Abstract
Policies for organ allocation can be based on medical urgency, utility or transplant benefit. With an urgency policy, patients with worse outcomes on the waiting list are given higher priority for transplantation [based on the Child–Turcotte–Pugh score or the Model for End-stage Liver Disease (MELD) score, or United Kingdom model for End-stage Liver Disease (UKELD) score]. The MELD and UKELD scores have statistical validation and use objective and widely available laboratory tests. However, both scores have important limitations. Adjustments to the original MELD equation and new scoring systems have been proposed to overcome these limitations; incorporation of serum sodium improves its predictive accuracy and is part of the UKELD score. The utility-based systems are based on post-transplant outcome taking into account donor and recipient characteristics. MELD and UKELD scores poorly predict outcomes after liver transplantation due to the absence of donor factors. The transplant benefit models rank patients according to the net survival benefit that they would derive from transplantation. These models would be based on the maximization of the lifetime gained through liver transplantation. Well-designed prospective studies and simulation models are necessary to establish the optimal allocation system in liver transplantation, as no current model has all the best characteristics.
Keywords: MELD score, UKELD score, liver transplantation, allocation, survival benefit
Introduction
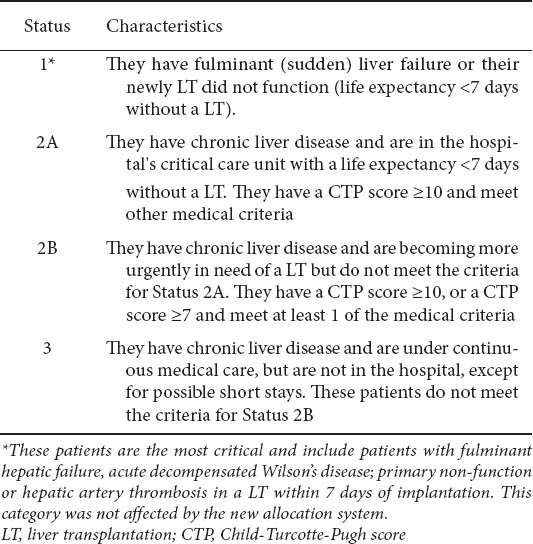
Prioritization for liver transplantation (LT) has evolved over the past 20 years [1]. Until 2002 transplant candidates were prioritized to undergo LT based on their United Network of Organ Sharing (UNOS) status (2A, 2B and 3) based on their Child–Turcotte–Pugh (CTP) scores [2] and the waiting time on the list. The UNOS status 2A, 2B and 3 (Table 1) was replaced by the model for end-stage liver disease (MELD) score adopting the “sickest first” policy for organ allocation [3,4]. In 2006, MELD was adopted by Eurotransplant, (https://www.eurotransplant.org), which allocates organs in seven countries of central Europe: Austria, Belgium, Croatia, Germany, Luxemburg, the Netherlands and Slovenia. In the United Kingdom, the UKELD score (www.uktransplant.nhs.uk) has been adopted for several years and recently published [5].
Table 1.
Classification of candidates for liver transplantation according to the old UNOS system
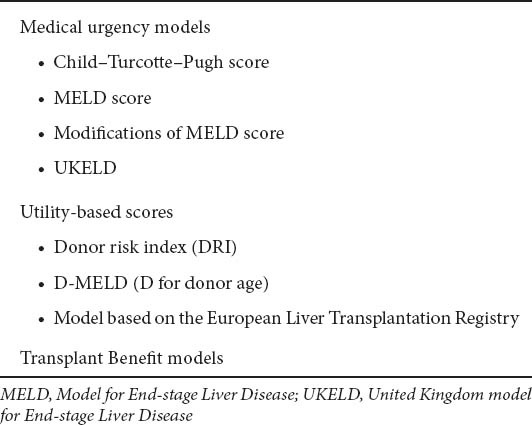
The use of MELD and UKELD scores reflect the adoption of mathematical models of prognosis for decompensated cirrhosis. However, as not all patients can be transplanted due to the shortage of available organs, prioritization of patients is necessary, but it strongly depends on the policy that is used for allocation of donor organs (Table 2). There are three possible policies for organ allocation [6]: a) medical urgency: patients with highest waiting list mortality have the higher priority for transplantation, b) utility system, based on expected post-transplant outcomes, and c) transplant benefit, in which both the waiting list and post-transplantation outcomes are taken into account. In the latter 2 policies, donor quality is an additional parameter for assessing transplant outcome [7]. In the following sections, we summarize the advantages and limitations of the current systems.
