Abstract
Patients with autoimmune disorders seem to have an elevated risk of lymphoma, especially non-Hodgkin’s lymphoma (NHL). The increased risk has been attributed to the disturbance of immune function found in these patients or to the immunosuppressive therapy used to treat the autoimmune disorders. However, little information exists about the estimated baseline risk for lymphoma in patients with primary biliary cirrhosis (PBC). In this case report, we describe a female patient who developed nodal diffuse large B-cell lymphoma ten years following PBC diagnosis. Twenty five additional case reports (19 NHL and 4 Hodgkin’s disease (HD), 2 without data about NHL or Hodgkin’s disease) predominantly females were identified in the English literature. B-cell lymphoma was the most common NHL type reported but beyond that no clear predisposition for any specific lymphoma subtype was documented. PBC usually preceded lymphoma diagnosis. Fifteen cases had extranodal localization and the most common site was the liver.
Keywords: PBC, non-Hodgkin’s lymphoma, autoimmune disorders
Introduction
Autoimmune disorders are increasingly recognized as risk factors for non-Hodgkin’s lymphoma (NHL) [1]. A recent pooled analysis demonstrated an increased risk of NHL in Sjögren’s syndrome, systemic lupus erythematosus, hemolytic anemia, celiac disease and psoriasis [2]. A specific subtype of NHL was found to be associated with each of the above mentioned autoimmune disorders. For example, parotid gland marginal zone lymphoma was associated with Sjögren’s syndrome. In the same study, many autoimmune disorders (rheumatoid arthritis, sarcoidosis, pernicious anemia etc) were assessed but the risk of NHL in primary biliary cirrhosis (PBC) was not evaluated. PBC is a chronic cholestatic autoimmune disease induced by destruction of small intrahepatic bile ducts probably by an immunologic process. Little information exists in the literature on the risk of lymphoma in PBC. We herein describe a patient presented 10 years following PBC diagnosis with nodal diffuse large B-cell lymphoma (DLBCL) and review the English literature.
Case report
A 75-year-old woman, was admitted with a 4-week-duration abdominal pain reflecting to the lumbar area bilaterally. She also mentioned malaise and loss of 5 Kg of weight. She had a past medical history of PBC diagnosed ten years previously, positive for antimitochondrial antibodies, histologically stage 3 at diagnosis. Portal hypertension (gastro-esophageal varices) was identified 2 years before current admission. She has been on ursodeoxycholic acid (VDCA) since PBC diagnosis. Pulmonary fibrosis was also documented 1 year previously. Physical examination on admission revealed hepatosplenomegaly and generalized lymphadenopathy (submandibular, right axillary and left inguinal-femoral nodes). Laboratory tests showed: hemoglobin 9.9 g/dL; white blood cells, 4,000 per mm3 (neutrophils 67%, lymphocytes 21%, monocytes 12%); platelets 200,000 per mm3 erythrocyte sedimentation rate 52mm/h; negative direct antiglobulin test; IgG, 1730g/dL (700-1600); IgM and IgA within normal limits; polyclonal gammopathy and ^-microglobulin 8.18mg/L. Antibodies for hepatitis virus B and C were negative. A lymph node biopsy revealed infiltration by large lymphocytes. Immunochemistry results were positive for CD45, CD20, PAX-5, CD79a and negative for Bcl2, Bcl6, MUM1, CD138, CD30, CD15, CD3, CD4, CD8 and ALK1. Bone marrow aspiration and biopsy showed infiltration by the same B cell population. Interestingly, the number of cell proliferation, Ki-67+, was increased to 60%. Imaging studies demonstrated enlarged mediastinal and, paraortic lymph nodes inducing ureter obstruction bilaterally, enlarged hilar nodes without focal lesions of the liver, left iliac and inguinal nodes. There was no evidence of ascitic fluid. A diagnosis of DLBCL, stage IV, was established and International Prognostic Index score (IPI) was calculated to 4 points. The patient received treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Clinical, laboratory and imaging improvement was ensued. The patient died from pneumonia five months after the diagnosis of lymphoma, following five cycles of chemotherapy.
Discussion
We identified all relevant English language reports by MEDLINE search using the following terms: (“Primary biliary cirrhosis” OR “PBC” OR “Biliary cirrhosis”) AND (“Lymphoma” OR “Non-Hodgkin lymphoma” OR “NHL” OR “Hodgkin disease” OR “HD” OR “Malignancy”). We also searched the references of selected articles to identify further reports.
