Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder which encompasses two forms of intestinal inflammation: ulcerative colitis and Crohn’s disease. It is reported that inflammation, carotid intima media thickness, homocysteine and insulin resistance, parameters that are all associated with atherosclerosis, are increased in IBD. On the contrary, it seems that IBD patients exhibit low levels of low density lipoprotein cholesterol and total cholesterol. This review highlights the lipid profiles that occur in IBD patients and summarizes data of studies using regimens that may affect lipid metabolism in these patients.
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides
Introduction
Inflammatory bowel disease (IBD) represents a chronic inflammatory disorder of the intestine, generally classified by histopathological and clinical features into two major entities: Crohn’s disease (CD) and ulcerative colitis (UC). UC is characterized by recurring episodes of inflammation limited to the mucosal layer of the colon; it almost invariably involves the rectum and may extend in a proximal and continuous fashion to involve other portions of the colon [1]. CD is characterized by transmural rather than superficial mucosal inflammation and by skip lesions rather than continuous disease [2]. The transmural inflammation often leads to fibrosis and to obstructive clinical presentations (not typically seen in UC), and can result in sinus tracts that burrow through and penetrate the serosa, giving rise to microperforations and fistulae. CD can occur throughout the gastrointestinal tract, most commonly in the terminal ileum and colon [2]. It is believed that IBD develops when intestinal microvascular endothelial cells are damaged by an abnormal immune response resulting in chronic intestinal inflammation [3]. Genome-wide association studies have firmly established that many genomic loci contribute to IBD, especially in CD. These studies have recently established the importance of the interleukin 23 and autophagy pathways in disease pathogenesis [4,5].
Several studies have reported that inflammation, carotid intima media thickness (cIMT), homocysteine and insulin resistance, parameters that are all associated with atherosclerosis, are increased in IBD [6-10]. In addition, some studies have discussed whether IBD is a risk factor for early atherosclerosis or not [6,10,11]. Furthermore, a study in patients with enterocutaneous fistulae howed that IBD is an independent predictor of hypertriglyceridemia [12]. Moreover, another study observed the prevalence of hypocholesterolemia in a hospital population [13]. This study concluded that IBD was one of the disorders where hypocholesterolemia was more commonly seen [13]. Long et al showed that approximately one in five children with CD and one in three with UC are overweight or obese and that obese IBD patients may have a more severe disease course, as indicated by increased need for surgery [14].
Herein, we present the available data with reference to the lipid profiles of patients suffering from IBD and summarize data regarding regimens that may affect these parameters in IBD patients.
Methods
We searched PubMed for eligible articles published from January 1970 to March 2011 using combinations of the following free-text keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, dyslipidemia and atherosclerosis. We performed a stepwise approach as we broke our research into different parts and combined them at the end. Randomized controlled trials, original papers, review articles and case reports are included in the present review. References of these articles were scrutinized for relevant articles. Language restrictions were not used.
Results
Studies evaluating lipid profile in IBD patients
In a recent retrospective study of Sappati et al lipoprotein profiles in an IBD patient population were described [15]. 393 patients with IBD (241 females, 190 CD patients) were compared with those of National Health and Nutrition Examination (NHANES) Survey 2005-2006 population database. This study showed that IBD patients exhibit lower total cholesterol (TCHOL) and high density lipoprotein cholesterol (HDL-C) levels while, on the other hand, low density lipoprotein cholesterol (LDL-C) levels were greater when compared with NHANES data patients [15] (Table 1).
Table 1.
