Abstract
The destruction of cellular targets during apoptosis is carried out by caspases, which are negatively regulated by the inhibitor of apoptosis proteins (IAP); however, death effector domain (DED) caspases of the extrinsic pathway are refractory to the IAP family. We have isolated a family of apoptotic inhibitors [caspases-8- and -10-associated RING proteins (CARPs)] that bind to and negatively regulate DED caspases. When overexpressed, CARPs, via an IAP-like RING domain, can contribute to the ubiquitin-mediated proteolysis of DED caspases. Furthermore, CARPs are rapidly cleaved during apoptosis. However, in tumors and tumor cell lines, they are overexpressed, and their silencing leads to restoration of efficient apoptosis via enhanced activation of DED caspases. Long-term inhibition of CARP expression results in suppression of cancer cell growth, highlighting their importance in tumor cell survival.
Programmed cell death is mediated by a family of cysteine proteases, termed caspases (1). Distinct initiator caspases are activated depending on the death stimulus via adaptor-induced caspase aggregation, culminating in executioner caspase activation and cell death. Cell death is initiated through two distinct pathways, the extrinsic pathway at the cell surface and the intrinsic pathway mediated by mitochondrial homeostasis. Binding of death ligands such as FasL to their cognate receptors triggers the extrinsic pathway, leading to death-inducing signaling complex (DISC) formation and activation of DED-containing caspases-8 and -10 (2). Cellular stresses such as UV irradiation result in engagement of the intrinsic cell death pathway leading to procaspase-9 and -2 activation (3). Both the intrinsic and extrinsic pathways engage a common set of executioner caspases (-3, -6, and -7), which dismantle the cell by cleaving intracellular proteins, altering or negating protein function (4).
Due to the central role of caspases in apoptosis, organisms have evolved numerous mechanisms to regulate their activity. All caspases are synthesized as inactive precursors, which require proteolytic cleavage to form the active enzyme. The activity of executioner caspases as well as initiator caspase-9 is negatively regulated by the inhibitor of apoptosis proteins (IAPs), which inhibit caspases until an appropriate apoptotic stimulus is received (5, 6). This idea is best demonstrated by Drosophila DIAP1 mutants, which undergo massive apoptosis due to constitutive caspase activation (7). Activation of the intrinsic pathway in higher organisms requires mechanisms to combat IAP-mediated caspase inhibition to allow cell death: these include caspase-mediated IAP cleavage (8, 9) and IAP-mediated autoubiquitination and degradation (10), as well as sequestration by resident mitochondrial proteins (Smac/DIABLO and Omi/HtrA2), which are released into the cytoplasm during apoptosis (11).
Despite the ability of IAPs to dampen the intrinsic pathway of cell death via caspase-9 inhibition, no IAP exists for the regulation of DED caspases of the extrinsic pathway, namely caspases-8 and -10. Due to the potential use of death ligands such as tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) as anti-cancer agents, we sought to identify molecules that may affect DED caspase activation and ultimately tumor cell survival. A gene family named caspase-8 and -10 associated RING protein (CARPs) was identified via yeast two-hybrid screening that interacts specifically with caspases-8 and -10. A C-terminal CARP RING domain with high homology to the IAP family contains E3 ubiquitin ligase activity and may contribute to the ubiquitin-mediated proteolysis of DED caspases in certain circumstances. Silencing of CARPs by RNA interference results in sensitization of cancer cells to death ligands by allowing more efficient caspase activation. Stable knockdown of CARPs results in inhibition of tumor cell growth, implying a role in tumor cell survival. CARP function is antagonized by caspase-mediated cleavage during cell death induction; however, CARPs are frequently overexpressed in human tumors, providing a mechanism of resistance against death ligand-induced apoptosis.
Materials and Methods
Yeast Two-Hybrid Screening. pGBKT7 (Clontech System 3) DNA-binding domain vector was used to express the death effector domains of caspase-8, caspase-10, FLIP, and Fas-associated death domain (FADD). The human cDNA libraries (Clontech) were supplied in pGAD10 or pACT2. Screens were conducted in the reporter strain HF7c. (see Supporting Text, which is published as supporting information on the PNAS web site).
