Abstract
Listeria monocytogenes produces severe fetoplacental infections in humans. How it targets and crosses the maternofetal barrier is unknown. We used immunohistochemistry to examine the location of L. monocytogenes in placental and amniotic tissue samples obtained from women with fetoplacental listeriosis. The results raised the possibility that L. monocytogenes crosses the maternofetal barrier through the villous syncytiotrophoblast, with secondary infection occurring via the amniotic epithelium. Because epidemiological studies indicate that the bacterial surface protein, internalin (InlA), may play a role in human fetoplacental listeriosis, we investigated the cellular patterns of expression of its host receptor, E-cadherin, at the maternofetal interface. E-cadherin was found on the basal and apical plasma membranes of syncytiotrophoblasts and in villous cytotrophoblasts. Established trophoblastic cell lines, primary trophoblast cultures, and placental villous explants were each exposed to isogenic InlA+ or InlA- strains of L. monocytogenes, and to L. innocua expressing or not InlA. Quantitative assays of cellular invasion demonstrated that bacterial entry into syncytiotrophoblasts occurs via the apical membrane in an InlA–E-cadherin dependent manner. In human placental villous explants, bacterial invasion of the syncytiotrophoblast barrier and underlying villous tissue and subsequent replication produces histopathological lesions that mimic those seen in placentas of women with listeriosis. Thus, the InlA–E-cadherin interaction that plays a key role in the crossing of the intestinal barrier in humans is also exploited by L. monocytogenes to target and cross the placental barrier. Such a ligand–receptor interaction allowing a pathogen to specifically cross the placental villous trophoblast barrier has not been reported previously.
Listeria monocytogenes is a food-borne pathogen that causes gastroenteritis, bacteremia, as well as CNS and maternofetal infections (1–3). Pregnant women constitute 60% of all cases of listeriosis in individuals <40 years of age (3). Their ≈20-fold higher risk of infection compared to otherwise healthy adults is presumed to be a consequence of the pregnancy-associated immunosuppression that allows tolerance of the fetoplacental allograft (4–7). Nonetheless, the predilection of the fetoplacental unit for infection raises the possibility that a specific mechanism may be responsible for targeting L. monocytogenes to the maternofetal barrier.
L. monocytogenes can enter cultured nonphagocytic human cells through a process mediated by the interaction of internalin (InlA), a bacterial surface protein, with E-cadherin, a cell surface transmembrane protein expressed by various epithelial lineages (8). InlA has a high degree of specificity for human E-cadherin: i.e., a single amino acid polymorphism between the orthologous human and mouse proteins is responsible for the relatively low pathogenicity of L. monocytogenes when administrated orally into mice (9). Studies of transgenic mice that express human E-cadherin in their intestinal epithelium and that have been orally inoculated with isogenic InlA+ or InlA-strains of L. monocytogenes, established that this bacterial protein plays an essential role in crossing the intestinal epithelial barrier (10).
A recent epidemiologic study indicates that InlA may also play a pivotal role in penetration of the maternofetal barrier (11). In this study, InlA expression was assessed by immunoblot assays of 300 clinical strains collected from sporadic cases of listeriosis occurring in France in a single year, plus a representative set of 150 strains obtained from food products during the same period. One hundred percent of L. monocytogenes isolates recovered from pregnant women (61 of 61) expressed the functional protein, whereas only 65% (98 of 150) of food isolates expressed it (P = 1 × 10-7) (11).
The human maternofetal barrier contains two anatomically distinct components: the chorioallantoic placenta and the chorioamnion (Fig. 5 A–E, which is published as supporting information on the PNAS web site). The barrier is formed at the placental level by the villous syncytiotrophoblast. This specialized epithelial lineage is in direct contact with maternal blood circulating through the intervillous space. In a subjacent layer, mononuclear cytotrophoblasts divide, differentiate, and fuse to renew overlying multinucleated syncytiotrophoblasts (Fig. 5 D and D′). A basement membrane separates these trophoblastic cells from a connective tissue core that contains fetal capillaries (Fig. 5 D and D′). The amniotic epithelium forms the maternofetal interface in the chorioamnion. The apical surface of this epithelium is exposed to amniotic fluid, whereas its basal surface sits on a basement membrane that overlies the amniotic mesoderm (Fig. 5 E and E′).
In contrast to the villous tree in the hemochorial placenta of the human (Fig. 5), rodent placentas are hemochorial and labyrinthine, with up to three layers of trophoblasts bathed by maternal blood and overlying the connective tissue of the labyrinthine core where fetal vessels reside (12). This arrangement results in a different maternofetal interface compared to human placental villi, with associated changes in maternal blood flow.