Lipid profiles of IBD patients
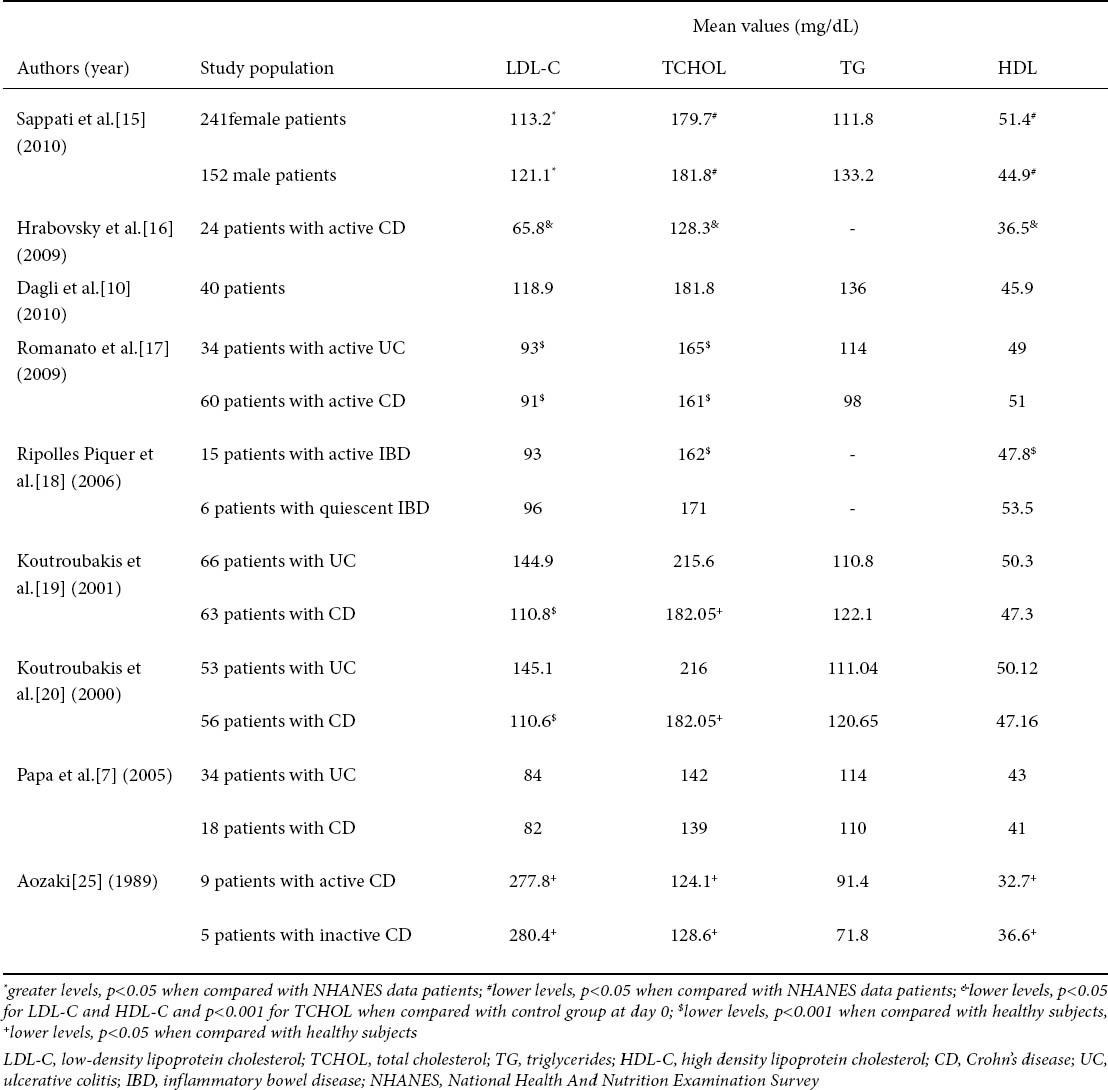
In another study, serum concetrations of TCHOL, LDL-C and HDL-C were evaluated in 24 patients with active CD at days 0, 3, 14 and 28 [16]. At day 0 patients received mesalazine per os 4 g per day and intravenous corticosteroids. In addition, vitamin E, antibiotics and treatment of symptoms were used. Hrabovsky et al showed that the levels of TCHOL, LDL-C and HDL-C at day 0 and 3 were significantly lower when compared with control group [16] (Table 1). On the contrary, there was no significant difference at days 14 and 28 in comparison with the control group. Moreover, markers of cholesterol synthesis and absorption, that can indicate the dominant process in cholesterol acquisition and its degree, were examined: plasma lathosterol and campesterol levels were significantly lower in active CD patients when compared with control group. There were no significant differences in sitosterol and squalene levels between active CD patients and controls [16].
A study in 40 IBD patients (8 patients with CD) concluded that IBD patients have an increased risk of early atherosclerosis as these patients exert greater values of cIMT (p=0.01), carotid artery stiffness (p=0.03), homocysteine (p=0.05), hsCRP (0.02) and insulin resistance (p=0.03) when compared with control patients. However, this study showed no significant difference between IBD patients and controls as far lipid profile is concerned [10] (Table 1).
Similar results and conclusions were made in another study where cIMT was significantly higher in patients with IBD in comparison with healthy subjects (p=0.008) [7] (Table 1).
Significantly lower levels of TCHOL and LDL-C were observed in 94 patients with active IBD (34 with UC and 60 with CD) when compared with healthy subjects [17](Table 1). On the other hand, there were no significant differences in HDL-C and triglycerides (TG) between IBD and control subjects. In this study, the percentage of palmitic acid (16:0) was higher in the UC patients while that of stearic acid (18:0) was lower in both UC and CD patients when compared with the healthy subjects. In addition, TCHOL values were inversely correlated to the number of bowel movements in all IBD patients but directly correlated with body mass index (BMI) only in UC patients. LDL-C and apolipoprotein (apo-B) levels were directly correlated to BMI in the UC patients, while LDL-C was inversely related to the number of bowel movements in the CD patients [17].