Cloning and Protein Expression. CARP1 and -2 cDNAs were cloned into pFLAG-CMV2 (Sigma). T7-tagged dominant negative caspase constructs were a generous gift of Emad Alnemri (Thomas Jefferson University, Philadelphia) (12). Point mutations were generated by using the QuikChange site-directed mutagenesis kit (Stratagene). Protein expression was carried out in DH10B by using pGEX-2T (Pharmacia). All constructs were fully sequenced to verify wild-type sequence or to check introduced point mutations.
Multiple Tissue Northern Blots and Cancer Arrays. Clontech Northern blots and the cancer profiling array II were probed and stripped per the manufacturer's instructions with human probes for CARP1, CARP2, β-actin, or ubiquitin, as shown.
In Vitro Ubiquitination Assay. E3 and E2 components were produced as GST fusion proteins after 3-h induction of bacteria at 37°C with 0.5 mM isopropyl β-d-thiogalactoside and isolated with glutathione-agarose beads. Eluted purified proteins were concentrated by using Centricon columns and dialyzed overnight by using Slide-a-lyzer cassettes (Pierce). Thirty-microliter reactions were carried out in 50 mM Tris, pH 7.5/2mMATP/5 mM MgCl2/2mMDTTwith 500 ng of GST-Ubc4/500 ng of E3 (GST, GST-CARP1, or GST H342A)/10 μg of ubiquitin/5 μl of crude bacterially expressed human E1 (a generous gift from Thanos Halazonetis, Wistar Institute, Philadelphia) at 30°C for 90 min. Reactions were separated by SDS/PAGE and probed with α-ubiquitin antibodies.
Immunofluorescence. U20S cells were transfected for 18–20 h. Cells were washed once with PBS, fixed for 15 min with 4% formaldehyde, permeabilized with 0.5% Trition X-100 for 10 min, and blocked for 20 min with goat serum. α-Flag primary antibody (5 μg/ml; 3 h) and goat α-mouse FITC secondary (The Jackson Laboratory; 1:100; 1 h) were washed extensively with PBS.
Quantitative TaqMan RT-PCR. The reactions were carried out (Applied Biosystems) as described (13) with a VIC-labeled human GAPDH probe on the ABI Prism 7700 Sequence Detector. The primer sequences for CARP1 are 5′-AACGCAGAGGATCGGAACC-3′ and 5′-TTCCACATCATCAAGGCTTGAC-3′ using a 6-carboxyf luorescin-labeled probe of 5′-CAGACAGTGAAGCTCTCACTCTCTCCTTGGA-3′. The primer sequences for CARP2 are 5′-AGCATGGTTCCACCTACCTCAC-3′ and 5′-CCTCTTGATCCTGAGACACATGG-3′ with a FAM-labeled probe of 5′-TGCACAAGCCACCTCTGTTCCCC-3′.
DISC Immunoprecipitation. After 72 h of siRNA transfections, cells were trypsinized and left untreated or treated with 500 ng/ml α-Fas IgM antibody for 10 or 45 min. Cells were washed and processed as described above for coimmunoprecipitation, except that 1 μg of goat α-mouse IgM (Sigma) was used for the immunoprecipitation, and isotype-specific horseradish peroxidase secondaries (mouse IgG1, Southern Biotechnology Associates) were used in Western blottings to avoid crossreacting bands.
Colony Formation Assay with Stable RNA Interference. siRNA hairpins were expressed from the pSuper-Puro vector. The knockdown sequences for CARP1 and -2 started at nucleotides 424 and 861, respectively. Cells were transfected and selected for puromycin resistance (1.5 μg/ml) for 3 days, followed by serial dilution plating in 24-well plates. Colonies were grown for 1–2 weeks followed by Coomassie staining.
