Abstract
Background
EUS, as it images the full thickness of the gastrointestinal tract wall, could provide more detailed information on benign esophageal strictures. Aim of this study was to evaluate the role of EUS in predicting the response to endoscopic dilatation in benign esophageal strictures.
Methods
27 patients with benign strictures (corrosive 14, peptic 10 & post-radiation 3) were prospectively studied with radial EUS.
Results
The maximum esophageal wall thickness was significantly greater in patients with corrosive and post-radiation strictures in comparison to patients with peptic strictures. In patients with peptic stricture, the mucosal thickness involved either the mucosa (n=2) or submucosa (n=8) and in none of the patients was the muscularis propria involved. However, muscularis propria was involved in all 3 patients with post-radiation strictures and in 11/14 (78.5%) patients with corrosive strictures. Two peptic stricture patients with only mucosal thickening required a single session of dilatation whereas patients with involvement of submucosa required 2-4 sessions of dilatation. Patients with corrosive stricture having only involvement of submucosa required significantly fewer sessions in comparison to patients having muscularis propria involvement (2.67±0.58 vs. 6.30±1.16 sessions, respectively; p=0.0003).
Conclusion
EUS by delineating the extent of wall involvement in benign esophageal strictures predicts the response to endoscopic dilatation.
Keywords: Corrosive, endoscopic ultrasound, endoscope, computed tomography
Introduction
Benign esophageal strictures can be acid reflux-induced (peptic), corrosive ingestion-induced, post-surgical (anastomotic), post-radiotherapy-induced, and drug-induced [1]. Peptic strictures are the most common cause of benign esophageal strictures in the West whereas corrosive strictures are the most common form of benign strictures in developing countries like India [2-4]. Because of its low morbidity, minimal invasiveness and good short- as well as long-term results, endoscopic dilatation with either a bougie or a balloon has been the standard of care for most patients with benign esophageal strictures [1-6]. Patients with peptic strictures usually require a few sessions of dilatation with the majority of patients responding to a single session of dilatation along with acid suppressive medicines, whereas patients with other benign causes of esophageal strictures, especially corrosive strictures, require repeated sessions of endoscopic dilation and may also recur [1-8]. It has been suggested that the extent of fibrosis in the esophageal wall is an important determinant of stricture severity and may influence the response to endoscopic dilation [9-10]. Lahoti et al have shown that the maximal esophageal thickness as measured on computed tomography (CT) can predict the response to endoscopic dilatation in patients with corrosive strictures [11]. Endoscopic ultrasound (EUS), as it images the full thickness of the gastrointestinal tract (GIT) wall, could provide more detailed information about the extent of the esophageal injury as compared with conventional endoscopy. In a case report, we have shown that EUS provides detailed information about the esophageal wall in a patient with corrosive stricture [12]. Therefore, we conducted this prospective study to evaluate the role of EUS in predicting the response to endoscopic dilatation in benign causes of esophageal strictures.
Patients and Methods
A total of 27 patients with benign esophageal strictures of various etiologies seen consecutively over the last 18 months were prospectively enrolled in the study. All patients had significant dysphagia requiring esophageal dilation. The esophageal strictures were characterized by barium contrast radiography and upper GI endoscopy. The etiology of the esophageal stricture was determined by the combination of clinical, endoscopic, radiological and histopathological evidence. A detailed history was taken from the patients as well as their attendants to exclude ingestion of non-steroidal anti-inflammatory drugs (NSAID’s) as well as corrosives. In patients with radiation-induced strictures multiple biopsy specimens were taken from the stricture site to exclude recurrent malignancy. In patients with corrosive stricture, endoscopic dilatation was done at least 4 weeks after the acute injury. Patients under 18 years of age, pharyngeal stenosis precluding endoscopic examination and dilation, multiple strictures precluding examination of all the strictures by EUS, tracheoesophageal fistula, malignancy, refusing consent and stomach cicatrization that precluded safe placement of a guidewire in the stomach were excluded from the study. Informed consent was obtained from each patient. The dysphagia was graded on a scale of 0 to 4: 0, normal diet possible; 1, unable to swallow certain solids; 2, able to swallow only semisolid soft diet; 3, able to swallow liquids only; 4, unable to swallow even liquids in adequate amounts [13].