Table 2.
The three possible policies for organ allocation
Urgency-based allocation systems (Table 3)
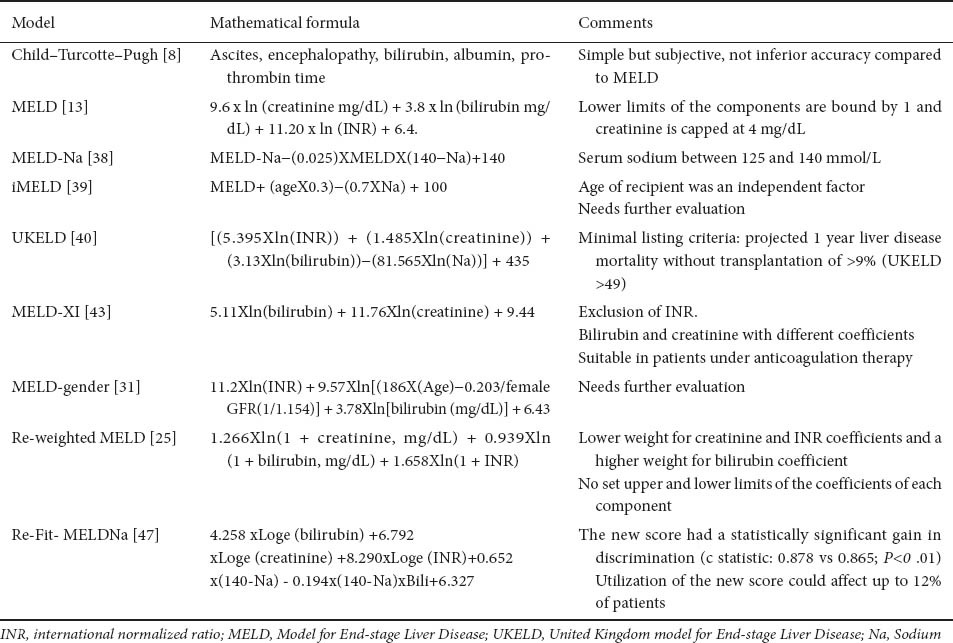
Table 3.
Prognostic models for urgency-based allocation systems
The CTP score
The CTP score [8] is based on 5 empirically selected vari- based on 5 empirically selected variables (ascites, encephalopathy, serum bilirubin, albumin and prothrombin time), with a range of 5 to 15 points derived originally for predicting the outcome of portal-caval shunt surgery and later transported to assess prognosis of cirrhosis across all etiologies. Although the CTP-based system represented a great improvement, its use for prioritization of candidates for LT had several drawbacks [9,10]. Firstly, ascites and encephalopathy are subjective variables. Secondly, patients were not sufficiently differentiated so that waiting time had great impact on prioritization; this was due to a “ceiling and floor effect” (minimum and maximum of laboratory values used in the scores). Thirdly, there was no variable reflecting renal function, a well-established prognostic marker in end-stage cirrhosis [11].
The MELD score
The MELD model was first published in 2000 to predict survival in patients undergoing transjugular intra-hepatic porto-systemic shunt (TIPS) [12]. In 2001, the same group [13], slightly modified this score to predict mortality for cirrhosis: the MELD score had discriminative ability for 3-month survival of greater than 80%, regardless of the severity of liver disease, without any significant improvement by adding etiology or complications of cirrhosis. The MELD was adopted in the USA from 27 February 2002 and has evolved following close audit and validation [14]. However, as we have published, CTP and MELD are equivalent in terms of their discriminative capacity as prognostic scores whether in LT candidates [15] or for cirrhosis in general [2], even with the addition of new studies [16,17]. Recent changes in UNOS policy require liver donor offers first to patients with MELD scores ≥15 within a region, before offers to local candidates with MELD <15 (Share 15 Policy) [18]. Patients are ranked according to their MELD score and stratified by blood type. MELD score may either increase or decrease with time and individual patient scores are forwarded regularly by each transplant center (http://www.unos.org). Despite its wide spread adoption, data on calibration of MELD score have not been published.
The advantages of the MELD score are its statistical validation, in contrast to CTP score, and use of objective and widely available laboratory tests [serum bilirubin, serum creatinine and the international normalized ratio of prothrombin time (INR)] [19] (Table 3). Several online calculators are available for calculating MELD. In addition, the “ceiling effect” is mini-the “ceiling effect” is minimized, since the ‘upper cap’ is set to 40 points. Furthermore, after adoption of MELD score, post-LT survival in the USA, remained unchanged [20], but more hepatocellular carcinoma (HCC) patients underwent LT (because of allocation of extra points) [19], and there was a small reduction in mortality on the waiting list (Table 4).
