Abstract
NK cells are effector lymphocytes that are under clinical investigation for the adoptive immunotherapy of hematologic malignancies, especially acute myeloid leukemia. Recent work in mice has identified innate memory-like properties of NK cells. Human NK cells also exhibit memory-like properties, and cytokine-induced memory-like (CIML) NK cells are generated via brief pre-activation with IL-12, IL-15, and IL-18, which later exhibit enhanced functionality upon restimulation. However, investigation of the optimal cytokine receptors and signals for maintenance of enhanced function and homeostasis following pre-activation remains unclear. Here, we show that IL-12, IL-15, and IL-18 pre-activation induces a rapid and prolonged expression of CD25, resulting in a functional high affinity IL-2 receptor (IL-2Rαβγ) that confers responsiveness to picomolar concentrations of IL-2. The expression of CD25 correlated with STAT5 phosphorylation in response to picomolar concentrations of IL-2, indicating the presence of a signal-competent IL-2Rαβγ. Furthermore, picomolar concentrations of IL-2 acted synergistically with IL-12 to co-stimulate IFN-γ production by pre-activated NK cells, an effect that was CD25-dependent. Picomolar concentrations of IL-2 also enhanced NK cell proliferation and cytotoxicity via the IL-2Rαβγ. Further, following adoptive transfer into immunodeficient NOD-SCID-γc−/− mice, human cytokine pre-activated NK cells expand preferentially in response to exogenous IL-2. Collectively, these data demonstrate that human CIML NK cells respond to IL-2 via IL-2Rαβγ with enhanced survival and functionality, and provide additional rationale for immunotherapeutic strategies that include brief cytokine pre-activation prior to adoptive NK cell transfer, followed by low dose IL-2 therapy.
Keywords: NK cell, adoptive immunotherapy, cytokine, IL-2, IL-2 receptor
INTRODUCTION
Natural killer (NK) cells are a subset of innate lymphoid cells critical for host anti-viral defense and mediate anti-tumor immunity.1–5 NK cells are of clinical interest and being explored as anti-tumor effectors in both the allogeneic hematopoietic stem cell transplantation (HSCT) setting, as well as adoptive cellular therapy of hematologic disease.6–8 Initial reports in the MHC-haploidentical transplantation setting indicated that NK cells may be harnessed for graft-versus-leukemia (GvL) effects, in the absence of graft-versus-host disease (GVHD).9 Subsequent studies have investigated the molecular basis of killer-cell immunoglobulin-like receptor (KIR) genetics and their MHC class I ligands on NK cell functional responses and outcomes following allogeneic HSCT.10–12 These studies highlight the importance of integrating new advances in basic NK cell biology, such as education and licensing, when applying NK cells as therapeutics in the HSCT or adoptive transfer setting.
NK cells are traditionally classified as innate immune lymphocytes, since they do not rearrange germline DNA to form a dominant clonal activation receptor, distinct from T and B cells. However, this paradigm has recently been challenged by several groups identifying innate memory mediated by mouse NK cells,13 in the setting of hapten-based sensitization,14 viral (murine cytomegalovirus, MCMV) infection,15 and following cytokine activation with IL-12, IL-15, and IL-18.16 Notably, NK cell memory that occurs following MCMV infection depends on pro-inflammatory cytokines,17 suggesting a common mechanistic link between virus- and cytokine-induced NK cell memory. Studies in humans have also shown that viral infection, in particular human CMV, results in imprinting on the NK cell compartment via altering the expression patterns of NKG2C and KIR that correlate with NK cell functional status. These studies include CMV re-activation post solid organ transplantation and HSCT, which may correlate with murine virus-induced memory NK cells.18,19 Human NK cell memory-like responses have been directly demonstrated in vitro following cytokine-activation with IL-12, IL-15, and IL-18.20 A brief (16 hour) pre-activation with IL-12, IL-15, and IL-18, followed by rest in vitro for 1–6 weeks, results in enhanced functionality including IFN-γ production following restimulation with cytokines, or exposure to leukemia targets. This enhanced functionality extended to both primary NK cell subsets present in peripheral blood (CD56bright and CD56dim). IL-15 was used as a survival cytokine during the in vitro rest period based on prior studies; however, additional cytokines that may contribute to the homeostasis and enhanced function of such cytokine-induced memory-like (CIML) NK cells has not been reported. Recent work has shown that murine IL-12, IL-15, and IL-18 pre-activated NK cells have an enhanced ability to control tumor cell line challenge, which in vivo in mice required T cell-derived IL-2.21
We therefore investigated the expression of CD25, a key component required to form the high affinity heterotrimeric IL-2Rαβγ, on human NK cells briefly activated with combinations of IL-12, IL-15, and IL-18. NK cells constitutively express two components of the high affinity IL-2R: the IL-2/15Rβ and γc, which form an intermediate affinity heterodimer that transduces signals in the presence of nanomolar concentrations of IL-2 or IL-15. In contrast, the heterotrimeric high affinity IL-2Rαβγ (including IL-2Rα, CD25) is ligated by picomolar concentrations of IL-2, and thus CD25 expression dictates high affinity IL-2 binding on NK cells.22,23 Previous work has demonstrated constitutive, low density expression of CD25 on CD56bright NK cells, facilitating cross-talk with T cells and antigen presenting cells.24 In addition, several studies have shown that IL-2 or IL-15 stimulation alone result in transient (hours to days) CD25 expression on the majority of NK cells.25,26 Here, we report that short-term (16 hour) combined cytokine activation of human NK cells with IL-12, IL-15, and IL-18 induced robust and prolonged CD25 expression that persisted for at least 7 days on CIML NK cells. This CD25 expression resulted in a signal-competent high affinity IL-2Rαβγ that stimulated STAT5 phosphorylation in NK cells. Moreover, picomolar concentrations of IL-2, acting via the IL-2Rαβγ, costimulated IFN-γ production, enhanced cytotoxicity, and induced proliferation of NK cells. Furthermore, IL-12, IL-15, and IL-18 pre-activated NK cells adoptively transferred into immunodeficient NOD-SCID-γc−/− (NSG) mice were selectively supported by exogenous rhIL-2. These findings provide a clear rationale for utilizing low dose IL-2 therapy as one method to support CIML NK homeostasis and functionality cells following adoptive transfer of IL-12, IL-15 and IL-18 pre-activated allogeneic NK cells.
MATERIALS AND METHODS
Reagents
The following anti-human mAbs were used, Beckman Coulter: CD56(N901), CD3(UCHT1); BD: CD16(3G8), IFN-γ(B27), CD25(M-A251), pSTAT5(pY694); purified anti-CD25 (B-B10, eBioscience) or isotype IgG1k (Biolegend). The following endotoxin-free cytokines were used: rhIL-2 (Chiron); rhIL-15 (CellGenix); rhIL-12, rhIL-18 (Peprotech). The K562 (ATCC) cell line was maintained in RPMI-1640 plus 10% FCS and supplements.20
NK cell purification and cell culture
Anonymous human platelet apheresis donor PBMCs were isolated by ficoll centrifugation. NK cells were purified to >95% CD56+CD3- purity using Rosettesep enrichment (StemCell Technologies) as described.20 In some experiments CD56dim NK cells were isolated by flow cytometric sorting (>99% pure). NK cells were cultured at 5×106/mL in complete RPMI-1640 media (Hyclone) with 10% human AB serum (Sigma) and supplements.20
Assays to assess CD25 expression and functions
Purified NK cells were pre-activated for 16 hours with individual cytokines or combinations for 16 hours, washed 3 times, and re-plated in IL-15 (1 ng/mL) for 2 or 6 additional days. At each time point, cells were harvested and assessed for CD25 expression by flow cytometry. For pSTAT5 experiments, cells were stimulated with varying concentrations of IL-2 for 15 minutes, and then stained for pSTAT5 as described.20 In some experiments, cells were pre-incubated for 1 hour at 37°C with either an isotype or anti-CD25 antibody (10 μg/mL). Pre-activated cells were re-stimulated after 3 days of in vitro culture with varying concentrations of IL-2 with or without IL-12. Cells were incubated for 6 hours in the presence of BFA/monensin (BD) and assayed for intracellular IFN-γ.20
Flow cytometric analysis
Cell staining was performed as described,20,27 and data were acquired on a Gallios flow cytometer (Beckman Coulter) and analyzed using FlowJo (TreeStar) or Kaluza (Beckman Coulter) software. Phospho-STAT5 flow cytometry assays were performed following the manufacturer’s instructions (BD).