After characterization of the esophageal stricture, EUS examination was performed with a radial echoendosocope (EG-3670 URK, Pentax Inc, Tokyo). The endosonologist was blinded to the etiology of the esophageal stricture. In patients with nonnegotiable stricture, EUS examination was performed from the mouth of the stricture. The echoendoscope was kept at the mouth of the stricture without exerting pressure so as to avoid stretching the esophagus. Thereafter, the maximum thickness of the esophageal wall was measured. After measuring the maximum thickness, minimal amount of water was insufflated into the balloon for better acoustic coupling so as to clearly characterize the various layers of the esophageal wall. On EUS, the extent of involvement of the esophageal wall was characterized in three categories: Category 1: Thickening of mucosa only; Category 2: Thickening of mucosa & submucosa, and Category 3: Involvement of muscularis propria.
After performing EUS examination, the patients underwent endoscopic bougie dilatation using Savary-Gilliard polyvinyl dilators (7, 9, 11, 12.8, 14 and 15 mm, Wilson-Cook Medical Inc., Winston-Salem, N.C.). The dilatation was performed under conscious sedation using intravenous midazolam and without the use of fluoroscopy. The dilators were passed over an endoscopically placed guidewire and 1-4 dilators were passed across the stricture during each endoscopic session depending upon the tightness of the stricture. The endoscopic dilation was done at three weekly intervals until dilatation was achieved with a 15 mm dilator and there was complete relief of dysphagia. The number of sessions required to achieve adequate dilation was also considered in assessing the response. Thereafter, dilation was repeated whenever the patient had recurrence of dysphagia. The dilatation was performed on an outpatient basis with each EUS in benign esophageal strictures patient after each session of endoscopic dilation being observed for 2 hours and being discharged the same day with instructions to immediately report to emergency in case of development of fever, chest or abdominal pain, subcutaneous emphysema or breathlessness. In addition, if patients remained asymptomatic for 4 hours, clear liquids were allowed, followed by oral diet 24 hours after dilatation. A liquid or mashed semi-solid diet was allowed until dilation of 12 mm was achieved and thereafter, normal diet was allowed. In patients with peptic stricture, proton pump inhibitors were given along with the endoscopic dilatation. Helicobacter pylori, whenever present, was eradicated.
Results
Twenty seven (20 males) patients with esophageal stricture of various benign etiologies were prospectively studied (Table 1). Fourteen patients had corrosive stricture, 10 had peptic stricture and 3 had post-radiation esophageal stricture. The locations of corrosive stricture was: upper esophagus (n=2), mid esophagus (n=7) and lower esophagus (n=5) whereas all the patients with peptic stricture had lower esophageal stricture. All the post-radiation strictures were located in the mid esophagus. The corrosive strictures were due to acid ingestion in 9 patients and alkali ingestion in 5 patients.
Stricture characteristics
All patients had dysphagia on presentation. Of the 14 patients with corrosive strictures, 9 had grade 3 dysphagia, 4 had grade 2 dysphagia and 1 had grade 4 dysphagia. Of the 10 patients with peptic stricture, 5 had grade 2 dysphagia, 3 had grade 3 dysphagia and 2 had grade 1 dysphagia. All 3 patients with post-radiation stricture had grade 2 dysphagia. The length of peptic and radiation strictures was shorter than 3 cm in length (mean length of peptic and post-radiation strictures was 1.98±0.52 cm and 2.6±0.20 cm, respectively) whereas the corrosive strictures were significantly longer with the mean length being 4.38±0.87 cm.
EUS characteristics (Fig. 1-5)
Figure 1.

EUS in a patient with peptic stricture showing thickened mucosa (between marks and arrow)
Figure 5.
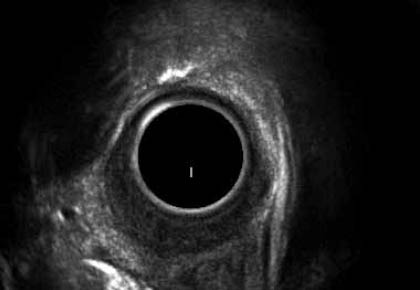
EUS in a patient with post-radiation stricture showing involvement of all the layers of esophagus with thickened esophageal wall
Figure 2.
