Abstract
Barrett’s esophagus (BE) is a condition that develops as a consequence of chronic gastroesophageal reflux disease in which stratified squamous epithelium is replaced by metaplastic columnar epithelium which in turn predisposes to the development of adenocarcinoma of esophagus. In this review article, we discuss recent advances in the endoscopic imaging techniques for the detection of dysplasia and early carcinoma in BE. This will include some of the current available novel technologies as well as future applications specifically concentrating on high-resolution endoscopy, narrow band imaging, chromoendoscopy, confocal laser endomicroscopy and autofluorescence imaging.
Keywords: Barrett’s esophagus, autofluorescence imaging, chromoendoscopy, confocal laser endomicroscopy, high-resolution endoscopy, narrow band imaging, optical coherence tomography
Introduction
Barrett’s esophagus (BE) is a premalignant condition which develops as a consequence of gastro-esophageal reflux disease (GERD) [1-3]. In the United States, BE is defined as a change in the distal esophageal epithelium of any length that can be recognized as columnar type mucosa at endoscopy and confirmed to have intestinal metaplasia (IM) by biopsies [4], whereas the British Society of Gastroenterology has excluded the need for IM. It is thought that BE progresses in a step wise manner from low-grade dysplasia (LGD) to high-grade dysplasia (HGD) and finally esophageal adenocarcinoma (EAC) [5] which has been attributed to DNA alterations in the mucosa [6].
Epidemiology
BE is usually seen in middle-aged and older adults whose mean age at the time of diagnosis by endoscopy is 55 years [7]. The male to female ratio is 2:1 [8]. Estimates of frequency of BE in general population has varied widely ranging from 0.9-20% [9-12]. This may be partly explained by the different populations studied and definitions used.
Screening
In order to decrease mortality from EAC, endoscopic screening for BE in patients having GERD symptoms has been recommended by the American College of Gastroenterology (ACG) [13,14]. It is however unclear if screening patients with GERD symptoms has any impact on identifying individuals at increased risk of EAC as 40% of patients diagnosed with EAC have no history of heartburn [15]. This presents a major limitation in screening patients with GERD symptoms for BE and EAC [16,17]. Although highly controversial, screening may be recommended in patients with the following risk factors:
Age 50 years or older
Male sex and white race with chronic GERD symptoms
Patients with evidence of a hiatal hernia
Patients with an elevated body mass index and intra-abdominal distribution of body fat
The American Gastroenterology Association (AGA) recommends against screening the general population with GERD symptoms [18].
Surveillance
The annual rate of incidence of cancer in patients having BE has been estimated at ranging between 0.12-2.0% [19-27]. A recent large population based Danish study reported that the annual risk of EAC among patients with BE was 0.12%, or 1 case of EAC per 860 patient years [27]. This was similar to another large population based study from Northern Ireland in which incidence rate of EAC was reported to be 1.3 cases per 1000 patient years (excluding cases that were diagnosed during the first year) [26]. These studies have brought into question the cost effectiveness of current surveillance protocols.
Surveillance is performed by taking four quadrant biopsies every 1-2 cm, however only a very tiny fraction of Barrett’s mucosa is sampled this way [28,29]. Lesions harboring dysplasia or EAC can be easily missed. Some studies suggest a risk of occult carcinoma in patients with HGD of around 40% [30-32]. The present recommended surveillance interval by the ACG is depicted in Table 1.
Table 1.
Dysplasia grade and surveillance interval, as per American College of Gastroenterology guidelines [4]
Endoscopic surveillance can potentially detect curable early neoplasia. Asymptomatic cancers found during surveillance are less advanced when compared with symptomatic cancer patients who have dysphagia and weight loss [33,34]. During the last decade, novel endoscopic techniques have enabled increased recognition of dysplasia and early cancers in BE. This review will discuss some of the advances in endoscopic imaging in BE.
Paris classification
Endoscopic appearance of lesions in BE may point towards the lesions’ potential to invade the submucosa (hence endoscopically unresectable). The updated Paris classification categorizes superficial lesions in esophagus into: protruding pedunculated (type 0–Ip), protruding sessile (0–Is), slightly elevated (0–IIa), completely flat (0–IIb), slightly depressed (0–IIc), excavated (0–III), or a mixed pattern [35]. A Danish retrospective study of endoscopic resection in BE suggested that type 0-I and 0-IIc lesions have higher submucosal infiltration rates [36]. There is surgical literature for patients with HGD in BE suggesting that a visible lesion on white light examination (WLE) is associated with an increased risk of coexisting cancer [37,38].