Results
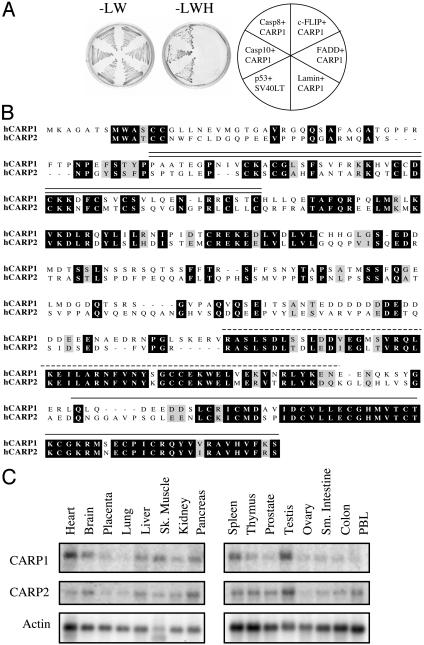
Identification of CARPs as DED-Caspase-Binding Proteins. Yeast two-hybrid screening with the prodomain of human caspase-10 using three different cDNA libraries (Table 1) resulted in the identification of two previously identified DED-caspase interacting proteins, FADD and FLASH (14), as well as a gene we named CARP1 (see below). Interaction studies in yeast revealed that CARP1 specifically interacts with both caspase-8 and -10 but not with c-FLIP or the adapter protein, FADD (Fig. 1A). Upon cloning of the full-length CARP1 cDNA using 5′ RACE, database searching revealed a second gene (CARP2) with high homology to CARP1, which contained all three functional domains: a FYVE domain, a caspase-interacting domain, and a RING domain with high homology to the IAP family (Fig. 1B, see below). Both CARP1 and -2 have highly conserved mouse homologues (88% and 83% identical, respectively); however, Drosophila contains only one CARP gene product. Northern blot analysis for human CARP1 and -2 expression (Fig. 1C) shows ubiquitous expression of both genes.
Table 1. Yeast two-hybrid screening with the prodomain of caspase-10.
| Library | No. clones screened | Clone identified |
|---|---|---|
| Fetal brain | 1.55 × 107 | FLASH (three) |
| HeLa | 0.55 × 106 | FADD (two) |
| Kidney | 0.23 × 106 | CARP1 (two) |
Numbers in parentheses represent the number of times the clone was recovered in the respective screen.
Fig. 1.
CARPs represent a gene family identified by a yeast two-hybrid screen and are found to interact with DED caspases. (A) Designated DED domains were cotransformed with CARP1 or Lamin into HF7c to test the specificity of the interaction. Transformed colonies were streaked onto plates lacking leucine and tryptophan (Left) to select for both plasmids, whereas plates lacking leucine, tryptophan, and histidine (Center) selected for interaction. p53/SV40 large T antigen served as a positive control. (B) Alignment of human CARP1 and -2 proteins highlights the three conserved functional domains. The FYVE domain is indicated by the double line, caspase interacting domain by the dashed line, and RING domain by the single solid line. (C) Human tissue expression of CARP1 and -2 with Actin loading control was determined by probing Clontech tissue poly(A)+ RNA blots.
CARP Functional Domains Include an IAP-Like RING Domain and a Phospholipid-Binding FYVE Domain. The presence of a C-terminal RING domain similar to IAP family members suggested that these genes may be novel IAPs that interact with DED caspases. Examination of the N terminus revealed eight cysteine residues with spacing reminiscent of zinc-binding domain architecture; however this region does not resemble a canonical BIR domain, which normally defines IAPs and contributes to their ability to interact with caspases (11). Instead, this motif has the highest similarity to a specialized RING domain called a FYVE domain (Fig. 2A), which has been demonstrated to bind phospholipids and contribute to subcellular localization (15). In addition to the conserved zinc-binding cysteine residues, this domain has a positively charged patch of amino acids responsible for interacting with negatively charged phospholipid head groups and two other motifs, the WXXD and RVC motif, which are thought to stabilize the interaction (Fig. 2A). Therefore, CARPs do not represent novel IAP proteins but rather define a separate gene family that interacts with caspases-8 and -10.