Flow based killing assay (FloKA)
Sorted CD56dim NK cells were pre-activated for 16 hours, washed 3 times, and then cultured in cytokine-free media for 2 days. Cells were then cultured for an additional 24 hours with or without the addition of IL-2, prior to harvesting for use as effectors in cytotoxicity assays. Flow-based killing assays were performed by co-incubation with CFSE-labeled K562 cells for 4 hours and assaying 7-AAD uptake as described.28 Spontaneous background K562 death (no effector control wells) was subtracted to yield percent specific killing, and in all cases was less than 5%. Flow based killing assay results consistently correlate with 51Cr release assays, and provide a direct method to count individual dead target cells.28,29
In vitro proliferation and survival assays
CFSE-labeled sorted CD56dim NK cells were pre-activated and cultured in IL-2 containing media, with media and cytokine changes every other day. On day 7, cells were harvested and assessed for CFSE dilution staining or 7-AAD by flow cytometry.
Human NK cell xenograft experiments
Purified human NK cells were pre-activated with IL-12, IL-15 and IL-18 or 1 ng/mL IL-15 alone (control) for 16 hours, washed 3 times, and then equivalent numbers of control or CIML NK cells from the same donor were transferred r.o. into groups of NSG mice (3–8×106/mouse). Mice were injected with rhIL-2 (75,000 IU/mouse) every other day x 3 doses. On day 7, mice were sacrificed and human cells were identified with human CD45 and CD56/CD16 expression, and all human CD45+ cells were NK cells. The relative abundance of NK cells was determined by calculating the ratio of mouse CD45 to human CD45 positive cells in the spleen, to control for variable absolute leukocyte numbers in different NSG mice.
Statistical analysis
Statistical comparisons were performed using student’s T test or ANOVA (*P<0.05; **P<0.005; ***P<0.0005), where appropriate.
RESULTS
Induction of CD25 on IL-12, IL-15 and IL-18 pre-activated human NK cells
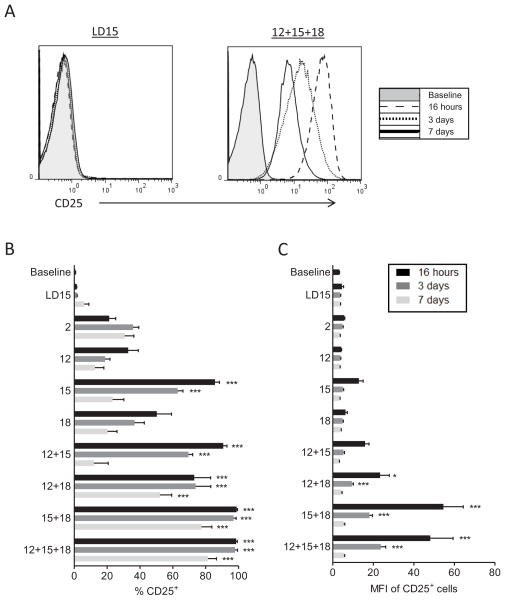
We hypothesized that combinations of IL-12, IL-15, and IL-18 stimulate NK cells to express CD25 thereby acquiring responsiveness to low concentrations of IL-2. To test this premise, cytokines known to bind to constitutively expressed cytokine receptors on resting NK cells were evaluated for the ability to induce cell surface CD25 expression. Purified human NK cells were cultured overnight in IL-2, IL-12, IL-15, IL-18, or combinations of these cytokines. Low dose IL-15 (LD15) was used as a control to maintain survival. At 16 hours, all cytokines tested, with the exception of LD15, resulted in increased expression of CD25 on both CD56dim and CD56bright NK cells. However, these stimuli resulted in varying percentages of CD25+ NK cells, which also varied markedly in expression density on a per cell basis (Figure 1). Stimulation with IL-2 or IL-12 alone modestly increased the percentage of CD25+ NK cells, but the expression of per cell CD25 (MFI) was low (Figure 1B, C). IL-15 or IL-18 alone enhanced the percentage of NK cells expressing CD25, but again resulted in a small increase in per cell expression (MFI). The combination of IL-15 and IL-18, with or without IL-12, resulted in the highest induction of CD25 per cell on NK cells (16-fold increase in MFI, Figure 1A–C). Withdrawal of cytokine stimulation after 16 hours resulted in decreased CD25 expression over the course of 7 days, although a large fraction of CD25+ cells persisted following pre-activation with IL-15 plus IL-18 or IL-12 plus IL-18, or IL-12 plus IL-15 plus IL-18, stimuli that have previously been reported to induce human CIML NK cells.20 We also observed increased expression of CD25 by both CD56bright and CD56dim NK cells, but notably, the mean fluorescence intensity (MFI) increase in CD56dim NK equaled or exceeded that of CD56bright NK cells in every cytokine condition tested (data not shown). Similar results were seen with NK cells cultured within PBMC (Figure S1). Since CD25 is not expressed in freshly isolated CD56dim NK cells, and at a low density on CD56bright NK cells, we evaluated whether the mechanism of induction of CD25 was at the IL2RA (CD25) mRNA level. There was a marked increase in IL2RA mRNA in NK cells following stimulation (Figure S2), suggesting that the mechanism of increased CD25 expression is primarily to enhance transcription or stability of CD25 mRNA. Thus, NK cells pre-activated with the combination of cytokines that induce CIML NK cells, also express CD25 for a prolonged time period, which may facilitate responsiveness to low concentrations of IL-2.
Figure 1. CD25 expression is induced on human NK cells by combined cytokine activation.
Freshly purified human NK cells were stained for baseline CD25 expression, and activated overnight with the following cytokines (1 ng/mL IL-15, LD15; 1 nM IL-2; 100 ng/mL IL-15; 10 ng/mL IL-12; 50 ng/mL IL-18) or combinations of the cytokines as indicated. After culture for 16 hours, cells were washed extensively and cultured for an additional 3 or 7 days in LD15 for all later time points. (A) Cell surface CD25 is induced following activation with IL-12, IL-15, and IL-18, compared to LD15, on NK cells. Representative flow histograms at baseline, 16 hours, 3 days, or 7 days after the initiation of IL-12, IL-15, and IL-18 pre-activation or culture in LD15, demonstrating the marked increase and prolonged expression of CD25. (B and C) CD25 percentage (B) and mean fluorescence intensity (MFI) (C) of NK cells at various time points following cytokine activation. Summary data represents n=6 donors (3 independent experiments). Data shown is mean ± SEM of percentage or the MFI of CD25+ NK cells. Significance is shown comparing each time point versus fresh (baseline) NK cells, and was calculated by one-way ANOVA. These data show that while individually IL-2, IL-12, IL-15, and IL-18 induce CD25+ CD56dim NK cells, the combinations of IL-15+IL-18 and IL-12+IL-18 result in higher per cell CD25 expression.
