Abstract
Almost one-third of patients with inflammatory bowel disease (IBD) develop skin lesions. Cutaneous disorders associated with IBD may be divided into 5 groups based on the nature of the association: specific manifestations (orofacial and metastatic IBD), reactive disorders (erythema nodosum, pyoderma gangrenosum, pyodermatitis-pyostomatitis vegetans, Sweet’s syndrome and cutaneous polyarteritis nodosa), miscellaneous (epidermolysis bullosa acquisita, bullous pemphigoid, linear IgA bullous disease, squamous cell carcinoma-Bowen’s disease, hidradenitis suppurativa, secondary amyloidosis and psoriasis), manifestations secondary to malnutrition and malabsorption (zinc, vitamins and iron deficiency), and manifestations secondary to drug therapy (salicylates, immunosupressors, biological agents, antibiotics and steroids). Treatment should be individualized and directed to treating the underlying IBD as well as the specific dermatologic condition. The aim of this review includes the description of clinical manifestations, course, work-up and, most importantly, management of these disorders, providing an assessment of the literature on the topic.
Keywords: Inflammatory bowel disease, skin disorders, treatment
Introduction
Inflammatory bowel disease (IBD) may be associated with extraintestinal manifestations that involve multiple organs (20-40%) [1]. After musculoskeletal involvement, dermatological lesions are the second most common extraintestinal disorders of IBD. Almost one-third of the patients with IBD develop skin lesions. The etiopathogenesis, classification, and natural history of cutaneous disorders related to IBD are not well defined. Sometimes skin lesions precede the development of gastrointestinal symptoms, but they can occur with or after the onset of IBD. Moreover, there is not always correlation between IBD activity and the onset and severity of skin disorders [2].
Cutaneous disorders associated with IBD may be divided into 5 groups based on the nature of the association: (a) specific manifestations, (b) reactive disorders, (c) miscellaneous, (d) manifestations secondary to malnutrition and malabsorption, and (e) manifestations secondary to drug therapy (see Table 1) [3].
Table 1.
Cutaneous manifestations in Inflammatory Bowel Disease [3]

The aim of this review includes the description of clinical manifestations, course, work-up and especially the management of these disorders, providing an assessment of the literature.
Because the incidence of the majority of these disorders is low, no prospective randomized controlled trials, and only a few studies with more than 10 cases, have been published; therefore, recommendations have to be interpreted with caution.
The treatment of the majority of dermatologic conditions associated with IBD is empiric, including topical and systemic immunosupressants, immunomodulatory and biological agents. Many of these disorders improve or resolve with control of IBD activity [4].
An interdisciplinary approach between dermatologists and gastroenterologists plays an essential role in the management of these patients.
Specific cutaneous manifestations
Orofacial Crohn’s disease and orofacial ulcerative colitis
In Crohn’s disease (CD), oral manifestations occur in 8-9% of the patients. They are more common in the pediatric population [5], and may precede intestinal involvement [6]. When there is oral involvement, the likelihood of other extraintestinal manifestations is greater. CD may appear in the oral cavity as aphthous ulcerations or superficial bleeding ulcers [7]. However, a wide variety of disease-specific oral lesions have been described in patients with intestinal CD (Table 2) [8,9].
Table 2.
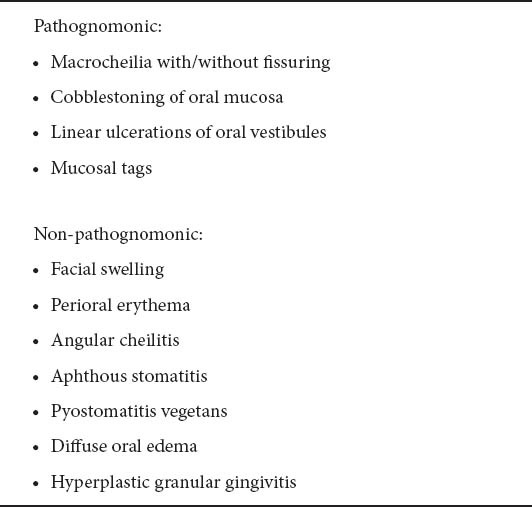
CD in the oral cavity usually takes the form of aphthous ulceration of the mucosal surface. Aphthae and mucosal tags are frequently seen if the oral cavity is carefully evaluated in patients with newly diagnosed CD. Deep tissue inflammation occurs uncommonly and may lead to labial, lingual, and facial swelling and fistula formation can result. CD sometimes shows only orofacial manifestations.
In ulcerative colitis (UC) patients, lesions of the oral cavity may appear as aphthous ulcerations, superficial hemorrhagic ulcers or angular cheilitis. In general, oral lesions coincide with exacerbations of the colonic disease. Similar ulcerations may arise on the buttocks, abdomen, thighs and face. Aphthous ulcers or angular stomatitis occur in as many as 5-10% of patients [7].
Indeed, classification of granulomatous orofacial disease is complex (particularly in the absence of inflammation elsewhere) and there is considerable overlap between orofacial CD, Melkersson Rosenthal syndrome, cheilitis granulomatoses, and the other orofacial granulomatosis [9]. Therefore, diagnosis should include tissue sampling of the involved orofacial region for histopathologic examination [10].
Effective treatment for specific orofacial CD has not been defined. Many patients who are found to have OCD manifestations may be completely asymptomatic, since the oral findings are not clinically problematic [11].
Therapy usually involves the use of agents with proven efficacy in enteric CD, including topical and systemic corticosteroids and immunomodulatory agents. A cinnamon- and benzoate-free diet may be the first approach [12]. Symptomatic treatment, including topical anesthetics, such as 2% viscous xylocaine, can help reduce the pain due to aphthous ulcers.
Beclamethasone mouthwashes (0.5 mg dissolved in water, several times a day) bring symptomatic relief. Lip swelling is sometimes helped by topical tacrolimus. Intralesional injection of steroids into swollen lips has been reported [6]. In patients with persistent pain, swelling, and cosmetic disfigurement, the use of immunosuppression at an early point is to be considered. Doses used in conventional CD are employed. There are no reliable outcome data on this approach.
Biologic agents that target tumor necrosis factor (TNF)-α have been established as effective therapies and are now the treatment of choice for enteric fistulizing CD. Case reports have suggested that infliximab and adalimumab [13,14] are effective therapies in orofacial CD [15,16]; orofacial fistula healing and closure have been described [17].
Metastatic CD
Metastatic CD (MCD) is an unusual cutaneous manifestation of CD, which appears as unspecific skin lesions such as plaques, nodules or crusts, which are erythematous and occasionally indurated or ulcerated. The histological study shows non-caseating granulomas, and there is no contiguity between the skin lesions and the intestinal involvement [18,19].
Lesions are most frequently located in flexures, genitalia and extremities, although lesions may appear in any other area of the skin, either alone or in groups (Fig. 1) [20-22].
Figure 1.
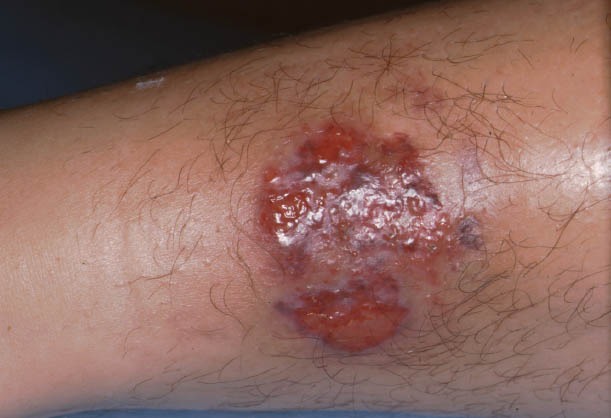
Metastatic Crohn’s disease. Ulcerative and crusting lesion
MCD is regarded as highly infrequent, although this is only based on isolated case series. It is a condition in which the heterogeneity of its overall appearance and clinical course may delay diagnosis. A clear correlation between the intestinal and the MCD activity has not been found. Mild and transient lesions may be the reason for consultation and may condition treatment [23,24]. Although MCD is well recognized in adults, it is extremely rare in children. It may develop simultaneously or precede gastro-intestinal involvement; most cases of MCD are seen before the diagnosis of gastrointestinal CD is made [23,25].
MCD diagnosis is based on a combination of clinical exploration and skin biopsies in the context of a patient with CD. However, there have been reports of increased diagnostic complexity, in which skin involvement precedes intestinal implication, with even an interval of several years between the two forms of presentation, or where the skin lesions appeared several years after a proctocolectomy [22,26,27].
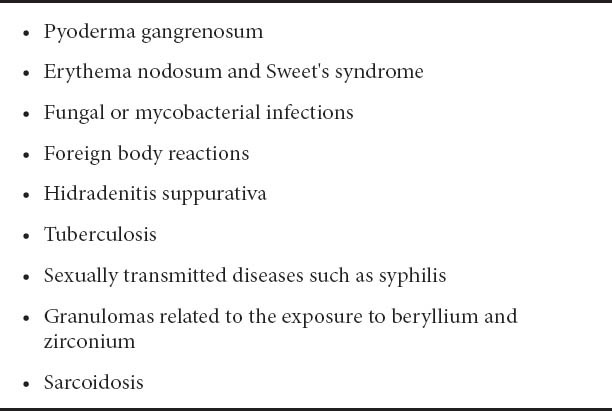
Other granulomatous skin diseases must be ruled out (Table 3) [28,29]. Some cases have shown good results after surgical treatment of the skin lesion [24]. Medical treatment is based on the use of topical and oral corticosteroids [20,28,29] associated or not with mesalazine [30] and/or azathioprine [14]. In cases that do not respond adequately, treatment with other immunosuppressants (cyclosporine or tacrolimus) [31-33] or biological agents (infliximab or adalimumab) [34-42] is used.
Table 3.
Reactive cutaneous disorders
Erythema nodosum
Erythema nodosum (EN) is a reactive cutaneous process that is most frequently associated with infections, sarcoidosis, rheumatologic diseases, IBD, drugs, autoimmune diseases, pregnancy, and malignancies (lymphomas). In up to 60% of the cases, no underlying cause is found [43,44].
EN is the most common form of septal panniculitis and the most frequent skin manifestation associated with IBD. It has been associated with IBD in 11% of the cases, occurring more frequently in patients with UC than CD. EN is mainly seen in females, and in patients with colonic involvement and/or arthritis, typically associated with an exacerbation of the colitis (90%) but not with the severity or colonic extent, although it may even occur before the diagnosis of IBD [4,44-46].
EN is typically present as multiple, deep-red tender, painful nodules, distributed usually over the shins. The lesions do not show suppuration, ulceration or scarring, and they change to a yellowish color, similar to a deep bruise. Outbreaks appear suddenly and may be accompanied by general symptoms (Fig. 2) [4,45-47].
Figure 2.
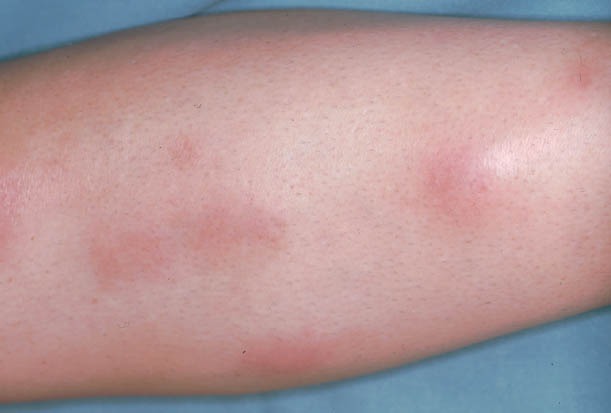
Erythema nodosum. Multiple red, warm, and tender nodules, classically located on pretibial surface of lower extremity
EN usually regresses spontaneously after a few weeks of supportive treatment with compression stockings, leg elevation, and rest [46]. It sometimes leaves a mild hyperpigmentation on the skin [44]. When associated with IBD, EN is usually resolved with control of the IBD and often recurs with exacerbations of the bowel disease [4].
Although the first-line therapy is nonsteroidal anti-inflammatory drugs (NSAIDs) [48], they should be avoided in IBD-related EN because they may trigger a flare; if needed, they should be used with caution. More aggressive pain management is reserved for clinical situations that become recurrent or unusually prolonged [43,49]. Intralesional injection of triamcinolone acetonide into the center of the nodules may cause them to heal [44].
Systemic steroids have been advocated as a relatively safe therapeutic option if underlying infection, risk of bacterial dissemination or sepsis, and malignancy has been excluded by a thorough evaluation [49].
Potassium iodide, colchicine, hydroxychloroquine, dapsone, and infliximab have been reported to be successful in treating severe or refractory lesions. Infliximab is highly effective in healing refractory lesions of EN [50]. However, EN has also paradoxically been reported to occur in patients treated with infliximab, with resolution of the lesions upon discontinuation of the medication [48].
Therapeutic agents useful in the treatment of EN have been used to treat IBD-associated EN: cyclosporin A, cyclophosphamide [51], or thalidomide [52]. They may be used in combination with steroids [53].