Table 4.
Advantages and disadvantages of MELD score

The disadvantages of MELD are firstly that it mainly reflects a “justice” system [15] (Table 4), and there are sig- (Table 4), and there are significant variations of MELD score due to different laboratory methodologies for INR [21,22], which may lead to differences of as much as 7 points in MELD score, and creatinine (Cr) measurements [23,24], leading to inequalities in prioritization of candidates, especially in those with the highest priority for LT (more jaundiced and greater renal dysfunction). Standardization of laboratory techniques would be necessary to avoid systematic biases.
Although the major advantage of MELD is the inclusion of renal dysfunction [10], Cr may weigh too heavily within the MELD score [25]. A further issue is that Cr provides only a rough estimation of renal function [i.e. glomerular filtration rate (GFR)] [26], since it is influenced by several extrarenal factors, such as total muscle mass, ethnicity and gender [27]. The latter has been highlighted by us [28], and found also by others [29] and can explain in part why women have higher waiting list mortality, compared to men, in the post-MELD era [29]. In fact, the Cr values in women, represent a worse GFR than in men, for the same Cr values [30]. Thus, a score corrected for female candidates (MELD-gender) has been suggested [31]. The use of “true” GFR could eliminate any gender or race bias, but it is expensive and impractical for routine use. Cystatin-C is considered a more accurate and clinically applicable serum marker for renal function [11], but we have shown that cystatin-based formulae have poor agreement with “true” GFR in patients with cirrhosis [32].
Lastly, the MELD system does not cater for transplant candidates who have complications of cirrhosis, which affect prognosis or quality of life, such as chronic encephalopathy, resistant ascites, recurrent cholangitis, or difficult-to-treat bleeding [33,34]. Ascites, determined by CT scan, improved the discrimination of MELD in a study of 1000 patients [33]. Infection, even if resolved, does affect prognosis in cirrhosis, contrary to the original MELD formulation [34]. Many of these complications are the “MELD exceptions”, which account for at least 15% of transplant candidates. The rules for accepting these patients are not standardized. The current policy for cirrhotics with HCC is no extra priority for the T1 stage, and 22 points for stage T2 (with a 3 monthly update). The justification of the extra MELD points is empirical and requires adjustment.
Proposed modifications of MELD score (Tables 3 and 5)
Table 5.
Proposed modifications of MELD score
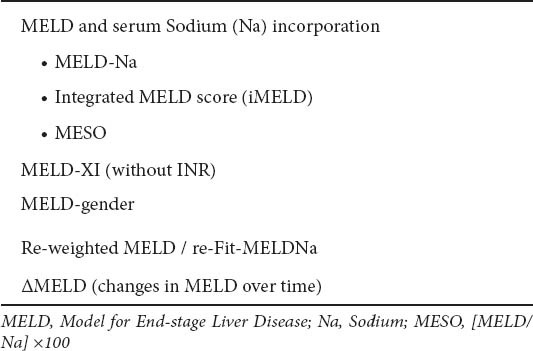
MELD-serum sodium score
Serum sodium has long been associated with hepatorenal syndrome, ascites and death in patients with decompensated cirrhosis [35,36]. In the LT setting, serum sodium is an independent factor of mortality, particularly for lower sodium values (120-135 mEq/L): within this range, a decrease of 1 mEq/L corresponds to a 12% increase in 3-month mortality independently of MELD score [37]. In addition, the MELD-Na, compared to standard MELD, provided better statistical performance for the risk of death among LT candidates: 7% of waiting-list deaths could be averted using MELD-Na score [38]. In Spain, MELD score, serum sodium, and recipient age were independently associated with mortality on the waiting list [39]: the new score integrating these variables into MELD (iMELD) was superior than standard MELD [39]. In 2008, the UK Liver Transplant Units developed a new scoring system (United Kingdom model for End-stage Liver Disease, UKELD score) [40], now instituted nationally. The constituent variables are serum bilirubin, Cr, sodium and INR (web-based calculator at www.uktransplant.nhs.uk). A UKELD score greater than 49 predicts a 1-year mortality of 9% or more without LT, and is the minimum score for listing for LT (current 1-year post-liver transplant mortality in the UK is approximately 9%) [5,41]. The UKELD score has had calibration as part of its validation [41].
