Abstract
Secondary rectal linitis plastica is a very rare malignancy with poor prognosis. Diagnosis is difficult because of nonspecific clinical and endoscopic findings and negative biopsies in most cases owing to the fact that the mucosa is frequently unaffected. We herein describe a 68-year-old man who presented with a six-month history of tenesmus and constipation. Endoscopy revealed a narrow distal rectum with an indurated, cobblestone appearance of mucosa. Multiple biopsies and fine-needle aspiration were negative for malignancy. Abdominal MRI and transrectal ultrasonography showed findings compatible with rectal linitis plastica. He underwent rectal extirpation with total cystectomy and lymph nodes dissection. Histology demonstrated secondary rectal linitis plastica due to a poorly differentiated urinary bladder carcinoma. We emphasize the endoscopic and endosonographic features and the difficulty to establish a preoperative diagnosis of secondary rectal linitis plastica.
Keywords: secondary rectal linitis plastica, urinary bladder carcinoma
Introduction
Linitis plastica is characterized by the diffuse infiltration of the submucosal and muscularis propria layers of a hollow organ with cancer cells, resulting in wall thickening that makes the involved organ constricted, inelastic and rigid. Although it is most commonly seen in the stomach, other organs, including small intestine, colon and rectum, are occasionally involved [1].
Rectal linitis plastica (RLP) is a rare tumor with an incidence of 1 in 1,000 colorectal carcinomas. Secondary RLP is more common than a primary one, having as a predominant cause for its development, previous gastric linitis plastica. Metastatic RLP of breast, prostate, gallbladder or bladder cancer is less frequent [2-6].
We herein describe a case of secondary RLP due to an asymptomatic urinary bladder cancer. To the best of our knowledge, secondary RLP as first manifestation of a urinary bladder cancer has as yet not been reported.
Case report
A 68-year-old man presented to the Department of Endoscopy with a six-month history of anal outlet obstruction, constipation and tenesmus. The patient had no relevant past medical history. Digital examination revealed a circumferential rectal narrowing with a firm, mass-like area, palpable all around the rectal wall and extending to a few centimeters of the anal margin. Laboratory tests, including urinalysis, were unremarkable. Subsequent endoscopy demonstrated a narrow distal rectum, with the overlying mucosa being indurated, non-ulcerated, with a cobblestone appearance (Fig. 1). Several biopsies and fine-needle aspiration were taken, but none was conclusive for diagnosis. T2-weighted MRI showed a double-layered thickening of the rectal wall with an inner iso-intense circumferential thickening of the submucosa and outer hypo-intense circumferential thickening of the muscular rectal wall, as well as a thickened bladder wall. Transrectal ultrasonography showed circumferential infiltration of the submucosa, whereas the other layers were normal (Fig. 2); an image compatible with “linitis plastica”. The patient underwent rectal extirpation and total cystectomy with lymph node dissection. Macroscopic examination of a sagittal section of resected rectum demonstrated an expanded submucosal layer due to infiltration with cancer cells, with intact mucosa and muscular layer (Fig. 3). Histological examination of the resected bladder showed a grade III papillary transitional cell carcinoma. In the rectum clusters of tumor cells of a poorly differentiated adenocarcinoma were observed. Immunostaining was positive for both CK7 and CK20; PSA was negative. Combining the cytokeratine profile histology and transrectal ultrasonography findings, the diagnosis of a primary urinary bladder tumor with hematogenous spread to rectal submucosal layer was established.
Figure 1.
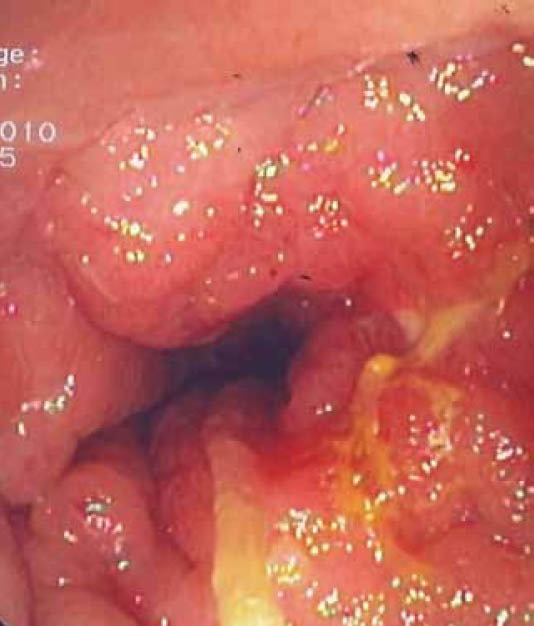
Endoscopic image showing circumferential narrowing of the rectal lumen, with intact overlying mucosa and cobblestone appearance
Figure 2.