Interestingly, another study showed that, in subjects with active IBD, inflammation led to alterations in lipid, apolipoprotein (apo), and lipoprotein profiles and reduced cholesterol efflux [18]. In brief, TCHOL, HDL-C, (apo) A-I, apoC-III, phospholipid, and phospholipids not bound to apoB levels were significantly lower, whereas serum triglyceride, serum amyloid A and C-reactive protein levels were significantly higher in patients with active IBD in comparison to controls (Table 1). Ripolles Piquer et al suggested that their results are similar to those proposed to promote atherogenesis and may contribute to the development of cardiovascular events [18].
A Greek study population showed alterations in lipid profile in 129 patients with IBD (63 CD patients) in comparison with 66 healthy controls [19]. More specifically, patients with CD had significantly lower TCHOL and LDL-C levels than both controls and UC patients (p<0.05 and p<0.001, respectively) (Table 1). Mean levels of HDL-C and TG were not significantly different between the three groups. Moreover, the concentrations of apoA-1 and apoB-100 in CD patients were significantly lower compared to controls (p=0.005 and p=0.001, respectively), while only apoB-100 levels in UC patients were significantly lower compared to controls (p=0.005). In addition, the ratio of apoB-100/apoA-1 was not significantly different between the three groups [19]. Finally, in CD patients, mean serum lipoprotein (a) levels were significantly higher compared to healthy controls (p=0.005). The differences in lipoprotein (a) levels between UC and control subjects did not reach significance. Elevated levels of lipoprotein (a) of more than 30 mg/dL were found in 29 CD patients (46%), 15 UC patients (23%) and 11 controls (17%) [19]. Koutroubakis et al suggested that these elevated levels of lipoprotein (a) in CD patients may expose them to a higher risk of thrombosis [19].
In another study of the same group, serum homocysteine levels were higher in patients with UC and CD in comparison with healthy controls (Table 1) [20].
In a case report, it was suggested that the presence of hyper-lipoprotein (a) [Lp(a)]-emia and small vessel thrombus formation may be associated with the development of UC [21].
In 22 pediatric CD patients, Levy et al examined the lipid profile, the lipoprotein composition and the oxidant-antioxidant status [22]. TCHOL and LDL-C were significantly lower in CD patients compared with controls (p<0.02), whereas TG levels were significantly higher in CD patients in comparison with control subjects (p<0.005). Plasma apoB (p< 0.02) and A-I (p<0.02) levels were also lower in CD patients. In addition, lipoprotein composition was altered in CD patients, with relative TG depletion and protein enrichment in very low density lipoprotein (VLDL). In contrast, intermediate-density lipoprotein of CD patients was characterized by an increased percentage of TG and protein (P < 0.005) and a reduced proportion of phospholipids (p<0.01). Furthermore, abnormalities were observed in the chemical distribution of HDL2 and HDL3 moieties. Lipid peroxidation was documented by higher plasma malondialdehyde concentrations in CD patients (p<0.05), accompanied by lower retinol concentrations (p<0.02) [22].
Capristo et al examined the metabolic features of 34 IBD patients in remission (18 patients with CD) [23]. They concluded that CD patients showed a decreased fat mass and enhanced utilization of lipids when compared with UC patients and controls. These results are probably due to the larger intestinal involvement in CD [23].
Stadnicki et al suggested that plasma lipid changes are not an independent risk factor for vascular complication in UC [24].
Finally, Aozaki suggested that abnormal lipid metabolism in patients with CD instead of Crohn’s disease led to a decrease in membrane fluidity and hence an alteration in membrane functions, not only in erythrocytes but also in other cells, and thus is related to the pathological status of the disease [25] (Table 1).
Lipid profiles after bowel resection in IBD patients
In CD patients, whose medical therapies have failed to produce a satisfactory response to treat complications, the predominant role for surgery is clear [26]. In addition, restorative proctocolectomy with ileal pouch-anal anastomosis is the first choice for the elective surgical treatment of patients affected by UC [27].
A prospective study in 24 patients who had intestinal surgery for CD showed that inflammatory status, impaired intestinal adsorption and bowel resection may have an impact on lipid metabolism [28]. More specifically, during the follow-up, only an increase in HDL-C (p=0.02) without any other modifications in the plasma phospholipid fatty acids (FA) composition were in evidence after surgery. On the other hand, significantly higher levels of TCHOL (p<0.01), HDL-C (p=0.01), and LDL-C (p=0.01) were observed in patients in remission compared with those with recurrent active CD [28].