However, there are concerns with using serum sodium in allocation systems, since the new scores (except for UKELD) were validated retrospectively, and although serum sodium measurement is considered objective and is widely available, it is also subject to laboratory variation [42] just as INR and Cr and can be altered by therapeutic interventions (e.g. vaptans and diuretics).
MELD-XI (MELD without INR)
A MELD-XI score (MELD excluding INR) has been proposed, which relies only on serum bilirubin and Cr, but with different coefficients for both variables [43]. However, the performance of this score is questionable and further validation is needed. Standardization of INR with a “liver INR” is impractical [44].
Re-weighting of MELD score components
Although the major advantage of MELD is the inclusion of serum Cr [10], transplant candidates with mild hepatic synthetic dysfunction and marked renal insufficiency may have “inappropriate” priority for LT, compared to those with severe liver disease, but “normal” renal function. Indeed, a higher proportion of patients with renal insufficiency have undergone LT in the post-MELD era [45,46]. Recently, the coefficients for the MELD components were re-estimated [25]. The proposed re-weighted MELD has lower relative weight for Cr and INR coefficients, and a higher relative weight for the bilirubin coefficient, compared to the original MELD score. This new MELD score had better performance than the standard MELD score to predict overall mortality (0.68 vs. 0.64), and 3-month waiting list mortality (0.77 vs. 0.75).
The recently published re-Fit-MELDNa score is another variation on the original MELD [47]. It is the only MELD variation which uses data from patients on LT waiting lists (as does UKELD), and also incorporates serum sodium. The co-efficients are changed in particular for Cr. However, as the accompanying Editorial points out, it does not resolve some of the inherent problems of MELD as outlined above.
Delta MELD
Changes in MELD score (ΔMELD) over time may add prognostic information. Although a rapid increase in MELD might be associated with worse outcome, compared to a stable or decreasing MELD score [48-52], data in the literature have given conflicting results [49,50].
Utility-based allocation systems (Table 2)
Utility-based systems are based on the expected post-transplant outcome. However, MELD and UKELD scores are weak predictors of mortality after LT [53-55], since post-LT outcomes depend on both the pre-LT parameters of recipient, and donor “quality”. In the face of organ shortage, optimization of donor/recipient pairing would have great importance for a better outcome after LT. A MELDD score -a second D for donor- [56] has been proposed by us for a utilitarian ap-[56] has been proposed by us for a utilitarian ap-utilitarian approach, which could lead to a transplant benefit model for allocation. A simple arithmetic product [57] of donor age and preoperative MELD (DMELD) has been proposed. A cut-off value of ≥1600 was derived for high-risk donor–recipient matches. However, implementation of DMELD could lead to waiting for ‘better’ donors for patients with high MELD scores and could potentially lead to an increase in waiting list mortality rates.
Donor risk index (DRI) has been developed based on seven donor factors to predict post-LT outcomes [7], which ranges from approximately 0.5 to 3.0 (with 3-year graft survival rates of 81% for organs with a DRI of less than 1.0 and 60% for organs with a DRI of greater than 2.0). However, this index is complex and it is not easily translated into clinical practice. To date, it has never been used as an allocation score, but just as a clinical decision-making tool.
A large and validated model predicting post-LT survival of 34,664 undergoing a first LT was published using the European Liver Transplantation Registry database from 23 European countries [58]. The derived prognostic models (based on donor age, total ischaemic time, and other operative and recipient factors not included in MELD), significantly and independently had an impact on the outcomes post-LT with very good calibration for 3- and 12-month mortality. Thus, these models could aid the subjective decision on donor and recipient matching which occurs at the time of organ procurement, and give clinicians some robust data for allocation. Furthermore, this study emphasized that disease-specific models (along with donor characteristics) are needed because particular diseases recur, and affect medium- and long-term survival (e.g. hepatitis C and HCC), and donor characteristics, such as donor age, can affect the severity of recurrence of the primary disease [59]. These disease specific models are yet to be developed, but would be an essential component of transplant benefit models.
Transplant-benefit allocation systems
An allocation scheme based on transplant benefit represents the balance between waiting list and post-LT outcomes, i.e. a liver graft is donated to the patient who is predicted to have neither the greatest post-LT lifetime nor the lowest waiting list lifetime, but the greatest difference between the two. Hence, patients are ranked according to the net survival benefit that they would derive from the transplant.