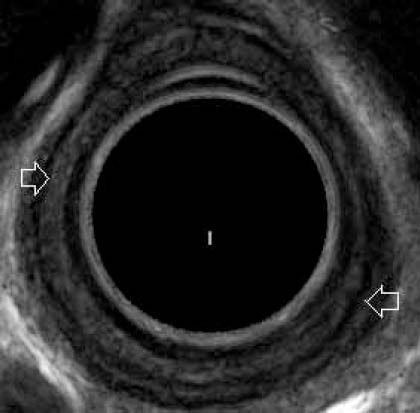
EUS in a patient with peptic stricture showing involvement of the mucosa and submucosa. Muscularis propria is seen as hypoechoic layer (arrows)
Figure 3.
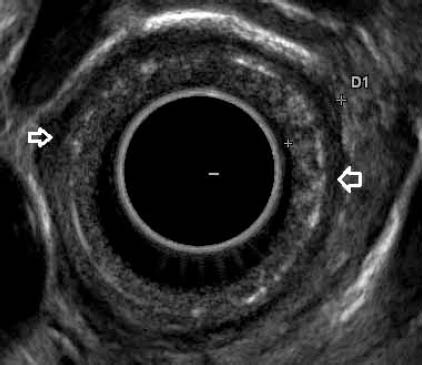
EUS in a patient with corrosive stricture showing involvement of the mucosa and submucosa. Muscularis propria is seen as hypoechoic layer (arrows)
Figure 4.
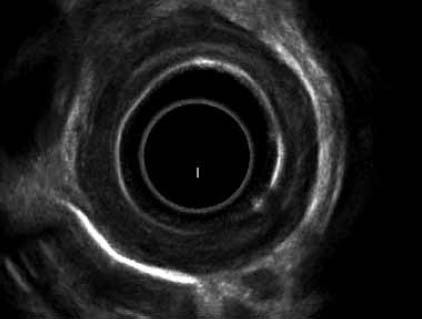
EUS in a patient with corrosive stricture showing involvement of the muscularis propria of esophagus with thickened esophageal wall
In none of the patients was the radial echoendosocope negotiable across the stricture and therefore the EUS examination was performed from the mouth of the stricture at a frequency of 10 MHz. The maximum esophageal wall thickness was significantly greater in patients with corrosive and post-radiation strictures in comparison to patients with peptic strictures (6.55±1.60 mm, 7.23±1.30 mm and 3.88±0.75 mm, respectively; p=0.0001). In patients with peptic stricture, the mucosal thickness involved either the mucosa (n=2) or submucosa (n=8) and in none of the patients was the muscularis propria involved. However, muscularis propria was involved in all the 3 patients with post-radiation strictures and in 11/14 (78.5%) patients with corrosive strictures. In the remaining 3 patients with corrosive stricture, the thickness involved the submucosa and muscularis propria was not involved.
Response to dilatation
Adequate dilatation could be achieved in all the patients except one patient with corrosive stricture in whom perforation occurred while dilating from 12 to 15 mm and the patient required emergency surgery. Patients with corrosive and post-radiation strictures required more dilating sessions in comparison to patients with peptic stricture (5.46±1.90, 5.67±1.53 and 2.10±0.88 sessions, respectively; p<0.05). Two patients with peptic stricture with only mucosal thickness required a single session of dilatation, whereas patients with involvement of submucosa required 2-4 sessions of dilatation. Patients with corrosive stricture having only involvement of submucosa required significantly fewer dilating sessions in comparison to patients having muscularis propria involvement (2.67±0.58 vs. 6.30±1.16 sessions, respectively; p=0.0003). Of the 13 patients with corrosive stricture and successful endoscopic dilatation, 5 patients required less than 6 endoscopic sessions for successful outcome. No significant difference in the esophageal wall thickness was found between the patients who required less than and more than 6 endoscopic sessions (6.00±1.35 mm and 7.07±1.72 mm, respectively).
The patients successfully dilated were subsequently followed up and 2 patients with corrosive stricture and one each with radiation and peptic stricture had recurrence of symptoms over a follow-up period of 3-21 months. These patients could be successfully treated with repeat endoscopic dilatation. Both patients with corrosive stricture who had recurrence of symptoms had involvement of muscularis propria.