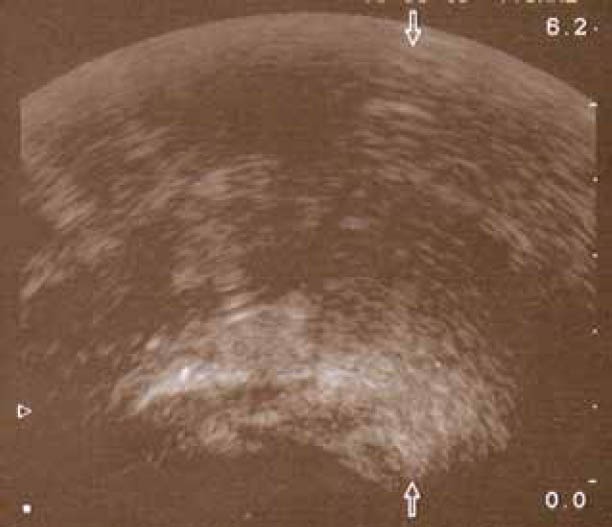
Transrectal ultrasonography showing marked thickening of the rectal wall with expansion of the submucosa
Figure 3.
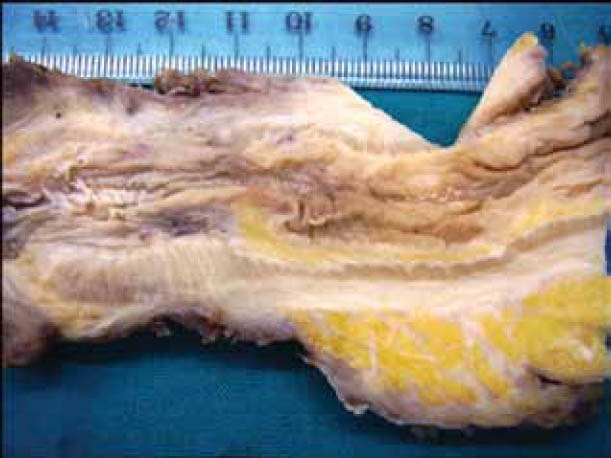
Macroscopic view of the resected specimen demonstrating an expanded submucosal space with intact mucosa and muscular layer
The patient received postoperative radiation followed by gemcitabine (100 g/m2 on days 1, 8, 15) and cisplatin (70 mg/ m2 on day 2) for a 28-day cycle for six months, but he died eight months later due to disseminated peritoneal carcinomatosis.
Discussion
RLP secondary to urinary bladder carcinoma is very rare [5,6]. Dressen et al described two patients who presented with changed bowel habits [5]. All diagnostic tests were inconclusive. In both patients pelvic MRI images revealed double-layered thickening of the rectal wall with an inner iso-intense circumferential thickening of the submucosal and hypo-intense circumferential thickening of the muscular rectal wall and a thickened bladder wall. Surgery disclosed a grade III papillary transitional cell carcinoma with infiltration of rectal wall and prostate gland in the first patient. In the second patient the RLP was due to recurrent transitional cell carcinoma of the urinary bladder. Gleeson et al described the EUS features and tissue diagnosis of secondary RLP in three patients with recurrent urinary bladder cancer [6].
Our patient was asymptomatic from the urinary tract and secondary RLP was caused by hematogenous spread of a small urinary bladder carcinoma. Our inability to establish a preoperative diagnosis, despite the fact that endoscopic and endorectal pictures were compatible with RLP, is quite common. There is usually a long delay between the onset of symptoms and final diagnosis [5-7], caused by the fact that RLP can mimic various diseases such as inflammatory bowel disease, stenosis related to diverticulosis, ischemia, radiation-induced strictures, solitary rectal ulcer, endometriosis and malignant lymphoma [1,8-11]. Moreover, mucosal biopsies are rarely positive, as in our case, because the disease is predominantly located in the submucosal and muscularis propria. With the advent of interventional endosonography, EUS-guided fine needle aspiration is performed more and more often, but both sensitivity and accuracy of the method are low [12,13]. Surgical deep biopsies may be needed if previous biopsies are negative.