WLE with high-resolution and magnification endoscopy
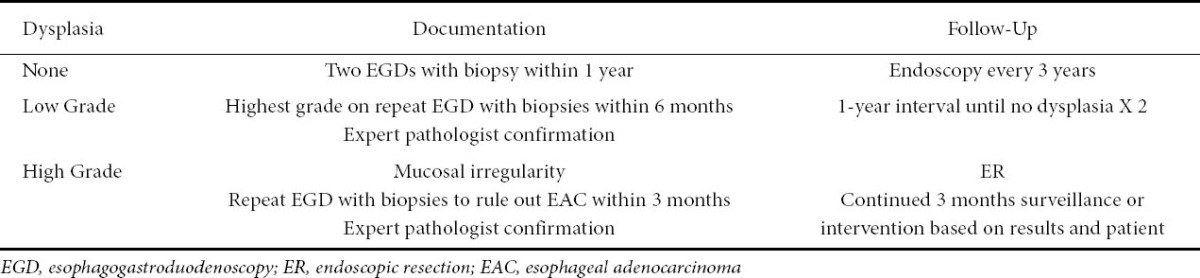
High-resolution endoscopes (HRE) are endoscopes equipped with high-density charged coupled device (CCD) chips enabling improved optics and images to be displayed at up to 850,000 pixels [39] (Fig. 1A). Magnification endoscopy enables the images to be magnified by up to 115 times by optical magnification [39]. These are major advancements in technology allowing for better visualization of the mucosa. Magnification endoscopy is best used in conjunction with chromoendoscopy [40-43]. A study by Sharma et al demonstrated that magnification chromoendoscopy helped to identify areas with IM and HGD [44]. The issue with this modality of imaging has been the high interbserver variability [45]. A study by Mayinger et al suggested that one reason for this is the difficulty in differentiating gastric cardiac mucosa from non-dysplastic Barrett’s mucosa [46].
Figure 1.
(A) Overview with high-resolution white light endoscopy: nodule in Barrett’s esophagus
Chromoendoscopy
Dyes can be used to better visualize the mucosal surface of the gastrointestinal tract. Methylene blue (MB) has been used to visualize the presence of IM/HGD, and cancer [47]. It is a vital stain that is actively absorbed by mucosa and is taken up by IM, but not gastric or squamous mucosa. Multiple reports have however shown discrepant results [48-51]. A meta-analysis by Ngamruengphong et al of 450 patients with BE in 9 studies concluded that MB chromoendoscopy had a yield for detection of specialized IM comparable to conventional four-quadrant random biopsies [52]. There is also a theoretical risk of acceleration of carcinogenesis with MB [53]. Indigo carmine has been used to evaluate dysplasia or cancer [54]. Acetic acid is another stain which has been used. However its utility in detecting dysplasia is not yet established [45,55,56]. The limitations to chromoendoscopy include the high interobserver variability secondary to lack of standardization of pit pattern classification systems [45], lack in training of general endoscopists, and a significant increase in the time taken for the examination. Studies looking at the impact of chromoendoscopy on patient outcomes are required.
NBI
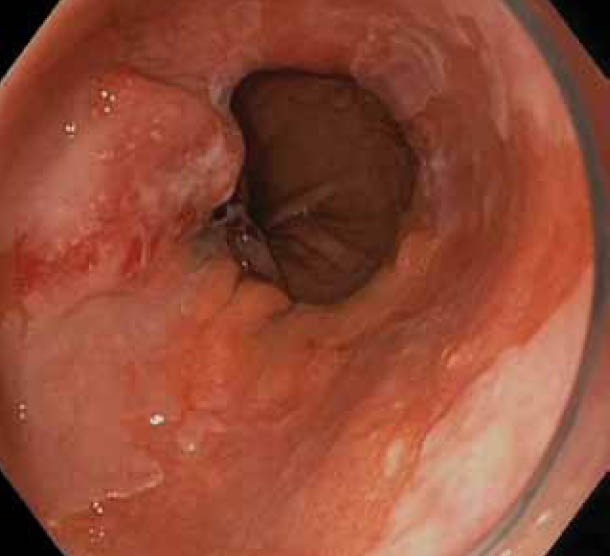
NBI was first described in 2004 [57]. It is a technique that uses filtered light where an increased contribution of the short wavelength blue light (440-460 nm) leads to better visualization of the mucosal surface pattern. The superficial capillary network is also highlighted as blue light has an increased affinity to and is better absorbed by hemoglobin in blood [58] (Fig. 1B/1C).
Figure 1.