Other alternative treatments have been tested in patients with UC-associated EN with good results, like proctocolectomy [52] and extracorporeal monocyte granulocytapheresis (M-GCAP) [53].
Pyoderma gangrenosum
Pyoderma gangrenosum (PG) is an idiopathic ulcerative, non-infectious, chronic inflammatory skin disorder of uncertain etiology and belongs to the neutrophilic reactive disease spectrum [54,55]. It is associated with systemic illnesses in 50% of the cases, such as IBD, systemic arthritis, hematological diseases and malignancies.
IBD is the most common underlying disorder and it is found in 10-15% of PG cases, being more frequent (5%) in UC patients. It is typically associated with more severe bowel disease [1] and the clinical course of PG is usually independent of IBD [56].
Characteristic lesions usually begin as a single pustule or vesiculopustule and progress to an ulcer or deep erosion with violaceous overhanging or undermined borders. Lesions are most commonly found on the lower legs. Lesions have been classified into 4 types: ulcerative (typical or classic), pustular, bullous (atypical) and vegetative PG. The diagnosis of PG is based primarily on the clinical presentation and course; histology can also be of value (Fig. 3) [57].
Figure 3.
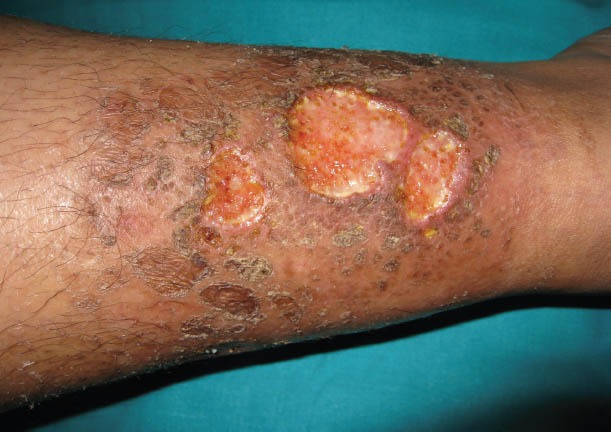
Pyoderma gangrenosum. Painful and irregularly shaped ulcers with violaceous edges
There are currently no standardized treatment guidelines for the management of PG [58]. General supportive therapy and treatment of any concomitant disease have been the standard of care (Table 4).
Table 4.
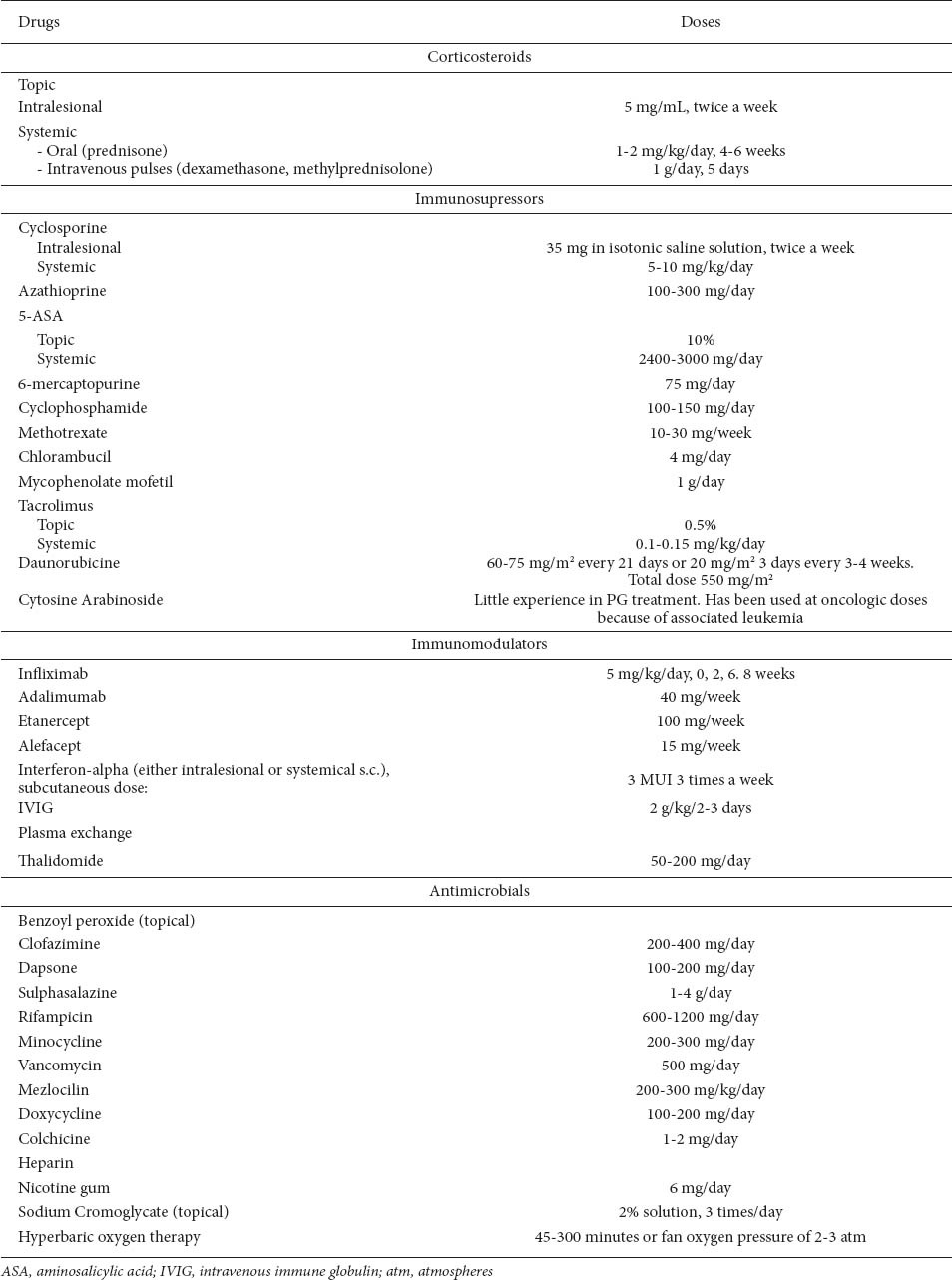
Local care includes wet compresses with sterile saline solution or Ringer-lactate solution, diluted potassium permanganate solution (1:20000), silver sulphadiazine cream, topical antibacterial creams, and moist dressings (such as hydrocolloid, allograft, Vaseline gauze) to relieve pain, facilitate autolytic debridement, promote re-epithelialization, prevent infections and trauma, and minimize the resulting scar. Occlusive hydrocolloid dressings may also be helpful in superficial ulcers [59], but in case of purulent lesions, semiocclusive dressings are contraindicated [60].
Bioengineered skin dressings may be beneficial in covering ulcers and preventing the need for skin grafts [61-63].
Topical therapy is variable and includes intralesional corticosteroids, topical corticosteroids, 5-aminosalicylic acid [61], sodium cromoglycate, intralesional cyclosporine, tacrolimus [64], pimecrolimus, benzoyl peroxide, nicotine patch, and nitrogen mustard [59,61,62,65]. Recombinant human epidermal growth factor (rhEGF) [66], human platelet-derived growth factor [54,56] and perilesional use of granulocyte macrophage colony stimulating factor [62] have also been reported to be effective. Local irradiation of the lesions, electron beam therapy and hyperbaric oxygen [60,61,65], have also been employed [67]. Topical therapy is most useful in early ulcers or if there are small isolated ulcers. It is usually ineffective as monotherapy, but it can support systemic medication [68].
Systemic treatment may be required for more severe cases or those that are refractory to local treatment. However, the first-line treatment is usually high-dose corticosteroids. In steroid-refractory cases, immunosuppressive drugs have been shown to be effective in individual patients, as well as various new drugs, including biologics.
Systemic therapies are also variable and include sulfa drugs and other antimicrobials such as dapsone, clofazimine, minocycline, and rifampicin, which have been used with different degrees of success. However, the mainstay of systemic treatment is still corticosteroids, administered as bolus doses [69] or in the form of pulse therapy. Other immunosuppressant agents in use include cyclophosphamide, azathioprine, chlorambucil, cyclosporine [70,71], mycophenolate mofetil [63], methotrexate, mercaptopurine, FK506 (tacrolimus) [72] and biologics in refractory cases of PG. Systemic combination therapy with oral or pulse intravenous corticosteroids and/or cyclosporine or azathioprine should be considered as first line therapy [54,73].
Other systemic treatments have also been used with varying success: plasma exchange [56], leukocytapheresis [60,74], intravenous immune globulin, interferon-α, potassium iodide, alefacept [55,56], cytosine arabinoside and daunorubicin, and Tripterygium wilfordii multiglycoside [62].
TNF-α inhibitors have improved and broadened the therapeutic options for IBD and have brought new perspectives to the treatment of patients with PG [75]. Three agents have been used in the treatment of PG: infliximab, adalimumab [76-78] and etanercept [79]. The effectiveness of infliximab for IBD-associated PG is reported in many articles [80-82] and a randomized placebo controlled trial showed a significant clinical response rate of PG to infliximab infusions. TNF-α inhibitors are used alone or in combination with azathioprine [83] or methotrexate [84].
Surgical treatment is useful only in extreme conditions because it can be complicated by pathergy in patients with PG [65,85]. Any surgical procedure has to be done as an adjunct measure to immunosuppression, and only in patients with stable disease or partial remission [60]. Options include split-skin grafts and autologous keratinocyte grafts [4,63].
Resolution of penile PG has been reported with therapeutic colectomy in ulcerative colitis [86]. Because the course of PG can be independent of the course of IBD and has even been reported years after proctocolectomy, bowel resection is not a primary therapy [4,56].
The prognosis is that of the associated disease. The control of the intestinal condition can resolve the skin problem and recurrences may occur at periods of exacerbation of IBD. In those patients who readily respond to treatment, the prognosis of the disease is good, but considerable scarring and disfigurement may eventually result [56].
Pyodermatitis-pyostomatitis vegetans
Pyodermatitis-pyostomatitis vegetans (PPV) is a benign and rare mucocutaneous dermatoses often associated with gastrointestinal disorders, especially with IBD. Some authors consider PPV in the spectrum of neutrophilic dermatoses, and others suggest that it is a form of PG [87,88].
There is a strong association of PPV with IBD, particularly with UC. Usually, the intestinal disease precedes the onset of oral lesions by months or years, but oral involvement in IBD could be previous or simultaneous to the gastrointestinal symptoms. The clinical course of oral lesions parallels the activity of IBD. There is general consensus that the bowel should be investigated in PPV, even if intestinal symptoms are absent at presentation [87-91].
Clinically, oral and cutaneous lesions are characteristic and distinctive (although any mucosal surface can be involved). Oral examination reveals multiple, non-painful, small yellowish pustules on an erythematous and edematous base; they rupture easily, producing an elongated superficial aspect, called ‘‘snail track’’ erosions. Cutaneous lesions are characterized by vesiculopustular, exudative, vegetating, annular plaques frequently affecting the scalp, axillae, and groins. Often, skin lesions of PD-PSV appear at the same time as or shortly after the oral disease [87-93].
Oral biopsy is important in establishing a correct diagnosis of the disease. Peripheral blood eosinophilia is associated in 90% of cases [87,91]. The differential diagnosis includes mainly pemphigus vegetans (the condition may also be caused by zinc deficiency) [88,91,92].
Management of PPV depends on the presence of coexisting IBD. The first course of action is the treatment of the underlying condition, which is usually sufficient to control oral and skin lesions.
Various treatments for PD-PSV have been reported, such as topical and oral corticosteroids, and systemic corticosteroids combined with antibiotics, sulfonamides, dapsone, sulphamethoxypiridazine, azathioprine, cyclosporine A or etanercept. The treatment of choice is systemic corticosteroids, starting with moderate to high dosage [91-94]. Bens et al reported that three injections of infliximab and successive maintenance therapy with methotrexate caused a rapid and complete regression of both the PV and the CD [95]. Multivitamin complexes and nutritional supplements can be provided [96]. Surgical treatment in severe cases IBD involves total colectomy and has resulted in complete remission of symptoms.
The oral lesions can be managed with local therapies using antiseptic mouthwashes, tetracycline mouth rinses, and topical corticosteroids. Treatment with topical tacrolimus ointment for PD-PSV has been beneficial in some patients [94]. However, local therapy is generally insufficient [95,97,98]. Unfortunately, the lesions can recur when treatment is tapered or stopped [88].
Sweet’s syndrome
Sweet’s syndrome (SS) (or acute febrile neutrophilic dermatosis) is a reactive neutrophilic dermatosis. It may be associated with infection of the upper respiratory tract and/or gastrointestinal tract, IBD, pregnancy, cancer, medications and connective tissue diseases [55,99].
The syndrome has been reported as an unusual extra-intestinal manifestation of IBD, principally associated with CD and less commonly with UC [99,100]. In these patients, SS is more common in women, with colonic and perianal involvement, and in patients with other extra-intestinal manifestations [101,102].