In another retrospective study, in 15 patients with UC after restorative proctocolectomy TCHOL and LDL-C levels were significantly lower than in healthy subjects (p<0.01 and p=0.05, respectively) [29]. Significantly lower levels in those parameters were observed in patients with UC at ileostomy closure when compared with healthy subjects. Interestingly, there was a significant difference between LDL-C and HDL-C levels between patients with UC at proctocolectomy and at ileostomy closure (HDL-C: from 43 to 56 mg/dL, p<0.01 and LDL-C: from 101 to 52 mg/dL, p=0.01) [29]. The authors suggest that the reduction in instead of LDL-C is an index of malabsorption probably due to the accelerated transit and to the exclusion of the terminal ileum caused by the covering ileostomy [29].
High and low-fiber diets were tested in 10 UC patients after proctocolectomy [30]. The fasting plasma free-cholesterol, TCHOL, TG and phospholipids were significantly higher when the subjects were on the low-fiber diet than on the high-fiber diet with the nibbling regimen (meal frequency: 7 times/day). On the contrary, high-fiber diet decreased insulin secretion [30].
Kuisma et al concluded that long term metabolic consequences after ileal pouch-anal anastomosis in UC patients are influenced by severity of inflammation, grade of villous atrophy, and extent of the disease in the remaining ileum [31]. In addition, these patients require long-term follow-up after ileal pouch-anal anastomosis for UC [31].
In 24 UC patients with ileal anal anastomosis serum TCHOL and LDL-C levels were significantly lower than controls (p<0.001), whereas those of VLDL and HDL cholesterol were similar to those of controls. Serum levels of TG were also noticeably reduced (p<0.05) [32]. This study concluded that cholesterol absorption is significantly impaired in patients with an ileal anal anastomosis, and is closely related to changes in serum and biliary lipids observed in these patients [32].
Plasma concentrations of TCHOL, LDL-C, and apoB decreased significantly, whereas plasma concentrations of total TG and VLDL triglycerides increased significantly in 12 patients with UC after ileal anal anastomosis [33]. The authors suggested that exclusion of about 95 cm of the ileum leads to an apparent selective malabsorption of bile acids [33]. It has been suggested that in patients with CD after ileum resection, malabsorption of bile acids leads to parallel stimulation of cholesterol synthesis, cholesterol degradation, and LDL-receptor expression in human liver [34]. The resulting effect in these patients was a significant reduction in LDL-C [34].
Treatment affecting lipid metabolism in IBD patients
Dietary intervention
Several studies have evaluated the effect of dietary intervention on IBD patients. Kawakami et al suggested that a diet restricting the intake of linoleic acid and supplemented with eicosapentanoic acid and antioxidative vitamins may be recommendable for the management of UC patients [35].
Tanaka et al showed that a decreased ratio of n-6 to n-3 polyunsaturated fatty acid was associated with a poor prognosis in CD patients [36]. This group suggested that effective prevention of relapse for CD patients might be achieved through moderate dietary temperance, particularly when the disease condition is unstable [36].
Another study suggested that hydrothermally processed cereals can induce antisecretory factor production in human IBD [37]. Although this diet significantly improved subjective ratings of clinical symptoms and increased plasma antisecretory factor levels compared with placebo, it did not affect plasma lipid levels [37].
A study showed that patients with inactive ileal CD had significantly higher diet-induced thermogenesis and lipid oxidation rate than do healthy volunteers [38]. Mingrone et al results may explain why CD patients have difficulty maintaining adequate nutritional status, and the findings also suggest that a diet relatively rich in fat may attain better energy balance [38].
Omega 3 Fatty acids
Omega-3 fatty acids mainly reduce TG concentration, but they exert many other anti-atherosclerotic properties [39]. Numerous prospective and retrospective trials from many countries have shown that moderate fish consumption decreases the risk of major cardiovascular events, such as myocardial infarction, sudden cardiac death, coronary heart disease, atrial fibrillation, and most recently, death in patients with heart failure [39,40].
It has been observed that, in patients with CD, there was a significant depletion of omega 3 fatty acids and that this decrease was greater in patients with more active disease [41]. In this context and considering the fact that omega 3 fatty acids have an anti-inflammatory effect, a lot of trials have examined the effect of omega 3 fatty acids supplementation on IBD. These studies showed controversial results since some of them found that omega 3 fatty acids delayed the first episode of relapse in UC and CD, while on the other hand, other studies found no benefit for the maintenance of remission [42-45].
Statins
Statins are hypolipidaemic drugs exerting beneficial effects for primary as well as for secondary prevention for the development of cardiovascular disease [46, 47]. These drugs may even protect the vasculature in the early stages of atherosclerotic disease [48]. Furthermore, statins improve survival and reduce the risk of major cardiovascular and cerebrovascular events in people with or without established cardiovascular disease [46-52]. Interestingly, it has been observed that long-term statin use was associated with reduced risk of colorectal cancer in patients with IBD [53].