CD25 induced on NK cells by cytokines results in a signal-competent IL-2Rαβγ
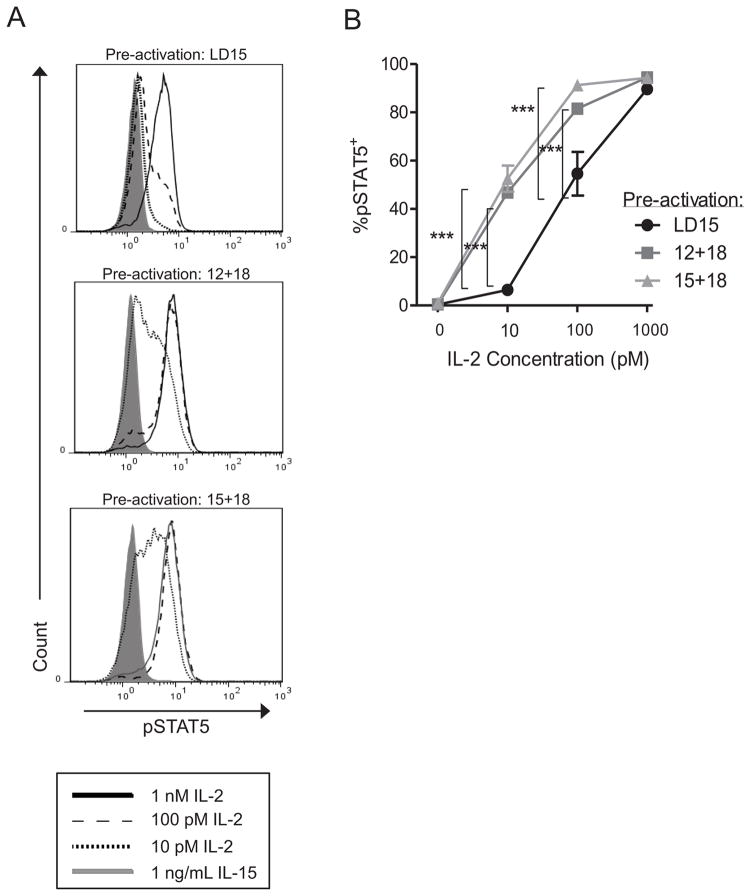
CD25 associates with the intermediate affinity IL-2Rβγ subunits to form the high affinity heterotrimeric IL-2Rαβγ.22,30 In response to ligation with IL-2, this complex signals through the IL-2Rβγ chains, resulting in phosphorylation of STAT5.22,23 We therefore evaluated whether cytokine-activated NK cells exhibited increased phospho-STAT5 when stimulated with picomolar concentrations of IL-2, which selectively ligate the high affinity IL-2Rαβγ. In these experiments, we focused on pre-activation cytokine combinations that result in CIML NK cells and the most pronounced induction of CD25: IL-15 plus IL-18 (also allowing assessment of IL-12-independent responses), or IL-12 plus IL-18 (Figure 1). NK cells were pre-activated for 16 hours with low doses of IL-15 (LD15, control), IL-12 plus IL-18, or IL-15 plus IL-18, washed, and rested in medium alone for an additional 2 days to allow the NK cells to return to a baseline state, but with maintained expression of CD25 (Figure 1). These rested NK cells were then evaluated for their ability to phosphorylate STAT5 in response to varying concentrations of IL-2. In these assays concentrations of 10–100 pM IL-2 selectively activate IL-2Rαβγ, whereas 1 nM IL-2 also activates the constitutively expressed intermediate affinity IL-2Rβγ.23 NK cells that were pre-activated with IL-12 plus IL-18 or IL-15 plus IL-18 demonstrated increased STAT5 phosphorylation beginning at 10 pM IL-2, compared to ≥100 pM IL-2 required to induce pSTAT5 in control LD15 pre-activated NK cells (Figure 2). Thus, cytokine-induced CD25 expression on NK cells results in signaling in response to picomolar concentrations of IL-2, indicating the presence of signal-competent heterotrimeric IL-2Rαβγ complexes. We next evaluated whether such signals result in enhanced NK cell function.
Figure 2. Picomolar concentrations of IL-2 induce phospho-STAT5 expression in pre-activated NK cells.
Purified NK cells were pre-activated with LD15 (control), IL-12+IL-18, or IL-15+IL-18, washed extensively, and re-plated in cytokine-free media for 2 days to allow recovery to a resting state. IL-2 was added at the indicated concentrations for 15 minutes and levels of phospho-STAT5 were analyzed by intracellular flow cytometry. Shown is one representative donor (A), or summary data of the mean ± SEM percentage pSTAT5 positive CD56dim NK cells from n=3 donors and experiments (B). Pre-activated NK cells respond to 10 and 100 pM IL-2 with enhanced STAT5 phosphorylation over control. Significance was calculated by ANOVA.
Signals via the induced IL-2Rαβγ on co-stimulate IFN-γ production
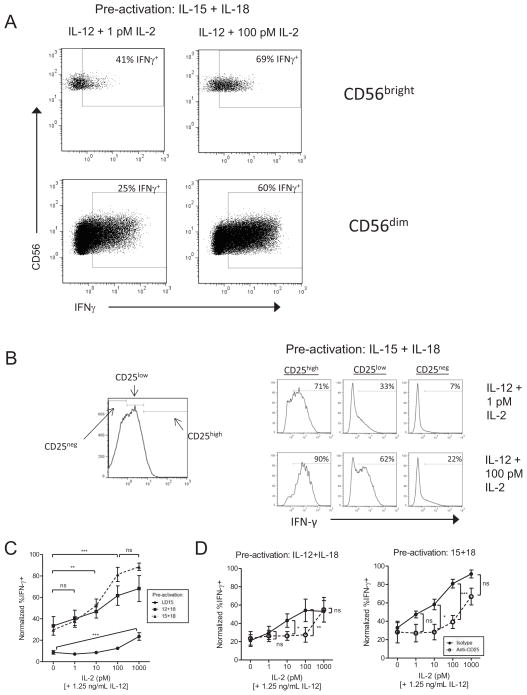
In resting CD56bright NK cells, CD25 is required for picomolar concentrations of IL-2 to act in concert with IL-12 for IFN-γ production.24 In contrast, resting CD56dim NK cells fail to produce IFN–γ in response to picomolar concentrations of IL-2 plus IL-12. Since both CD56bright and CD56dim NK cells exhibit memory-like properties, we evaluated the ability of low dose IL-2 to co-stimulate IFN-γ production in these NK cell subsets following combined cytokine-induction of CD25. In these experiments, purified NK cells were pre-activated with cytokine combinations that result in CIML NK cells20 and CD25 induction (IL-12 plus IL-18 or IL-15 plus IL-18) for 16 hours, washed, and then rested for 2 days in cytokine-free media to allow the NK cells to return to a baseline state (not producing IFN-γ protein). In the setting of cytokine pre-activation, we observed a moderate fraction of NK cells producing IFN-γ in response to IL-2 alone, indicating that prior cytokine activation primes an enhanced IFN-γ response to individual cytokines (Figure S3).20 Similarly, a modest fraction of pre-activated NK cells produce IFN-γ in response to IL-12 alone, again due to the initial pre-activation (0 pM IL-2, Figure 3C). When IL-2 and IL-12 were combined, concentrations of 10 or 100 pM IL-2 were able to significantly co-stimulate IFN-γ production in cytokine pre-activated NK cells. Concentrations above 100 pM failed to further increase IFN-γ production, suggesting that additional signaling via intermediate affinity IL-2Rβγ was not required for maximal IFN-γ in this setting. Control NK cells that were not pre-activated with cytokines but were cultured in medium alone (i.e., 72 hours in medium only) excluded trypan blue and 7-AAD, but failed to produce IFN-γ, even in response to established co-stimulatory conditions in resting CD56dim NK cells (e.g., 1 nM IL-2 plus IL-12),31 likely due to prolonged growth factor starvation (data not shown). Therefore, we cultured NK cells in low dose IL-15 (1 ng/mL), which preserves the functionality of these cells but does not induce CD25 expression (Figure 1B, C). Low dose IL-15 activated cells did not produce increased IFN-γ with addition of IL-12 and IL-2, with the exception of 1nM IL-2 (Figure 3C). Thus, low concentrations of IL-2 stimulate and co-stimulate (in cooperation with IL-12) IFN-γ production by combined cytokine pre-activated NK cells. We next evaluated the absolute requirement for CD25 and the high affinity IL-2Rαβγ for these IL-2 effects.