Fig. 2.
Conserved functional domains of CARP family demonstrate phopholipid binding and ubiquitin ligase activities. (A) FYVE domain alignment highlighting a lack of conservation compared to canonical domains. The WXXD and RVC motifs are underlined with solid and dotted lines, respectively, whereas the negatively charged phospholipid-binding residues are denoted by the dashed line. (B) Phospholipid membranes were incubated with GST-CARP1, GST-Hrs FYVE, or GST alone. GST alone yielded no lipid-binding activity after overnight exposure. The diagram denotes the specific phospholipids spotted onto the membranes. (C) U2OS cells were transfected with Flag-CARP1 or -2 for 18–24 h followed by Flag primary and FITC secondary antibody staining. Intense lamel-lapodia staining at the periphery is characteristic of plasma membrane staining. No endosomal localization was seen, as judged by punctate staining or colocalization with transferrin or transferrin receptor (data not shown). (D) RING domain alignment demonstrating a high degree of similarity between CARPs and IAPs. (E) In vitro ubiquitination assay reconstituted with ATP, ubiquitin, crude E1, and purified GST-E2 and -E3 proteins. GST alone and a RING point mutant of CARP1 (GST-H342A) were used as negative controls.
Most FYVE domain proteins bind to phosphoinositol-3-phosphate (PI3P) and, in conjunction with a second targeting sequence (i.e., palmitoylation or protein/protein interactions), often confer an endosomal localization. However, some FYVE domain-containing proteins do not localize to the endosome such as the Fgd1 family of proteins, located at the plasma membrane (15). Alignment of known FYVE domains with CARP1 and -2 (Fig. 2A) revealed the highest similarity to the Fgd1 subgroup of proteins due to the lack of conservation of the WXXD motif. An in vitro protein/phospholipid-binding assay revealed that CARP1 binds phosphoinositol-5-phosphate (PI5P) and PI3P with high affinity, whereas Hrs, a classical endosomally located FYVE domain protein, demonstrates strong PI3P binding (Fig. 2B). The binding profile for CARP1 with increased affinity for PI5P mimics that seen for the Fgd1 subfamily of FYVE domain proteins (16), suggesting that the CARPs may not localize to endosomes. Immunoflourence of Flag-tagged CARP1 and -2 in U2OS cells confirmed this idea and demonstrated a cytosolic as well as a plasma membrane distribution (Fig. 2C and Fig. 7, which is published as supporting information on the PNAS web site).
The discovery that RING domains confer E3 ubiquitin ligase activity suggests their involvement in the regulation of protein stability through ubiquitin-mediated proteolysis (17). Although the role of the RING domain of IAPs in the suppression of mammalian apoptosis is controversial, studies have confirmed the E3 ubiquitin ligase activity of this domain (10). In vitro studies suggest that these IAPs can monoubiquitinate caspases (18); however, in vivo evidence of this activity is still forthcoming. The RING domain of the CARP family (Fig. 2D) is most homologous to that of the IAPs. Reconstitution of an in vitro ubiquitination reaction with purified GST-CARP1 as the E3 generated a high-molecular-weight smear of ubiquitinated proteins characteristic of this reaction (Fig. 2E). As predicted by studies with IAPs (18), Ubc4 was able to act as the E2-conjugating enzyme in this reaction. Mutation of a conserved zinc-binding histidine residue within the RING domain (CARP1 H342A) abolished all E3 activity associated with CARP1, demonstrating the necessity of an intact RING domain to mediate this activity (Fig. 2E, lane 6).