Discussion
Endoscopic dilatation is the preferred treatment modality for patients with benign esophageal strictures, with peptic stricture patients usually requiring few sessions of dilatation in contrast to patients with other benign causes of esophageal strictures especially corrosive strictures who require multiple sessions of endoscopic dilation [1-8]. Even some patients with corrosive strictures require fewer endoscopic dilatations whereas many other patients with corrosive strictures require multiple sessions of endoscopic dilatation. The factors that predict the response to endoscopic dilatation in patients with benign esophageal strictures have not been adequately elucidated. Lahoti et al [11] studied 21 patients with corrosive strictures and attempted to correlate the esophageal wall thickness, as determined by CT, with the number of endoscopic dilatation sessions required for complete response. They found that maximal esophageal wall thickness was independently associated with the number of sessions required for adequate dilation. The stricture length was not associated with the number of endoscopic sessions required for dilatation. EUS has been widely used to stage various GI malignancies because of its excellent ability to image the GI tract wall. The cross-sectional 5-layer structure of the GI wall and, consequently, the depth of invasion of a malignant lesion are well demonstrated by EUS [14-16]. Therefore EUS, as it images the full thickness of the GIT wall, could provide more detailed information about the extent of the esophageal injury as compared with conventional endoscopy and help in predicting the response to endoscopic dilatation.
In the current study, we have demonstrated that patients with corrosive and post-radiation strictures have thicker esophageal wall in comparison to patients with peptic strictures. Also, patients with corrosive and post-radiation strictures have deeper esophageal injury as compared to patients with peptic stricture as demonstrated by higher frequency of muscularis propria involvement. We have also shown that rather than the esophageal wall thickness, it is the extent of the depth of involvement of the esophageal wall that predicts the response to endoscopic dilatation. Patients who have involvement of mucosa and submucosa only will respond easily to endoscopic dilatation, whereas involvement of muscularis propria makes the endoscopic dilatation difficult and these patients require multiple endoscopic sessions. Chiu et al [17] using catheter probe at 12 MHz performed EUS in acute corrosive ingestion in 16 patients within 24 hours of ingestion. They demonstrated that involvement of muscularis propria on EUS predicts stricture formation with an accuracy of 100%. We encountered perforation following dilatation in one patient with corrosive stricture. This patient had muscularis propria involvement but the esophageal wall thickness was only 5.2 mm. This suggests that patients with deeper esophageal wall involvement but thinner wall are at increased risk of perforation. However, further studies are needed to confirm this hypothesis.
Another potential advantage of EUS in chronic corrosive strictures could be identifying patients with deeper involvement of the esophageal wall along with marked wall thickness, who would be expected to have poor response to dilatation, and thus intralesional steroids along with endoscopic dilatation could be offered to these patients, as intralesional steroids have been shown to augment the response to endoscopic dilatation in patients with refractory strictures [18]. Also, it has been shown that intralesional steroids can be precisely injected in the most thickened GI tract wall under EUS guidance [19].
As we did not have catheter EUS probes and in none of our patients was the radial echoendosocope negotiable across the esophageal stricture, we did the EUS examination from the mouth of the stricture. Incomplete EUS examination of the stricture was a limitation of our study but EUS examination from the mouth of the stricture itself has yielded interesting and clinically relevant results. The results of EUS examination with a miniprobe would be interesting.
In conclusion, EUS, as it images the full thickness of the GIT wall, provides more detailed information about the esophageal wall in patients with benign esophageal strictures of various etiologies. EUS by delineating the extent of esophageal wall involvement predicts the response to dilatation with patients having involvement of muscularis propria requiring more number of sessions of dilatation in comparison to patients having involvement of mucosa and submucosa.
Summary Box.
What is already known:
Benign esophageal strictures can be effectively treated with endoscopic dilatation
Corrosive strictures require repeated dilatations and may also recur
What the new findings are:
EUS provides more detailed information about the esophageal wall in patients with benign esophageal strictures of various etiologies
EUS by delineating the extent of esophageal wall involvement predicts the response to dilatation with patients having involvement of muscularis propria requiring more number of sessions of dilatation in comparison to patients having involvement of mucosa and submucosa.