The skin manifestations of SS appear concurrently with the relapse of CD (75% of the cases) although SS may also precede or follow the digestive symptoms by many years. It has been also described after proctocolectomy [103,104].
Cutaneous lesions may exhibit pathergy and include a sudden eruption of painful tender red papules, plaques, pustules, or vesicles. They are commonly located on the upper extremities, face and neck. Patients with SS show severe systemic symptoms [99,105,106]. The diagnosis of SS is based on the clinical and histopathological manifestations (Fig. 4) [55].
Figure 4.
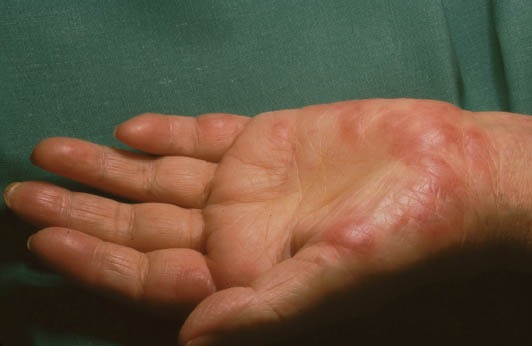
Sweet’s syndrome. Painful, red, tender edematous papules with pseudovesiculation
The onset can be acute with abrupt resolution of cutaneous symptoms upon treatment or the disease can take a chronic, recurrent course. The natural history of untreated SS is the resolution of skin lesions within 6-8 weeks. Recurrences are common [101,107].
Many different treatments have been used in SS, with different rates of success and relapse. Systemic corticosteroids have been found to be the most common first-line therapy in use [105,106,108]. Localized SS lesions may be treated topically with high-potency corticosteroids, and intralesional corticosteroid. Potassium iodide [109] and colchicine [110,111] are also available as first-line agents to treat SS.
Cyclosporine [112] and dapsone [113] have been used as either monotherapy or in combination therapy (usually as corticosteroid-sparing agents). They should be considered as an alternative to corticosteroids in the treatment of severe, steroid-dependent or steroid-resistant SS cases.
Indomethacin [108,114], chlorambucil, clofazimine, cyclophosphamide [115], methotrexate, etretinate, pentoxifylline [116], danazol, intralesional and systemic interferon-alpha [117], aspirin, naproxen and anakinra [118] have been reported to be effective treatment agents in uncontrolled studies and single case reports.
There have been occasional SS patients whose dermatosis has resolved after treatment with antimicrobials, such as doxycycline [119,120], minocycline, tetracycline, penicillin, pyrimethamine, sulfonamide and metronidazole. Some patients benefit from the combination of steroids and metronidazole [99,103,105,121].
Other treatments for SS such as tacrolimus [103] or infliximab have been described, obtaining worse results, in cases of corticosteroid refractory IBD [104,122].
It is useful to consider that azathioprine, a drug used for maintenance of remission in IBD patients, may cause SS, and, therefore, this possibility must be discarded in patients with SS starting azathioprine treatment [103,123-125].
Cutaneous polyarteritis nodosa
Cutaneous polyarteritis nodosa (CPN) is a distinct clinical entity characterized by a chronic, relapsing, benign course and the absence of systemic involvement. Clinically, it presents with painful subcutaneous nodules, erythema and livedo reticularis that mainly affects the lower extremities. Systemic manifestations are usually associated symptoms [126-128]. Ulcers may develop frequently, without altering the clinical evolution [127].
CPN is a rare cutaneous complication in IBD patients; the relationship between CPN and UC [129] or CD is debatable [128,130,131]. The clinical and histopathological findings confirm the diagnosis. Treatment is directed toward controlling inflammation and treating any associated systemic illness or infection, but the prolonged course of the disease, with periodic exacerbations and remissions, makes response to treatment difficult to evaluate [126-128].
Low to moderate doses of systemic corticosteroids for 3 to 6 months may induce a remission during an acute flare of the disease. Mild cases can be treated with NSAIDs, like salicylates, alone or in combination with prednisone.
The combination of cyclophosphamide and prednisone results in a good clinical response [129,131,132]. Intravenous immunoglobulins, IFN-α and low-dose methotrexate and other immunosuppressants have also been used [127,132]. A long course of treatment with antibiotics can be effective in patients with streptococcal or other bacterial infections. Sulfapyridine, dapsone, pentoxifyllin and nicotinic acid can also be effective [126,127].
Improvement in cutaneous PAN and CD after treatment with steroids, immunosuppressive agents, aminosalicylates or surgery, has been reported [128], although the course of CPN is considered independent of the bowel disease [133].
Miscellaneous
Epidermolysis bullosa acquisita
Epidermolysis bullosa acquisita (EBA) is a rare nonhereditary mechanobullous disorder of the skin, characterized by IgG autoantibodies to type VII collagen. IBD is noted in approximately 30% of reported cases of EBA, most of which are CD [134,135]. EBA may present only as a GI disease such as cases restricted to the esophagus. The activity of both IBD and EBA is independent [135,136]; however, an improvement in the skin lesions upon remission of the intestinal involvement has been reported in some cases [136-138].
EBA is often refractory to the conventional treatment approach of high-dose corticosteroids and corticosteroid-sparing agents. Many treatments have been reported with inconsistent benefits, such as topical corticosteroids, azathioprine, vitamin E, sulfasalazine, mesalamine, phenytoin, gold sodium thiomalate, cyclosporine, colchicine, extracorporeal photochemotherapy, intravenous immunoglobulin (IVIg), mycophenolate mofetil, dapsone, rituximab, and other immunosuppressive medications [134,137-139]. Lehman et al affirmed that conventional therapy with high-dose corticosteroids for long-term has many side effects without consistent benefit and proposed that colchicine or IVIg be considered as first line treatments in mild or advanced cases, respectively [140].
Bullous pemphigoid
Bullous pemphigoid (BP) is an uncommon, chronic, bullous skin disease, mainly affecting the elderly. It is often described in association with other autoimmune disorders and it has seldom been observed in association with IBD [141].
Some authors consider that the autoimmune disorders associations are the consequence of an autoimmune state [142] and the co-existence of ulcerative colitis and BP is more likely to be incidental rather than etiopathogenic [141,143]. Another hypothesis for the association of both disorders is drug-induced BP, such as by mesalamine [141] or sulfasalazine [144].
Clinically, BP is characterized by subepidermal blisters and intensive pruritus. Lesions commonly involve the extremities, lower abdomen, groin, and axillae. BP affects the mucous membranes in about one-third of cases.
The selection of treatment options is based on clinical experience. Oral steroids are considered the standard treatment and they have been used alone or combined; the adjuvant use of immunosuppressant shows some steroid-sparing effect (dapsone and azathioprine). Systemic antibiotics (tetracycline) are needed [141,142,145].
Moreover, colectomy has been reported to ameliorate or even cure immunobullous skin disease in the setting of UC [141], but in another case, improvement was temporary [143].
Linear IgA bullous disease
Linear IgA bullous disease (LABD) is a rare acquired subepidermal autoimmune blistering disorder associated with the deposition of IgA in a linear pattern at the dermoepidermal junction [146,147]. The etiology of LABD is still unknown, although it has been associated with malignancy, autoimmune diseases, infections and drugs. Its association with IBD, particularly UC, has been reported. UC prevalence in LABD patients was 7.1% compared to the 0.05% prevalence in the general population [146-148]. UC usually precedes the onset of the skin disease, although the opposite may also occur [148,149].
Clinical lesions are tense vesicles or bullae. This eruption is usually confined to the skin, but it may involve the mucous membranes in up to 80% of patients [150,151]. Diagnosis of LABD is based on the histopathological and immunopathological findings.
The primary therapy in LABD is still dapsone. Sulfapyridine may be used, if the patient is intolerant to dapsone. Small doses of corticosteroids are often needed in addition to dapsone or sulfapyridine in cases where high-dose dapsone or sulfapyridine alone do not adequately control the disease. Sulfamethoxypyridazine, colchicine, cotrimoxazole, erythromycin, mycophenolate mofetil, oxacillin, dicloxacillin, flucloxacillin, erythromycin, IgIV and a combination of tetracycline and nicotinamide have also been tried with variable results [148,149,151].
Complete remission of LABD after total colectomy for UC has been reported [152].
The course of UC and LABD is not considered to be parallel [146,153], but, in some case reports, both disorders improved after therapy [146,148,153,154].
Squamous cell carcinoma - Bowen’s disease
Squamous cell carcinoma (SCC) is one of the most common skin malignancies. Bowen’s disease (BD) is an in situ form of SCC of the skin [155].
CD and UC have been reported as the cause in a few cases of SCC [156]. The perineal area is the most frequent location of these lesions in IBD, but they can appear at other sites, like photodamaged zones and skin-grafted ileostomy stoma [157]. In patients with CD, SCC was found to be significantly more common (14%) than anal cancer in patients without IBD (1.4%) [158-161].
Cutaneous SCC appears as an erythematous friable papule, plaque, or non-healing ulcer. BD presents with scaly lesions and erythema [162-164].
Most of the squamous tumors develop after prolonged periods [159,165], so it is important to take biopsies in a long-standing perianal IBD with non-healing lesions or when changes in habitual symptoms occur [158,159,166,167].
Surgical excision, with adjuvant radiation in aggressive lesions, is the treatment of choice for most skin SCC and BD [162].
The prognosis of anal carcinoma is generally poor, reflecting the advanced stage at the time of diagnosis; therefore, early diagnosis is essential [168].
Hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a chronic recurrent disease that usually affects areas of the skin with a high density of apocrine glands (axillae, groin, perianal and perineal regions), characterized by the development of subcutaneous nodules, sinus tracts, fistulae formation, and dermal scarring (Fig. 5). There are reports in the literature associating this condition with CD. The prevalence of HS in UC is unknown [169]. The pathogenesis of this condition is attributed to follicular hyperkeratosis, with occlusion, dilatation, and disruption of the follicle resulting in local inflammatory response [170]. Medical treatment includes general measures (antiseptic soaps, warm baths, etc.), pharmacological therapy, including local and/or systemic antibiotics, retinoids, and anti-TNF agents, with promising results especially in mild, early-stage disease. Several case reports describe the successful treatment of HS concomitant to CD using anti-TNF-α-agents. Infliximab appears to be an effective medical approach to management and also prepares the patient for “curative” surgery [171-177].
Figure 5.
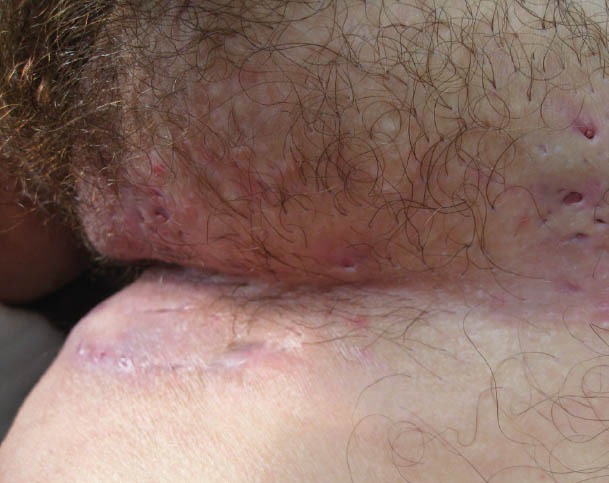
Hidradenitis suppurativa. Subcutaneous nodules, sinus tracts, fistulae formation and dermal scarring
Case series and controlled trials with adalimumab [178] and etanercept obtained favorable results, although further studies are needed for standard practice [179]. Alternative treatment with high dose vitamin B12 has been used where conventional therapies have failed [180]. Extensive surgical removal of the affected areas with healing by secondary intention is the appropriate treatment for severe refractory cases [181].
Secondary amyloidosis
Amyloidosis is a clinical condition resulting from abnormal folding of human proteins, which precipitate as insoluble fibrillar polypeptide aggregates in the extracellular tissues, thereby interfering with their normal function. Based on the biochemical nature of the protein itself, systemic amyloidosis had been historically classified as primary (AL) or secondary (AA). AA-amyloidosis is associated to several chronic inflammatory diseases. Systemic amyloidosis is a rare but life-threatening complication of IBD, most cases being reported among CD patients [181-184]. The association of IBD with AL amyloidosis, is very infrequent as it is confined to a case report [185].
The therapeutic approach of these patients should be dual: minimizing the activity of the underlying disease (mitigating or preventing AA production, the precursor of plasma amyloid deposit and responsible for tissue malfunction) and treating the disease once it is established [186]. Although drugs such as azathioprine, colchicine, and dimethylsulphoxide are suggested to delay the progression of renal amyloidosis, their efficacy in patients with high serum amyloid level and acute progression of the renal dysfunction has not been fully elucidated [187].
Case series or single cases treated with TNF-α inhibitors have demonstrated their effectiveness in patients with an acute progression of the renal dysfunction with high serum amyloid levels [188-192].