Figure 3. Induced CD25 on pre-activated NK cells results in a functional high affinity IL-2Rαβγ that signals for enhanced IFN-γ production.
Purified NK cells were activated for 16 hours with IL-12+IL-18 or IL-15+IL-18, washed extensively, and re-plated in cytokine-free media for 2 days to allow recovery to a resting state. IL-2 was then added at the indicated doses in combination with IL-12 (1.25 ng/mL). After 6 hours, IFN-γ was measured by intracellular flow cytometry. (A) Representative flow cytometric plots from one pre-activated donor depicting dose-dependent IFN-γ production co-stimulated by picomolar IL-2. (B) Representative flow plots from IL-15+IL-18 pre-activated NK cells that were subgated based on CD25 expression and IFN-γ expression with stimulation by IL-12 + 1 or 100 pM IL-2. (C) Summary data of pre-activated CD56dim NK cell IFN-γ production with IL-12 combined with various doses of IL-2, shown as mean ± SEM normalized IFN-γ percentage (n=4 donors). (D) In a separate assay, cells were pre-treated for one hour with either an anti-CD25 blocking antibody or IgG1 isotype control, followed by stimulation with 1.25 ng/mL IL-12 and 10-fold dilutions of IL-2. In (B,C), data is shown with the maximum IFN-γ positive percentage in each donor (n=4) normalized to 100%, since absolute IFN-γ+ percentages between donors are variable (range of maximum %IFN-γ+:14–66% for IL-12+IL-18 pre-activated cells, 28–86% for IL-15+IL-18 pre-activated cells). Significance was calculated by ANOVA.
IFN-γ production in response to picomolar concentrations of IL-2 requires CD25 and the high affinity IL-2Rαβγ
Selective responsiveness to 10–100 pM IL-2 suggests action through the high affinity IL-2Rαβγ. Furthermore, increased CD25 induction mediated by IL-15+IL-18 versus IL-12+IL-18 enhanced IL-2 co-stimulated IFN-γ production (mean 53% vs 32%, p<0.05), and populations that expressed more CD25 per cell also produced more IFN-γ (Figure 3B). However, to definitively establish the requirement for CD25 and high affinity binding, we used a blocking anti-CD25 mAb. This approach selectively blocks the formation of the high affinity IL-2Rαβγ since CD25 is not available to the complex, but allows stimulation via the intermediate affinity IL-2Rβγ. The enhanced IFN-γ production in response to 10 or 100 pM IL-2 by cytokine pre-activated NK cells was abrogated by pre-incubation with an anti-CD25 mAb (Figure 3D). In contrast, 1 nM IL-2 co-stimulated IFN-γ production regardless of CD25 blockade. Collectively, these data indicate that picomolar concentrations of IL-2, signaling through an induced IL-2Rαβγ, stimulate enhanced IFN-γ in pre-activated NK cells.
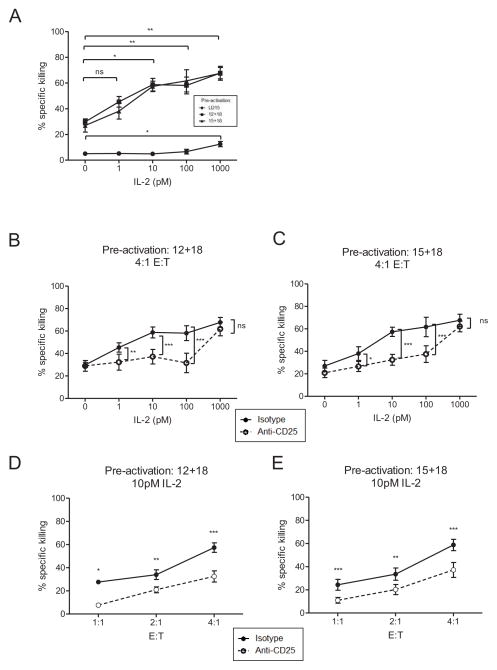
Picomolar concentrations of IL-2 act via IL-2Rαβγ to augment cytotoxicity by cytokine pre-activated NK cells
Resting CD56dim NK cells mediate cytotoxicity when triggered by the appropriate target cell.1,32 We hypothesized that similar to the IFN-γ results, picomolar concentrations of IL-2 would augment pre-activated NK cell cytotoxicity. To investigate this, we pre-activated CD56dim NK cells with low dose IL-15, IL-12 plus IL-18, or IL-15 plus IL-18 for 16 hours, rested these cells in medium only (without IL-2) for 48 hours, and then stimulated with varying concentrations of IL-2. After 24 hours a standard flow based cytotoxicity assay was performed with K562 leukemia cells as targets. Consistent with the IFN-γ experiments, we observed significantly enhanced killing with 10 pM IL-2 stimulation, an effect that was prevented by pre-incubation with an anti-CD25 antibody (Figure 4). Notably, incubation with 1 nM IL-2 failed to enhance cytotoxicity above levels induced by 10 pM IL-2 in pre-activated CD56dim NK cells, consistent with expression of IL-2Rαβγ and saturation of IL-2 signaling. Low dose IL-15 activated NK cells had enhanced killing only when cultured with 1 nM IL-2. Importantly, CD25 blockade abrogated enhanced cytotoxicity in response to picomolar concentrations of IL-2, but not at 1 nM, suggesting that the intermediate affinity IL-2Rβγ was being utilized at this IL-2 concentration. In each pre-activation condition, the observed killing was dependent on the effector:target ratio, as expected (Figure 4D, E and Figure S4).
Figure 4. Low concentrations of IL-2 enhance pre-activated CD25+ CD56dim NK cell killing of K562 leukemia target cells.