The species-specificity of InlA–E-cadherin interactions (9), the difference between placental structures of mice and humans (12), the absence of a transgenic mouse model where human E-cadherin is expressed in all epithelial lineages (13), plus our epidemiological findings (11) prompted us to examine the role of the InlA–E-cadherin interaction in crossing of the maternofetal barrier in humans by immunohistopathological studies of placentas from women with listeriosis, and by incubating strains of L. monocytogenes and Listeria innocua that did or did not express a functional InlA with (i) an established human trophoblast cell line (BeWo) that differentiates in vitro into syncytiotrophoblasts when exposed to cAMP, (ii) primary cultures of human cytotrophoblasts that spontaneously differentiate into syncytiotrophoblasts, and (iii) human placental villous explants. Our results demonstrate that InlA mediates attachment to E-cadherin receptors in syncytiotrophoblasts and subsequent invasion of the underlying villous tissue. Therefore, L. monocytogenes ability to target and cross the human maternofetal barrier relies on the interaction between the bacterial protein internalin and its cellular ligand E-cadherin at the villous trophoblast barrier level. Such a ligand–receptor interaction allowing a pathogen to specifically target and cross the placental villous trophoblast barrier has not been reported previously.
Materials and Methods
Bacterial Strains, Cell Lines, and Antibodies. See Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Placental Sections from Women with Pregnancy-Associated Listeriosis. Formalin-fixed, paraffin-embedded blocks of placental tissues and chorioamnion from seven women with maternofetal listeriosis were retrieved from the archived tissue collection of the Histopathology Department of the Cochin–Port Royal Hospital (Assistance Publique-Hôpitaux de Paris). Five-micrometer-thick sections of tissues were stained with hematoxylin and eosin. Adjacent sections were stained with R11 primary antibodies [final dilution = 1:500 in blocking buffer (1% BSA/0.3% Triton X-100 in PBS)], followed by horseradish peroxidase-conjugated monkey anti-rabbit Ig (1:400). Antigen–antibody complexes were visualized by using reagents supplied in the Envision kit (Dakocytomation, Carpinteria, CA). Sections were counterstained with hematoxylin.
Culture of Trophoblastic Cell Lines. See Supporting Materials and Methods.
Isolation and Culture of Primary Cytotrophoblasts/Syncytiotrophoblasts and Placental Villous Explants. Informed consent for the use of human placentas was obtained by using a protocol approved by the Institutional Review Board of Washington University School of Medicine. Placentas were obtained immediately after a repeat cesarean section delivery at the end an uncomplicated full-term pregnancy. None of the patients received antibiotics before the placental harvest.
Cytotrophoblasts were isolated by trypsin-DNase gradient centrifugation described by Kliman (14) with modifications (15). Cells were subsequently seeded in 24-well plates, at a density of 3 × 105 cells per cm2, in DMEM (Invitrogen/GIBCO) supplemented with 10% FBS (HyClone), incubated for 8 h or 96 h at 37°C in 5% CO2, and exposed to various bacterial strains.
A 0.5-cm-thick section of decidual basalis was removed from the placenta, and villous tissue was dissected from multiple cotyledons, avoiding the chorionic plate (see Fig. 6, which is published as supporting information on the PNAS web site). A single villous explant (average weight, 100 ± 10 mg; average size, 125 ± 5 mm3) was then placed in each well of a 24-well plate containing DMEM, and exposed to various bacterial strains.
Infections of Cells and Tissue Explants. See Supporting Materials and Methods.
Cell and Tissue Immunolabeling. See Supporting Materials and Methods.
Results
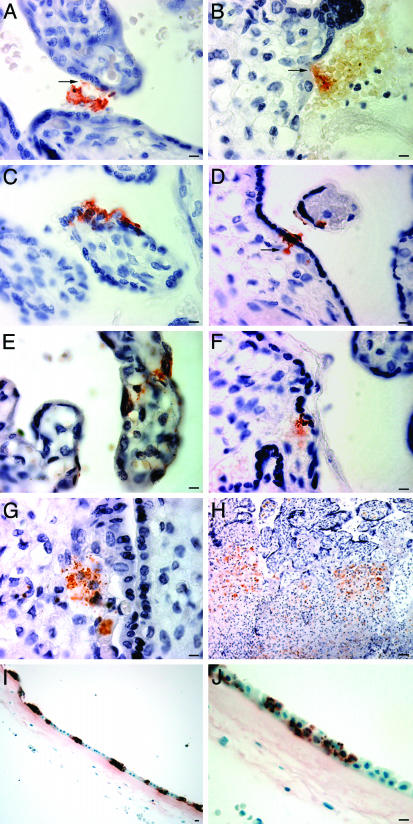
Immunohistologic Studies of Human Placentas from Patients with Listeriosis Suggest That Bacterial Invasion Follows a Transplacental Rather than a Transamniotic Route. We analyzed the location of L. monocytogenes in placentas obtained from women with listeriosis (n = 7). Immunohistochemical studies of multiple sections prepared from each placenta revealed blood-borne bacteria in intervillous spaces (Fig. 1A). Bacteria were also detected on the surfaces of syncytiotrophoblasts and cytotrophoblasts (Fig. 1 A and B), in the cytoplasm of syncytiotrophoblasts (Fig. 1C), and in villous core adjacent to fetal capillaries (Fig. 1 D–F). Isolated villi (G) and villous clusters (H) contained foci of bacteria-forming abscesses (Fig. 1 G and H). Bacteria were also detected on and in amniotic epithelial cells (Fig. 1I), but were not apparent in the connective tissue of the subjacent chorion that overlies the maternal decidua (Fig. 1J).