Psoriasis
Psoriasis is a chronic multisystem, inflammatory disorder which most commonly manifests itself on the skin of the elbows, knees, scalp, lumbosacral and intertriginous areas. Diseases like neoplasms, cardiovascular diseases, IBD and the metabolic syndrome are significantly associated with psoriasis [193,194]. Several studies have described the epidemiological, pathogenic, and genetic association between psoriasis and CD [195-197].
In both diseases, anti-TNF-α antibodies such as infliximab [198-203] and adalimumab [203,204] have a proven beneficial effect. Etanercept has failed to show significant benefit in IBD clinical trials [205]. Novel anti-TNF-α agents, golimumab and certolizumab pegol [206], recently entered the market for the treatment of CD and psoriasis [207]. Several reviews referred to the use of ustekinumab (anti-IL12/23 IgG1 kappa human monoclonal antibody) for the treatment of CD (phase II studies) and psoriasis [208-210].
Non-controlled studies have shown favorable results for tacrolimus and pimecrolimus, (especially for inverse psoriasis) [211], abatacept (T cell co-stimulation blocker) [212], efalizumab (monoclonal antibody targeting CD11a) [213].
Manifestations secondary to malnutrition and malabsorption
The cutaneous manifestations due to malabsorption include diseases that are secondary to deficits in vitamins and trace elements. In all these disorders, the substitution of the deficient factor leads to a complete resolution of the cutaneous lesions [3] (Table 5):
Table 5.

Zinc. Acrodermatitis enteropathica (AE) is a rare, inherited form of zinc deficiency with periorofacial and acral dermatitis (erythematous, dry and scaly patches and plaques that may evolve into crusted vesiculobullous erosive psoriasiform and pustular lesions), alopecia (diffuse, may affect eyebrows and eyelashes) and diarrhea. The skin lesions may become secondarily infected. Glossitis, hair discoloration, paronychia and nail dystrophy are frequent. Skin biopsy may be useful for diagnostic purposes, but the diagnosis is mainly clinical, with the confirmation of low levels of zinc and alkaline phosphatase in plasma. The differential diagnosis includes atopic dermatitis, candidiasis and seborrheic dermatitis [214,215].
Vitamin A. Vitamin A promotes cell proliferation and differentiation, and its deficiency is manifested by metaplasia of epithelia throughout the body. The skin lesion due to this deficiency is known as “Phrynodermia”, which is manifested as follicular hyperkeratosis distributed symmetrically in the dorsal and lateral surfaces of the extremities. It can also cause widespread xerosis and delayed healing [216].
Vitamin E. Vitamin E deficiency causes a seborrheic-type dermatitis and edema [217].
Vitamin K. Its deficiency causes bleeding, bruising and petechiae on the skin [216].
Iron. Iron deficiency causes angular stomatitis, brittle nails with koilonychia, a painful smooth tongue and diffuse alopecia [216].
Cutaneous manifestations secondary to drug therapy
The cutaneous side-effects of drugs used in the treatment of IBD [3,218] are listed in Table 6.
Table 6.
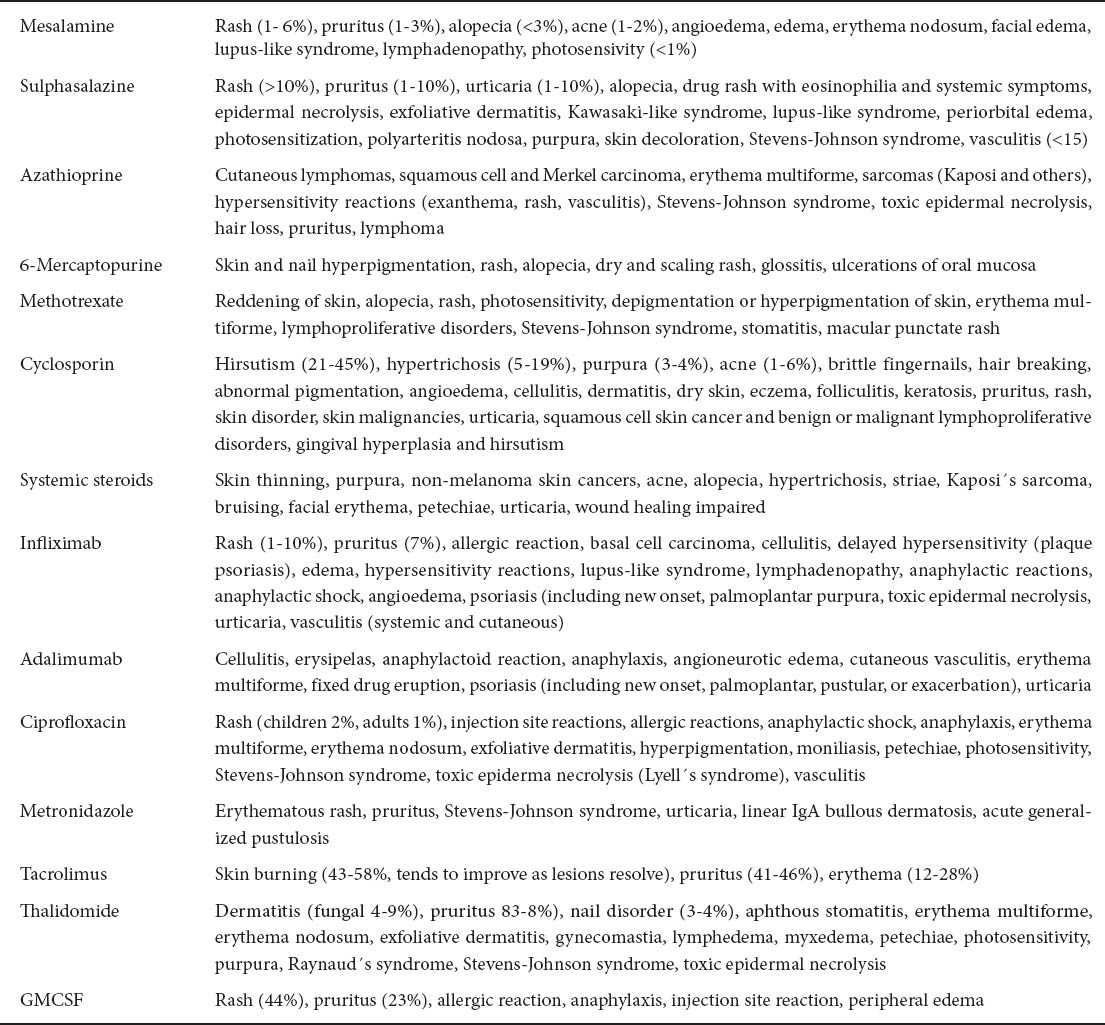
It is quite frequent to observe changes in the skin or mucosa triggered by the drugs used to treat IBD. The occurrence of these disorders raises questions about whether to continue or discontinue a drug, in part due to the absence of clear guidelines regarding drug-induced skin reactions. Therefore, the decision depends on the characteristics of the eruption, based on the clinical morphology (bullous forms are the most serious and require immediate discontinuation of treatment), extent (localized or generalized) and symptoms (lesions may be asymptomatic or cause severe itching, burning and / or pain) [219].
The timing of skin reactions is often a useful diagnostic tool. In general, the onset occurs within a few weeks of the introduction of the causative drug. If a medication has been taken for many years with no problem, it is less likely to be responsible. When examining a list of medications taken by a patient with a rash, new drugs taken within the previous month are the most likely cause [220].
On visiting a patient with a supposed drug-induced skin reaction, all recently taken medications should be noted, including herbal drugs, vaccines, etc. We should also take into account if the patient has taken the drug previously and the commercial name of the medicine, for various skin reactions (such as some urticarias) are secondary to the excipients [220].
Many aspects influence the appearance and severity of the drug-induced skin reactions. Baumgart et al recently published the characteristics of skin reactions induced by adalimumab in a cohort of patients. They observed that longer disease duration, lower dose induction schedule, and concomitant use of steroids or immunosuppressants were more often associated with an unfavorable skin outcome.
As in all aspects of IBD, a multidisciplinary view of the patient should be undertaken. In Baumgart’s study, skin outcomes differed significantly between patients who saw a dermatologist and/or had a dermatological intervention. They recommended consultation with a dermatologist when skin reaction appears in the context of recent administration of adalimumab [221].
Generally, drug-induced side effects disappear on removing the drug, although sometimes specific treatment or no treatment at all is required. For example, to prevent the appearance of skin rash with infliximab, premedication with corticosteroids and antihistamines is recommended. When rash appears, the extent and, especially, the accompanying symptoms, will indicate the need to stop infliximab or not.
Anti-TNF agents have also been reported to induce the appearance of psoriasis or exacerbate a pre-existing disease, even though they are part of the treatment armamentarium for that same disorder. Wollina et al studied patients with rheumatoid arthritis, IBD, psoriasis, etc, who had pustular lesions during TNF-α inhibitor treatment. He observed that they most frequently had psoriasis which disappeared after removing the drug, sometimes by changing to a different anti-TNF, and occasionally just with topical treatment, but that other times cessation of the drug only achieved stabilization of the disease [222].
Some mild skin reactions such as local reddening of the skin at the injection site of methotrexate or adalimumab, do not require treatment. Other side effects like erythema multiforme, erythema nodosum etc, require specific treatment, in addition to removing the responsible drug, as is done in drug-unrelated cases. Case reports of specific drug desensitization have been described. Akahoshi et al reported the successful desensitization of a sulfasalazine-induced skin rash in a patient with UC [223].
In conclusion, dermatologic lesions are frequent in IBD patients, who should be examined for cutaneous alterations upon presentation and during the course of the diseas e, not only because of the large variety of associated skin disorders, but also because of the different type of medications that are used in the IBD treatment. The long-term toxicity and carcinogenicity of immunomodulators and biologic agents are still being investigated [224]. The etiology of cutaneous disorders associated to IBD is quite diverse and, therefore, treatment should be individualized and directed to treating the underlying IBD as well as the specific dermatologic condition.
The intention of this review has been to offer the therapeutic recommendations for IBD-related skin disorders that are present in the literature. However, in the absence of prospective and randomized studies, treatment should always be individualized and include the multidisciplinary focus of both the Dermatologist and Gastroenterologist.