Flow sorted CD56dim NK cells were pre-activated with LD15, IL-12+IL-18, or IL-15+IL-18, washed, and re-plated in cytokine-free media for 2 days. Cells were then incubated for 1 hour with an isotype or anti-CD25 blocking antibody prior to activation with dilutions of IL-2. After 24 hours, cells were harvested and plated in a four-hour in vitro flow-based killing assay with CFSE-labeled K562 cells. Shown is mean ± SEM percent specific killing at a 4:1 effector:target ratio pre-incubated with (A) an isotype control or (B,C) an anti-CD25 blocking antibody. (D,E) Killing by pre-activated CD56dim NK cells is NK cell dose (effector:target ratio) dependent, shown at 10 pM IL-2, with anti-CD25 or isotype control pre-incubation. Data is summarized from 3–5 donors in 2 independent experiments.
Low concentrations of IL-2 facilitate cytokine-activated NK cell proliferation via the IL-2Rαβγ
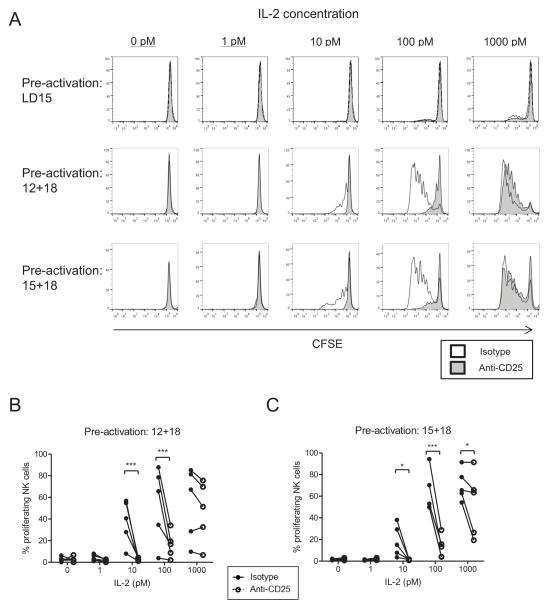
T cell secretion of IL-2 acts in an autocrine or paracrine capacity to induce activated T cell proliferation.23 Additionally, activated mouse NK cells, either in the context of in vivo viral infection or in vitro cytokine stimulation, undergo substantial proliferation.4,33 Resting CD56dim NK cells have been characterized as less proliferative compared to CD56bright NK cells following cytokine stimulation;1,34–36 however, proliferation by CD56dim NK cells after combined cytokine pre-activation has been observed.20 We evaluated whether combined cytokine-activated CD56dim NK cells proliferated in response to low doses of IL-2 using the high affinity IL-2Rαβγ. Flow sorted CD56dim NK cells were labeled with CFSE, and pre-activated with low dose IL-15, IL-12 plus IL-18, or IL-15 plus IL-18. After 16 hours the cells were washed, and incubated with anti-CD25 or isotype control mAbs for 1 hour. Following this blockade, IL-2 was supplemented at the indicated concentrations. In medium only (without IL-2, IL-15, or other cytokines), we observed minimal proliferation (Figure 5A). However, with IL-2 added at 10 pM or greater, CD56dim NK cells were induced to proliferate in a dose-dependent manner (Figure 5A–C). Furthermore, when cells were pre-cultured with an anti-CD25 blocking antibody, the proliferation induced by low dose IL-2 (10–100 pM) was abrogated (Figure 5B,C). We observed substantial donor-dependent variability in the degree of proliferation by CD56dim NK cells as demonstrated in Figure 5B and C, presumably due to reported differences in the proliferative capacity of human NK subsets37–39. However, all donors displayed a significant enhancement in proliferation with ≥10 pM IL-2 stimulation, as well as abrogation of this signaling following CD25 blockade.
Figure 5. Low concentrations of IL-2 stimulate the proliferation of pre-activated CD56dim NK cells.
CFSE-labeled, flow sorted CD56dim NK cells were pre-activated with low dose IL-15, IL-12+IL18, or IL-15+IL-18 for 16 hours, washed extensively, and blocked with an isotype or anti-CD25 blocking antibody. Dilutions of IL-2 were then added, with subsequent media, cytokine, and blocking antibody additions occurring every other day. After 7 days, cells were harvested and analyzed for CFSE dilution by flow cytometry. Analysis was restricted to live cells by 7-AAD exclusion. (A) Data shown represents histograms from one donor. Both isotype-blocked (open histograms) and anti-CD25 blocked (shaded histograms) NK cell proliferation is shown. (B) Summary data depicting the variability of proliferation between CD56dim NK cells from different donors, and the dependence of picomolar IL-2 mediated proliferation on CD25 / IL-2Rαβγ. Significance was assessed by two-way ANOVA.
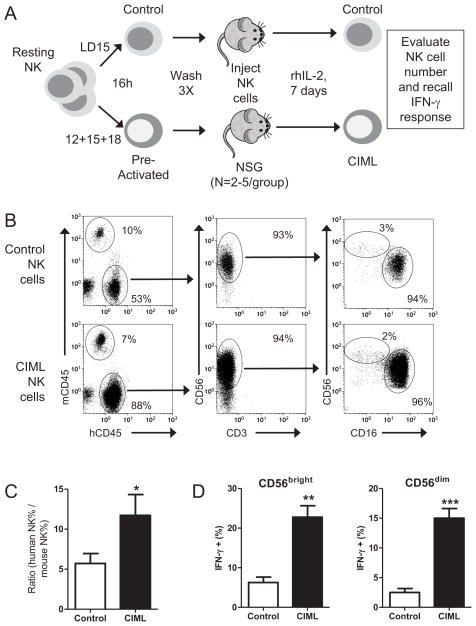
IL-2 supports cytokine pre-activated CIML NK cells in vivo in NSG mice
Pre-activation of human NK cells with IL-12, IL-15, and IL-18 for 16 hours followed by prolonged culture in vitro with IL-15 for survival results in CIML NK cells. Based on the induction of a functional IL-2Rαβγ on pre-activated NK cells, we hypothesized that rhIL-2 would selectively support cytokine pre-activated (IL-12, IL-15, IL-18) compared to control (IL-15 only) activated NK cells in vivo. Cytokine pre-activated or control NK cells were generated from the same donor, and following extensive washing, were transferred into NSG mice, and rhIL-2 was administered every other day. After one week, mice were analyzed for human NK cell numbers, and evaluated for CIML NK cell recall responses. Cytokine pre-activated NK cells had significantly improved engraftment in NSG mice in this system, compared to control (IL-15) NK cells (Figure 6). Both CD56bright and CD56dim NK cells we identified, and enhanced recall functionality to cytokine or tumor target based restimulation by CIML NK cells was also evident ex vivo. These data suggest that administration of rhIL-2 following adoptive transfer of IL-12, IL-15, and IL-18 pre-activated NK cells is a novel approach to administer and support function-enabled NK cells as adoptive immunotherapy for patients with hematologic malignancies.
Figure 6. Exogenous IL-2 supports CIML NK cells in a NSG xenograft model.
(A) Purified human NK cells were pre-activated with IL-12, IL-15, and IL-18 (CIML) or low dose IL-15 only (control) for 16 hours, washed, and identical numbers of NK cells were transferred into NSG mice, followed by injections of 75,000 IU i.p. of rhIL-2 every other day. After 7 days, mice were assessed for human NK cell content, and CIML NK cell recall responses. (B) Representative flow plots from the peripheral blood of mice injected with the same number of CIML or control NK cells from the same donor 7 days earlier. Human CD45+ cells are CD56+CD3− NK cells with preserved CD56bright and CD56dim NK cells subsets. (C) Summary data from 3 different NK cell donors transferred into a total of 12 NSG mice demonstrating superior engraftment of CIML, compared to control, NK cells. The ratio of human: mouse CD45+ cells, representing the relative abundance of human NK cells, is used to control for differences in blood volume obtained. (D) Mouse splenocytes containing adoptively transferred human NK cells were evaluated for a recall IFN-γ response following IL-12+IL-15 restimulation, demonstrating that CIML NK cells exhibit preserved, enhanced functionality in this xenograft model. Significance was assessed by T test, with *P<0.05, **P<0.01, and ***P<0.001.
