelerated phase-chronic myeloid leukemia. Leukemia. 2012;26(10):2254–2259. doi: 10.1038/leu.2012.92. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981–1987. doi: 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brave M, Goodman V, Kaminskas E, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res 2008. 2008;14(2):352–359. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 9.Hochhaus A, Giles F, Apperley J, et al. Nilotinib in chronic myeloid leukemia patients in accelerated phase (CML-AP) with imatinib resistance or intolerance: 24-month follow-up results of a phase 2 study [abstract] Haematologica. 2009;94(suppl 2):256, Abstract 0631. [Google Scholar]
- 10.Palandri F, Castagnetti F, Alimena G, et al. The long-term durability of cytogenetic responses in patients with accelerated phase chronic myeloid leukemia treated with imatinib 600 mg: the GIMEMA CML Working Party experience after a 7-year follow-up. Haematologica. 2009;94(2):205–212. doi: 10.3324/haematol.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahaye T, Riehm B, Berger U, et al. Response and resistance in 300 patients with BCR-ABL-positive leukemias treated with imatinib in a single center: a 4.5-year follow-up. Cancer. 2005;103(8):1659–1669. doi: 10.1002/cncr.20922. [DOI] [PubMed] [Google Scholar]
- 12.Jabbour E, Kantarjian HM, Abruzzo LV, et al. Chromosomal abnormalities in Philadelphia chromosome negative metaphases appearing during imatinib mesylate therapy in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood. 2007;110(8):2991–2995. doi: 10.1182/blood-2007-01-070045. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JE, Talpaz M, Giles F, et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101(10):3794–3800. doi: 10.1182/blood-2002-09-2790. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian HM, O’Brien S, Cortes JE, et al. Treatment of philadelphia chromosome-positive, accelerated-phase chronic myelogenous leukemia with imatinib mesylate. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8(7):2167–2176. [PubMed] [Google Scholar]
- 15.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Marrow Donor Program. [Accessed 12-22-12];Chronic Myelogenous Leukemia (CML) Outcomes. http://marrow.org/Physicians/Unrelated_Search_and_Transplant/NMDP_Outcomes/CML_Outcomes/Chronic_Myelogenous_Leukemia_(CML)_Outcomes.aspx-ref1.
- 17. [Accessed 1-17-13];Public summary of positive opinion for orphan designation of imatinib mesilate for treatment of chronic myeloid leukaemia. http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2009/10/WC500006437.pdf.
- 18. [Accessed 1-17-13];Highlights of prescribing. http://www.pharma.us.novartis.com/product/pi/pdf/gleevec_tabs.pdf.
- 19.Johnson JR, Bross JR, Cohen M, et al. Approval Summary: Imatinib Mesylate Capsules for Treatment of Adult Patients with Newly Diagnosed Philadelphia Chromosome-positive Chronic Myelogenous Leukemia in Chronic Phase. Clin Cancer Res. 2003;9(6):1972–1979. [PubMed] [Google Scholar]
- 20.Palandri F, Iacobucci I, Castagnetti F, et al. Front-line treatment of Philadelphia positive chronic myeloid leukemia with imatinib and interferon-alpha: 5-year outcome. Haematologica. 2008;93(5):770–774. doi: 10.3324/haematol.12265. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. The New England journal of medicine. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 22.Deininger M, O’Brien S, Guilhot F, Goldman J, Hochhaus A, Hughes T. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract] Blood. 2009;114(22):462. Abstract 1126. [Google Scholar]
- 23.Apperley J, Cortes J, Kim D. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START-A trial. J Clin Oncol. 2009;27(21):3472–3479. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99(6):1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 25.le Coutre PD, Giles FJ, Hochhaus A, et al. Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 2012;26(6):1189–1194. doi: 10.1038/leu.2011.323. [DOI] [PubMed] [Google Scholar]
- 26.Gratwohl A, Brand R, Apperley J, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2006;91(4):513–521. [PubMed] [Google Scholar]
- 27.Jiang Q, Xu LP, Liu DH, et al. Imatinib mesylate versus allogeneic hematopoietic stem cell transplantation for patients with chronic myelogenous leukemia in the accelerated phase. Blood. 2011;117(11):3032–3040. doi: 10.1182/blood-2010-09-308510. [DOI] [PubMed] [Google Scholar]
- 28.Jain P, Kantarjian H, Nazha A, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121(24):4867–4874. doi: 10.1182/blood-2013-03-490128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Dwyer ME, Mauro MJ, Kurilik G, et al. The impact of clonal evolution on response to imatinib mesylate (STI571) in accelerated phase CML. Blood. 2002;100(5):1628–1633. doi: 10.1182/blood-2002-03-0777. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian HM, Keating MJ, Estey EH, et al. Treatment of advanced stages of Philadelphia chromosome-positive chronic myelogenous leukemia with interferon-alpha and low-dose cytarabine. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1992;10(5):772–778. doi: 10.1200/JCO.1992.10.5.772. [DOI] [PubMed] [Google Scholar]
- 31.Cortes J, O’Dwyer ME. Clonal evolution in chronic myelogenous leukemia. Hematology/oncology clinics of North America. 2004;18(3):671–684. x. doi: 10.1016/j.hoc.2004.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.