Biography
University Clinic Hospital of Valencia, Spain
Footnotes
Conflict of Interest: None
References
- 1.Kethu SR. Extraintestinal manifestations of inflammatory bowel diseases. J Clin Gastroenterol. 2006;40:467–475. doi: 10.1097/00004836-200607000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:546–550. doi: 10.1002/ibd.20807. [DOI] [PubMed] [Google Scholar]
- 3.Georgiou S, Pasmatzi E, Monastirli A, Tsambaos D. Cutaneous manifestations of inflammatory bowel disease. Hosp Chron. 2006;1:158–168. [Google Scholar]
- 4.Gregory B, Ho VC. Cutaneous manifestations of gastrointestinal disorders. Part II. J Am Acad Dermatol. 1992;26:371–383. doi: 10.1016/0190-9622(92)70059-o. [DOI] [PubMed] [Google Scholar]
- 5.Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn's disease. J Pediatr. 2001;138:767–771. doi: 10.1067/mpd.2001.113008. [DOI] [PubMed] [Google Scholar]
- 6.Rowland M, Fleming P, Bourke B. Looking in the mouth for Crohn´s disease. Inflamm Bowel Dis. 2010;16:332–337. doi: 10.1002/ibd.20983. [DOI] [PubMed] [Google Scholar]
- 7.Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. JEADV. 2008;22:1033–1043. doi: 10.1111/j.1468-3083.2008.02741.x. [DOI] [PubMed] [Google Scholar]
- 8.Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn's disease. An analysis of 79 cases. J Clin Gastroenterol. 1991;13:29–37. doi: 10.1097/00004836-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Fatahzadeh M, Schwartz RA, Kapila R, Rochford C. Orofacial Crohn's disease: an oral enigma. Acta Dermatovenerol Croat. 2009;17:289–300. [PubMed] [Google Scholar]
- 10.Quezada S, Turner PL, Alexiev B, Daly B, Cross R. Severe refractory orofacial Crohn's disease: report of a case. Dig Dis Sci. 2009;54:2290–2295. doi: 10.1007/s10620-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 11.Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn's disease. Clin Gastroenterol Hepatol. 2005;3:886–891. doi: 10.1016/s1542-3565(05)00424-6. [DOI] [PubMed] [Google Scholar]
- 12.White A, Nunes C, Escudier M, et al. Improvement in orofacial granulomatosis on a cinnamon- and benzoate-free diet. Inflamm Bowel Dis. 2006;12:508–514. doi: 10.1097/00054725-200606000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Bressler B, Sands BE. Review article: medical therapy for fistulizing Crohn's disease. Aliment Pharmacol Ther. 2006;24:1283–1293. doi: 10.1111/j.1365-2036.2006.03126.x. [DOI] [PubMed] [Google Scholar]
- 14.Brunner B, Hirschi C, Weimann R, et al. Treatment-resistant lingual Crohn's disease disappears after infliximab. Scand J Gastroenterol. 2005;40:1255–1259. doi: 10.1080/00365520510023512. [DOI] [PubMed] [Google Scholar]
- 15.Mahadevan U, Sandborn WJ. Infliximab for the treatment of orofacial Crohn's disease. Inflamm Bowel Dis. 2001;7:38–42. doi: 10.1097/00054725-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Doherty G, Kalachand R, Patchett S. Adalimumab as therapy for fistulizing orofacial Crohn's disease. Inflamm Bowel Dis. 2010;16:184–185. doi: 10.1002/ibd.20958. [DOI] [PubMed] [Google Scholar]
- 17.Ploysangam T, Heubi JE, Eisen D, Balistreri WF, Lucky AW. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997;36:697–704. doi: 10.1016/s0190-9622(97)80320-9. [DOI] [PubMed] [Google Scholar]
- 18.Yu JTHT, Chong LY, Lee KC. Metastatic Crohn's disease in a Chinese girl. Hong Kong Med J. 2006;12:467–469. [PubMed] [Google Scholar]
- 19.Farhi D, Duriez P, Aractingi S, Cosnes J, Khosrotehrani K. Misleading pustular plaques of the lower limbs during Crohn´s disease: two cases reports. J Med Case Reports. 2007;1:109–112. doi: 10.1186/1752-1947-1-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González Gómez JM, Sierra Salinas C, Alonso Usabiaga I, Barco Gálvez A, del Río Mapelli L, García Lorenzo C. Enfermedad de Crohn metastásica. An Esp Pediatr. 2001;55:165–168. [PubMed] [Google Scholar]
- 21.Emmanuel PO, Phelps RG. Metastatic Crohn´s disease: a histopathologic study of 12 cases. J Cutan Pathol. 2008;35:457–461. doi: 10.1111/j.1600-0560.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 22.Delgado J, Delgado B, Cagnano E, Sperber AD, Fich A. Presentation of Crohn´s disease as metastatic cutaneous non-caseating granulomatous lesions. IMAJ. 2003;5:897–898. [PubMed] [Google Scholar]
- 23.Graham DB, Jafer DL, Borum ML. Metastatic Crohn´s disease of the face. Dig Dis Sci. 2006;51:2062–2063. doi: 10.1007/s10620-006-9533-2. [DOI] [PubMed] [Google Scholar]
- 24.Goyal A, Mansel RE, Young HL, Douglas-Jones A. Metastatic cutaneous Crohn´s disease of the nipple: report of a case. Dis Colon Rectum. 2006;49:1324. doi: 10.1007/s10350-005-0218-2. [DOI] [PubMed] [Google Scholar]
- 25.Staines KS, Green R, Felix DH. The management of fistulizing oral Crohn's disease with infliximab. J Oral Pathol Med. 2007;36:444–446. doi: 10.1111/j.1600-0714.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 26.Eames T, Landthaler M, Karrer S. Crohn´s disease: an important differential diagnosis of granulomatous skin diseases. Eur J Dermatol. 2009;19:360–364. doi: 10.1684/ejd.2009.0674. [DOI] [PubMed] [Google Scholar]
- 27.Guest GD, Fink RL. Metastatic Crohn´s disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000;43:1764–1766. doi: 10.1007/BF02236866. [DOI] [PubMed] [Google Scholar]
- 28.Kafity AA, Pellegrini AE, Fromkes JJ. Metastatic Crohn´s disease. A rare cutaneous manifestation. J Clin Gastroenterol. 1993;17:300–303. [PubMed] [Google Scholar]
- 29.McGillis ST, Huntley AC. Metastatic Crohn´s disease. West J Med. 1989;151:203–205. [PMC free article] [PubMed] [Google Scholar]
- 30.Gilson MR, Elston LC, Pruitt CA. Metastatic Crohn´s disease: remission induced by mesalamine and prednisone. J Am Acad Dermatol. 1999;41:476–479. doi: 10.1016/s0190-9622(99)70125-8. [DOI] [PubMed] [Google Scholar]
- 31.Carranza DC, Young L. Successful treatment of metastatic Crohn´s disease with cyclosporine. J Drugs Dermatol. 2008;7:789–791. [PubMed] [Google Scholar]
- 32.Sierra C, Barco A, del Río L. Treatment of metastatic Crohn´s disease. J Pediatr Gastroenterol Nutr. 2002;35:708–709. doi: 10.1097/00005176-200211000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Casson DH, Eltumi M, Tomlin S, Walker-Smith JA, Murch SH. Topical tacrolimus may be effective in the treatment of oral and perineal Crohn's disease. Gut. 2000;47:436–440. doi: 10.1136/gut.47.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dullemen HM, de Jong E, Slors F, Tytgat GN, van Deventer SJ. Treatment of therapy-resistant perineal metastatic Crohn's disease after proctectomy using anti-tumor necrosis factor chimeric monoclonal antibody, cA2: Report of two cases. Dis Colon Rectum. 1998;41:98–102. doi: 10.1007/BF02236903. [DOI] [PubMed] [Google Scholar]
- 35.Miller AM, Elliott PR, Fink R, Connell W. Rapid response of severe refractory metastatic Crohn's disease to infliximab. J Gastroenterol Hepatol. 2001;16:940–942. doi: 10.1046/j.1440-1746.2001.02439.x. [DOI] [PubMed] [Google Scholar]
- 36.Escher JC, Stoof TJ, van Deventer SJ, van Furth AM. Successful treatment of metastatic Crohn disease with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:420–423. doi: 10.1097/00005176-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Rispo A, Lembo G, Insabato L, Cozzolino A, Pesce G, Castiglione F. Successful treatment of therapy-resistant metastatic Crohn's disease with infliximab. Br J Dermatol. 2004;150:1045–1046. doi: 10.1111/j.1365-2133.2004.05958.x. [DOI] [PubMed] [Google Scholar]
- 38.Graham DB, Jager DL, Borum ML. Metastatic Crohn's disease of the face. Dig Dis Sci. 2006;51:2062–2063. doi: 10.1007/s10620-006-9533-2. [DOI] [PubMed] [Google Scholar]
- 39.Konrad A, Seibold F. Response of cutaneous Crohn's disease to infliximab and methotrexate. Dig Liver Dis. 2003;35:351–356. doi: 10.1016/s1590-8658(03)00080-x. [DOI] [PubMed] [Google Scholar]
- 40.Martín de Carpi J, Chávez Caraza K, Vicente Villa MA, González Ensenat MA, Vilar Escrigas P, Vila Miravet V. Manifestaciones cutáneas de la enfermedad inflamatoria intestinal. An Pediatr. 2009;70:570–577. doi: 10.1016/j.anpedi.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Petrolati A, Altavilla N, Cipolla R, et al. Cutaneous Metastatic Crohn´s Disease Responsive to Infliximab. Am J Gastroenterol. 2009;104:1058–1059. doi: 10.1038/ajg.2008.171. [DOI] [PubMed] [Google Scholar]
- 42.Makhija S, Trotter M, Wagner E, Coderre S, Panaccione R. Refractory Crohn´s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835–837. doi: 10.1155/2007/737640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Velden JJ, van Marion AM, Kremer B, Straetmans JM, Henquet CJ, Frank J. Erythema nodosum as an early sign of Crohn's disease. Int J Dermatol. 2007;46(Suppl 3):27–29. doi: 10.1111/j.1365-4632.2007.03507.x. [DOI] [PubMed] [Google Scholar]
- 44.Requena L, Sánchez Yus E. Erythema nodosum. Semin Cutan Med Surg. 2007;26:114–125. doi: 10.1016/j.sder.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Gregory B, Ho VC. Cutaneous manifestations of gastrointestinal disorders. Part II. J Am Acad Dermatol. 1992;26:371–383. doi: 10.1016/0190-9622(92)70059-o. [DOI] [PubMed] [Google Scholar]
- 46.Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol. 2008;26:265–273. doi: 10.1016/j.clindermatol.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Mana J, Marcoval J. Erythema nodosum. Clin Dermatol. 2007;25:288–294. doi: 10.1016/j.clindermatol.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320–327. doi: 10.1111/j.1529-8019.2010.01332.x. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician. 2007;75:695–700. [PubMed] [Google Scholar]
- 50.Tavarela Veloso F. Review article: skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):50–53. doi: 10.1111/j.1365-2036.2004.02055.x. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt KJ, Fellermann K, Wellhöner P, et al. Clinical trial: cyclophosphamide pulse therapy - a promising therapeutic alternative in refractory Crohn's disease. Aliment Pharmacol Ther. 2009;29:1230–1239. doi: 10.1111/j.1365-2036.2009.03999.x. [DOI] [PubMed] [Google Scholar]
- 52.Goudet P, Dozois RR, Kelly KA, Ilstrup DM, Phillips SF. Characteristics and evolution of extraintestinal manifestations associated with ulcerative colitis after proctocolectomy. Dig Surg. 2001;18:51–55. doi: 10.1159/000050097. [DOI] [PubMed] [Google Scholar]
- 53.Fukunaga K, Sawada K, Fukuda Y, et al. Extracorporeal monocyte granulocytapheresis was effective for a patient of erythema nodosum concomitant with ulcerative colitis. Ther Apher Dial. 2003;7:122–126. doi: 10.1046/j.1526-0968.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 54.Conrad C, Trüeb RM. Pyoderma gangrenosum. J Dtsch Dermatol Ges. 2005;3:334–342. doi: 10.1111/j.1610-0387.2005.05022.x. [DOI] [PubMed] [Google Scholar]
- 55.Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10:301–312. doi: 10.2165/11310730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008–1017. doi: 10.1111/j.1468-3083.2009.03199.x. [DOI] [PubMed] [Google Scholar]
- 57.Miller J, Yentzer BA, Clark A, Jorizzo JL, Feldman SR. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646–654. doi: 10.1016/j.jaad.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 58.Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273–283. doi: 10.1016/j.jaad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Ahmadi S, Powell FC. Pyoderma gangrenosum: uncommon presentations. Clin Dermatol. 2005;23:612–620. doi: 10.1016/j.clindermatol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Wollina U. Pyoderma gangrenosum--a review. Orphanet J Rare Dis. 2007;2:19. doi: 10.1186/1750-1172-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saigal R, Singh Y, Mittal M, Kansal A, Maharia HR. Pyoderma gangrenosum. J Assoc Physicians India. 2010;58:378–383. [PubMed] [Google Scholar]
- 62.Bhat RM. Management of pyoderma gangrenosum--an update. Indian J Dermatol Venereol Leprol. 2004;70:329–335. [PubMed] [Google Scholar]