Overexpression of CARPs Leads to the Proteasomal Degradation of DED Caspases. The ability of CARPs to act as E3 ubiquitin ligases in vitro prompted us to test the effect of CARP overexpression on the stability of exogenously expressed caspases. Catalytically inactive caspases-8, -9, and -10 were used to avoid activation of cell death. Overexpression of full-length CARP1 or the C-terminal domain containing the caspase-interacting and RING domains was sufficient to lead to the disappearance of full length caspases-8 and -10 but not caspase-9 (Fig. 3A). Furthermore, mutants of CARP1 lacking a functional RING domain either by deletion or point mutation were unable to mediate this effect (Fig. 3 C and D and Fig. 8, which is published as supporting information on the PNAS web site). The IAP family has been demonstrated to bind to executioner caspases-3 and -7 and also the initiator caspase-9 in addition to having ubiquitin ligase activity. Recently, X-linked inhibitor of apoptosis (XIAP) has been shown to ubiquitinate and degrade a constitutively active mutant of caspase-3, but not procaspase-3, in similar studies, which contributes to the antiapoptotic effect of XIAP on Fas-mediated cell death (19). Experiments using XIAP, c-IAP1, c-IAP2, and CARP1 demonstrated that only CARP1 is able to degrade caspases-8 and -10, whereas caspase-9 levels remain unaffected (Fig. 3B). Furthermore, the maintenance of caspase-9 levels in the presence of the IAPs suggests that pro-caspase-9 is not an E3 target of the IAPs.
Fig. 3.
Overexpression of CARP1 and -2 leads to ubiquitin-mediated proteolysis of DED caspases. (A) 293 cells were cotransfected for 24 h with equivalent amounts of caspase and CARP constructs. Levels of transfected caspase were examined by T7 Western blot. (B) 293 cells were transfected as in A, along with a GFP transfection marker (1/10 of the total) to serve as a transfection loading control. (C) 293 cells were transfected as in B for 12 h followed by either 5 μM lactacystin or 0.5 μM epoxomicin to inhibit proteasomal degradation for 8 h. (D) 293 cells were transfected as in B followed by immunoprecipitation with α-Flag antibody, washing, and subsequent Western blot analysis. (Top) Coimmunoprecipitation of caspase-8 with E3-deficient CARP mutants (IP; Middle). (Bottom) Levels of transfected caspase and CARPs in the total cell lysate.
Treatment of cells with the proteasome inhibitors, lactacystin or epoxomicin, during coexpression of CARP1 and caspase-8 results in the rescue of caspase-8 expression, implicating ubiquitin-mediated proteolysis (Fig. 3C). Due to the strong degradative phenotype when CARPs and DED caspases are coexpressed, the use of E3-deficient CARP1 and -2 mutants was necessary to demonstrate an interaction in mammalian cells (Fig. 3D). Coexpression of wild-type CARPs and caspase-8 led to the disappearance of the caspase, whereas a point mutation within the RING domain (CARP1 H342A or CARP2 H333A) or a RING deletion preserves caspase-8 levels (Fig. 3D Bottom). The rescue of caspase-8 levels with the CARP point mutants, H342A and H333A, allowed for the detection of caspase-8/CARP complexes. Reciprocal coimmunoprecipitations as well as experiments with caspase-10 (Fig. 9, which is published as supporting information on the PNAS web site) demonstrate CARP1 and -2 can interact with the prodomain of both caspases-8 and -10.
siRNA-Mediated Down-Regulation of CARPs Leads to Sensitization to Death Ligand Treatment. Based on overexpression studies that demonstrate the ability of CARPs to interact with and degrade DED caspases, CARP1 and -2 potentially represent novel negative regulators of cell death. To test this theory, we designed siRNA against both CARP1 and -2 and investigated the effect on DED caspases and their response to death ligands. Quantitative TaqMan RT-PCR demonstrated that siRNA against CARP1 or -2 leads to a 60% and 70% decrease in message, respectively in H460 human lung cancer cells, consistent with transfection efficiency. Surprisingly, transient knockdown of CARP1 or -2 had no effect on basal levels of caspase-8 or -10 expression (Fig. 4A). Despite an inability to effect overall caspase stability after transient CARP knockdown, we tested the ability of CARP proteins to regulate the activity of caspases-8 and -10 in response to an apoptotic stimulus such as death ligand treatment. CARP1 or -2 siRNA treatment of H460 cells followed by TNF-α/cycloheximide or TRAIL exposure led to enhanced caspase-8 and -10 cleavage in response to the ligands (Fig. 4B). Consistent with enhanced cleavage and activation of these DED caspases, a downstream target of executioner caspases, poly ADP-ribose polymerase (PARP), showed enhanced cleavage. Knockdown of CARP2 expression consistently demonstrated a more dramatic effect on DED caspase activation and cell death compared to CARP1 in all cell lines tested.