Fig. 1.
Immunohistological study of the location of L. monocytogenes in the placenta and amnion of women with listeriosis. Bacteria are labeled with polyclonal antibodies to L. monocytogenes and appear red-brown after immunoenzymatic color development. Sections are counterstained with hematoxylin. Bloodborne L. monocytogenes are located in the intervillous space (A), unassociated with macrophages or polymorphonuclear cells. Some bacteria associated with the syncytiotrophoblast (A, arrow) or cytotrophoblast (B, arrow). Other bacteria have invaded the syncytiotrophoblast cytoplasm (C) or penetrated through this trophoblast layer to the villous core adjacent to fetal capillaries (D, arrow). Bacteria are seen throughout the cross section of some villi, either adhering to or localized within the syncytiotrophoblast (E) or contained within the villous core connective tissue where fetal vessels are in close proximity (E and F). Isolated villi (G) or villous clusters (H) contain foci of bacteria and microscopic abscesses, with fibrin deposition in the intervillous space (H). The amnion epithelium (I) is markedly infected, yet there are no bacteria detectable on its chorionic surface (I and J). (Scale bars, 10 μm, except for H, 200 μm.)
These findings suggested that L. monocytogenes infection of the human fetoplacental unit follows a transplacental route. They also suggested a testable hypothesis: namely, that extracellular bacteria present in the maternal blood that bathes placental villi recognize and bind to a specific surface receptor, leading to penetration of the syncytiotrophoblast layer and subsequent invasion of the fetal vascular compartment in the villous core.
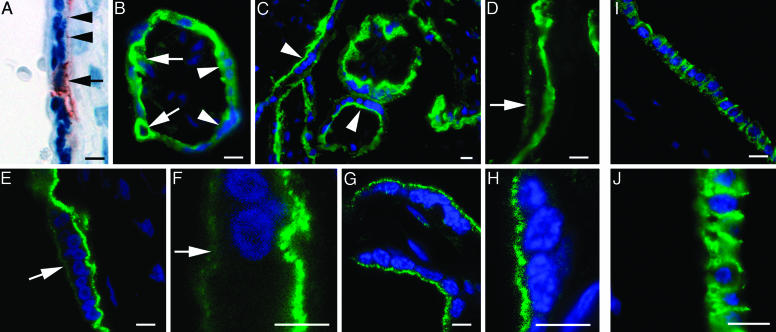
Human Villous Trophoblasts Express the InlA Receptor, E-Cadherin. As mentioned in the Introduction, epidemiological evidence suggests a role for internalin (InlA), an E-cadherin ligand, in maternofetal listeriosis (11). Immunohistochemical studies of sections of paraffin-embedded placental tissue, using a well characterized mouse mAb raised against human E-cadherin (HECD-1), revealed the protein in villous cytotrophoblasts and localized areas of the basal plasma membrane of syncytiotrophoblasts (Fig. 2A). This finding was consistent with previously reported analyses (16–18), but was inconsistent with the hypothesis that blood-borne L. monocytogenes would interact with E-cadherin at the apical surface of syncytiotrophoblasts. However, our previous work on intestinal tissue had established that immunofluorescent labeling of E-cadherin with HECD-1 of fresh cryosections yielded a specific and far more intense signal than with immunoenzymatic labeling of formalin fixed, paraffin-embedded tissue (10). Therefore, we combined epifluoresence techniques with scanning confocal microscopy to survey cryo-sections prepared from placentas delivered by women after normal-term pregnancies. We found that HECD-1 labeled the surface of cytotrophoblasts and produced prominent staining of the basal and, to a lesser extent, the apical surface membranes of syncytiotrophoblasts (Fig. 2 B–F).
Fig. 2.