Grip et al showed that 80 mg of atorvastatin reduced plasma chemokine (CXCL10) levels in 10 patients with CD after 13 months of treatment [54]. The authors suggested that this reduction by atorvastatin may represent a candidate for an approach to the treatment of CD in the future. In addition, The same team showed that atorvastatin therapy reduces inflammation in patients with CD and, therefore, suggested further investigations of statin-mediated protective effects in IBD [55]. Moreover, in an older study, Grip et al found that CD patients have increased plasma levels of certain proinflammatory biomarkers, such as tumor necrosis factor (TNF)-a, monocyte chemoattractant protein 1 and oxidized LDL [56]. Furthermore they suggested that treatment with atorvastatin may be a potential strategy to reduce oxLDL and inhibit monocyte migration to inflamed tissue, in order to attenuate the inflammatory response [56].
Infliximab
Infliximab is an anti-TNF-a agent and is the most widely used biological therapy for IBD. Several studies have evaluated the effects of infliximab in lipid profile and insulin sensitivity in different study populations such as rheumatoid arthritis and ankylosing spondylitis [57-62]. However, the effect of infliximab therapy on lipid profiles in patients with IBD has not been adequately examined.
In a prospective study, lipid profile was assessed in 111 CD patients receiving infliximab infusions every 8 weeks, with a mean follow-up of 41 weeks [63]. There was a significant increase in TCHOL and HDL-C levels (p=0.02 and p=0.008, respectively) over time during infliximab maintenance therapy, while, on the other hand, no significant changes in LDL-C and TG levels were observed throughout the study [63]. The authors concluded that infliximab induction therapy is associated with a significant increase in abdominal fat tissue in CD patients and that infliximab maintenance therapy has no deleterious effects on lipid profile [63]. In addition, inf liximab abnormalities in IBD liximab therapy was accompanied by a decrease in glycemia and HbA1c concentrations, probably by reversing the impairment of TNF-induced insulin-mediated glucose uptake [63].
Similar results were shown in another study, where infliximab therapy in 22 IBD patients led to a significant increase in TCHOL, HDL-C and apoA1 levels after 14 weeks, while no difference was observed in TG, LDL-C, apoB100 and lipoprotein (a) levels [64].
Metronidazole
Some studies have shown a lipid lowering effect of metronidazole [65,66]. Indeed, administration of 400 mg of metronidazole in 5 CD patients led to a 20% reduction in TCHOL levels after one year of treatment [66].
Parenteral nutrition
In 12 patients with IBD, the effect of parenteral nutrition with 20% glucose, 4.25% amino-acids and 10% intralipid for 3 weeks on plasma lipids was investigated [67]. At the end of the 3rd week TCHOL and phospholipids were significantly increased (p<0.03 and p<0.01, respectively) while LDL-C and HDL-C were significantly lowered (p<0.01 and p<0.005, respectively) [67]. The authors suggest that patients undergoing long-term treatment with glucose and intralipid should be closely monitored for the occurrence of dyslipoproteinemia [67].
After 3 months of home parenteral nutrition with two lipid emulsions, no change has been observed in plasma lipid concentration. However, some modifications were observed in the composition of lipoprotein fractions demonstrating a redistribution of lipid components [68].
Conclusion
IBD patients exhibit lower levels of LDL-C and TCHOL compared to healthy subjects with that finding being more profound in CD than UC patients. In addition, no significant alterations have been reported in these patients as far as HDL-C and TG levels are concerned. These findings remain stable independently of disease activity and are also observed post-operatively. Moreover, IBD patients exhibit lower levels of LDL-C even after intestinal resection. Hypocholesterolemia is a common feature in patients with various types of acute disease, including surgery, trauma, burn injury and sepsis [69]. It has been related to severity of illness [70]. Chemokine production could affect lipoprotein metabolism and might in part be held responsible for lipid derangements in patients with IBD. However, more large-scale and epidemiological studies should be carried out in order to reach more robust conclusions about the lipid alterations reported in IBD patients.