DISCUSSION
Freshly isolated human CD56dim NK cells are thought to require high concentrations of IL-2 (or IL-15), signaling through the IL-2/15Rβγ, to enhance or prime their functional capacity through this receptor complex. Here, we show that combined cytokine activation of both CD56bright and CD56dim NK cells with IL-15 plus IL-18 or IL-12 plus IL-18 results in robust CD25 expression, which remains present on CIML NK cells after 7 days. This CD25 expression combines with constitutively present IL-2/15Rβ and γc to form a functional high affinity IL-2Rαβγ on pre-activated NK cells. Such NK cells have increased STAT5 phosphorylation when re-stimulated with picomolar concentrations of IL-2, and exhibit augmented functional responses including IFN-γ production, cytotoxicity, and proliferation. Further, IL-2 supports cytokine pre-activated NK cells in vivo in NSG xenograft models. Thus, both primary peripheral blood NK cell subsets (CD56bright and CD56dim) are induced to express IL-2Rαβγ after combined cytokine pre-activation, expanding the potential of low concentrations of IL-2 to stimulate and support CIML NK cells. These data suggest that low dose IL-2 therapy can support survival, proliferative, and effector functions of IL-12, IL-15, and IL-18 pre-activated allogeneic NK cells following adoptive transfer.
What role does the high affinity IL-2Rαβγ on CD56dim NK cells play during a physiologic immune response? During the host response to a pathogen, NK cells have a complex set of interactions with other members of the innate and adaptive immune system.2,40,41 Following infection with murine cytomegalovirus (MCMV), a model virus infection with a prominent early NK cell response, an initial wave of cytokines are produced that drive NK cell proliferation and enhances their functional capacity. In this setting, IL-12, IL-15, and IL-18 are induced over 1–2 days, a time frame consistent with the induction of CD25 on mouse NK cells.42,43 In a recent report from the Biron laboratory, induced CD25 was identified on NK1.1+TCRβ− NK cells peaking approximately 3.5 days post MCMV infection. In vitro, NK cells within bulk murine splenocytes were induced to express CD25 after 24 hours by the combination of IL-12 plus IL-18, which absolutely required IL-12-induced STAT4 signals.43 Thus, we suspect that during human responses to viruses and potentially other pathogens, IL-12, IL-15, and IL-18 are produced, and CD56dim NK cells induced to express CD25 (Figure S5). In our studies, we identified that human NK cells are optimally stimulated by IL-15 plus IL-18 (in the presence or absence of IL-12) to express CD25, differing from mouse NK cell requirement for IL-12. Since all doublet combinations of IL-12, IL-15, and IL-18 resulted in CD25 expression, human NK cells may have an expanded flexibility to express the high affinity IL-2Rαβγ, in different inflammatory cytokine responses. Notably, IL-18 appeared to be necessary, but not sufficient for substantial CD25 expression in our experiments.
Recently, combined cytokine pre-activation with IL-12, IL-15, and IL-18 has been shown in mouse16,21,44 and human20 NK cells to result in memory-like NK cell functions, with long lived, enhanced functionality to re-stimulation. Cytokine-induced memory-like (CIML) NK cell function refers to NK cells that are pre-activated (e.g., IL-12+IL-15+IL-18), return to a resting baseline state, and then exhibit enhanced functional responses to a wide variety of re-stimulation triggers, including cytokines, activating NK cell receptors, or tumor cells. This was first described in mouse systems,16,45 and human NK cells were also found to exhibit similar CIML responses.20 In addition, Ly49H-mediated ‘memory’ NK cell function in the context of MCMV infection was found to depend on early pro-inflammatory cytokine signals.17 For human NK cells, it is noteworthy that the same cytokine combinations of IL-12 plus IL-18 or IL-15 plus IL-18 that pre-activate memory-like NK functionality are also the optimal cytokine inducers of CD25 expression. While human CIML NK cells were supported with IL-15 in vitro following pre-activation,20 low concentrations of IL-2 may also have a role in supporting these memory-like NK cell functions, either endogenously or exogenously.21 In addition, mouse IL-12, IL-15, and IL-18-pre-activated NK cells were also recently shown to effectively clear mouse tumor cell line challenges in vivo.21 Following transfer into irradiated syngeneic mice, NK cells were found to require CD4+ T cell derived IL-2 in vivo in order to effectively eliminate lymphoma and melanoma cell line challenges. Both murine and human NK cells were also shown to express CD25 immediately following IL-12, IL-15, and IL-18 activation, which is consistent with our findings in human NK cell subsets.21 Thus, both human and mouse NK cells can be induced to express CD25 and a high affinity IL-2Rαβγ, which may be important to support NK cell functional competency following viral infection or after exogenous IL-12, IL-15, and IL-18 pre-activation.
What are the clinical implications of peripheral blood NK cell acquisition of a functional high affinity IL-2Rαβγ? Allogeneic NK cell therapy has evidence of anti-leukemic activity in clinical trials in AML patients.46 Low dose or ultra-low dose IL-2 therapy has been shown to expand CD56bright NK cells selectively over CD56dim NK cells in patients with HIV infection or cancer,47,48 most likely due to lack of the high affinity IL-2Rαβγ on CD56dim NK cells that promotes survival in the resting state.49 As CD56dim NK cells represent 80–95% of the mature peripheral blood NK population, adoptive transfer of NK cells pre-activated with combinations of IL-12, IL-15, and IL-18, followed by low dose IL-2 therapy in vivo, may be a useful strategy to further enhance anti-tumor and/or anti-viral NK cell responses. Such an approach would include strategies to limit Treg numbers or function.
In summary, NK cells that are pre-activated with combinations of IL-12, IL-15, and IL-18 are induced to express CD25 and a high affinity IL-2Rαβγ, which persists on CIML NK cells. This results in an acquired ability of all peripheral blood NK cells to proliferate, kill tumor targets, and produce IFN-γ in response to picomolar concentrations of IL-2. IL-2 supports CIML NK cells in vivo in xenograft models. Thus, our findings provide a rationale for low dose IL-2 therapy to enhance expansion and NK cell function following IL-12, IL-15, and IL-18-pre-activated NK cell adoptive transfer as immunotherapy for cancer patients.
Supplementary Material
Supplemental Figure 1. NK cells cultured within PBMC express CD25 upon IL-12 + IL-18 or IL-15 + IL-18 stimulation and subsequently respond to low dose IL-2 stimulation through enhanced IFN-γ production. (A) Representative FACS plots of CD25 expression on gated CD56bright or CD56dim NK cells at baseline, or cultured for 16 hours with low dose IL-15, IL-12+18, or IL-15+18. (B) Summary CD25 expression data from 4 donors. (C) Pre-activated NK cells within PBMC cultures were stimulated overnight with cytokines, washed, and rested for 2 days before stimulation with IL-12 and IL-2 to induce IFN-γ production.