(B) Nodule in Barrett’s esophagus in Figure 1A seen with narrow band imaging: some overlying squamous mucosa clearly visible as well
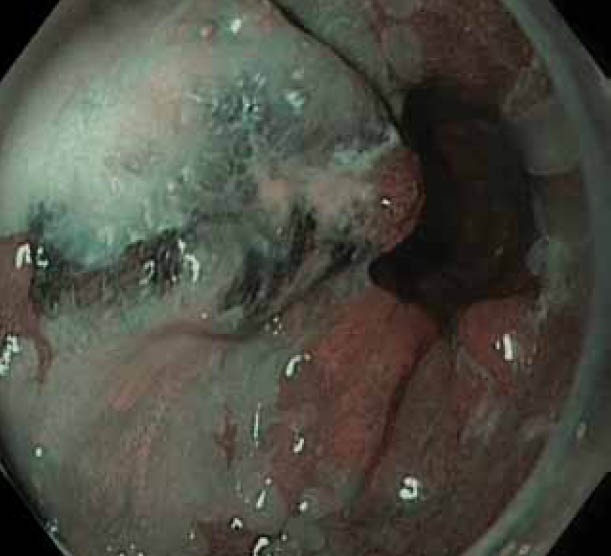
Figure 1.
(C) Nodule on narrow band imaging with optical magnification: distorted pit pattern and vasculature in keeping with adenocarcinoma
Different pit pattern classifications have been described with this technology in BE [58-60]. These classification systems are in itself a limitation in the use of NBI as they make reproducibility in the community a problem. It requires further validation in large randomized multicenter trials [61]. Sharma et al described NBI images with mucosal patterns (ridge/ villous, circular, irregular) and vascular patterns (normal and abnormal) [59]. They demonstrated that presence of irregular mucosal or vascular patterns had a high sensitivity, specificity and positive predictive value for the diagnosis of HGD and cancer [59]. A prospective cohort study of 109 patients with BE exhibited that the presence of villous/ridged/absent pit patterns were highly suggestive of specialized IM (SIM) [60]. In another study it was found that NBI with optical magnification was superior to WLE and optical magnification in the prediction of dysplastic tissue in BE (p<0.05) [62], whereas in a randomized crossover study comparing WLE with NBI by Kara et al no difference was found in the detection of HGD/intramucosal cancer (IMC) [63]. In another study by Wolfsen et al, NBI was found to have a higher detection rate of dysplasia, compared with WLE (57% vs. 43%) [64]. However an important bias in this study was that NBI was used with a high-resolution system when compared with WLE which used standard resolution. A systematic review by Curvers et al found good sensitivity (77-100%), specificity (79-94%), and accuracy (88-96%) of NBI in differentiating gastric mucosa from IM [65].
The advantage of NBI is that both the mucosal and vascular pattern can be studied. It can easily be combined with other modalities for better mucosal examination. Some of the other advantages of NBI include its wide availability, ease of use and integration into standard endoscopy with no additional risks to the patient.
Autofluorescence imaging (AFI)
AFI takes advantage of the phenomenon in which tissue after exposure to light of a shorter wavelength emits fluorescent light with a longer wavelength. Examples of tissue fluorophores are collagen, amino acids, flavins, etc. This phenomenon is called auto fluorescence [66,67] (Fig. 2A, 2B). Initial studies using point spectroscopy techniques demonstrated a difference between fluorescence pattern of Barrett’s dysplasia/cancer and normal tissue [68-70]. Initial systems using fiber optic endoscopy with AFI were compared to WLE, and showed no difference in the number of patients detected with HGD/ early stage cancer [71,72]. A plausible reason could be the substandard image quality of fiber optic endoscopy.
Figure 2.
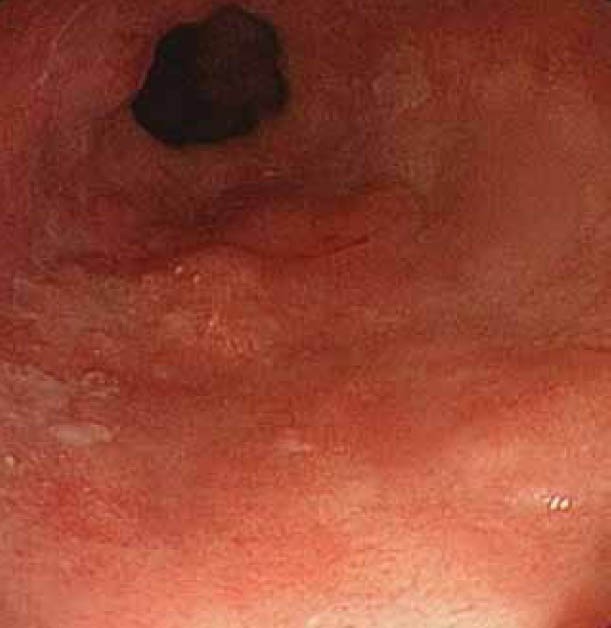
(A) Overview with white light of inconspicuous flat areas harboring dysplasia
Figure 2.