Biography
University of Ioannina, Greece
Footnotes
Conflict of Interest: None
References
- 1.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Hatoum OA, Binion DG. The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflamm Bowel Dis. 2005;11:304–313. doi: 10.1097/01.mib.0000160772.78951.61. [DOI] [PubMed] [Google Scholar]
- 4.Cho JH. Inflammatory bowel disease: genetic and epidemiologic considerations. World J Gastroenterol. 2008;14:338–347. doi: 10.3748/wjg.14.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara S, Aziz MM, Yuki T, Kazumori H, Kinoshita Y. Inflammatory bowel disease: review from the aspect of genetics. J Gastroenterol. 2009;44:1097–1108. doi: 10.1007/s00535-009-0141-8. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 7.Papa A, Santoliquido A, Danese S, et al. Increased carotid intima-media thickness in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:839–846. doi: 10.1111/j.1365-2036.2005.02657.x. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Sgambato A, Papa A, et al. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol. 2005;100:886–895. doi: 10.1111/j.1572-0241.2005.41469.x. [DOI] [PubMed] [Google Scholar]
- 9.Bregenzer N, Hartmann A, Strauch U, Scholmerich J, Andus T, Bollheimer LC. Increased insulin resistance and beta cell activity in patients with Crohn's disease. Inflamm Bowel Dis. 2006;12:53–56. doi: 10.1097/01.mib.0000195975.97673.f5. [DOI] [PubMed] [Google Scholar]
- 10.Dagli N, Poyrazoglu OK, Dagli AF, et al. Is inflammatory bowel disease a risk factor for early atherosclerosis? Angiology. 2010;61:198–204. doi: 10.1177/0003319709333869. [DOI] [PubMed] [Google Scholar]
- 11.Maharshak N, Arbel Y, Bornstein NM, et al. Inflammatory bowel disease is not associated with increased intimal media thickening. Am J Gastroenterol. 2007;102:1050–1055. doi: 10.1111/j.1572-0241.2007.01086.x. [DOI] [PubMed] [Google Scholar]
- 12.Visschers RG, Olde Damink SW, Schreurs M, Winkens B, Soeters PB, van Gemert WG. Development of hypertriglyceridemia in patients with enterocutaneous fistulas. Clin Nutr. 2009;28:313–317. doi: 10.1016/j.clnu.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Crook MA, Velauthar U, Moran L, Griffiths W. Hypocholesterolaemia in a hospital population. Ann Clin Biochem. 1999;36(Pt 5):613–616. doi: 10.1177/000456329903600508. [DOI] [PubMed] [Google Scholar]
- 14.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis 2010. 2010 Dec 17; doi: 10.1002/ibd.21585. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sappati Biyyani RS, Putka BS, Mullen KD. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J Clin Lipidol. 2010;4:478–482. doi: 10.1016/j.jacl.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Hrabovsky V, Zadak Z, Blaha V, et al. Cholesterol metabolism in active Crohn's disease. Wien Klin Wochenschr. 2009;121:270–275. doi: 10.1007/s00508-009-1150-6. [DOI] [PubMed] [Google Scholar]
- 17.Romanato G, Scarpa M, Angriman I, et al. Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment Pharmacol Ther. 2009;29:298–307. doi: 10.1111/j.1365-2036.2008.03886.x. [DOI] [PubMed] [Google Scholar]
- 18.Ripolles Piquer B, Nazih H, Bourreille A, et al. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism. 2006;55:980–988. doi: 10.1016/j.metabol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Koutroubakis IE, Malliaraki N, Vardas E, et al. Increased levels of lipoprotein (a) in Crohn's disease: a relation to thrombosis? Eur J Gastroenterol Hepatol. 2001;13:1415–1419. doi: 10.1097/00042737-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Koutroubakis IE, Dilaveraki E, Vlachonikolis IG, et al. Hyperhomocysteinemia in Greek patients with inflammatory bowel disease. Dig Dis Sci. 2000;45:2347–2351. doi: 10.1023/a:1005583606647. [DOI] [PubMed] [Google Scholar]
- 21.Kawabata S, Katagiri S, Negoro H, et al. Elevated serum lipoprotein levels associated with ulcerative colitis in a young Japanese patient. Intern Med. 1997;36:389–391. doi: 10.2169/internalmedicine.36.389. [DOI] [PubMed] [Google Scholar]
- 22.Levy E, Rizwan Y, Thibault L, et al. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr. 2000;71:807–815. doi: 10.1093/ajcn/71.3.807. [DOI] [PubMed] [Google Scholar]
- 23.Capristo E, Mingrone G, Addolorato G, Greco AV, Gasbarrini G. Metabolic features of inflammatory bowel disease in a remission phase of the disease activity. J Intern Med. 1998;243:339–347. doi: 10.1046/j.1365-2796.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 24.Stadnicki A, Bojko B, Myczkowska K, Witalinska-Labuzek J. [Selected risk factors of thrombotic complications in patients with ulcerative colitis] Wiad Lek. 2003;56:341–347. [PubMed] [Google Scholar]