Supplemental Figure 2. IL2RA (CD25) mRNA increases in NK cells following IL-15 plus IL-18 activation. Purified NK cells were treated with LD15 or IL-15 + IL-18 for 16 hours, washed, and then cultured in LD15 for the 3 or 7 days. At the indicated time point total RNA was isolated using Trizol. The relative expression of IL2RA/CD25 was assessed by real-time RT-qPCR with 18s rRNA used as the calibrator. Data is expressed as mean ± SEM fold change in CD25 mRNA compared to freshly isolated NK cells. Summarizes N=3 donors.
Real-time qPCR was performed using the high capacity cDNA RT kit (ABI) on total RNA and amplified using, fwd: GACGAGGCAGGAAGTCTCAC; rev: ATCAGTGCGTCCAGGGATAC; probe: CTGAGAGCGTCTGCAAAATG, specific for CD25/IL2RA.
Supplemental Figure 3. IL-2 induces IFN-g production by pre-activated CD56dim NK cells. Purified NK cells were treated with IL-12 + IL-18 or IL-15 + IL-18 for 16 hours, washed, and then rested in medium only for 2 days. IL-2 was added at the indicated concentrations, and after 6 hours, IFN-g was measured by intracellular flow cytometry. Data is expressed as mean ± SEM normalized IFN-g (as described in Figure 3). Distinct from freshly isolated NK cells, IL-2 stimulated IFN-g production without additional cytokines present, in pre-activated CD56dim NK cells. Summarizes N=4 donors.
Supplemental Figure 4. IL-2-enhanced cytotoxicity by pre-activated CD56dim NK cells depends on the effector: target cell ratio. Complete cytotoxicity data from experiments shown in Figure 4; see Figure 4 for description.
Supplemental Figure 5. Schema summarizing how induced CD25 and IL-2Rabg on pre-activated NK cells impacts immunotherapy.
Acknowledgments
This was supported by American Society of Hematology (ASH) Trainee Award (JMC), T32 HL708836 from the NIH (RPS), and K08HL093299 from the NIH, ASH Scholar Award from the ASH Foundation, and a V Scholar Award from the V Foundation for Cancer Research (TAF).
The authors thank the Siteman Cancer Center Flow Cytometry Core (P30 CA091842) and the Washington University Pathology & Immunology Flow Cytometry Core. We also thank Dr. Anthony French for insightful discussion.
Footnotes
Financial Disclosure Statement:
The authors have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caligiuri MA. Human natural killer cells. [Accessed July 25, 2011];Blood. 2008 112(3):461–9. doi: 10.1182/blood-2007-09-077438. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2481557&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. [Accessed July 22, 2010];Nat Immunol. 2008 9(5):503–10. doi: 10.1038/ni1582. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18425107. [DOI] [PubMed] [Google Scholar]
- 3.Di Santo JP. Natural killer cells: diversity in search of a niche. [Accessed July 19, 2011];Nat Immunol. 2008 9(5):473–5. doi: 10.1038/ni.f.201. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18425102. [DOI] [PubMed] [Google Scholar]
- 4.Vidal SM, Khakoo SI, Biron CA. Natural Killer Cell Responses during Viral Infections: Flexibility and Conditioning of Innate Immunity by Experience. [Accessed January 9, 2012];Curr Opin Virol. 2011 1(6):497–512. doi: 10.1016/j.coviro.2011.10.017. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3237675&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orr MT, Lanier LL. Natural killer cell education and tolerance. [Accessed July 2, 2011];Cell. 2010 142(6):847–56. doi: 10.1016/j.cell.2010.08.031. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2945212&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy WJ, Parham P, Miller JS. NK cells--from bench to clinic. [Accessed August 28, 2013];Biol Blood Marrow Transpl. 2012 18(1 Suppl):S2–7. doi: 10.1016/j.bbmt.2011.10.033. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3260456&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljunggren H-G, Malmberg K-J. Prospects for the use of NK cells in immunotherapy of human cancer. [Accessed July 19, 2011];Nat Rev Immunol. 2007 7(5):329–39. doi: 10.1038/nri2073. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17438573. [DOI] [PubMed] [Google Scholar]
- 8.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. [Accessed July 19, 2011];Curr Opin Immunol. 2009 21(5):525–30. doi: 10.1016/j.coi.2009.07.015. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19717293. [DOI] [PubMed] [Google Scholar]
- 9.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (80-) 2002;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 10.Cooley S, Weisdorf DJ, Guethlein La, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. [Accessed September 7, 2011];Blood. 2010 116(14):2411–9. doi: 10.1182/blood-2010-05-283051. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2953880&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. [Accessed August 22, 2011];Blood. 2009 113(3):726–32. doi: 10.1182/blood-2008-07-171926. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2628378&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venstrom JM, Pittari G, Gooley Ta, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. [Accessed January 30, 2013];N Engl J Med. 2012 367(9):805–16. doi: 10.1056/NEJMoa1200503. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22931314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. [Accessed March 19, 2013];Trends Immunol. 2013 doi: 10.1016/j.it.2013.02.005. Epub Mar 1Available at: http://www.ncbi.nlm.nih.gov/pubmed/23499559. [DOI] [PMC free article] [PubMed]
- 14.O’Leary JG, Goodarzi M, Drayton DL, Yu H, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. [Accessed July 7, 2011];Nat Immunol. 2006 7(5):507–16. doi: 10.1038/ni1332. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16617337. [DOI] [PubMed] [Google Scholar]
- 15.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. [Accessed July 20, 2011];Nature. 2009 457(7229):557–61. doi: 10.1038/nature07665. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2674434&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106(6):1915–9. doi: 10.1073/pnas.0813192106. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2644138&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JC, Madera S, Bezman Na, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. [Accessed April 13, 2012];J Exp Med. 2012 209(5):947–954. doi: 10.1084/jem.20111760. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22493516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Verges S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57+NKG2C high natural killer cell subset during acute human cytomegalovirus infection. [Accessed August 9, 2011];Proc Natl Acad Sci USA. 2011 108(36) doi: 10.1073/pnas.1110900108. Available at: http://www.pnas.org/cgi/doi/10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. [Accessed December 21, 2011];Blood. 2011 :612–626. doi: 10.1182/blood-2011-10-386995. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22180440. [DOI] [PMC free article] [PubMed]
- 20.Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. [Accessed September 17, 2012];Blood. 2012 120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. Available at: http://www.bloodjournal.org/cgi/doi/10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. [Accessed December 5, 2012];J Exp Med. 2012 209(13):2351–2365. doi: 10.1084/jem.20120944. Available at: http://www.jem.org/cgi/doi/10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. [Accessed March 4, 2013];Nat Rev Immunol. 2006 6(8):595–601. doi: 10.1038/nri1901. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16868550. [DOI] [PubMed] [Google Scholar]
- 23.Malek TR. The biology of interleukin-2. [Accessed March 9, 2013];Ann Rev Immunol. 2008 26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18062768. [DOI] [PubMed] [Google Scholar]
- 24.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. [Accessed July 17, 2012];Blood. 2003 101(8):3052–7. doi: 10.1182/blood-2002-09-2876. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12480696. [DOI] [PubMed] [Google Scholar]