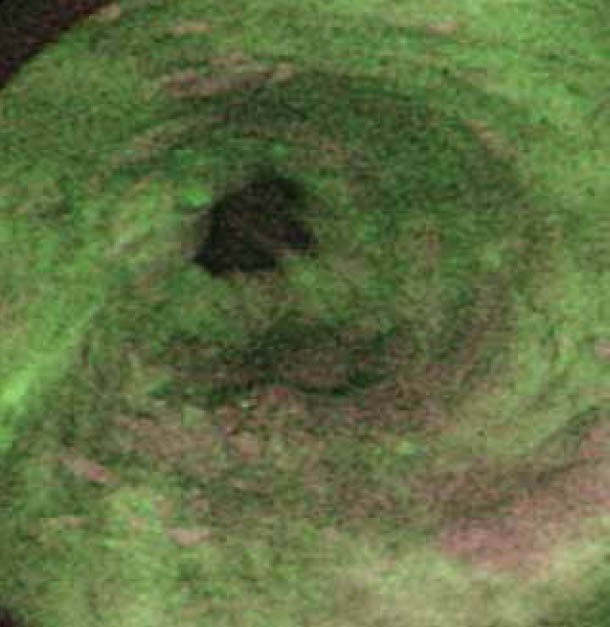
(B) Dysplastic areas on Figure 2A which clearly delineated by autofluorescence imaging as purplish patches
More recently endoscopic trimodal imaging (ETMI) system has been developed incorporating autofluorescence, HRE, and NBI. A prospective multi-center study in 4 tertiary referral centers demonstrated that AFI increases the detection rate of high grade intraepithelial neoplasia (HGIN), and early neoplasia (EN) from 53% to 90%. The problem with AFI is its false positive rate of 81%. In this study, the use of NBI reduced the false positive rate of AFI to 26% [73]. Another study using ETMI demonstrated that overall detection of patients with HGIN/EN was not statistically different from standard endoscopy (84% vs. 73%) [74]. In a recent multicenter, randomized, cross over study in a community practice setting, no significant difference in the overall histological yield between ETMI and standard video endoscopy was observed [75].
Confocal laser endomicroscopy (CLE)
CLE is a technique that allows the gastroenterologist to perform real-time histological assessment of the gastrointestinal lining. There are two systems available, endoscope based confocal system (eCLE) and a probe based system (pCLE). Both systems uses blue laser light which is focused on the mucosa while an intra venous (IV) contrast agent (fluorescein sodium) is injected [76]. The microscopic images of the mucosa are magnified up to 1250-fold, up to 250 µm below the mucosal surface [77]. It is essential for the gastroenterologist performing CLE to have basic histopathology knowledge and ability to differentiate between normal and dysplastic mucosa [78]. Classification systems for BE have been described for both eCLE and pCLE [79,80].
Kiesslich et al reported an accuracy of 97.4%, sensitivity and specificity of 94.1% and 98.5% respectively for the prediction of BE associated neoplasia [79]. Dunbar et al in their single center, randomized, cross over study with eCLE reported that, when compared with standard endoscopy, eCLE increases the yield of unlocalized neoplasia from 17% to 34%. It was also reported that eCLE required fewer biopsies to achieve a comparable overall diagnosis [81]. Pohl et al, in a study using pCLE to distinguish dysplastic and non-dysplastic BE, demonstrated a very good negative predictive value of 98%, with good interobserver agreement (k=0.6) [80]. A blinded multi-center study by Wallace et al demonstrated a high sensitivity and specificity (91% and 100% respectively) and a very accurate interobserver agreement of (k=0.83) amongst endoscopists with prior pCLE experience for diagnosis of HGIN and cancer [82].
Optical coherence tomography (OCT)
OCT uses short coherence length broadband light for cross-sectional imaging of esophageal mucosa. It is similar to ultrasonography but uses light waves rather than acoustic waves [83]. There are several studies which have described the normal and abnormal esophageal mucosa on OCT during endoscopy. In normal esophagus, the epithelium, lamina propria and muscularis mucosa are clearly identified [83,84]. One of the earliest prospective studies to establish the sensitivity and specificity of OCT for the diagnosis of SIM following a specific criterion found the sensitivity and specificity to be 97% and 92% respectively [85]. Qi et al, using a computer aided diagnostic algorithm with histology as a reference standard, reported the sensitivity, specificity, and accuracy of 82%, 74%, and 83% respectively [86]. Evans et al developed an algorithm for diagnosis of SIM using 2 blinded investigators [89]. They reported a sensitivity of 81% and a specificity of 66% for both OCT readers. The interobserver agreement was good (k=0.53) [87]. OCT however is not widely available currently [88].
Spectroscopy
Spectroscopy-based devices can assess the interaction between light and mucosal surface to provide information about the nuclear size, crowding, vascularity, and organization of glands. This technology can only examine potentially suspicious regions and is currently being investigated to differentiate between normal and abnormal tissue [88]. The different spectroscopic modalities are light scattering, reflectance, and Raman-based. Light scattering spectroscopy gives information about cell nuclei characteristics. Various studies have demonstrated dysplasia detection in BE using this technique [89-91]. Reflectance spectroscopy also assists in differentiating normal from neoplastic tissue [92,93]. A new system called Endoscopic Polarized Scanning Spectroscopy (EPSS) shows great promise in the detection of dysplasia in BE. Unlike other spectroscopic modalities, it scans the entire esophagus and combines polarized light scattering spectroscopy with diffuse reflectance spectroscopy in the same instrument [94]. Raman spectroscopy is used to study the different characteristics of molecular vibrations in cancerous and normal cells in BE thus differentiating one from the other [95]. A major drawback of Raman scattering is that the signal is typically very weak and the differences may be too small to be appreciated.