E-cadherin is expressed in placental cytotrophoblasts and syncytiotrophoblasts, and in the amniotic epithelium. Formalin-fixed, paraffin-embedded (A) or methanol-fixed (B–J) cryosections of human placenta and amnion were stained with an E-cadherin mAb (HECD-1). Immunoreactive E-cadherin appears red (A) or green (B–J). Sections have been counterstained with hematoxylin (A) or a blue nuclear marker (Bis-benzimide in B–D and To-Pro-3 iodide blue in E–H) for identification of tissue organization. E-cadherin is apparent in formalin-fixed cytotrophoblast (A, arrow). A lower intensity, discontinuous signal is also seen along the basal plasma membrane of the syncytiotrophoblast (arrowhead). In the immunostained cryosections shown in B and C, cytotrophoblasts (e.g., arrows) and the basal plasma membrane of syncytiotrophoblasts (e.g., arrowheads) contain detectable E-cadherin. At higher magnification, a less intense E-cadherin signal is apparent on the apical microvillous surface of syncytiotrophoblasts (see arrow in D). Confocal microscopic images of cryosections with HECD-1 (E and F) confirm the apical surface membrane localization of E-cadherin (e.g., arrows) and the absence of cytoplasmic staining in the syncytiotrophoblast. When placental tissue was formalin fixed to eliminate the possibility of endocytosis and immunostained with HECD-1 before embedding (see Materials and Methods), a clearly detectable apical, microvillous surface signal for E-cadherin remains on the syncytiotrophoblast (G and H). As expected, no cytotrophoblast or basal syncytiotrophoblast plasma membrane signal is evident. The amnion epithelium (I and J) contains immnuoreactive E-cadherin, in contrast to the associated mesenchyme of the chorion, adjacent to the maternal deciduas (positioned to the left of the epithelium). (Scale bar, 10 μm.)
We were also able to label the E-cadherin pool that is accessible from the intervillous space by fixing placental tissue fragments before incubating with the HECD-1 mAb, before embedding and sectioning. The results revealed a clear signal on the apical surface membrane of syncytiotrophoblasts (Fig. 2 G and H). Control experiments that omitted the primary antibody did not produce any signal (data not shown). Moreover, staining placental fragments with a mAb specific for the intracytoplasmic protein, Bcl-2, produced no surface staining in either cytotrophoblast or syncytiotrophoblasts (data not shown).
E-cadherin was detected by HECD-1 in the amniotic epithelium but was absent from the mesenchyme of the chorion positioned adjacent to the maternal decidua (Fig. 2 I and J). Together, these results demonstrate that E-cadherin is expressed at the human maternofetal interface.
Studies of the Human Trophoblastic Cell Line BeWo Indicate That L. monocytogenes InlA Mediates Attachment to and Entry into E-Cadherin-Expressing Syncytiotrophoblasts. Apical membrane-associated E-cadherin is positioned to act as an accessible receptor for InlA produced by virulent strains of L. monocytogenes that may have entered the maternal blood space. We used a well established model of in vitro syncytiotrophoblastic differentiation to test this hypothesis (19).
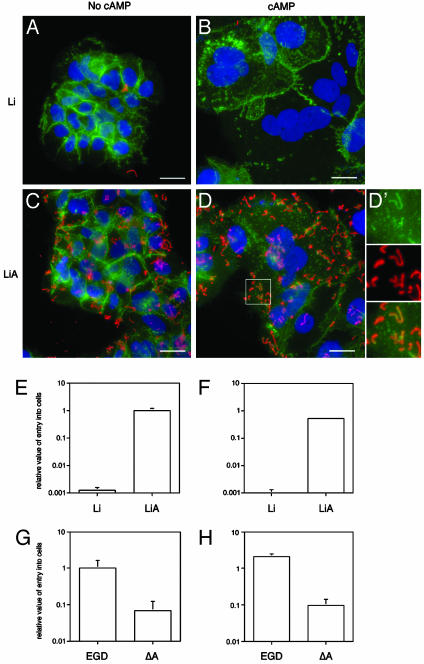
The human BeWo cell line reproducibly converts from a cytotrophoblast phenotype to a syncytiotrophoblast phenotype when cultured in presence of 1 mM 8-Br-cAMP (19) (Fig. 3 A and B). We found that the apical membranes of syncytiotrophoblasts formed after a 72-h incubation of BeWo cells with cAMP contain E-cadherin (Fig. 3 A and B). The levels of E-cadherin on apical membranes are lower than in untreated cytotrophoblast-like BeWo cells (n = 6 comparisons; each performed in a blinded fashion) (Fig. 3 A and B) (20).
Fig. 3.
Listeria-binding and invasion assays of BeWo cells. (A–D′) Immunohistochemical study of cells cultured in the presence or absence of 1 mM 8-Br-cAMP and infected with L. innocua (Li) or L. innocua expressing InlA (LiA). Bacteria appear in red, E-cadherin appears in green, and nuclei appear in blue in each panel. The enlargement (D′) of the boxed region in D shows that L. innocua expressing internalin is able to recruit E-cadherin on the surface of syncytiotrophoblasts. (E–H) Histograms displaying the results of invasion assays. Data have been normalized and are expressed as the number of intracellular colony-forming units (CFU) of bacteria relative to the number in added to the plate, per condition tested. Mean values ± SD are presented (each assay/strain/condition was done in triplicate; n = 3 independent experiments). EGD refers to the wild-type L. monocytogenes strain, ΔA refers to its isogenic InlA-deficient mutant derivative. Results for untreated BeWo cells (mononucleated cytotrophoblasts) are presented in E and G, whereas data obtained from cAMP-treated cultures (syncytiotrophoblasts) are presented in F and H. (Bars in A–D, 10 μm.)