Recently, Schaubel et al confirmed [60] that a “transplant benefit” system should take into account donor and/or operative factors, and matching of donor to recipient characteristics for optimal outcomes. Subsequently, the same group [61] showed that “matching” could have a great impact on survival benefit from LT. The authors created a waiting list survival model (utilizing recipient characteristics) with reasonable discriminative ability (c statistic 0.74), but the post-LT survival model (consisting of donor and recipient characteristics) had a poorer performance (c statistic 0.63). This suggests that accurate evaluation of risk of death before LT is more important than after LT, presumably because the risk of death is always higher without a transplant. Indeed, in a recent paper from a single center [62], patients with MELD scores higher than 20, always had a survival benefit with LT, regardless of any donor or recipient factors. Although further studies are needed, it is encouraging that a “transplant survival benefit” allocation system is currently under serious consideration in the USA in order to maximize lifetime gained through LT [61].
Conclusions
An ideal donor liver allocation model should not only be able to allocate according to the highest probability of dying before LT, but should also be able to predict which patients have the lowest post-LT mortality in order to improve utility (i.e. a survival benefit system) [56]. This policy is currently under serious consideration for LT in the USA, while it is already used for lung allocation in the USA [63]. MELD score was instituted in 2002 for liver organ allocation in the USA, and has been adopted in several European, Asian and South America countries. Although there is no clear evidence that MELD is superior to CTP score for prognosis (the latter remains more convenient to use at the bedside), MELD score is more suitable for liver allocation [2,15]. Scores that incorporate serum sodium such as UKELD and MELD-sodium have better prediction than the standard MELD [64,65].
However, the MELD score has several drawbacks. The accuracy of MELD score has been based on its discriminative ability, but the evaluation of its calibration (i.e., the observed versus predicted outcome), which is a better index of model performance, has seldom been performed [65]. This is in contrast to UKELD score which has had its calibration evaluated as part of its validation [41]. In addition, although the three components of the MELD score were selected statistically, the initial variables entered in the model were selected empirically. The application of an artificial neural network also needs further consideration [66-68]. In addition, end-points other than survival may also be important. The MELD score at the time of LT is not related to quality of life during the first months after LT [69]. Thus, quality of life requires different modeling to incorporate into well-designed prospective studies to optimize decisions about allocation [70-72].
Urgency-based allocation systems (such as MELD and UKELD scores) are weak predictors of mortality after LT, as they do not take into account donor characteristics or quality, which influence post-LT outcomes. In fact, MELD use may have adverse effect on post-LT outcomes, as has been shown in a recent study from a Eurotransplant center [73], possibly due to accepting “any donor” for the top of the list recipients. A similar issue is the increase in resource utilization as a consequence of the use of MELD score, an important consequence as cost-effectiveness becomes even more important in health care systems. Foxton et al [74] found that patients with MELD score higher than 24 at LT had significantly longer post-LT stay in the intensive care unit (ICU) (p<0.0001), total post-LT hospital stay (p=0.008) and more frequently need for renal replacement therapy (p<0.001) [74].
The utility-based systems take into account the expected post-LT outcome based on donor and recipient characteristics. Although their performance has not been evaluated officially, in clinical practice many transplant surgeons have generally utilized organs with a higher than average risk of failure for candidates with the least risk of death without transplant. The D-MELD [57] seems very attractive, but potential limitations must be taken into account, particularly for HCV-related cirrhosis, where age is of great importance with respect to recurrent disease, such that D-MELD may need to be developed for each liver disease etiology.
The transplant benefit models are based on the difference between expected lifetime with a transplant versus without a transplant, and they have already been used for lung transplantation in the United States since 2005. In LT, a recent study has presented interesting results [61], but the proposed benefit model was based on complex statistical analysis and its performance in terms of discrimination was relatively poor (c-statistic between 0.63 and 0.74). In addition, the net survival gain mainly depends on the waiting list mortality and much less on mortality after LT diminishing the utility but not the fairness of the transplant benefit model. Thus, more data are needed before changing the current urgency-based allocation policy, but clinicians should be aware of their limitations, and allocation systems should allow for flexibility according to clinical judgment and experience [75].