Conclusion
There has been great advancement in the imaging techniques used for the detection of dysplasia in BE. Most of these techniques have been studied in tertiary centers with investigators having special interest in BE. It would be interesting to see if these results could be reproduced in the hands of the general endoscopist. Ease of availability, cost, procedural time, and medico-legal issues associated with image interpretation are some of the concerns which need addressing. Currently, however, a detailed examination of the BE segment with WLE and random 4-quadrant biopsies is probably still the best approach, with other imaging modalities used in addition to increase the yield of detecting dysplastic areas in BE.
Biography
Gosford Hospital, NSW Australia; Lyell McEwin Hospital, University of Adelaide, SA Australia
Footnotes
Conflict of Interest: None
References
- 1.Spechler SJ, Fitzgerald RC, Prasad GA, Wang KK. History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus. Gastroenterology. 2010;198:854–869. doi: 10.1053/j.gastro.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Richter JE. Barrett's oesophagus. Lancet. 2009;373:850–861. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P. Barrett's esophagus. N Engl J Med. 2009;361:2548–2556. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 4.Wang KK, Sampliner RE. Updated Guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 5.Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 6.Spechler SJ. Disputing dysplasia. Gastroenterology. 2001;120:1864–1868. doi: 10.1053/gast.2001.25291. [DOI] [PubMed] [Google Scholar]
- 7.Spechler SJ. Barrett's esophagus. Semin Gastrointest Dis. 1996;7:51–60. [PubMed] [Google Scholar]
- 8.Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162:1050–1061. doi: 10.1093/aje/kwi325. [DOI] [PubMed] [Google Scholar]
- 9.Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology. 1999;116:277–285. doi: 10.1016/s0016-5085(99)70123-x. [DOI] [PubMed] [Google Scholar]
- 10.Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99:918–922. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 11.Ormsby AH, Kilgore SP, Goldblum JR, et al. The location and frequency of intestinal metaplasia at the esophagogastric junction in 223 consecutive autopsies: implications for patient treatment and preventive strategies in Barrett's esophagus. Mod Pathol. 2000;13:614–620. doi: 10.1038/modpathol.3880106. [DOI] [PubMed] [Google Scholar]
- 12.Ward EM, Wolfsen HC, Achem SR, et al. Barrett's esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol. 2006;101:12–17. doi: 10.1111/j.1572-0241.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 13.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 14.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:1434–1442. doi: 10.1111/j.1572-0241.1999.1123_a.x. [DOI] [PubMed] [Google Scholar]
- 15.Sampliner RE. A population prevalence of Barrett's esophagus- finally. Gastroenterology. 2005;129:2101–2103. doi: 10.1053/j.gastro.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastrooesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222–1227. doi: 10.1111/j.1365-2036.2010.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 18.American Gastroenterological Association. Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Spechler SJ. The frequency of esophageal cancer in patients with Barrett's esophagus. Acta Endoscopica. 1992;22:541–544. [Google Scholar]
- 20.Eckardt VF, Kanzler G, Bernhard G. Life expectancy and cancer risk in patients with Barrett's esophagus: a prospective controlled investigation. Am J Med. 2001;111:33–37. doi: 10.1016/s0002-9343(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 21.Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett's esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–1939. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 22.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–398. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 24.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2006;4:566–572. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 28.Falk GW, Rice TW, Goldblum JR, Richter JE. Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 1999;49:170–176. doi: 10.1016/s0016-5107(99)70482-7. [DOI] [PubMed] [Google Scholar]
- 29.Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett's esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology. 1994;107:945–949. [PubMed] [Google Scholar]