- 25.Pillet A-H, Thèze J, Rose T. Interleukin (IL)-2 and IL-15 have different effects on human natural killer lymphocytes. [Accessed October 3, 2012];Hum Immunol. 2011 72(11):1013–7. doi: 10.1016/j.humimm.2011.07.311. Available at: http://dx.doi.org/10.1016/j.humimm.2011.07.311. [DOI] [PubMed] [Google Scholar]
- 26.Pillet A-H, Bugault F, Thèze J, Chakrabarti LA, Rose T. A programmed switch from IL-15- to IL-2-dependent activation in human NK cells. [Accessed October 3, 2012];J Immumol. 2009 182(10):6267–77. doi: 10.4049/jimmunol.0801933. Available at: http://www.jimmunol.org/content/182/10/6267.short. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan RP, Leong JW, Schneider SE, et al. MicroRNA deficient NK cells exhibit decreased survival but enhanced function. J Immunol. 2012;188(7):3019–30. doi: 10.4049/jimmunol.1102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehniger TA, Cai SF, Cao X, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. [Accessed January 31, 2011];Immunity. 2007 26(6):798–811. doi: 10.1016/j.immuni.2007.04.010. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17540585. [DOI] [PubMed] [Google Scholar]
- 29.White DW, Keppel CR, Schneider SE, et al. Latent herpesvirus infection arms NK cells. [Accessed July 26, 2011];Blood. 2010 115(22):4377–83. doi: 10.1182/blood-2009-09-245464. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2881492&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang BYH, Smith KA. The IL-2 Receptor: Functional Consequences of its Bimolecular Structure. J Exp Med. 1987;166:1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immumol. 1999;162(8):4511–20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10201989. [PubMed] [Google Scholar]
- 32.Orange JS. Formation and function of the lytic NK-cell immunological synapse. [Accessed June 15, 2011];Nat Rev Immunol. 2008 8(9):713–25. doi: 10.1038/nri2381. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2772177&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22(11):405–29. doi: 10.1146/annurev.immunol.22.012703.104711. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15032583. [DOI] [PubMed] [Google Scholar]
- 34.Caligiuri MA, Zmuidzinas A, Manley T, Levine H, Smith K, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity. [Accessed October 30, 2012];J Exp Med. 1990 171:1509–1526. doi: 10.1084/jem.171.5.1509. Available at: http://jem.rupress.org/content/171/5/1509.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagler A, Lanier L, Phillips J. Constitutive expression of high affinity interleukin 2 receptors on human CD16- natural killer cells in vivo. J Exp Med. 1990;171:1527–1533. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romagnani C, Juelke K, Falco M, et al. CD56 bright CD16 - Killer Ig-Like Receptor - NK Cells Display Longer Telomeres and Acquire Features of CD56 dim NK Cells upon Activation. J Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 37.Björkström NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. [Accessed July 23, 2012];Blood. 2010 116(19):3853–64. doi: 10.1182/blood-2010-04-281675. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20696944. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Mao HC, Wei M, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115(2):274–81. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juelke K, Killig M, Luetke-Eversloh M, et al. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. [Accessed August 25, 2011];Blood. 2010 116(8):1299–307. doi: 10.1182/blood-2009-11-253286. Available at: http://bloodjournal.hematologylibrary.org/cgi/content/abstract/116/8/1299. [DOI] [PubMed] [Google Scholar]
- 40.Lee S-H, Miyagi T, Biron Ca. Keeping NK cells in highly regulated antiviral warfare. [Accessed September 7, 2011];Trends Immunol. 2007 28(6):252–9. doi: 10.1016/j.it.2007.04.001. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17466596. [DOI] [PubMed] [Google Scholar]
- 41.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 42.French AR, Sjo H, Kim S, et al. DAP12 signaling directly augments proprolfierative cytokine stimulation of NK cells during viral infection. J Immunol. 2006;177(29):4981–4990. doi: 10.4049/jimmunol.177.8.4981. [DOI] [PubMed] [Google Scholar]
- 43.Lee S-H, Fragoso MF, Biron CA. Cutting Edge: A Novel Mechanism Bridging Innate and Adaptive Immunity: IL-12 Induction of CD25 To Form High-Affinity IL-2 Receptors on NK Cells. [Accessed October 29, 2012];J Immunol. 2012 189(6):2712–6. doi: 10.4049/jimmunol.1201528. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22888135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keppel M, Yang L, Cooper M. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J Immumol. 2013;190(9):4754–62. doi: 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. [Accessed June 14, 2011];EMBO Rep. 2009 10(10):1103–10. doi: 10.1038/embor.2009.203. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2759738&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. [Accessed July 19, 2011];Blood. 2005 105(8):3051–7. doi: 10.1182/blood-2004-07-2974. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15632206. [DOI] [PubMed] [Google Scholar]
- 47.Caligiuri MA, Murray C, Soiffer R, et al. Extended Continuous Infusion Low-Dose Recombinant Interleukin-2 in Advanced Cancer: Prolonged Immunomodulation Without Significant Toxicity. J Clin Invest. 1991;9(12):2110–9. doi: 10.1200/JCO.1991.9.12.2110. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1960552. [DOI] [PubMed] [Google Scholar]
- 48.Caligiuri MA, Murray C, Robertson M, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91(1):123–32. doi: 10.1172/JCI116161. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=330005&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fehniger T, Bluman E, Porter M, et al. Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy. J Clin Invest. 2000;106(1):117–24. doi: 10.1172/JCI6218. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=314354&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. NK cells cultured within PBMC express CD25 upon IL-12 + IL-18 or IL-15 + IL-18 stimulation and subsequently respond to low dose IL-2 stimulation through enhanced IFN-γ production. (A) Representative FACS plots of CD25 expression on gated CD56bright or CD56dim NK cells at baseline, or cultured for 16 hours with low dose IL-15, IL-12+18, or IL-15+18. (B) Summary CD25 expression data from 4 donors. (C) Pre-activated NK cells within PBMC cultures were stimulated overnight with cytokines, washed, and rested for 2 days before stimulation with IL-12 and IL-2 to induce IFN-γ production.
Supplemental Figure 2. IL2RA (CD25) mRNA increases in NK cells following IL-15 plus IL-18 activation. Purified NK cells were treated with LD15 or IL-15 + IL-18 for 16 hours, washed, and then cultured in LD15 for the 3 or 7 days. At the indicated time point total RNA was isolated using Trizol. The relative expression of IL2RA/CD25 was assessed by real-time RT-qPCR with 18s rRNA used as the calibrator. Data is expressed as mean ± SEM fold change in CD25 mRNA compared to freshly isolated NK cells. Summarizes N=3 donors.
Real-time qPCR was performed using the high capacity cDNA RT kit (ABI) on total RNA and amplified using, fwd: GACGAGGCAGGAAGTCTCAC; rev: ATCAGTGCGTCCAGGGATAC; probe: CTGAGAGCGTCTGCAAAATG, specific for CD25/IL2RA.
Supplemental Figure 3. IL-2 induces IFN-g production by pre-activated CD56dim NK cells. Purified NK cells were treated with IL-12 + IL-18 or IL-15 + IL-18 for 16 hours, washed, and then rested in medium only for 2 days. IL-2 was added at the indicated concentrations, and after 6 hours, IFN-g was measured by intracellular flow cytometry. Data is expressed as mean ± SEM normalized IFN-g (as described in Figure 3). Distinct from freshly isolated NK cells, IL-2 stimulated IFN-g production without additional cytokines present, in pre-activated CD56dim NK cells. Summarizes N=4 donors.
Supplemental Figure 4. IL-2-enhanced cytotoxicity by pre-activated CD56dim NK cells depends on the effector: target cell ratio. Complete cytotoxicity data from experiments shown in Figure 4; see Figure 4 for description.
Supplemental Figure 5. Schema summarizing how induced CD25 and IL-2Rabg on pre-activated NK cells impacts immunotherapy.