The role of InlA in attachment and invasion of syncytiotrophoblasts was subsequently tested by incubating 8-Br-cAMP-induced BeWo syncytiotrophoblasts with L innocua harboring an InlA expression vector or the vector alone, or with isogenic InlA+ or InlA- strains of L. monocytogenes. Invasion was assessed by gentamicin survival assay (21).
As with cultured enterocytes (22), the results confirmed that bacteria attach to syncytiotrophoblasts in an InlA-dependant manner and recruit E-cadherin to their sites of attachment (Fig. 3 D and D′). The level of invasion of cAMP-induced syncytiotrophoblasts by L. innocua expressing InlA (LiA) was 436 ± 112 greater than with L. innocua that did not express internalin (Li) (n = 3 independent experiments, each performed in triplicate; P < 0.001; Student's t test) (Fig. 3 B, D, and F). The level of invasion of wild-type L. monocytogenes (strain EGD) was 27 ± 10 greater than an isogenic InlA- strain (ΔA) (Fig. 3H).
Similar differences were noted when cytotrophoblast-like BeWo cells that had not been exposed to cAMP were used; i.e., strain LiA exhibited 600 ± 57 greater entry compared to Li (P < 0.001) (Fig. 3 A, C, and E), whereas wild-type L. monocytogenes (strain EGD) manifested 18 ± 5-fold greater internalization compared to the isogenic InlA- strain ΔA (Fig. 3G).
InlA Mediates Bacterial Invasion of Primary Syncytiotrophoblasts. BeWo cells are derived from a malignant choriocarcinoma (23), and thus may have a number of differences when compared to normal cytotrophoblasts and syncytiotrophoblasts. Therefore, we used primary cultures of trophoblasts, obtained from placentas harvested after normal-term pregnancies, to examine E-cadherin expression and the role of InlA in bacterial attachment and entry. Harvested cells are known to differentiate from cytotrophoblasts to syncytiotrophoblasts over the course of a 4-day culture in DMEM supplemented with 10% FCS (15, 24, 25).
We found that E-cadherin expression in the differentiated syncytiotrophoblasts was lower than in cytotrophoblasts (Fig. 7 A and B, which is published as supporting information on the PNAS web site) (20). Attachment and internalization was markedly higher for L. innocua expressing InlA compared to L. innocua (10.4 ± 1.7-fold; P < 0.001) (Fig. 7 B, D, and F), and for the wild-type EGD strain of L. monocytogenes compared to the InlA- isogenic strain ΔA (16.7 ± 7-fold; P < 0.001) (Fig. 7H). Multilabel immunohistochemical studies also established that InlA-directed attachment to the cell surface was accompanied by recruitment of E-cadherin (Fig. 7 D and D′).
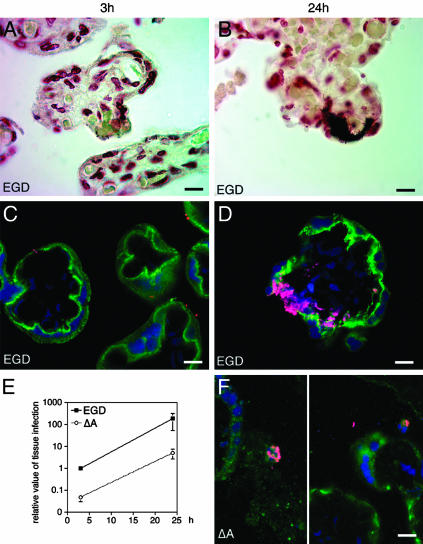
InlA Mediates Crossing of the Trophoblastic Barrier in Human Placental Explants. Having demonstrated that InlA directs E-cadherin recruitment, cell surface attachment, and entry into cultured cAMP-treated BeWo-derived, as well as primary syncytiotrophoblasts, we asked whether the InlA–E-cadherin interaction was critical for bacterial targeting and crossing of the trophoblastic barrier in the normal intact placenta. To address this question, we exposed placental villous explants to the EGD strain and to its InlA-deficient isogenic mutant derivative, ΔA.