- 30.Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett's esophagus overestimated? Clin Gastroenterol Hepatol. 2008;6:159–164. doi: 10.1016/j.cgh.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson MK, Naunheim KS. Resection for Barrett's mucosa with high-grade dysplasia: implications for prophylactic photodynamic therapy. J Thorac Cardiovasc Surg. 1997;114:824–829. doi: 10.1016/S0022-5223(97)70087-4. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrini CA, Pohl D. High-grade dysplasia in Barrett's esophagus: surveillance or operation? J Gastrointest Surg. 2000;4:131–134. doi: 10.1016/s1091-255x(00)80048-7. [DOI] [PubMed] [Google Scholar]
- 33.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 34.Streitz JM, Jr, Andrews CW, Jr, Ellis FH., Jr Endoscopic surveillance of Barrett's esophagus. Does it help? J Thorac Cardiovasc Surg. 1993;105:383–387. discussion 387-388. [PubMed] [Google Scholar]
- 35.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 36.Peters FP, Brakenhoff KP, Curvers WL, et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett's esophagus. Gastrointest Endosc. 2008;67:604–609. doi: 10.1016/j.gie.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 37.Nigro JJ, Hagen JA, DeMeester TR, et al. Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg. 1999;230:433–438. doi: 10.1097/00000658-199909000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tharavej C, Hagen JA, Peters JH, et al. Predictive factors of coexisting cancer in Barrett's high-grade dysplasia. Surg Endosc. 2006;20:439–443. doi: 10.1007/s00464-005-0255-x. [DOI] [PubMed] [Google Scholar]
- 39.Reddymasu SC, Sharma P. Advances in endoscopic imaging of the esophagus. Gastroenterol Clin North Am. 2008;37:763–774. doi: 10.1016/j.gtc.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Guelrud M, Herrera I, Essenfeld H, et al. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett's esophagus. Gastrointest Endosc. 2001;53:559–565. doi: 10.1067/mge.2001.114059. [DOI] [PubMed] [Google Scholar]
- 41.Kiesslich R, Mergener K, Naumann C, et al. Value of chromoendoscopy and magnification endoscopy in the evaluation of duodenal abnormalities: a prospective, randomized comparison. Endoscopy. 2003;35:559–563. doi: 10.1055/s-2003-40240. [DOI] [PubMed] [Google Scholar]
- 42.Toyoda H, Rubio C, Befrits R, et al. Detection of intestinal metaplasia in distal esophagus and esophagogastric junction by enhanced-magnification endoscopy. Gastrointest Endosc. 2004;59:15–21. doi: 10.1016/s0016-5107(03)02527-6. [DOI] [PubMed] [Google Scholar]
- 43.Rey JF, Inoue H, Guelrud M. Magnification endoscopy with acetic acid for Barrett's esophagus. Endoscopy. 2005;37:583–586. doi: 10.1055/s-2005-861321. [DOI] [PubMed] [Google Scholar]
- 44.Sharma P, Weston AP, Topalovski M, et al. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett's oesophagus. Gut. 2003;52:24–27. doi: 10.1136/gut.52.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meining A, Rösch T, Kiesslich R, et al. Inter- and intra-observer variability of magnification chromoendoscopy for detecting specialized intestinal metaplasia at the gastroesophageal junction. Endoscopy. 2004;36:160–164. doi: 10.1055/s-2004-814183. [DOI] [PubMed] [Google Scholar]
- 46.Mayinger B, Oezturk Y, Stolte M, et al. Evaluation of sensitivity and inter- and intra- observer variability in the detection of intestinal metaplasia and dysplasia in Barrett's esophagus with enhanced magnification endoscopy. Scand J Gastroenterol. 2006;41:349–356. doi: 10.1080/00365520510024016. [DOI] [PubMed] [Google Scholar]
- 47.Connor MJ, Sharma P. Chromoendoscopy and magnification endoscopy for diagnosing esophageal cancer and dysplasia. Thorac Surg Clin. 2004;14:87–94. doi: 10.1016/S1547-4127(04)00042-8. [DOI] [PubMed] [Google Scholar]
- 48.Canto MI, Setrakian S, Petras RE, et al. Methylene blue selectively stains intestinal metaplasia in Barrett's esophagus. Gastrointest Endosc. 1996;44:1–7. doi: 10.1016/s0016-5107(96)70221-3. [DOI] [PubMed] [Google Scholar]
- 49.Canto MI, Setrakian S, Willis JE, et al. Methylene blue staining of dysplastic and nondysplastic Barrett's esophagus: an in vivo and ex vivo study. Endoscopy. 2001;33:391–400. doi: 10.1055/s-2001-14427. [DOI] [PubMed] [Google Scholar]
- 50.Dave U, Shousha S, Westaby D. Methylene blue staining: is it really useful in Barrett's esophagus? Gastrointest Endosc. 2001;53:333–335. doi: 10.1016/s0016-5107(01)70408-7. [DOI] [PubMed] [Google Scholar]
- 51.Wo JM, Ray MB, Mayfield-Stokes S, et al. Comparison of methylene blue-directed biopsies and conventional biopsies in the detection of intestinal metaplasia and dysplasia in Barrett's esophagus: a preliminary study. Gastrointest Endosc. 2001;54:294–301. doi: 10.1067/mge.2001.115732. [DOI] [PubMed] [Google Scholar]
- 52.Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc. 2009;69:1021–1028. doi: 10.1016/j.gie.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 53.Olliver JR, Wild CP, Sahay P, et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet. 2003;362:373–374. doi: 10.1016/s0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]