Biography
Hippokration General Hospital of Thessaloniki, Greece; Royal Free Hospital and UCL, London, UK
Footnotes
Conflict of Interest: None
References
- 1.Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3–18. doi: 10.1055/s-0029-1192052. [DOI] [PubMed] [Google Scholar]
- 2.Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: the model for end-stage liver disease - should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RB, Jamieson N, Schaubel DE, et al. Who should get a liver graft? J Hepatol. 2009;50:664–673. doi: 10.1016/j.jhep.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110–122. doi: 10.1055/s-2008-1040325. [DOI] [PubMed] [Google Scholar]
- 5.Neuberger J, Gimson A, Davies M, et al. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008;57:252–257. doi: 10.1136/gut.2007.131730. [DOI] [PubMed] [Google Scholar]
- 6.Asrani S, Kim WR. Organ allocation for chronic liver disease: model for end-stage liver disease and beyond. Curr Opin Gastroenterol. 2010;26:209–213. doi: 10.1097/MOG.0b013e32833867d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 8.Pugh R, Murray-lyon I, Dawson J. Transcection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 9.Christensen E. Prognostic models including the Child-Pugh, MELD and Mayo risk scores--where are we and where should we go? J Hepatol. 2004;41:344–350. doi: 10.1016/j.jhep.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42(Suppl (1)):S100–S107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Cholongitas E, Shusang V, Marelli L, et al. Review article: renal function assessment in cirrhosis - difficulties and alternative measurements. Aliment Pharmacol Ther. 2007;26:969–978. doi: 10.1111/j.1365-2036.2007.03443.x. [DOI] [PubMed] [Google Scholar]
- 12.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 13.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 14.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 15.Cholongitas E, Marelli L, Shusang V, et al. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049–1061. doi: 10.1002/lt.20824. [DOI] [PubMed] [Google Scholar]
- 16.Attia KA, Ckoundou-N’guessan KC, N’dri-Yoman AT, et al. Child-Pugh-Turcott versus Meld score for predicting survival in a retrospective cohort of black African cirrhotic patients. World J Gastroenterol. 2008;14:286–291. doi: 10.3748/wjg.14.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boursier J, Cesbron E, Tropet AL, et al. Comparison and improvement of MELD and Child-Pugh score accuracies for the prediction of 6-month mortality in cirrhotic patients. J Clin Gastroenterol. 2009;43:580–585. doi: 10.1097/MCG.0b013e3181889468. [DOI] [PubMed] [Google Scholar]
- 18.Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiesner RH. Patient selection in an era of donor liver shortage: current US policy. Nat Clin Pract Gastroenterol Hepatol. 2005;2:24–30. doi: 10.1038/ncpgasthep0070. [DOI] [PubMed] [Google Scholar]
- 20.Freeman R, Wiesner R, Edwards E. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 21.Trotter JF, Brimhall B, Arjal R, et al. Specific laboratory methodologies achieve higher model for endstage liver disease (MELD) scores for patients listed for liver transplantation. Liver Transpl. 2004;10:995–1000. doi: 10.1002/lt.20195. [DOI] [PubMed] [Google Scholar]
- 22.Porte RJ, Lisman T, Tripodi A, et al. The International Normalized Ratio (INR) in the MELD score: problems and solutions. Am J Transplant. 2010;10:1349–1353. doi: 10.1111/j.1600-6143.2010.03064.x. [DOI] [PubMed] [Google Scholar]
- 23.Goulding C, Cholongitas E, Nair D, et al. Assessment of reproducibility of creatinine measurement and MELD scoring in four liver transplant units in the UK. Nephrol Dial Transplant. 2010;25:960–966. doi: 10.1093/ndt/gfp556. [DOI] [PubMed] [Google Scholar]
- 24.Cholongitas E, Marelli L, Kerry A, et al. Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl. 2007;13:523–529. doi: 10.1002/lt.20994. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Schaubel DE, Sima CS, et al. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575–1581. doi: 10.1053/j.gastro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 27.Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 28.Cholongitas E, Marelli L, Kerry A, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7:685–692. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 29.Moylan CA, Brady CW, Johnson JL, et al. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cholongitas E, Germani G, Tsochatzis E, et al. Towards a better liver allocation system. J Hepatol. 2009;51:827–828. doi: 10.1016/j.jhep.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Huo S, Huo T, Lin H, et al. Is the corrected-creatinine model for end-stage liver disease a feasible strategy to adjust gender difference in organ allocation for liver transplantation? Transplantation. 2007;84:1406–1412. doi: 10.1097/01.tp.0000282867.92367.d0. [DOI] [PubMed] [Google Scholar]