- 63.Wollina U, Karamfilov T. Treatment of recalcitrant ulcers in pyoderma gangrenosum with mycophenolate mofetil and autologous keratinocyte transplantation on a hyaluronic acid matrix. J Eur Acad Dermatol Venereol. 2000;14:187–190. doi: 10.1046/j.1468-3083.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- 64.Lyon CC, Smith AJ, Beck MH, Wong GA, Griffiths CE. Parastomal pyoderma gangrenosum: clinical features and management. J Am Acad Dermatol. 2000;42:992–1002. [PubMed] [Google Scholar]
- 65.Rozen SM, Nahabedian MY, Manson PN. Management strategies for pyoderma gangrenosum: case studies and review of literature. Ann Plast Surg. 2001;47:310–315. doi: 10.1097/00000637-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 66.Kim TY, Han DS, Eun CS, Chung YW. Recombinant human epidermal growth factor enhances wound healing of pyoderma gangrenosum in a patient with ulcerative colitis. Inflamm Bowel Dis. 2008;14:725–727. doi: 10.1002/ibd.20327. [DOI] [PubMed] [Google Scholar]
- 67.Chow RK, Ho VC. Treatment of pyoderma gangrenosum. J Am Acad Dermatol. 1996;34:1047–1060. doi: 10.1016/s0190-9622(96)90285-6. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel J, Gerdsen R, Phillipp-Dormston W, Bieber T, Uerlich M. Topical treatment of pyoderma gangraenosum. Dermatology. 2002;205:221–223. doi: 10.1159/000065843. [DOI] [PubMed] [Google Scholar]
- 69.Aseni P, Di Sandro S, Mihaylov P, Lamperti L, De Carlis LG. Atypical presentation of pioderma gangrenosum complicating ulcerative colitis: rapid disappearance with methylprednisolone. World J Gastroenterol. 2008;14:5471–5473. doi: 10.3748/wjg.14.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friedman S, Marion JF, Scherl E, Rubin PH, Present DH. Intravenous cyclosporine in refractory pyoderma gangrenosum complicating inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:1–7. doi: 10.1097/00054725-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Schöfer H, Baur S. Successful treatment of postoperative pyoderma gangrenosum with cyclosporin. J Eur Acad Dermatol Venereol. 2002;16:148–151. doi: 10.1046/j.1468-3083.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 72.Baumgart DC, Wiedenmann B, Dignass AU. Successful therapy of refractory pyoderma gangrenosum and periorbital phlegmona with tacrolimus (FK506) in ulcerative colitis. Inflamm Bowel Dis. 2004;10:421–424. doi: 10.1097/00054725-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 73.Hasselmann DO, Bens G, Tilgen W, Reichrath J. Pyoderma gangrenosum: clinical presentation and outcome in 18 cases and review of the literature. J Dtsch Dermatol Ges. 2007;5:560–564. doi: 10.1111/j.1610-0387.2007.0328.x. [DOI] [PubMed] [Google Scholar]
- 74.Fujimoto E, Fujimoto N, Kuroda K, Tajima S. Leukocytapheresis treatment for pyoderma gangrenosum. Br J Dermatol. 2004;151:1090–1092. doi: 10.1111/j.1365-2133.2004.06249.x. [DOI] [PubMed] [Google Scholar]
- 75.Reguiaï Z, Grange F. The role of anti-tumor necrosis factor-alpha therapy in Pyoderma gangrenosum associated with inflammatory bowel disease. Am J Clin Dermatol. 2007;8:67–77. doi: 10.2165/00128071-200708020-00002. [DOI] [PubMed] [Google Scholar]
- 76.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 77.Barrie A, Regueiro M. Biologic therapy in the management of extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1424–1429. doi: 10.1002/ibd.20196. [DOI] [PubMed] [Google Scholar]
- 78.Alkhouri N, Hupertz V, Mahajan L. Adalimumab treatment for peristomal pyoderma gangrenosum associated with Crohn's disease. Inflamm Bowel Dis. 2009;15:803–806. doi: 10.1002/ibd.20748. [DOI] [PubMed] [Google Scholar]
- 79.Juillerat P, Mottet C, Pittet V, et al. Extraintestinal manifestations of Crohn's disease. Digestion. 2007;76:141–148. doi: 10.1159/000111029. [DOI] [PubMed] [Google Scholar]
- 80.Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505–509. doi: 10.1136/gut.2005.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kouklakis G, Moschos J, Leontiadis GI, et al. Infliximab for treatment of pyoderma gangrenosum associated with clinically inactive Crohn's disease. A case report. Rom J Gastroenterol. 2005;14:401–403. [PubMed] [Google Scholar]
- 82.Ermis F, Ozdil S, Akyüz F, Pinarbasi B, Mungan Z. Pyoderma gangrenosum treated with infliximab in inactive ulcerative colitis. Inflamm Bowel Dis. 2008;14:1611–1613. doi: 10.1002/ibd.20481. [DOI] [PubMed] [Google Scholar]
- 83.Gelber AC. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;363:1086. doi: 10.1056/NEJMc1005805. [DOI] [PubMed] [Google Scholar]
- 84.Tada M, Nakanishi T, Hirata C, et al. Use of infliximab in a patient with pyoderma gangrenosum and rheumatoid arthritis. Mod Rheumatol. 2010;20:598–601. doi: 10.1007/s10165-010-0336-0. [DOI] [PubMed] [Google Scholar]
- 85.Chia MW, Teo L, Tay YK, Poh WT. Pustular pyoderma gangrenosum: an uncommon variant which is easily misdiagnosed. Dermatol Online J. 2008;14:21. [PubMed] [Google Scholar]
- 86.Badgwell C, Rosen T. Penile pyoderma gangrenosum. Dermatol Online J. 2006;12:8. [PubMed] [Google Scholar]
- 87.Scarfe WC. “All that glitters is not gold”: standards for cone-beam computerized tomographic imaging. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:402–408. doi: 10.1016/j.tripleo.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Lourenço SV, Hussein TP, Bologna SB, Sipahi AM, Nico MM. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204–207. doi: 10.1111/j.1468-3083.2009.03304.x. [DOI] [PubMed] [Google Scholar]
- 89.Hegarty AM, Barrett AW, Scully C. Pyostomatitis vegetans. Clin Exp Dermatol. 2004;29:1–7. doi: 10.1111/j.1365-2230.2004.01438.x. [DOI] [PubMed] [Google Scholar]
- 90.VanHale HM, Rogers RS, 3rd, Zone JJ, Greipp PR. Pyostomatitis vegetans. A reactive mucosal marker for inflammatory disease of the gut. Arch Dermatol. 1985;121:94–98. doi: 10.1001/archderm.121.1.94. [DOI] [PubMed] [Google Scholar]
- 91.Konstantopoulou M, O’Dwyer EM, Steele JC, Field EA, Lewis MA, Macfarlane AW. Pyodermatitis-pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666–668. doi: 10.1111/j.1365-2230.2005.01906.x. [DOI] [PubMed] [Google Scholar]
- 92.Soriano ML, Martínez N, Grilli R, Fariña MC, Martín L, Requena L. Pyodermatitis-pyostomatitis vegetans: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:322–326. doi: 10.1016/s1079-2104(99)70216-7. [DOI] [PubMed] [Google Scholar]
- 93.Storwick GS, Prihoda MB, Fulton RJ, Wood WS. Pyodermatitis-pyostomatitis vegetans: a specific marker for inflammatory bowel disease. J Am Acad Dermatol. 1994;31:336–341. doi: 10.1016/s0190-9622(94)70167-9. [DOI] [PubMed] [Google Scholar]
- 94.Yasuda M, Amano H, Nagai Y, Tamura A, Ishikawa O, Yamaguchi S. Pyodermatitis-pyostomatitis vegetans associated with ulcerative colitis: successful treatment with total colectomy and topical tacrolimus. Dermatology. 2008;217:146–148. doi: 10.1159/000135708. [DOI] [PubMed] [Google Scholar]
- 95.Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn's disease. Br J Dermatol. 2003;149:181–184. doi: 10.1046/j.1365-2133.2003.05385.x. [DOI] [PubMed] [Google Scholar]
- 96.Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. Report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447–454. doi: 10.1016/j.tripleo.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 97.Femiano F, Lanza A, Buonaiuto C, Perillo L, Dell’Ermo A, Cirillo N. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114–E117. [PubMed] [Google Scholar]
- 98.Calobrisi SD, Mutasim DF, McDonald JS. Pyostomatitis vegetans associated with ulcerative colitis. Temporary clearance with fluocinonide gel and complete remission after colectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:452–454. doi: 10.1016/s1079-2104(05)80126-x. [DOI] [PubMed] [Google Scholar]
- 99.Paoluzi OA, Crispino P, Amantea A, et al. Diffuse febrile dermatosis in a patient with active ulcerative colitis under treatment with steroids and azathioprine: a case of Sweet's syndrome. Case report and review of literature. Dig Liver Dis. 2004;36:361–366. doi: 10.1016/j.dld.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 100.Anglada Pintado JC, Michán Doña A, Silva Abad A, et al. Síndrome de Sweet y enfermedad inflamatoria intestinal. Aportación de un nuevo caso y revisión de la literatura. An Med Interna (Madrid) 2002;19:419–422. [PubMed] [Google Scholar]
- 101.Malheiros AP, Teixeira MG, Takahashi MD, de Almeida MG, Kiss DR, Cecconello I. Sweet syndrome associated with ulcerative colitis. Inflamm Bowel Dis. 2007;13:1583–1584. doi: 10.1002/ibd.20227. [DOI] [PubMed] [Google Scholar]
- 102.Pérez Rodríguez MT, Martínez-Ares D, Martín-Granizo I, Pallarés Peral A. Sweet's syndrome and erythema nodosum in a Crohn's disease patient. Med Clin (Barc) 2007;128:156–157. doi: 10.1016/s0025-7753(07)72519-8. [DOI] [PubMed] [Google Scholar]
- 103.Catalán-Serra I, Martín-Moraleda L, Navarro-López L, et al. Crohn's disease and Sweet's syndrome: an uncommon association. Rev Esp Enferm Dig. 2010;102:331–337. doi: 10.4321/s1130-01082010000500009. [DOI] [PubMed] [Google Scholar]
- 104.Foster EN, Nguyen KK, Sheikh RA, Prindiville TP. Crohn's disease associated with Sweet's syndrome and Sjögren's syndrome treated with infliximab. Clin Dev Immunol. 2005;12:145–149. doi: 10.1080/17402520500134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen PR, Kurzrock R. Sweet's syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761–778. doi: 10.1046/j.1365-4362.2003.01891.x. [DOI] [PubMed] [Google Scholar]
- 106.Cohen PR, Kurzrock R. Sweet's syndrome: a review of current treatment options. Am J Clin Dermatol. 2002;3:117–131. doi: 10.2165/00128071-200203020-00005. [DOI] [PubMed] [Google Scholar]
- 107.Vij A, Modi GM, Suwattee P, Cockerell CJ, Hsu S. Chronic, recurrent neutrophilic dermatosis: a case report. Dermatol Online J. 2010;16:1. [PubMed] [Google Scholar]
- 108.Kaur MR, Bazza MA, Ryatt KS. Neutrophilic dermatosis of the dorsal hands treated with indomethacin. Br J Dermatol. 2006;155:1089–1090. doi: 10.1111/j.1365-2133.2006.07491.x. [DOI] [PubMed] [Google Scholar]
- 109.Smith HR, Ashton RE, Beer TW, Theaker JM. Neutrophil-poor Sweet's syndrome with response to potassium iodide. Br J Dermatol. 1998;139:555–556. doi: 10.1046/j.1365-2133.1998.02439.x. [DOI] [PubMed] [Google Scholar]
- 110.Ritter S, George R, Serwatka LM, Elston DM. Long-term suppression of chronic Sweet's syndrome with colchicine. J Am Acad Dermatol. 2002;47:323–324. doi: 10.1067/mjd.2002.121028. [DOI] [PubMed] [Google Scholar]
- 111.Maillard H, Leclech C, Peria P, Avenel-Audran M, Verret JL. Colchicine for Sweet's syndrome. A study of 20 cases. Br J Dermatol. 1999;140:565–566. doi: 10.1046/j.1365-2133.1999.02747.x. [DOI] [PubMed] [Google Scholar]
- 112.von den Driesch P, Steffan C, Zöbe A, Hornstein OP. Sweet's syndrome--therapy with cyclosporin. Clin Exp Dermatol. 1994;19:274–277. doi: 10.1111/j.1365-2230.1994.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 113.Spencer B, Nanavati A, Greene J, Butler DF. Dapsone-responsive histiocytoid Sweet's syndrome associated with Crohn's disease. J Am Acad Dermatol. 2008;59(2 Suppl 1):S58–S60. doi: 10.1016/j.jaad.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 114.Jeanfils S, Joly P, Young P, Le Corvaisier-Pieto C, Thomine E, Lauret P. Indomethacin treatment of eighteen patients with Sweet's syndrome. J Am Acad Dermatol. 1997;36:436–439. doi: 10.1016/s0190-9622(97)80222-8. [DOI] [PubMed] [Google Scholar]
- 115.Joe EK. Sweet syndrome. Dermatol Online J. 2003;9:28. [PubMed] [Google Scholar]
- 116.Cohen PR, Holder WR. Pentoxifylline for Sweet's syndrome. J Am Acad Dermatol. 1995;32:533–535. doi: 10.1016/0190-9622(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 117.Su WP, Fett DL, Gibson LE, Pittelkow MR. Sweet syndrome: acute febrile neutrophilic dermatosis. Semin Dermatol. 1995;14:173–178. doi: 10.1016/s1085-5629(05)80015-x. [DOI] [PubMed] [Google Scholar]