- 54.Sharma P, Marcon N, Wani S, et al. Nonbiopsy detection of intestinal metaplasia and dysplasia in Barrett's esophagus: a prospective multicenter study. Endoscopy. 2006;38:1206–1212. doi: 10.1055/s-2006-944974. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman A, Kiesslich R, Bender A, et al. Acetic acid-guided biopsies after magnifying endoscopy compared with random biopsies in the detection of Barrett's esophagus: a prospective randomized trial with crossover design. Gastrointest Endosc. 2006;64:1–8. doi: 10.1016/j.gie.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 56.Fortun PJ, Anagnostopoulos GK, Kaye P, et al. Acetic acid-enhanced magnification endoscopy in the diagnosis of specialized intestinal metaplasia, dysplasia and early cancer in Barrett's oesophagus. Aliment Pharmacol Ther. 2006;23:735–742. doi: 10.1111/j.1365-2036.2006.02823.x. [DOI] [PubMed] [Google Scholar]
- 57.Gono K, Obi T, Yamaguchi M, Ohyama N, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 58.Kara MA, Ennahachi M, Fockens P, et al. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett's esophagus by using narrow band imaging. Gastrointest Endosc. 2006;64:155–166. doi: 10.1016/j.gie.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 59.Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett's esophagus. Gastrointest Endosc. 2006;64:167–175. doi: 10.1016/j.gie.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 60.Singh R, Anagnostopoulos GK, Yao K, et al. Narrow-band imaging with magnification in Barrett's esophagus: validation of a simplified grading system of mucosal morphology patterns against histology. Endoscopy. 2008;40:457–463. doi: 10.1055/s-2007-995741. [DOI] [PubMed] [Google Scholar]
- 61.Lee MM, Enns R. Narrow band imaging in gastroesophageal reflux disease and Barrett's esophagus. Can J Gastroenterol. 2009;23:84–87. doi: 10.1155/2009/732481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh R, Karageorgiou H, Owen V, et al. Comparison of high resolution magnification narrow band imaging and whiltelight endoscopy in the prediction of histology in Barett's esophagus. Scand J Gastroenterol. 2009;44:85–92. doi: 10.1080/00365520802400818. [DOI] [PubMed] [Google Scholar]
- 63.Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett's esophagus: a prospective randomized crossover study. Endoscopy. 2005;37:929–936. doi: 10.1055/s-2005-870433. [DOI] [PubMed] [Google Scholar]
- 64.Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett's esophagus. Gastroenterology. 2008;135:24–31. doi: 10.1053/j.gastro.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 65.Curvers WL, van den Broek FJ, Reitsma JB, et al. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach. Gastrointest Endosc. 2009;69:307–317. doi: 10.1016/j.gie.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 66.Kara MA, Peters FP, Ten Kate FJ, et al. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett's esophagus. Gastrointest Endosc. 2005;61:679–685. doi: 10.1016/s0016-5107(04)02577-5. [DOI] [PubMed] [Google Scholar]
- 67.Kara M, DaCosta RS, Wilson BC, et al. Autofluorescence-based detection of early neoplasia in patients with Barrett's esophagus. Dig Dis. 2004;22:134–141. doi: 10.1159/000080312. [DOI] [PubMed] [Google Scholar]
- 68.Panjehpour M, Overholt BF, Vo-Dinh T, et al. Endoscopic fluorescence detection of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 1996;111:93–101. doi: 10.1053/gast.1996.v111.pm8698231. [DOI] [PubMed] [Google Scholar]
- 69.Bourg-Heckly G, Blais J, Padilla JJ, et al. Endoscopic ultraviolet-induced autofluorescence spectroscopy of the esophagus: tissue characterization and potential for early cancer diagnosis. Endoscopy. 2000;32:756–765. doi: 10.1055/s-2000-7704. [DOI] [PubMed] [Google Scholar]
- 70.Georgakoudi I, Jacobson BC, Van DJ, et al. Fluorescence, reflectance, and lightscattering spectroscopy for evaluating dysplasia in patients with Barrett's esophagus. Gastroenterology. 2001;120:1620–1629. doi: 10.1053/gast.2001.24842. [DOI] [PubMed] [Google Scholar]
- 71.Kara MA, Smits ME, Rosmolen WD, et al. A randomized crossover study comparing light-induced fluorescence endoscopy with standard videoendoscopy for the detection of early neoplasia in Barrett's esophagus. Gastrointest Endosc. 2005;61:671–678. doi: 10.1016/s0016-5107(04)02777-4. [DOI] [PubMed] [Google Scholar]
- 72.Borovicka J, Fischer J, Neuweiler J, et al. Autofluorescence endoscopy in surveillance of Barrett's esophagus: a multicenter randomized trial on diagnostic efficacy. Endoscopy. 2006;38:867–872. doi: 10.1055/s-2006-944726. [DOI] [PubMed] [Google Scholar]