- 32.Xirouchakis E, Marelli L, Cholongitas E, et al. Comparison of cystatin C and creatinine based glomerular filtration rate formulae with 51Cr-EDTA clearance in patients with cirrhosis. Clin J Am Soc Nephrol. 2010;6:84–92. doi: 10.2215/CJN.03400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somsouk M, Guy J, Biggins SW, et al. Ascites improves upon [corrected] serum sodium plus [corrected] model for end-stage liver disease (MELD) for predicting mortality in patients with advanced liver disease. Aliment Pharmacol Ther. 2009;30:741–748. doi: 10.1111/j.1365-2036.2009.04096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality 4-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson M, Paton A, Sherlock S. Control of ascites in hepatic cirrhosis. Lancet. 1954;266:128–129. doi: 10.1016/s0140-6736(54)90980-0. [DOI] [PubMed] [Google Scholar]
- 36.Sherlock S, Summerkill WH, White LP, et al. Portal-systemic encephalopathy; neurological complications of liver disease. Lancet. 1954;267:454–457. [PubMed] [Google Scholar]
- 37.Londono MC, Cardenas A, Guevara M, et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56:1283–1290. doi: 10.1136/gut.2006.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luca A, Angermayr B, Bertolini G, et al. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174–1180. doi: 10.1002/lt.21197. [DOI] [PubMed] [Google Scholar]
- 40.Barber K, Pioli S, Blackwell J. Development of a UK score for patients with end-stage liver disease. Hepatology. 2007;46:510A. [Google Scholar]
- 41.Barber K, Madden S, Allen J, et al. Elective liver transplant list mortality: development of a United Kingdom end-stage liver disease score. Transplantation. 2011;92:469–476. doi: 10.1097/TP.0b013e318225db4d. [DOI] [PubMed] [Google Scholar]
- 42.Xiol X, Gines P, Castells L, et al. Clinically relevant differences in the model for end-stage liver disease and model for end-stage liver disease-sodium scores determined at three university-based laboratories of the same area. Liver Transpl. 2009;15:300–305. doi: 10.1002/lt.21688. [DOI] [PubMed] [Google Scholar]
- 43.Heuman DM, Mihas AA, Habib A, et al. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007;13:30–37. doi: 10.1002/lt.20906. [DOI] [PubMed] [Google Scholar]
- 44.Tripodi A, Chantarangkul V, Primignani M, et al. The international normalized ratio calibrated for cirrhosis (INR(liver)) normalizes prothrombin time results for model for end-stage liver disease calculation. Hepatology. 2007;46:520–527. doi: 10.1002/hep.21732. [DOI] [PubMed] [Google Scholar]
- 45.Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States 1999-2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 46.Gonwa TA, McBride MA, Anderson K, et al. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651–2659. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 47.Leise MD, Kim WR, Kremers WK, et al. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology. 2011;140:1952–1960. doi: 10.1053/j.gastro.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huo TI, Wu JC, Lin HC, et al. Evaluation of the increase in model for end-stage liver disease (DeltaMELD) score over time as a prognostic predictor in patients with advanced cirrhosis: risk factor analysis and comparison with initial MELD and Child-Turcotte-Pugh score. J Hepatol. 2005;42:826–832. doi: 10.1016/j.jhep.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Merion RM, Wolfe RA, Dykstra DM, et al. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003;9:12–18. doi: 10.1053/jlts.2003.50009. [DOI] [PubMed] [Google Scholar]
- 50.Bambha K, Kim WR, Kremers WK, et al. Predicting survival among patients listed for liver transplantation: an assessment of serial MELD measurements. Am J Transplant. 2004;4:1798–1804. doi: 10.1111/j.1600-6143.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 51.Choi PC, Kim HJ, Choi WH, et al. Model for end-stage liver disease, model for end-stage liver disease-sodium and Child-Turcotte-Pugh scores over time for the prediction of complications of liver cirrhosis. Liver Int. 2009;29:221–226. doi: 10.1111/j.1478-3231.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 52.Bae WK, Lee JS, Kim NH, et al. [Usefulness of DeltaMELD/month for prediction of the mortality in the first episode of variceal bleeding patients with liver cirrhosis: comparison with CTP, MELD score and DeltaCTP/month] Korean J Hepatol. 2007;13:51–60. [PubMed] [Google Scholar]