Twenty five additional cases including case reports and case series were found [3]. The median age at diagnosis of lymphoma was 64 years (range 27-82) with a female predominance [Table 1]. Lymphoma was diagnosed after the diagnosis of PBC in 14 patients including index case, (median time interval between two diagnoses 5 years) [3-9], both diseases were diagnosed simultaneously in 6 [3, 10-12] and lymphoma preceded PBC diagnosis in 5 patients (median time interval between two diagnoses, 9.5 years) [3,13-15]. There was no information about the timing of diagnosis in one case [3].
Table 1.
Characteristics of patients with concomitant primary biliary cirrhosis and lymphoma reported in the literature
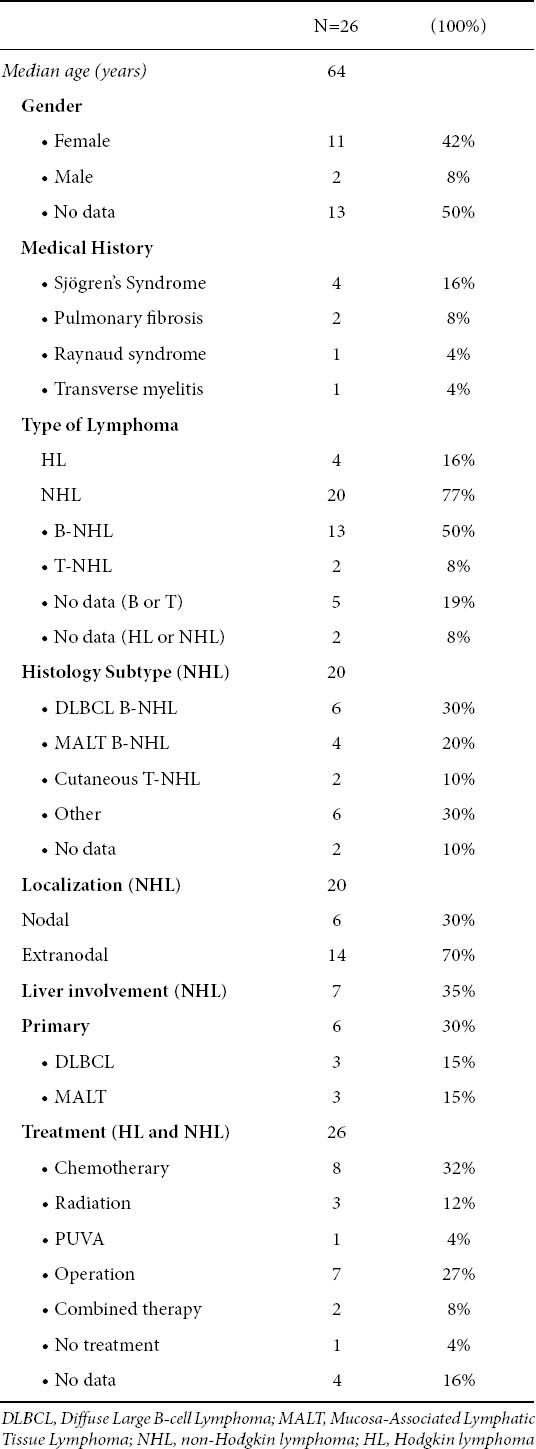
At the time of lymphoma diagnosis, clinical examination disclosed palpable lymphadenopathy in 11 patients [3,5,10,15], splenomegaly in 4 [3,8], hepatomegaly in 3 [7-8,14], cutaneous lesions in 3 [3,7,13]. Four patients were reported to have clinical signs of Sjögren’s syndrome [5,7-8,10], two of pulmonary fibrosis [7], one of Raynaud syndrome [14], and one of transverse myelitis [5].
NHL was diagnosed in 20 cases: B-cell in 13 (including index case) [3-6,8-12,14], T-cell in 2 [3,7], while in the remaining 5 there was no information about type of lymphocyte population involved [3]. Hodgkin’s disease (HD) was diagnosed in 4 cases [3,13,15]. There was no data about the type of lymphoma (Hodgkin or NHL) in 2 cases [3]. Fifteen cases of NHL had extranodal localization [3-5,7,9-14]. The most common site was the liver (7 cases) [4-6,8,9, 11,14], followed by thyroid and skin (2 cases each) [3], spleen (1) [3], eyelid (1) [10], lung (1) [3], bone marrow (index case). In 6 cases primary lymphoma of the liver was diagnosed [4,6,8,9,11,14]. No predisposition for any specific NHL subtype was identified as it was shown in Table 1.