- 25.Aozaki S. Decreased membrane fluidity in erythrocytes from patients with Crohn's disease. Gastroenterol Jpn. 1989;24:246–254. doi: 10.1007/BF02774321. [DOI] [PubMed] [Google Scholar]
- 26.Parkes M, Jewell DP. Review article: the management of severe Crohn's disease. Aliment Pharmacol Ther. 2001;15:563–573. doi: 10.1046/j.1365-2036.2001.00963.x. [DOI] [PubMed] [Google Scholar]
- 27.Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120–127. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanato G, Scarpa M, Ruffolo C, et al. Lipid and phospholipid profile after bowel resection for Crohn's disease. Int J Colorectal Dis. 2008;23:931–938. doi: 10.1007/s00384-008-0503-3. [DOI] [PubMed] [Google Scholar]
- 29.Scarpa M, Romanato G, Manzato E, et al. Restorative proctocolectomy for ulcerative colitis: impact on lipid metabolism and adipose tissue and serum fatty acids. J Gastrointest Surg. 2008;12:279–287. doi: 10.1007/s11605-007-0380-z. [DOI] [PubMed] [Google Scholar]
- 30.Lundin EA, Zhang JX, Lairon D, et al. Effects of meal frequency and high-fibre rye-bread diet on glucose and lipid metabolism and ileal excretion of energy and sterols in ileostomy subjects. Eur J Clin Nutr. 2004;58:1410–1419. doi: 10.1038/sj.ejcn.1601985. [DOI] [PubMed] [Google Scholar]
- 31.Kuisma J, Nuutinen H, Luukkonen P, Jarvinen H, Kahri A, Farkkila M. Long term metabolic consequences of ileal pouch-anal anastomosis for ulcerative colitis. Am J Gastroenterol. 2001;96:3110–3116. doi: 10.1111/j.1572-0241.2001.05256.x. [DOI] [PubMed] [Google Scholar]
- 32.Hakala K, Vuoristo M, Luukkonen P, Jarvinen HJ, Miettinen TA. Impaired absorption of cholesterol and bile acids in patients with an ileoanal anastomosis. Gut. 1997;41:771–777. doi: 10.1136/gut.41.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akerlund JE, Bjorkhem I, Angelin B, Liljeqvist L, Einarsson K. Apparent selective bile acid malabsorption as a consequence of ileal exclusion: effects on bile acid, cholesterol, and lipoprotein metabolism. Gut. 1994;35:1116–1120. doi: 10.1136/gut.35.8.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akerlund JE, Reihner E, Angelin B, et al. Hepatic metabolism of cholesterol in Crohn's disease. Effect of partial resection of ileum. Gastroenterology. 1991;100:1046–1053. doi: 10.1016/0016-5085(91)90281-o. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami Y, Okada H, Murakami Y, et al. Dietary intake, neutrophil fatty acid profile, serum antioxidant vitamins and oxygen radical absorbance capacity in patients with ulcerative colitis. J Nutr Sci Vitaminol (Tokyo) 2007;53:153–159. doi: 10.3177/jnsv.53.153. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka M, Iwao Y, Sasaki S, et al. Moderate dietary temperance effectively prevents relapse of Crohn disease: a prospective study of patients in remission. Gastroenterol Nurs. 2007;30:202–210. doi: 10.1097/01.SGA.0000278169.35930.f8. [DOI] [PubMed] [Google Scholar]
- 37.Bjorck S, Bosaeus I, Ek E, et al. Food induced stimulation of the antisecretory factor can improve symptoms in human inflammatory bowel disease: a study of a concept. Gut. 2000;46:824–829. doi: 10.1136/gut.46.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mingrone G, Capristo E, Greco AV, et al. Elevated diet-induced thermogenesis and lipid oxidation rate in Crohn disease. Am J Clin Nutr. 1999;69:325–330. doi: 10.1093/ajcn/69.2.325. [DOI] [PubMed] [Google Scholar]
- 39.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 40.Gazi I, Liberopoulos EN, Saougos VG, Elisaf M. Beneficial effects of omega-3 fatty acids: the current evidence. Hellenic J Cardiol. 2006;47:223–231. [PubMed] [Google Scholar]
- 41.Kuroki F, Iida M, Matsumoto T, Aoyagi K, Kanamoto K, Fujishima M. Serum n3 polyunsaturated fatty acids are depleted in Crohn's disease. Dig Dis Sci. 1997;42:1137–1141. doi: 10.1023/a:1018873217192. [DOI] [PubMed] [Google Scholar]
- 42.Endres S, Lorenz R, Loeschke K. Lipid treatment of inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 1999;2:117–120. doi: 10.1097/00075197-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Turner D, Zlotkin SH, Shah PS, Griffiths AM. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009:CD006320. doi: 10.1002/14651858.CD006320.pub2. [DOI] [PubMed] [Google Scholar]
- 44.John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:602–606. doi: 10.1097/MEG.0b013e3283352d05. [DOI] [PubMed] [Google Scholar]
- 45.Feagan BG, Sandborn WJ, Mittmann U, et al. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA. 2008;299:1690–1697. doi: 10.1001/jama.299.14.1690. [DOI] [PubMed] [Google Scholar]
- 46.Prinz V, Endres M. The acute (cerebro) vascular effects of statins. Anesth Analg. 2009;109:572–584. doi: 10.1213/ane.0b013e3181a85d0e. [DOI] [PubMed] [Google Scholar]