As in primary cell cultures, E-cadherin was detected on the apical surfaces of syncytiotrophoblasts (Fig. 4). After a 1-h infection, InlA-dependent attachment to and invasion of the syncytiotrophoblast layer was evident and the InlA-expressing EGD strain was prominently represented in vasculo-syncytial areas of the trophoblast layer, where the maternal and fetal circulations are in close proximity (Fig. 4 A and C), whereas the InlA-deficient mutant was noticeably absent. Quantitative assessment of bacterial invasion using the gentamicin survival assay disclosed that the level of EGD invasion was >10-fold higher than that of inlA mutant at the 1-h and 24-h time points, respectively (P < 0.001 in each case) (Fig. 4E). At the 24-h time point, which corresponds to a 1-h infection with L. monocytogenes, a 2-h incubation with gentamicin to kill extracellular bacteria, and an overnight culture in DMEM without antibiotics to allow intratissular bacterial growth, bacterial replication produced histological lesions indistinguishable from those observed in the villi of placentas harvested from women with maternofetal listeriosis (compare Figs. 1 C–F and 4 B and D). In contrast, placental villitis was not observed in explants infected with the ΔA mutant: at the 24-h time point, ΔA bacteria were only detected in nontrophoblastic components of the placenta (e.g., in fibrin-containing deposits and in maternal neutrophils and phagocytes in the intervillous space blood; Fig. 4F).
Fig. 4.
E-cadherin localization and Listeria invasion assays of placental explants. Shown are the results of Gram stains of tissue sections (A and B), immunohistochemical assays of E-cadherin (green) and bacterial (magenta) localization (C, D, and F), and the number of gentamicin-resistant viable bacteria (E) after incubation of explants with isogenic wild-type (EGD) (A–D) or InlA-deficient (ΔA) (F) strains of L. monocytogenes. (A and C) Findings obtained after a 1-h infection and a 2-h incubation with gentamicin. (B, D, and F) Representative results after a 1-h infection, followed by a 2-h incubation with gentamicin and a 21-h incubation in culture medium without antibiotics. The graph in E displays the relative level of infection after 3 and 24 h with either EGD or ΔA strains (mean values ± SD are plotted, n = 3 independent experiments, each done in quadruplicate). (Bars in A–D and F, 10 μm.)
Analogous invasion assays performed with amnion tissue (n = 3 independent assays, each performed in triplicate) revealed that the fetal-facing E-cadherin-positive surface of the amniotic epithelium is susceptible to InlA-dependant invasion, whereas the connective tissue of the maternal face of the chorio-amnion does not exhibit this dependency (Fig. 8, which is published as supporting information on the PNAS web site).
Taken together, our results demonstrate that InlA mediates attachment to E-cadherin receptors in human placental syncytiotrophoblasts. The resulting bacterial invasion of the syncytiotrophoblast barrier and underlying villous tissue allows L. monocytogenes to replicate and recapitulate in this ex vivo model the histopathological lesions seen in intact placentas of women with listeriosis.
Discussion
Our studies of the location of L. monocytogenes in placental and amniotic tissue samples from women with documented fetoplacental listeriosis suggested that L. monocytogenes crosses the maternofetal barrier at the villous syncytiotrophoblast level, with secondary infection via the amnion epithelium. Because our recent epidemiological studies indicated that InlA plays a role in human fetoplacental listeriosis (11), we explored the pattern of expression of the InlA receptor, E-cadherin, at the maternofetal interface and found that it is present in villous cytotrophoblasts and at the basal and apical plasma membranes of the syncytiotrophoblasts. We used established trophoblastic cell lines, primary trophoblast cultures, and placental villous explants to show that L. monocytogenes invades syncytiotrophoblasts via the apical membrane in an InlA-dependent manner.
Pregnancy-associated infections are often silent for the mother but can have devastating effects on the fetus. A wide range of intracellular pathogens, ranging from Toxoplasma gondii to rubella virus and L. monocytogenes, are able to cross the maternofetal barrier. Host microbial interactions that mediate placental tropism have been described. For example, Plasmodium falciparum-infected erythrocytes express the malarial protein PfEMP1, which interacts with chondroitin sulfate A expressed on placental villi (26). To our knowledge, the bacterial InlA–trophoblast E-cadherin interaction described in the current study is the first ligand–receptor interaction that has been identified that allows a bacterial pathogen to specifically cross the placental villous trophoblast barrier.
The physiological significance of apical E-cadherin in syncytiotrophoblasts is unknown. Its presence may simply reflect the presence of remnant surface membranes left over from the fusion of mononuclear cytotrophoblasts to multinucleated syncytiotrophoblasts during normal villous trophoblast differentiation (27, 28). The syncytiotrophoblast is the only syncytial epithelium in humans. The absence of lateral cell membranes distinguishes this barrier from other epithelial barriers. Differences in the expression of proteins in the apical and basal membranes of the syncytiotrophoblasts, compared to other epithelia, have been described. For example, Na+,K(+)-ATPase is apically and basally expressed in syncytiotrophoblasts, but is restricted to the basolateral surfaces of other epithelia (29). Another unique aspect of syncytiotrophoblasts is their direct exposure to maternal blood in the intervillous space, effectively making the syncytiotrophoblast a specialized form of endothelium. L. monocytogenes appears to take advantage of this unique feature so that blood-borne bacteria can adhere directly to the trophoblast epithelium, invade the trophoblast layer, and thereby gain access to the core of placental villi. We propose that this particular feature provides a molecular explanation for the striking placental tropism of L. monocytogenes in humans.