- 118.Delluc A, Limal N, Puéchal X, Francès C, Piette JC, Cacoub P. Efficacy of anakinra, an IL1 receptor antagonist, in refractory Sweet syndrome. Ann Rheum Dis. 2008;67:278–279. doi: 10.1136/ard.2006.068254. [DOI] [PubMed] [Google Scholar]
- 119.Lear JT, Atherton MT, Byrne JP. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet's syndrome. Postgrad Med J. 1997;73:65–68. doi: 10.1136/pgmj.73.856.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Joshi RK, Atukorala DN, Abanmi A, al Khamis O, Haleem A. Successful treatment of Sweet's syndrome with doxycycline. Br J Dermatol. 1993;128:584–586. doi: 10.1111/j.1365-2133.1993.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 121.Banet DE, McClave SA, Callen JP. Oral metronidazole, an effective treatment for Sweet's syndrome in a patient with associated inflammatory bowel disease. J Rheumatol. 1994;21:1766–1768. [PubMed] [Google Scholar]
- 122.Rahier JF, Lion L, Dewit O, Lambert M. Regression of Sweet's syndrome associated with Crohn's disease after anti-Tumour Necrosis Factor therapy. Acta Gastroenterol Belg. 2005;68:376–379. [PubMed] [Google Scholar]
- 123.El-Azhary RA, Brunner KL, Gibson LE. Sweet syndrome as a manifestation of azathioprine hypersensitivity. Mayo Clin Proc. 2008;83:1026–1030. doi: 10.4065/83.9.1026. [DOI] [PubMed] [Google Scholar]
- 124.Treton X, Joly F, Alves A, Panis Y, Bouhnik Y. Azathioprine-induced Sweet's syndrome in Crohn's disease. Inflamm Bowel Dis. 2008;14:1757–1758. doi: 10.1002/ibd.20518. [DOI] [PubMed] [Google Scholar]
- 125.Yiasemides E, Thom G. Azathioprine hypersensitivity presenting as a neutrophilic dermatosis in a man with ulcerative colitis. Australas J Dermatol. 2009;50:48–51. doi: 10.1111/j.1440-0960.2008.00503.x. [DOI] [PubMed] [Google Scholar]
- 126.Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706–713. [PubMed] [Google Scholar]
- 127.Díaz-Pérez JL, De Lagrán ZM, Díaz-Ramón JL, Winkelmann RK. Cutaneous polyarteritis nodosa. Semin Cutan Med Surg. 2007;26:77–86. doi: 10.1016/j.sder.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 128.Komatsuda A, Kinoshita K, Togashi M, et al. Cutaneous polyarteritis nodosa in a patient with Crohn's disease. Mod Rheumatol. 2008;18:639–642. doi: 10.1007/s10165-008-0110-8. [DOI] [PubMed] [Google Scholar]
- 129.Matsumara Y, Mizuno K, Okamoto H, Imamura S. A case of cutaneous polyarteritis nodosa associated with ulcerative colitis. Br J Dermatol. 2000;142:561–562. doi: 10.1046/j.1365-2133.2000.03380.x. [DOI] [PubMed] [Google Scholar]
- 130.Gibson LE. Cutaneous vasculitis: approach to diagnosis and systemic associations. Mayo Clin Proc. 1990;65:221–229. doi: 10.1016/s0025-6196(12)65016-2. [DOI] [PubMed] [Google Scholar]
- 131.Goslen JB, Graham W, Lazarus GS. Cutaneous polyarteritis nodosa. Report of a case associated with Crohn's disease. Arch Dermatol. 1983;119:326–329. doi: 10.1001/archderm.119.4.326. [DOI] [PubMed] [Google Scholar]
- 132.Uthman I. Pharmacological therapy of vasculitis: an update. Curr Opin Pharmacol. 2004;4:177–182. doi: 10.1016/j.coph.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Volk DM, Owen LG. Cutaneous polyarteritis nodosa in a patient with ulcerative colitis. J Pediatr Gastroenterol Nutr. 1986;5:970–972. doi: 10.1097/00005176-198611000-00027. [DOI] [PubMed] [Google Scholar]
- 134.Ray TL, Levine JB, Weiss W, Ward PA. Epidermolysis bullosa acquisita and inflammatory bowel disease. J Am Acad Dermatol. 1982;6:242–252. doi: 10.1016/s0190-9622(82)70017-9. [DOI] [PubMed] [Google Scholar]
- 135.Hughes BR, Horne J. Epidermolysis bullosa acquisita and total ulcerative colitis. J R Soc Med. 1988;81:473–475. doi: 10.1177/014107688808100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chouvet B, Guillet G, Perrot H, Descos L. Acquired epidermolysis bullosa with Crohn's disease. Report of two cases and review of literature. Ann Dermatol Venereol. 1982;109:53–63. [PubMed] [Google Scholar]
- 137.Gluck M, Kayne A. Acquired epidermolysis bullosa and Crohn's disease. Gastrointest Endosc. 2003;57:563–564. doi: 10.1067/mge.2003.96. [DOI] [PubMed] [Google Scholar]
- 138.Raab B, Fretzin DF, Bronson DM, Scott MJ, Roenigk HH, Jr, Medenica M. Epidermolysis bullosa acquisita and inflammatory bowel disease. JAMA. 1983;250:1746–1748. [PubMed] [Google Scholar]
- 139.Chen M, O’Toole EA, Sanghavi J, et al. The epidermolysis bullosa acquisita antigen (type VII collagen) is present in human colon and patients with crohn's disease have autoantibodies to type VII collagen. J Invest Dermatol. 2002;118:1059–1064. doi: 10.1046/j.1523-1747.2002.01772.x. [DOI] [PubMed] [Google Scholar]
- 140.Lehman JS, Camilleri MJ, Gibson LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227–235. doi: 10.1111/j.1365-4632.2009.03886.x. [DOI] [PubMed] [Google Scholar]
- 141.Selby L, De Castro F, De Villiers WJ. The association of bullous pemphigoid and ulcerative colitis. Dig Dis Sci. 2004;49:1768–1770. doi: 10.1007/s10620-004-9567-2. [DOI] [PubMed] [Google Scholar]
- 142.Tripodi G, Risso M, Tenerini L, Gandullia P, Castellano E, Rivabella L. Drug-resistant bullous pemphigoid and inflammatory bowel disease in a pediatric case successfully treated by plasma exchange and extracorporeal photochemotherapy. J Clin Apher. 2007;22:26–30. doi: 10.1002/jca.20115. [DOI] [PubMed] [Google Scholar]
- 143.Ahmed AR, Kaplan RP, Hardy D, Feldman E, Pitt H. Bullous pemphigoid and ulcerative colitis. Int J Dermatol. 1982;21:594–598. doi: 10.1111/j.1365-4362.1982.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 144.Vaccaro M, D’Amico D, Borgia F, Guarneri F, Cannavò S. Bullous pemphigoid following use of sulphasalazine for ulcerative colitis: drug-induced eruption or true association? Dermatology. 2001;203:194–195. doi: 10.1159/000051744. [DOI] [PubMed] [Google Scholar]
- 145.Tirado-Sánchez A, Díaz-Molina V, Ponce-Olivera RM. Efficacy and safety of azathioprine and dapsone as an adjuvant in the treatment of bullous pemphigoid. Allergol Immunopathol (Madr) 2011 doi: 10.1016/j.aller.2010.12.009. in press. [DOI] [PubMed] [Google Scholar]
- 146.Birnie AJ, Perkins W. A case of linear IgA disease occurring in a patient with colonic Crohn's disease. Br J Dermatol. 2005;153:1050–1052. doi: 10.1111/j.1365-2133.2005.06888.x. [DOI] [PubMed] [Google Scholar]
- 147.Nanda A, Dvorak R, Al-Sabah H, Madda JP, Anim JT, Alsaleh QA. Association of linear IgA bullous disease of childhood with Crohn's disease. Int J Dermatol. 2006;45:1184–1186. doi: 10.1111/j.1365-4632.2006.02563.x. [DOI] [PubMed] [Google Scholar]
- 148.Keller AS, Bouldin MB, Drage LA, Hauser SC, Davis MD. Linear IgA bullous dermatosis: an association with ulcerative colitis versus renal cell carcinoma. Dig Dis Sci. 2003;48:783–789. doi: 10.1023/a:1022805329847. [DOI] [PubMed] [Google Scholar]
- 149.Egan CA, Zone JJ. Linear IgA bullous dermatosis. Int J Dermatol. 1999;38:818–827. doi: 10.1046/j.1365-4362.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- 150.Fernández-Guarino M, Sáez EM, Gijón RC, García BP, Olasolo PJ. Linear IGA dermatosis associated with ulcerative colitis. Eur J Dermatol. 2006;16:692–693. [PubMed] [Google Scholar]
- 151.Kenani N, Mebazaa A, Denguezli M, et al. Childhood linear IgA bullous dermatosis in Tunisia. Pediatr Dermatol. 2009;26:28–33. doi: 10.1111/j.1525-1470.2008.00817.x. [DOI] [PubMed] [Google Scholar]
- 152.Walker SL, Banerjee P, Harland CC, Black MM. Remission of linear IgA disease associated with ulcerative colitis following panproclocolectomy. Br J Dermatol. 2000;143:1341–1342. doi: 10.1046/j.1365-2133.2000.03928.x. [DOI] [PubMed] [Google Scholar]
- 153.Torres T, Sanches M, Selores M. Linear IgA bullous disease in a patient with Crohn's disease. Acta Dermatovenerol Alp Panonica Adriat. 2010;19:29–31. [PubMed] [Google Scholar]
- 154.Taniguchi T, Maejima H, Saito N, Katsuoka K, Haruki S. Case of linear IgA bullous dermatosis-involved ulcerative colitis. Inflamm Bowel Dis. 2009;15:1284–1285. doi: 10.1002/ibd.20795. [DOI] [PubMed] [Google Scholar]
- 155.Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33–57. doi: 10.1016/j.yadr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 156.Frisch M, Johansen C. Anal carcinoma in inflammatory bowel disease. Br J Cancer. 2000;83:89–90. doi: 10.1054/bjoc.2000.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O’Connell PR, Dozois RR, Irons GB, Scheithauer BW. Squamous cell carcinoma occurring in a skin-grafted ileostomy stoma. Report of a case. Dis Colon Rectum. 1987;30:475–478. doi: 10.1007/BF02556501. [DOI] [PubMed] [Google Scholar]
- 158.Devon KM, Brown CJ, Burnstein M, McLeod RS. Cancer of the anus complicating perianal Crohn's disease. Dis Colon Rectum. 2009;52:211–216. doi: 10.1007/DCR.0b013e318197d0ad. [DOI] [PubMed] [Google Scholar]
- 159.Slater G, Greenstein A, Aufses AH., Jr Anal carcinoma in patients with Crohn's disease. Ann Surg. 1984;199:348–350. doi: 10.1097/00000658-198403000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010;105:1480–1487. doi: 10.1038/ajg.2009.760. [DOI] [PubMed] [Google Scholar]
- 161.Kang J, Min BS, Lee KY, Jang SJ, Kim WH, Kim NK. Squamous cell carcinoma of the anus in a patient with perianal Crohn's disease. Int J Colorectal Dis. 2010;25:411–413. doi: 10.1007/s00384-009-0778-z. [DOI] [PubMed] [Google Scholar]
- 162.Gurudutt VV, Genden EM. Cutaneous squamous cell carcinoma of the head and neck. J Skin Cancer 2011. 2011:502723. doi: 10.1155/2011/502723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cleary RK, Schaldenbrand JD, Fowler JJ, Schuler JM, Lampman RM. Perianal Bowen's disease and anal intraepithelial neoplasia: review of the literature. Dis Colon Rectum. 1999;42:945–951. doi: 10.1007/BF02237107. [DOI] [PubMed] [Google Scholar]
- 164.Sarmiento JM, Wolff BG, Burgart LJ, Frizelle FA, Ilstrup DM. Perianal Bowen's disease: associated tumors, human papillomavirus, surgery, and other controversies. Dis Colon Rectum. 1997;40:912–918. doi: 10.1007/BF02051198. [DOI] [PubMed] [Google Scholar]
- 165.Kuhlgatz J, Golas MM, Sander B, Füzesi L, Hermann RM, Miericke B. Human papilloma virus infection in a recurrent squamous cell carcinoma associated with severe Crohn's disease. Inflamm Bowel Dis. 2005;11:84–86. doi: 10.1097/00054725-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 166.Fox LP, Pasternack FR, Geyer AS, Grossman ME. Perineal squamous cell cancer in a patient with fistulizing and ulcerating Crohn's disease. Clin Exp Dermatol. 2005;30:718–719. doi: 10.1111/j.1365-2230.2005.01884.x. [DOI] [PubMed] [Google Scholar]
- 167.Buchman AL, Ament ME, Doty J. Development of squamous cell carcinoma in chronic perineal sinus and wounds in Crohn's disease. Am J Gastroenterol. 1991;86:1829–1832. [PubMed] [Google Scholar]
- 168.Seya T, Tanaka N, Shinji S, et al. Squamous cell carcinoma arising from recurrent anal fistula. J Nippon Med Sch. 2007;74:319–324. doi: 10.1272/jnms.74.319. [DOI] [PubMed] [Google Scholar]
- 169.Van der Zee HH, van der Woude CJ, Florencia EF, Prens EP. Hidradenitis suppurativa and inflammatory bowel disease: are they associated? Results of a pilot study. Br J Dermatol. 2010;162:195–197. doi: 10.1111/j.1365-2133.2009.09430.x. [DOI] [PubMed] [Google Scholar]
- 170.Koilakou S, Karapiperis D, Tzathas C. A case of hidradenitis suppurativa refractory to anti-TNFalpha therapy in a patient with Crohn's disease. Am J Gastroenterol. 2010;105:231–232. doi: 10.1038/ajg.2009.489. [DOI] [PubMed] [Google Scholar]
- 171.Moschella SL. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? A report of three treated cases. Int J Dermatol. 2007;46:1287–1291. doi: 10.1111/j.1365-4632.2007.03293.x. [DOI] [PubMed] [Google Scholar]
- 172.Goertz RS, Konturek PC, Naegel A, et al. Experiences with a long-term treatment of a massive gluteal acne inversa with infliximab in Crohn's disease. Med Sci Monit. 2009;15:CS14–CS18. [PubMed] [Google Scholar]