- 73.Curvers WL, Singh R, Wong Kee Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett's oesophagus; a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2007;57:167–172. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]
- 74.Curvers WL, Herrero LA, Wallace MB, et al. Endoscopic Tri-Modal Imaging Is More Effective Than Standard Endoscopy in Identifying Early-Stage Neoplasia in Barrett's Esophagus. Gastroenterology. 2010;139:1106–1114. doi: 10.1053/j.gastro.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 75.Curvers WL, van Vilsteren FG, Baak LC, et al. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett's neoplasia: a multicenter, randomized, crossover study in general practice. Gastrointest Endosc. 2011;73:195–203. doi: 10.1016/j.gie.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548–552. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- 77.Kiesslich R, Goetz M, Vieth M, et al. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2005;15:715–731. doi: 10.1016/j.giec.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen NQ, Leong RW. Current application of confocal endomicroscopy in gastrointestinal disorders. J Gastroenterol Hepatol. 2008;23:1483–1491. doi: 10.1111/j.1440-1746.2008.05469.x. [DOI] [PubMed] [Google Scholar]
- 79.Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Pohl H, Rösch T, Vieth M, et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett's oesophagus. Gut. 2008;57:1648–1653. doi: 10.1136/gut.2008.157461. [DOI] [PubMed] [Google Scholar]
- 81.Dunbar KB, Okolo P, 3rd, Montgomery E, et al. Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. 2009;70:645–654. doi: 10.1016/j.gie.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett's esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19–24. doi: 10.1016/j.gie.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouma BE, Tearney GJ, Compton CC, et al. High-resolution imaging of the human esophagus and stomach in vivo using optical coherence tomography. Gastrointest Endosc. 2000;51:467–474. doi: 10.1016/s0016-5107(00)70449-4. [DOI] [PubMed] [Google Scholar]
- 84.Sivak MV, Jr, Kobayashi K, Izatt JA, et al. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest Endosc. 2000;51:474–479. doi: 10.1016/s0016-5107(00)70450-0. [DOI] [PubMed] [Google Scholar]
- 85.Poneros JM, Brand S, Bouma BE, et al. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology. 2001;120:7–12. doi: 10.1053/gast.2001.20911. [DOI] [PubMed] [Google Scholar]
- 86.Qi X, Sivak MV, Isenberg G, et al. Computer-aided diagnosis of dysplasia in Barrett's esophagus using endoscopic optical coherence tomography. J Biomed Opt. 2006;11:044010. doi: 10.1117/1.2337314. [DOI] [PubMed] [Google Scholar]
- 87.Evans JA, Bouma BE, Bressner J, et al. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest Endosc. 2007;65:50–56. doi: 10.1016/j.gie.2006.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shahid MW, Wallace MB. Endoscopic imaging for the detection of esophageal dysplasia and carcinoma. Gastrointest Endosc Clin N Am. 2010;20:11–24. doi: 10.1016/j.giec.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 89.Backman V, Wallace MB, Perelman LT, et al. Detection of preinvasive cancer cells. Early-warning changes in precancerous epithelial cells can be spotted in situ. Nature. 2000;406:35–36. doi: 10.1038/35017638. [DOI] [PubMed] [Google Scholar]
- 90.Gurjar RS, Backman V, Perelman LT, et al. Imaging human epithelial properties with polarized light scattering spectroscopy. Nat Med. 2001;7:1245–1248. doi: 10.1038/nm1101-1245. [DOI] [PubMed] [Google Scholar]
- 91.Wallace MB, Perelman LT, Backman V, et al. Endoscopic detection of dysplasia in patients with Barrett's Esophagus using light scattering spectroscopy: a prospective study. Gastroenterology. 2000;119:677–682. doi: 10.1053/gast.2000.16511. [DOI] [PubMed] [Google Scholar]
- 92.Georgakoudi I, Van DJ. Characterization of dysplastic tissue morphology and biochemistry in Barrett's esophagus using diffuse reflectance and light scattering spectroscopy. Gastrointest Endosc Clin N Am. 2003;13:297–308. doi: 10.1016/s1052-5157(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 93.Badisadegan Backman V, Boone CW, et al. Spectroscopic diagnosis and imaging of invisible pre-cancer. Faraday Discuss. 2004;126:265–279. doi: 10.1039/b305410a. [DOI] [PubMed] [Google Scholar]
- 94.Qiu L, Pleskow DK, Chuttani R, et al. Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett's esophagus. Nat Med. 2010;16:603–606. doi: 10.1038/nm.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kendall C, Stone N, Shepherd N, et al. Raman spectroscopy, a potential tool for the objective identification and classification of neoplasia in Barrett's oesophagus. J Pathol. 2003;200:602–609. doi: 10.1002/path.1376. [DOI] [PubMed] [Google Scholar]