The endoscopic picture, associated with the endorectal ultrasonography findings, was compatible with RLP in our patient. Endoscopic features of RLP include luminal stenosis and indurated folds with an infiltrated or cobblestone appearance. Large cerebroid folds and ulcerations are present only in advanced cases [1,3,6]. Dumontier et al reported the main EUS finding of secondary RLP, which consists of a circumferential wall thickening, predominantly affecting the submucosal and muscularis propria layers [7].
In general, prognosis is poor because of the late recognition of the disease and the absence of any effective treatment. Even in patients with early diagnosis and limited RLP who were treated surgically, long-term survival was extremely low [14]. Lymphatic invasion with lymph nodes metastases and peritoneal carcinomatosis are usually present in 50-80% of cases at the time of diagnosis and account for the poor prognosis [14].
Biography
G. Gennimatas General Hospital, Thessaloniki, Greece
Footnotes
Conflict of Interest: None
References
- 1.Wei SC, Su WC, Chang MC, Chang YT, Wang CY, Wong JM. Incidence, endoscopic morphology and distribution of metastatic lesions in the gastrointestinal tract. J Gastroenterol Hepatol Rectal linitis plastica and urinary bladder carcinoma 175. 2007;22:827–831. doi: 10.1111/j.1440-1746.2006.04532.x. [DOI] [PubMed] [Google Scholar]
- 2.Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: II. The colon and rectum. Gastrointest Endosc. 1992;38:136–141. doi: 10.1016/s0016-5107(92)70378-2. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani MS. EUS and EUS-guided fine-needle aspiration for the diagnosis of rectal linitis plastica secondary to prostate carcinoma. Gastrointest Endosc. 1999;50:117–119. doi: 10.1016/s0016-5107(99)70361-5. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf TE, Levy MJ, Wiersema MJ. EUS features of recurrent transitional cell bladder cancer metastatic to the GI tract. Gastrointest Endosc. 2005;61:314–316. doi: 10.1016/s0016-5107(04)02578-7. [DOI] [PubMed] [Google Scholar]
- 5.Dresen RC, Beets GH, Vliegen RF, Creytens DH, Beets-Tan RG. Linitis plastica of the rectum secondary to bladder carcinoma: a report of two cases and its MR features. Br J Radiol. 2008;81:e249–e251. doi: 10.1259/bjr/59924178. [DOI] [PubMed] [Google Scholar]
- 6.Gleeson FC, Clain JE, Rajan E, et al. Secondary linitis plastica of the rectum: EUS features and tissue diagnosis. Gastrointest Endosc. 2008;68:591–596. doi: 10.1016/j.gie.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Dumontier I, Roseau G, Palazzo L, Barbier JP, Couturier D. Endoscopic ultrasonography in rectal linitis plastica. Gastrointest Endosc. 1997;46:532–536. doi: 10.1016/s0016-5107(97)70009-9. [DOI] [PubMed] [Google Scholar]
- 8.Caramella E, Bruneton JN, Roux P, Aubanel D, Lecomte P. Metastases of the digestive tract. Report of 77 cases and review of the literature. Eur J Radiol. 1983;3:331–338. [PubMed] [Google Scholar]
- 9.Cox MR, Heinz AW, Fisher AL, Smart P. Linitis plastica carcinoma of the colon mimicking Crohn's colitis. Aust N Z J Surg. 1992;62:654–657. doi: 10.1111/j.1445-2197.1992.tb07540.x. [DOI] [PubMed] [Google Scholar]
- 10.Melnick GS, Rosenholtz MJ. Metastatic breast carcinoma simulating ulcerative colitis: report of a case. Am J Roentgenol Radium Ther Nucl Med. 1961;86:702–706. [PubMed] [Google Scholar]
- 11.Zrihen E, Aziza G, Crespon B, Rougier P. Rectal lymphoma: endorectal ultrasound aspect. Gastroenterol Clin Biol. 1992;16:375–376. [PubMed] [Google Scholar]
- 12.Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 13.Wittmann J, Kocjan G, Sgouros SN, Deheraqoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 14.Ha HK, Jee KR, Yu E, et al. CT features of metastatic linitis plastica to the rectum in patients with peritoneal carcinomatosis. Am J Roentgenol. 2000;174:463–466. doi: 10.2214/ajr.174.2.1740463. [DOI] [PubMed] [Google Scholar]