There were no adequate data about treatment of both diseases and follow up. The available data about PBC treatment was the following: Six patients were treated with ursodeoxycholic acid (UDCA) [3,7-9,13], one with D-penicillamine [3], one with cyclosporine [3] and one underwent liver transplantation [6]. Three patients were treated with corticosteroids after diagnosis of PBC due to co-existence of Sjögren’s syndrome [7-8,10]. Eight patients received chemotherapy alone for treatment of lymphoma [3-4,9,12,15], 7 underwent a surgical procedure (2 liver segmentectomy, 1 liver transplantation, 2 thyroidectomy, 3 lymph node resection) [3,6,11], 3 radiation [2 patients with HD, 1 with Mucosa-Associated Lymphatic Tissue (MALT) of eyelid] [3,10], 1 Psoralen and Ultraviolet A light (PUVA) [7] and 2 patients combined therapy (chemotherapy plus radiation or operation) [3,10]. Nine patients died [3,9,12,15].
The potential association between lymphoma and PBC has been suggested on the basis of individual reports in the literature. Panjala et al, in a retrospective study of 2,912 patients with PBC evaluated over a 22-year-period, found that 13 patients (0.6%) had also evidence of lymphoma [3].
The risk of malignancy in patients with PBC have been reported in literature. Deutsch et al. reported that in a study of 212 patients with PBC 10.8% patients were diagnosed with malignancy. 3.8% had hepatocellular carcinoma (HCC) and 7% had several extrahepatic malignancies. In this study lymphoproliferative disease developed in 2 patients (0.9%) [16,17]. A leading article about malignancies in PBC, provided interesting information for a gross comparison between incidence ratio of malignancies in the general population in Italy and incidence ratio of malignancies in PBC in Greece [18]. Considering the total incidence of malignancies, a slightly higher incidence is apparent in the study by Deutsch et al [16]. More specifically a slightly higher incidence rate is also evaluated for lymphoproliferative diseases (157/100,000 persons/year versus 102-128/100,000 persons/year for PBC and general population respectively) [18].
In a recent report, the investigators found that an increased risk of NHL was associated with only a few autoimmune disorders and these associations were stronger for some NHL subtypes than others suggesting that different immune mechanisms were involved [1]. Secondary Sjögren’s syndrome occurs in 70% of cases of PBC and it was shown to be associated with a 1000-fold increased risk of parotid gland marginal zone lymphoma and with diffuse large B cell and follicular lymphomas [19]. However, we did not identify any case of parotid gland marginal zone type in the review of patients with PBC and lymphoma, even though many patients had extranodal disease. Some patients had diffuse large B cell lymphomas but no clear predisposition for any specific lymphoma subtype was documented.
However, we paradoxically found that primary hepatic lymphoma was reported in the literature in six PBC cases [4,6,8,9,11,14]. Primary hepatic lymphoma is a rare malignancy since it accounts for less than 1% of all extranodal lymphomas and 0.016% of all cases of NHL. The predominant histology is B-cell lymphoma, most commonly diffuse large B-cell type [20,21]. It has been reported to occur with increased frequency in patients with chronic hepatitis C infection [22]. The possible association of PBC with primary lymphoma of the liver is of interest as some other autoimmune diseases are associated with an increased incidence of lymphoma in the affected organs [2]. However, it is possible that coexistence of primary hepatic lymphoma with PBC was overestimated in the literature because only rare cases were described and the vast majority of various NHL types may have been underreported. In addition, in the series of Panjala et al [3] there was no evidence of primary hepatic lymphoma.
Examples of local antigenic immune stimulation for NHL development are the parotid gland in Sjögren’s syndrome and the small intestine in celiac disease [1,2]. Whether the higher than expected association between PBC and primary hepatic lymphomas is incidental or the result of higher risk of lymphoma originating in the affected organ i.e. the liver in patients with PBC, is not yet clear.