Fig. 4.
siRNA against CARP1 or -2 accelerates DED caspase activation and sensitizes cancer cells to death ligands and chemotherapy. (A) H460 lung cancer cells are transfected with siRNA against control luciferase (L), CARP1 (C1), or CARP2 (C2) using Oligofectamine for 48 h. Gene knockdown efficiency for CARP1- and -2-specific siRNA duplexes is shown (Left) as measured by Quantitative TaqMan RT-PCR. Effects on caspase-8, -10, and c-FLIP protein levels as determined by Western blot showed no significant changes. Additionally, no effects on FADD or c-FLIPs were noted (data not shown). (B) H460 cells were transfected with siRNA as in A. Subsequently cells were either treated with TRAIL (50 ng/ml) and crosslinking α-His antibody (1 μg/ml) for 2 h or were pretreated for 1 h with 10 μg/ml cycloheximide (CHX) followed by a 6-h 10 ng/ml TNF-α treatment. Kinetics of caspase-8 and -10 activation as well as PARP cleavage was determined by Western blotting. Ras-related nuclear protein is supplied as a loading control. (C) After siRNA transfection and TNF/CHX treatment (2 or 6 h) as in B, cells were trypsinized and resuspended with 1× CaspaTag caspase-8 specific FAM labeled LETD-fmk and incubated for 1hat37°C. Live cells were then washed, and caspase-8 activity was measured by flow cytometry. (D) H460 cells were transfected with a constant amount (15 μl from 20 μM stocks) of various combinations of siRNA duplexes. The cells were subsequently treated and processed as in B. (E) SW480 cells were transfected with siRNA as in A and subsequently treated with 500 ng/ml α-Fas IgM antibody for 2 or 4 h. Protein lysates were examined for caspase-8 cleavage kinetics (Upper). (Lower) A Fas DISC IP of siRNA-treated SW480 cells after 10 or 45 min of 500 ng/ml α-Fas IgM treatment. DISC-associated caspase-8 and FADD were measured by Western blot analysis. (F) H460 cells were transfected with siRNA as in A and then treated with TNF-α/CHX (6 h), 0.5 μM etoposide (24 h), 1 μg/ml adriamycin (24 h), or 1 μM Taxol (24 h). Apoptosis was assessed by SubG1 content. Data are representative of three independent experiments.
To conclude that enhanced processing of caspase-8 and -10 did indeed lead to higher levels of caspase activity, we conducted a caspase-8 activity assay in siRNA-treated cells after death ligand exposure. Although no increase in basal levels of caspase-8 activity was observed, treatment with death ligand led to enhanced caspase-8 activity after transient CARP1 or -2 siRNA treatment (Fig. 4C). Combined knockdown of CARP1 and -2 in H460 led to the most dramatic effect on caspase-8 activation (Fig. 4D) and cell death in response to TRAIL treatment strengthening the hypothesis that the CARP family acts as a negative regulatory mechanism for DED caspases.
Similar results were also observed in response to agonist α-Fas antibody treatment in SW480 colon cancer cells, which displayed similar CARP knockdown efficiency as H460 (data not shown). In CARP1 or -2 siRNA-treated SW480 cells, enhanced kinetics of caspase-8 activation were observed after 2 and4hof α-Fas antibody treatment (Fig. 4E Upper). To determine whether the effect of CARP proteins functioned at the level of DISC formation and activation or during more downstream DED caspase activation, we assessed the effect of siRNA treatment on the Fas DISC in SW480 cells (Fig. 4E Lower). Diminution of CARP1 or -2 appeared to have no effect on FADD or caspase-8 recruitment or activation at the DISC, suggesting that CARP1 and -2 normally act downstream of the Fas DISC in SW480 cells. Similar effects were also observed in H460 cells. Due to the phospholipid-binding activity and potential plasma membrane localization of the CARP family of proteins, it is possible in some cell types that CARPs regulate DED caspase activation at the level of the DISC. Most notably, Type II cells have decreased caspase-8 activation due to impaired DISC formation (20), presenting one example where elimination of CARP proteins may augment caspase-8 activation at the DISC effectively converting a Type II to a Type I cell.