- 47.Watson KE. The JUPITER trial: How will it change clinical practice? Rev Cardiovasc Med. 2009;10:91–96. [PubMed] [Google Scholar]
- 48.Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 50.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 51.Wei L, Ebrahim S, Bartlett C, Davey PD, Sullivan FM, MacDonald TM. Statin use in the secondary prevention of coronary heart disease in primary care: cohort study and comparison of inclusion and outcome with patients in randomised trials. BMJ. 2005;330:821. doi: 10.1136/bmj.38398.408032.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strippoli GF, Navaneethan SD, Johnson DW, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and metaregression of randomised controlled trials. BMJ. 2008;336:645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samadder NJ, Mukherjee B, Huang SC, et al. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer 2010. 2011;117:1640–1648. doi: 10.1002/cncr.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn's disease. PLoS One. 2009;4:e5263. doi: 10.1371/journal.pone.0005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grip O, Janciauskiene S, Bredberg A. Use of atorvastatin as an anti-inflammatory treatment in Crohn's disease. Br J Pharmacol. 2008;155:1085–1092. doi: 10.1038/bjp.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grip O, Janciauskiene S, Lindgren S. Circulating monocytes and plasma inflammatory biomarkers in active Crohn's disease: elevated oxidized low-density lipoprotein and the anti-inflammatory effect of atorvastatin. Inflamm Bowel Dis. 2004;10:193–200. doi: 10.1097/00054725-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Peters MJ, Vis M, van Halm VP, et al. Changes in lipid profile during infliximab and corticosteroid treatment in rheumatoid arthritis. Ann Rheum Dis. 2007;66:958–961. doi: 10.1136/ard.2006.059691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spanakis E, Sidiropoulos P, Papadakis J, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol. 2006;33:2440–2446. [PubMed] [Google Scholar]
- 59.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1495–1498. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 60.Kiortsis DN, Mavridis AK, Filippatos TD, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33:921–923. [PubMed] [Google Scholar]
- 61.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–766. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Derdemezis CS, Filippatos TD, Voulgari PV, Tselepis AD, Drosos AA, Kiortsis DN. Leptin and adiponectin levels in patients with ankylosing spondylitis. The effect of infliximab treatment. Clin Exp Rheumatol. 2010;28:880–883. [PubMed] [Google Scholar]
- 63.Parmentier-Decrucq E, Duhamel A, Ernst O, et al. Effects of infliximab therapy on abdominal fat and metabolic profile in patients with Crohn's disease. Inflamm Bowel Dis. 2009;15:1476–1484. doi: 10.1002/ibd.20931. [DOI] [PubMed] [Google Scholar]
- 64.Koutroubakis IE, Oustamanolakis P, Malliaraki N, et al. Effects of tumor necrosis factor alpha inhibition with infliximab on lipid levels and insulin resistance in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21:283–288. doi: 10.1097/MEG.0b013e328325d42b. [DOI] [PubMed] [Google Scholar]
- 65.Davis JL, Schultz TA, Mosley CA. Metronidazole lowers serum lipids. Ann Intern Med. 1983;99:43–44. doi: 10.7326/0003-4819-99-1-43. [DOI] [PubMed] [Google Scholar]
- 66.von Bergmann K, Streicher U, Leiss O, Jensen C, Gugler R. Serum-cholesterol-lowering effect of metronidazole and possible mechanisms of action. Klin Wochenschr. 1985;63:279–281. doi: 10.1007/BF01731475. [DOI] [PubMed] [Google Scholar]
- 67.Weinberg RB, Singh KK. Short-term parenteral nutrition with glucose and Intralipid: effects on serum lipids and lipoproteins. Am J Clin Nutr. 1989;49:794–798. doi: 10.1093/ajcn/49.5.794. [DOI] [PubMed] [Google Scholar]
- 68.Richelle M, Rubin M, Kulapongse S, Deckelbaum RJ, Elwyn DH, Carpentier YA. Plasma lipoprotein pattern during long-term home parenteral nutrition with two lipid emulsions. JPEN J Parenter Enteral Nutr. 1993;17:432–437. doi: 10.1177/0148607193017005432. [DOI] [PubMed] [Google Scholar]
- 69.Giovannini I, Boldrini G, Chiarla C, Giuliante F, Vellone M, Nuzzo G. Pathophysiologic correlates of hypocholesterolemia in critically ill surgical patients. Intensive Care Med. 1999;25:748–751. doi: 10.1007/s001340050940. [DOI] [PubMed] [Google Scholar]
- 70.Fraunberger P, Nagel D, Walli AK, Seidel D. Serum cholesterol and mortality in patients with multiple organ failure. Crit Care Med. 2000;28:3574–3575. doi: 10.1097/00003246-200010000-00047. [DOI] [PubMed] [Google Scholar]