Biography
Hippokration General Hospital, Athens, Greece
Footnotes
Conflict of interest: None
References
- 1.Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smedby KE, Hjalgrim H, Askling J, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 3.Panjala C, Talwalkar JA, Lindor KD. Risk of lymphoma in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:761–764. doi: 10.1016/j.cgh.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama S, Yokote T, Kobayashi K, et al. Primary hepatic MALT lymphoma associated with primary biliary cirrhosis. Leuk Res. 2010;34:e17–20. doi: 10.1016/j.leukres.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Wakatsuki T, Miyata M, Shishido S, et al. Sjögren's syndrome with primary biliary cirrhosis, complicated by transverse myelitis and malignant lymphoma. Intern Med. 2000;39:260–265. doi: 10.2169/internalmedicine.39.260. [DOI] [PubMed] [Google Scholar]
- 6.Ye MQ, Suriawinata A, Black C, et al. Primary hepatic marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type in a patient with primary biliary cirrhosis. Arch Patho. Lab Med. 2000;124:604–608. doi: 10.5858/2000-124-0604-PHMZBC. [DOI] [PubMed] [Google Scholar]
- 7.Stroehmann A, Dorner T, Lukowsky A, et al. Cutaneous T cell lymphoma in a patient with primary biliary cirrhosis and secondary Sjogren's syndrome. J Rheumatol. 2002;29:1326–1329. [PubMed] [Google Scholar]
- 8.Sato S, Masuda T, Oikawa H, et al. Primary hepatic lymphoma associated with primary biliary cirrhosis. Am J Gastroenterol. 1999;94:1669–1673. doi: 10.1111/j.1572-0241.1999.01160.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldin R, Sayer J, Wilkins M, et al. Primary liver lymphoma associated with primary biliary cirrhosis. Histopathology. 1993;22:184–185. doi: 10.1111/j.1365-2559.1993.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 10.Hahn JS, Kim C, Min YH, et al. Non-Hodgkin's lymphoma & primary biliary cirrhosis with Sjögren's syndrome. Yonsei Med J. 2001;42:258–263. doi: 10.3349/ymj.2001.42.2.258. [DOI] [PubMed] [Google Scholar]
- 11.Prabhu RM, Medeiros LJ, Kumar D, et al. Primary hepatic low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) associated with primary biliary cirrhosis. Mod Pathol. 1998;11:404–410. [PubMed] [Google Scholar]
- 12.Chan CY, Lee SD, Huang YS, et al. Primary biliary cirrhosis in Taiwan. J Gastroenterol Hepatol. 1990;5:560–565. doi: 10.1111/j.1440-1746.1990.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoneda K, Sugimoto K, Shiraki K, et al. Primary biliary cirrhosis following chemotherapy for Hodgkin's lymphoma. Intern Med. 2008;47:419–420. doi: 10.2169/internalmedicine.47.0306. [DOI] [PubMed] [Google Scholar]
- 14.Lizarralde E, Martinez P, Ibanez T, et al. Primary hepatic lymphoma and primary biliary cirrhosis. Am J Gastroenterol. 2000;95:562–563. doi: 10.1111/j.1572-0241.2000.t01-1-01811.x. [DOI] [PubMed] [Google Scholar]
- 15.Jansen PL, van der Lelie H. Intrahepatic cholestasis and biliary cirrhosis associated with extrahepatic Hodgkin's disease. Neth J Med. 1994;44:99–102. [PubMed] [Google Scholar]
- 16.Deutsch M, Papatheodoridis GV, Tzakou A, et al. Risk of hepatocellular carcinoma and extrahepatic malignancies in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:5–9. doi: 10.1097/MEG.0b013e3282f163ed. [DOI] [PubMed] [Google Scholar]
- 17.Papatheodoridis GV, Hadziyannis ES, Deutsch M, et al. Ursodeoxycholic acid for primary biliary cirrhosis: final results of a 12-year, prospective, randomized, controlled trial. Am J Gastroenterol. 2002;97:2063–2070. doi: 10.1111/j.1572-0241.2002.05923.x. [DOI] [PubMed] [Google Scholar]
- 18.Pisscaglia F, Sagrini E. Malignancies in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:1–4. doi: 10.1097/MEG.0b013e3282f16436. [DOI] [PubMed] [Google Scholar]
- 19.Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjögren's syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766–775. [PubMed] [Google Scholar]
- 20.Masood A, Kairouz S, Hudhud KH, et al. Primary non-Hodgkin lymphoma of liver. Curr Oncol. 2009;16:74–77. doi: 10.3747/co.v16i4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noronha V, Shafi NQ, Obando JA, et al. Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53:199–207. doi: 10.1016/j.critrevonc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 22.de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6:451–458. doi: 10.1016/j.cgh.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