Although we found that InlA can bind to the apical surface of amniotic epithelium, this effect was only detected when infection was initiated on the fetal side of the amnion. Consequently, InlA-dependent infection of the amnion may amplify a preexisting infection acquired via a transplacental route and thereby increase the known risk for premature delivery in women with listeriosis.
The listerial surface is decorated with an exceptionally high number of surface proteins (30, 31). Would proteins other than InlA also be implicated in crossing the placental barrier? Another well documented protein also involved in entry into mammalian cells in vitro is InlB. This protein mediates bacterial entry into a wide variety of nonphagocytic cell types including epithelial, endothelial, and fibroblastic cell lines (8). The three known InlB receptors, c-Met, gC1q-R, and glycosaminoglycans, are expressed in virtually all human tissues, including the placenta (32–34). Invasion assays with isogenic InlB+ or InlB- L. monocytogenes strains indicated that InlB does not play a role in bacterial entry into cultured BeWo-derived or primary syncytiotrophoblasts (data not shown). Nevertheless, we found in villous explants a lower level of infection of the inlB mutant, compared to the parental EGD strain: the level of invasion was comparable to that of the inlA mutant and that of an inlAB double mutant (data not shown). This finding is not surprising because virtually all cell types tested so far, in the presence or absence of a functional InlA pathway, internalize InlB-expressing bacteria (8). However, in contrast to the InlA–E-cadherin interaction, the ubiquitous expression of InlB receptors does not make InlB a good candidate for mediating the specific targeting of L. monocytogenes to the human placenta. Accordingly, maternofetal infections do not appear to occur in mice as electively as in humans, an observation that correlates with the fact that the mouse is permissive for the InlB pathway and nonpermissive for InlA–E-cadherin interactions (9).
In conclusion, our results show that L. monocytogenes uses a common strategy to target and cross the intestinal and placental barriers. This raises the possibility that L. monocytogenes placental tropism may be a consequence of its evolved mechanism for targeting the intestinal epithelium. Interestingly, the blood–brain barrier is composed of E-cadherin-expressing microvascular endothelium and choroid plexus epithelium (13). Thus, it is tempting to speculate that L. monocytogenes targeting to and invasion of the CNS may also be mediated by the interaction between InlA and E-cadherin. Other human pathogens such as T. gondii or human cytomegalovirus exhibit a similar tropism for these three barriers. Future studies focused on deciphering the similarities and specificities of these barriers may extend our understanding of the strategies microbes deploy to breach them.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Pasteur Institute (to P.C. and M.H.) and National Institutes of Health Grants HD29190 (to D.M.N.) and DK30292 (to J.I.G.). M.L. was the recipient of a European Molecular Biology Organization postdoctoral fellowship. P.C. is an International Research Scholar of the Howard Hughes Medical Institute.
References
- 1.Lorber, B. (1997) Clin. Infect. Dis. 24, 1-9. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez-Boland, J. A., Kuhn, M., Berche, P., Chakraborty, T., Dominguez-Bernal, G., Goebel, W., Gonzalez-Zorn, B., Wehland, J. & Kreft, J. (2001) Clin. Microbiol. Rev. 14, 584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing, E. J. & Gregory, S. H. (2002) J. Infect. Dis. 185, Suppl. 1, S18-S24. [DOI] [PubMed] [Google Scholar]
- 4.Redline, R. W. & Lu, C. Y. (1987) J. Clin. Invest. 79, 1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline, R. W. & Lu, C. Y. (1988) J. Immunol. 140, 3947-3955. [PubMed] [Google Scholar]
- 6.Redline, R. W., Shea, C. M., Papaioannou, V. E. & Lu, C. Y. (1988) Am. J. Pathol. 133, 485-497. [PMC free article] [PubMed] [Google Scholar]