- 53.Jacob M, Copley LP, Lewsey JD, et al. Pretransplant MELD score and post liver transplantation survival in the UK and Ireland. Liver Transpl. 2004;10:903–907. doi: 10.1002/lt.20169. [DOI] [PubMed] [Google Scholar]
- 54.Desai NM, Mange KC, Crawford MD, et al. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99–106. doi: 10.1097/01.TP.0000101009.91516.FC. [DOI] [PubMed] [Google Scholar]
- 55.Nagler E, Van Vlierberghe H, Colle I, et al. Impact of MELD on short-term and long-term outcome following liver transplantation: a European perspective. Eur J Gastroenterol Hepatol. 2005;17:849–856. doi: 10.1097/00042737-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Burroughs AK, Marelli L, Cholongitas E, et al. Towards a better liver transplant allocation system. Liver Transpl. 2007;13:935–936. doi: 10.1002/lt.21110. [DOI] [PubMed] [Google Scholar]
- 57.Halldorson JB, Bakthavatsalam R, Fix O, et al. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318–326. doi: 10.1111/j.1600-6143.2008.02491.x. [DOI] [PubMed] [Google Scholar]
- 58.Burroughs AK, Sabin CA, Rolles K, et al. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367:225–232. doi: 10.1016/S0140-6736(06)68033-1. [DOI] [PubMed] [Google Scholar]
- 59.Ioannou GN. Development and validation of a model predicting graft survival after liver transplantation. Liver Transpl. 2006;12:1594–1606. doi: 10.1002/lt.20764. [DOI] [PubMed] [Google Scholar]
- 60.Schaubel DE, Sima CS, Goodrich NP, et al. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419–425. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 61.Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(4 Pt 2):970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravaioli M, Grazi GL, Dazzi A, et al. Survival benefit after liver transplantation: a single European center experience. Transplantation. 2009;88:826–834. doi: 10.1097/TP.0b013e3181b26807. [DOI] [PubMed] [Google Scholar]
- 63.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 64.Huo TI, Lin HC, Huo SC, et al. Comparison of four model for end-stage liver disease-based prognostic systems for cirrhosis. Liver Transpl. 2008;14:837–844. doi: 10.1002/lt.21439. [DOI] [PubMed] [Google Scholar]
- 65.Biselli M, Gitto S, Gramenzi A, et al. Six score systems to evaluate candidates with advanced cirrhosis for orthotopic liver transplant: which is the winner? Liver Transpl. 2010;16:964–973. doi: 10.1002/lt.22093. [DOI] [PubMed] [Google Scholar]
- 66.Cucchetti A, Vivarelli M, Heaton ND, et al. Artificial neural network is superior to MELD in predicting mortality of patients with end-stage liver disease. Gut. 2007;56:253–258. doi: 10.1136/gut.2005.084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lucey MR. How will patients be selected for transplantation in the future? Liver Transpl. 2004;10(10 Suppl 2):S90–S92. doi: 10.1002/lt.20256. [DOI] [PubMed] [Google Scholar]
- 68.Martin AP, Bartels M, Hauss J, et al. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc. 2007;39:3169–3174. doi: 10.1016/j.transproceed.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 69.Byrne T, Douglas D, Lopez J, et al. Does MELD score influence post-transplant quality of life? Hepatology. 2004;40:765A. [Google Scholar]
- 70.Kamath PS, Kim WR. Is the change in MELD score a better indicator of mortality than baseline MELD score? Liver Transpl. 2003;9:19–21. doi: 10.1053/jlts.2003.50031. [DOI] [PubMed] [Google Scholar]
- 71.Neuberger J. Allocation of donor livers--is MELD enough? Liver Transpl. 2004;10:908–910. doi: 10.1002/lt.20166. [DOI] [PubMed] [Google Scholar]
- 72.Freeman RB. Mathematical models and behavior: assessing delta MELD for liver allocation. Am J Transplant. 2004;4:1735–1736. doi: 10.1111/j.1600-6143.2004.00644.x. [DOI] [PubMed] [Google Scholar]
- 73.Weismuller TJ, Negm A, Becker T, et al. The introduction of MELD-based organ allocation impacts 3-month survival after liver transplantation by influencing pretransplant patient characteristics. Transpl Int. 2009;22:970–978. doi: 10.1111/j.1432-2277.2009.00915.x. [DOI] [PubMed] [Google Scholar]
- 74.Foxton MR, Al-Freah MA, Portal AJ, et al. Increased model for end-stage liver disease score at the time of liver transplant results in prolonged hospitalization and overall intensive care unit costs. Liver Transpl. 2010;16:668–677. doi: 10.1002/lt.22027. [DOI] [PubMed] [Google Scholar]
- 75.Cholongitas E, Germani G, Burroughs AK. Prioritization for liver transplantation. Nat Rev Gastroenterol Hepatol. 2010;7:659–668. doi: 10.1038/nrgastro.2010.169. [DOI] [PubMed] [Google Scholar]