- 173.Rosi YL, Lowe L, Kang S. Treatment of hidradenitis suppurativa with infliximab in a patient with Crohn's disease. J Dermatolog Treat. 2005;16:58–61. doi: 10.1080/09546630410024547. [DOI] [PubMed] [Google Scholar]
- 174.Lebwohl B, Sapadin AN. Infliximab for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2003;49(5 Suppl):S275–276. doi: 10.1016/s0190-9622(03)01132-0. [DOI] [PubMed] [Google Scholar]
- 175.Roussomoustakaki M, Dimoulios P, Chatzicostas C, et al. Hidradenitis suppurativa associated with Crohn's disease and spondyloarthropathy: response to anti-TNF therapy. J Gastroenterol. 2003;38:1000–1004. doi: 10.1007/s00535-003-1185-9. [DOI] [PubMed] [Google Scholar]
- 176.Katsanos KH, Christodoulou DK, Tsianos EV. Axillary hidradenitis suppurativa successfully treated with infliximab in a Crohn's disease patient. Am J Gastroenterol. 2002;97:2155–2156. doi: 10.1111/j.1572-0241.2002.05950.x. [DOI] [PubMed] [Google Scholar]
- 177.Martínez F, Nos P, Benlloch S, Ponce J. Hidradenitis suppurativa and Crohn's disease: response to treatment with infliximab. Inflamm Bowel Dis. 2001;7:323–326. doi: 10.1097/00054725-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 178.Moul DK, Korman NJ. The cutting edge. Severe hidradenitis suppurativa treated with adalimumab. Arch Dermatol. 2006;142:1110–1112. doi: 10.1001/archderm.142.9.1110. [DOI] [PubMed] [Google Scholar]
- 179.Giamarellos-Bourboulis EJ, Pelekanou E, Antonopoulou A, et al. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol. 2008;158:567–572. doi: 10.1111/j.1365-2133.2007.08372.x. [DOI] [PubMed] [Google Scholar]
- 180.Mortimore M, Florin TH. A role for B₁₂ in inflammatory bowel disease patients with suppurative dermatoses? An experience with high dose vitamin B₁₂ therapy. J Crohns Colitis. 2010;4:466–470. doi: 10.1016/j.crohns.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 181.Bleiziffer O, Dragu A, Kneser U, Horch RE. Solving acne inversa (hidradenitis suppurativa) in Crohn disease with buried chip skin grafts. J Cutan Med Surg. 2009;13:164–168. doi: 10.2310/7750.2008.08008. [DOI] [PubMed] [Google Scholar]
- 182.Greenstein AJ, Sachar DB, Panday AK, et al. Amyloidosis and inflammatory bowel disease. A 50-year experience with 25 patients. Medicine (Baltimore) 1992;71:261–270. doi: 10.1097/00005792-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 183.Wester AL, Vatn MH, Fausa O. Secondary amyloidosis in inflammatory bowel disease: a study of 18 patients admitted to Rikshospitalet University Hospital, Oslo, from 1962 to 1998. Inflamm Bowel Dis. 2001;7:295–300. doi: 10.1097/00054725-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 184.Serra I, Oller B, Mañosa M, Naves JE, Zabana Y, Cabré E. Systemic amyloidosis in inflammatory bowel disease: retrospective study on its prevalence, clinical presentation, and outcome. J Crohns Colitis. 2010;4:269–274. doi: 10.1016/j.crohns.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 185.Janczewska I, Mejhert M, Hast R, Runarsson G, Sandstedt B. Primary AL-amyloidosis, ulcerative colitis and collagenous colitis in a 57-year-old woman: a case study. Scand J Gastroenterol. 2004;39:1306–1309. doi: 10.1080/00365520410008105. [DOI] [PubMed] [Google Scholar]
- 186.Seijo Ríos S, Barreiro de Acosta M, Vieites Pérez-Quintela B, et al. Secondary amyloidosis in Crohn's disease. Rev Esp Enferm Dig. 2008;100:792–797. doi: 10.4321/s1130-01082008001200011. [DOI] [PubMed] [Google Scholar]
- 187.Iizuka M, Konno S, Horie Y, Itou H, Shindo K, Watanabe S. Infliximab as a treatment for systemic amyloidosis associated with Crohn's disease. Gut. 2006;55:744–745. doi: 10.1136/gut.2005.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Verschueren P, Lensen F, Lerut E, et al. Benefit of anti-TNFalpha treatment for nephrotic syndrome in a patient with juvenile inflammatory bowel disease associated spondyloarthropathy complicated with amyloidosis and glomerulonephritis. Ann Rheum Dis. 2003;62:368–369. doi: 10.1136/ard.62.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fernández-Nebro A, Ureña I, Irigoyen MV, García-Vicuña R. Anti-TNF-alpha for treatment of amyloidosis associated with Crohn's disease. Gut. 2006;55:1666–1667. [PMC free article] [PubMed] [Google Scholar]
- 190.Boscá MM, Pérez-Baylach CM, Solis MA, et al. Secondary amyloidosis in Crohn's disease: treatment with tumour necrosis factor inhibitor. Gut. 2006;55:294–295. doi: 10.1136/gut.2005.082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hatakeyama T, Komatsuda A, Matsuda A, et al. Renal amyloidosis associated with extracapillary glomerulonephritis and vasculitis in a patient with inflammatory bowel disease treated with infliximab. Clin Nephrol. 2008;70:240–244. doi: 10.5414/cnp70240. [DOI] [PubMed] [Google Scholar]
- 192.Park YK, Han DS, Eun CS. Systemic amyloidosis with Crohn's disease treated with infliximab. Inflamm Bowel Dis. 2008;14:431–432. doi: 10.1002/ibd.20289. [DOI] [PubMed] [Google Scholar]
- 193.Lotti T, Hercogova J, Prignano F. The concept of psoriatic disease: can cutaneous psoriasis any longer be separated by the systemic comorbidities? Dermatol Ther. 2010;23:119–122. doi: 10.1111/j.1529-8019.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 194.Kocak E, Ozer E, Filik L. Differential diagnosis of inflammatory bowel diseases in psoriasis. Acta Clin Belg. 2010;65:208–209. doi: 10.1179/acb.2010.047. [DOI] [PubMed] [Google Scholar]
- 195.Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn's disease. J Am Acad Dermatol. 2003;48:805–821. doi: 10.1067/mjd.2003.540. [DOI] [PubMed] [Google Scholar]
- 196.Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn's disease. J Eur Acad Dermatol Venereol. 2009;23:561–565. doi: 10.1111/j.1468-3083.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 197.Lee FI, Bellary SV, Francis C. Increased occurrence of psoriasis in patients with Crohn's disease and their relatives. Am J Gastroenterol. 1990;85:962–963. [PubMed] [Google Scholar]
- 198.Oh CJ, Das KM, Gottlieb AB. Treatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol. 2000;42:829–830. doi: 10.1067/mjd.2000.105948. [DOI] [PubMed] [Google Scholar]
- 199.Andreakos E, Taylor PC, Feldmann M. Monoclonal antibodies in immune and inflammatory diseases. Curr Opin Biotechnol. 2002;13:615–620. doi: 10.1016/s0958-1669(02)00355-5. [DOI] [PubMed] [Google Scholar]
- 200.Kuek A, Hazleman BL, Ostör AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83:251–260. doi: 10.1136/pgmj.2006.052688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mössner R, Schön MP, Reich K. Tumor necrosis factor antagonists in the therapy of psoriasis. Clin Dermatol. 2008;26:486–502. doi: 10.1016/j.clindermatol.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 202.De Vries HS, van Oijen MG, Driessen RJ, et al. Appropriate infliximab infusion dosage and monitoring: results of a panel meeting of rheumatologists, dermatologists and gastroenterologists. Br J Clin Pharmacol. 2011;71:7–19. doi: 10.1111/j.1365-2125.2010.03760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Karampetsou MP, Liossis SN, Sfikakis PP. TNF-α antagonists beyond approved indications: stories of success and prospects for the future. QJM. 2010;103:917–928. doi: 10.1093/qjmed/hcq152. [DOI] [PubMed] [Google Scholar]
- 204.Piérard GE, Piérard-Franchimont C, Szepetiuk G, Paquet P, Quatresooz P. The therapeutic potential of TNF-alpha antagonists for skin psoriasis comorbidities. Expert Opin Biol Ther. 2010;10:1197–1208. doi: 10.1517/14712598.2010.500283. [DOI] [PubMed] [Google Scholar]
- 205.Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088–1094. doi: 10.1053/gast.2001.28674. [DOI] [PubMed] [Google Scholar]
- 206.Schreiber S, Rutgeerts P, Fedorak RN, et al. CDP870 Crohn's Disease Study Group. A randomized, placebo-controlled trial of certolizumabpegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807–818. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 207.Gisondi P, Del Giglio M, Cozzi A, Girolomoni G. Psoriasis, the liver, and the gastrointestinal tract. Dermatol Ther. 2010;23:155–159. doi: 10.1111/j.1529-8019.2010.01310.x. [DOI] [PubMed] [Google Scholar]
- 208.Cingoz O. Ustekinumab. MAbs. 2009;1:216–221. doi: 10.4161/mabs.1.3.8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ryan C, Thrash B, Warren RB, Menter A. The use of ustekinumab in autoimmune disease. Expert Opin Biol Ther. 2010;10:587–604. doi: 10.1517/14712591003724670. [DOI] [PubMed] [Google Scholar]
- 210.Scherl EJ, Kumar S, Warren RU. Review of the safety and efficacy of ustekinumab. Therap Adv Gastroenterol. 2010;3:321–328. doi: 10.1177/1756283X10374216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lin AN. Innovative use of topical calcineurin inhibitors. Dermatol Clin. 2010;28:535–545. doi: 10.1016/j.det.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 212.Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4-Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.James DG, Seo DH, Chen J, Vemulapalli C, Stone CD. Efalizumab, a Human Monoclonal Anti-CD11a Antibody, in the Treatment of Moderate to Severe Crohn's Disease: An Open-Label Pilot Study. Dig Dis Sci. 2010 doi: 10.1007/s10620-010-1525-6. in press. [DOI] [PubMed] [Google Scholar]
- 214.Sehgal VN, Jain S. Acrodermatits enteropathica. Clin Dermatol. 2000;18:745–748. doi: 10.1016/s0738-081x(00)00150-4. [DOI] [PubMed] [Google Scholar]
- 215.Park CH, Lee MJ, Kim HJ, Lee G, Park JW, Cinn YW. Congenital zinc deficiency from mutations of the SLC39A4 gene as the genetic background of acrodermatitis enteropathica. J Korean Med Sci. 2010;25:1818–1820. doi: 10.3346/jkms.2010.25.12.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Medina-Murillo R, Rodríguez-Wong U. Manifestaciones cutáneas de las enfermedades inflamatorias intestinales. Rev Hosp Jua Mex. 2007;74:11–15. [Google Scholar]
- 217.Mariotti C, Gellera C, Rimoldi M, et al. Ataxia with isolated vitamin E deficiency: neurological phenotype, clinical follow-up and novel mutations in TTPA gene in Italian families. Neurol Sci. 2004;25:130–137. doi: 10.1007/s10072-004-0246-z. [DOI] [PubMed] [Google Scholar]
- 218.Rose BD, editor. UpToDate. Walham, MA: 2011. [access June 2011]. Lexicomp Inc. Mesalamine,Sulphasalazine, Azathioprine, 6-Mercaptopurine, Methotrexate, Cyclosporine, Systemic steroids, Infliximab, Adalimumab, Ciprofloxacine, Metronidazole, Tacrolimus, Thalidomide, GMCSF: Drug information [Internet] UpToDate. Available at: http://www.uptodate.com . [Google Scholar]
- 219.Fernández Herrera J, Requena Caballero L. Erupciones Medicamentosas Cutáneas. Área Científica Menarini, Signament Edics. 2004 [Google Scholar]
- 220.Lee A, Thomson J. Adverse Drug Reactions. 2nd Edition. Pharmaceutical Press; 2006. Drug-induced Skin Reactions. Chapter 5. [Google Scholar]
- 221.Baumgart DC, Grittner U, Steingräber A, Azzaro M, Philipp S. Frequency, phenotype, outcome, and therapeutic impact of skin reactions following initiation of adalimumab therapy: Experience from a consecutive cohort of inflammatory bowel disease patients. Inflamm Bowel Dis. 2011;17:2512–2520. doi: 10.1002/ibd.21643. [DOI] [PubMed] [Google Scholar]
- 222.Wollina U, Hansel G, Koch A, Schönlebe J, Köstler E, Haroske G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol. 2008;9:1–14. doi: 10.2165/00128071-200809010-00001. [DOI] [PubMed] [Google Scholar]
- 223.Akahoshi K, Chijiwa Y, Kabemura T, Okabe H, Akamine Y, Nawata H. Desensitization for sulfasalazine-induced skin rash in a patient with ulcerative colitis. J Gastroenterol. 1994;29:772–775. doi: 10.1007/BF02349286. [DOI] [PubMed] [Google Scholar]
- 224.Katsanos KH, Christodoulou DK, Kitsanou M, Basioukas K, Bai M, Tsianos EV. Perianal condylomata and HPV-negative ileostomal papillomatous lesion in Crohn's disease during Infliximab therapy Ann Gastroenterol. 2005;18:80–83. [Google Scholar]