- 7.Abram, M., Schluter, D., Vuckovic, D., Wraber, B., Doric, M. & Deckert, M. (2003) FEMS Immunol. Med. Microbiol. 35, 177-182. [DOI] [PubMed] [Google Scholar]
- 8.Cossart, P., Pizarro-Cerda, J. & Lecuit, M. (2003) Trends Cell Biol. 13, 23-31. [DOI] [PubMed] [Google Scholar]
- 9.Lecuit, M., Dramsi, S., Gottardi, C., Fedor-Chaiken, M., Gumbiner, B. & Cossart, P. (1999) EMBO J. 18, 3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecuit, M., Vandormael-Pournin, S., Lefort, J., Huerre, M., Gounon, P., Dupuy, C., Babinet, C. & Cossart, P. (2001) Science 292, 1722-1725. [DOI] [PubMed] [Google Scholar]
- 11.Jacquet, C., Doumith, M., Gordon, J. I., Martin, P. M. V., Cossart, P. & Lecuit, M. (2004) J. Infect. Dis., in press. [DOI] [PubMed]
- 12.Georgiades, P., Ferguson-Smith, A. C. & Burton, G. J. (2002) Placenta 23, 3-19. [DOI] [PubMed] [Google Scholar]
- 13.Lecuit, M. & Cossart, P. (2002) Trends Mol. Med. 8, 537-542. [DOI] [PubMed] [Google Scholar]
- 14.Kliman, H. J., Nestler, J. E., Sermasi, E., Sanger, J. M. & Strauss, J. F., III (1986) Endocrinology 118, 1567-1582. [DOI] [PubMed] [Google Scholar]
- 15.Nelson, D. M., Johnson, R. D., Smith, S. D., Anteby, E. Y. & Sadovsky, Y. (1999) Am. J. Obstet. Gynecol. 180, 896-902. [DOI] [PubMed] [Google Scholar]
- 16.Shimoyama, Y., Hirohashi, S., Hirano, S., Noguchi, M., Shimosato, Y., Takeichi, M. & Abe, O. (1989) Cancer Res. 49, 2128-2133. [PubMed] [Google Scholar]
- 17.Floridon, C., Nielsen, O., Holund, B., Sunde, L., Westergaard, J. G., Thomsen, S. G. & Teisner, B. (2000) Mol. Hum. Reprod. 6, 943-950. [DOI] [PubMed] [Google Scholar]
- 18.Getsios, S., Chen, G. T. & MacCalman, C. D. (2000) J. Reprod. Fertil. 119, 59-68. [DOI] [PubMed] [Google Scholar]
- 19.Wice, B., Menton, D., Geuze, H. & Schwartz, A. L. (1990) Exp. Cell. Res. 186, 306-316. [DOI] [PubMed] [Google Scholar]
- 20.Coutifaris, C., Kao, L. C., Sehdev, H. M., Chin, U., Babalola, G. O., Blaschuk, O. W. & Strauss, J. F., III (1991) Development (Cambridge, U.K.) 113, 767-777. [DOI] [PubMed] [Google Scholar]
- 21.Vaudaux, P. & Waldvogel, F. A. (1979) Antimicrob. Agents Chemother. 16, 743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecuit, M., Hurme, R., Pizarro-Cerda, J., Ohayon, H., Geiger, B. & Cossart, P. (2000) Proc. Natl. Acad. Sci. USA 97, 10008-10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattillo, R. A., Gey, G. O., Delfs, E. & Mattingly, R. F. (1968) Science 159, 1467-1469. [DOI] [PubMed] [Google Scholar]
- 24.Farmer, D. R. & Nelson, D. M. (1992) Placenta 13, 163-177. [DOI] [PubMed] [Google Scholar]
- 25.Church, S. L., Farmer, D. R. & Nelson, D. M. (1992) Dev. Biol. 149, 177-184. [DOI] [PubMed] [Google Scholar]
- 26.Pouvelle, B., Buffet, P. A., Lepolard, C., Scherf, A. & Gysin, J. (2000) Nat. Med. 6, 1264-1268. [DOI] [PubMed] [Google Scholar]
- 27.Mayhew, T. M. (2001) Histol. Histopathol. 16, 1213-1224. [DOI] [PubMed] [Google Scholar]
- 28.Potgens, A. J., Schmitz, U., Bose, P., Versmold, A., Kaufmann, P. & Frank, H. G. (2002) Placenta 23, Suppl. A, S107-S113. [DOI] [PubMed] [Google Scholar]
- 29.Johansson, M., Jansson, T. & Powell, T. L. (2000) Am. J. Physiol. 279, R287-R294. [DOI] [PubMed] [Google Scholar]
- 30.Glaser, P., Frangeul, L., Buchrieser, C., Rusniok, C., Amend, A., Baquero, F., Berche, P., Bloecker, H., Brandt, P., Chakraborty, T., et al. (2001) Science 294, 849-852. [DOI] [PubMed] [Google Scholar]
- 31.Cabanes, D., Dehoux, P., Dussurget, O., Frangeul, L. & Cossart, P. (2002) Trends Microbiol. 10, 238-245. [DOI] [PubMed] [Google Scholar]
- 32.Kauma, S., Hayes, N. & Weatherford, S. (1997) J. Clin. Endocrinol. Metab. 82, 949-954. [DOI] [PubMed] [Google Scholar]
- 33.Ghebrehiwet, B., Lim, B. L., Kumar, R., Feng, X. & Peerschke, E. I. (2001) Immunol. Rev. 180, 65-77. [DOI] [PubMed] [Google Scholar]
- 34.Muhlhauser, J., Marzioni, D., Morroni, M., Vuckovic, M., Crescimanno, C. & Castellucci, M. (1996) Cell Tissue Res. 285, 101-107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.