Biography
Post Graduate Institute of Medical Education and Research, Chandigarh, India
Footnotes
Conflict of Interest: None
References
- 1.Miller LS, Jackson W, McCray W, Chung CY. Benign nonpeptic esophageal strictures: diagnosis and treatment. Gastrointest Endosc Clin N Am. 1998;8:329–355. [PubMed] [Google Scholar]
- 2.Patterson DJ, Graham DY, Smith JL, et al. Natural history of benign esophageal stricture treated by dilation. Gastroenterology. 1983;89:346–350. [PubMed] [Google Scholar]
- 3.Williamson RCN. The management of peptic esophageal stricture. Br J Surg. 1975;62:448–454. doi: 10.1002/bjs.1800620607. [DOI] [PubMed] [Google Scholar]
- 4.Broor SL, Kumar A, Chari ST, et al. Corrosive esophageal strictures following acid ingestion: clinical profile results of endoscopic dilatation. J Gastroenterol Hepatol. 1989;4:56–61. doi: 10.1111/j.1440-1746.1989.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 5.Zargar SA, Kochhar R, Mehta S, Mehta SK. The role of fiberoptic endoscopy in the management of corrosive ingestion and modified endoscopic classification of burns. Gastrointest Endosc. 1991;37:165–169. doi: 10.1016/s0016-5107(91)70678-0. [DOI] [PubMed] [Google Scholar]
- 6.Broor SL, Raju GS, Bose PP, et al. Long term results of endoscopic dilatation for corrosive esophageal strictures. Gut. 1993;34:1498–1501. doi: 10.1136/gut.34.11.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirsch M, Blue M, Desai RK, Sivak MV., Jr Intralesional steroid injections for peptic esophageal strictures. Gastrointest Endosc. 1991;37:180–182. doi: 10.1016/s0016-5107(91)70681-0. [DOI] [PubMed] [Google Scholar]
- 8.Marks RD, Richter JE. Peptic strictures of the esophagus. Am J Gastroenterol. 1993;88:1160–1173. [PubMed] [Google Scholar]
- 9.Postelthwait RW. Chemical burns of the esophagus. In: Postelthwait RW, editor. Surgery of the esophagus. Norwalk, Connecticut: Appleton Century Crofts; 1986. pp. 317–344. [Google Scholar]
- 10.Marchand P. Caustic strictures of the esophagus. Thorax. 1955;10:171–175. doi: 10.1136/thx.10.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahoti D, Broor SL, Basu PP, Gupta A, Sharma R, Pant CS. Corrosive esophageal strictures: predictors of response to endoscopic dilation. Gastrointest Endosc. 1995;41:196–200. doi: 10.1016/s0016-5107(95)70337-3. [DOI] [PubMed] [Google Scholar]
- 12.Rana SS, Bhasin DK, Sinha SK, Nagi B, Singh K. Endoscopic ultrasonography (EUS) in corrosive stricture. J Gastroenterol Hepatol. 2010;25:840. doi: 10.1111/j.1440-1746.2010.06314.x. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson M, Ferguson R, Ogilvie AL. Management of malignant dysphagia by intubation at endoscopy. J R Soc Med. 1979;72:894–897. doi: 10.1177/014107687907201206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scotiniotis IA, Kochman ML, Lewis JD, Furth EE, Rosato EF, Ginsberg GG. Accuracy of EUS in the evaluation of Barrett's esophagus and high grade dysplasia or intramucosal carcinoma. Gastrointest Endosc. 2001;54:689–696. doi: 10.1067/mge.2001.119216. [DOI] [PubMed] [Google Scholar]
- 15.May A, Gunter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective and blinded trial. Gut. 2004;53:634–640. doi: 10.1136/gut.2003.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastroesophageal carcinoma. Gut. 2001;49:534–539. doi: 10.1136/gut.49.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu HM, Lin JT, Huang SP, Chen CH, Yang CS, Wang HP. Prediction of bleeding and stricture formation after corrosive ingestion by EUS concurrent with upper endoscopy. Gastrointest Endosc. 2004;60:827–833. doi: 10.1016/s0016-5107(04)02031-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Kubik CM, Polhamus CD, Brady CD, Kadakia SE. Preliminary experience with endoscopic intralesional steroid injection therapy for refractory upper gastrointestinal strictures. Gastrointest Endosc. 1995;41:598–601. doi: 10.1016/s0016-5107(95)70199-0. [DOI] [PubMed] [Google Scholar]
- 19.Bhutani MS, Usman N, Shenoy V, et al. Endoscopic ultrasound miniprobe-guided steroid injection for treatment of refractory esophageal strictures. Endoscopy. 1997;29:757–759. doi: 10.1055/s-2007-1004304. [DOI] [PubMed] [Google Scholar]