Despite the fact that caspase-8 is not required for chemotherapy-induced apoptosis in mice (21), numerous reports suggest that caspase-8 can act as an executioner caspase and enhance the death kinetics after chemotherapy treatment (22, 23). Because CARP1 and -2 appear to regulate DED caspase activation downstream of the Fas DISC in H460 and SW480 cells, this suggests that agents that use caspase-8 as an executioner caspase might also be affected by CARPs. siRNA against CARP1 and -2 enhanced the death kinetics of chemotherapeutic agents such as etoposide, adriamycin, and taxol as assessed by increased SubG1 content in H460 cells (Fig. 4E). These results are consistent with a model in which CARP1 and -2 serve to inhibit DED caspase activation.
CARPs Represent Caspase Cleavage Targets. The cell has adopted multiple mechanisms to counteract the negative influence of IAPs when confronted with a potent death stimulus. Because of the antiapoptotic nature of CARP1 and -2, we investigated whether death ligand treatment had any effect on CARP stability. TRAIL treatment of transfected HeLa cells led to the rapid disappearance of both CARP1 and -2 proteins. Cotreatment with a Pan caspase inhibitor, ZVAD-fmk, blocked this disappearance, whereas addition of a proteasome inhibitor did not (Fig. 5A). Furthermore, the combination of caspase-8 and -10 inhibitors completely blocked CARP cleavage, consistent with inhibition of death receptor signaling. The ability of caspase-3-specific inhibitors to block these cleavage events suggests that executioner caspases may be involved in CARP processing (Fig. 5A). In support of this, the kinetics of Flag-tagged CARP1 cleavage correlates with another caspase-3 target, PARP (Fig. 5B). Attempts to make a noncleavable CARP mutant have been unsuccessful to this point. Twelve aspartic acid to alanine substitution mutations in potential caspase cleavage sites in CARP1 have been generated without identifying a critical target site (data not shown). Nevertheless, these experiments suggest that CARPs are negatively regulated by caspase-dependent cleavage, providing the cell with a mechanism to alleviate the death repression CARPs impose.
Fig. 5.
CARP proteins undergo caspase-dependent cleavage during death receptor-mediated apoptosis. (A) HeLa cells were transfected overnight followed by 12-h TRAIL (50 ng/ml) and α-His antibody (1 μg/ml) treatment. ZVAD-fmk, caspase-3 inhibitor, or a combination of caspase-8 and -10 inhibitors (20 μM;R& D Systems), and MG132 (10 μM; Sigma) were added 1 h before TRAIL treatment. CARP1 and -2 expression was measured by α-Flag Western, and Ras-related nuclear protein is provided as a loading control. (B) SW480 cells were transfected overnight and treated with TRAIL and α-His antibody for 2 and 4 h. The kinetics of caspase-8, CARP1, and PARP cleavage were compared by Western blot analysis.
CARPs Are Overexpressed in Human Tumors and Tumor Cell Lines. Because CARPs negatively regulate DED-caspase activation, deregulation of CARPs in tumor cells would be predicted to be advantageous to avoid elimination via apoptosis. SAGE on the NCI 60 cell line tumor panel revealed CARP1 or -2 expression to be at the highest levels detectable in this assay for 20% of the cell lines (Fig. 6A). Cell lines with high levels of CARP expression via SAGE were used for RNA interference studies to access the role of CARPs in resistance to death. siRNA-mediated knockdown of CARP2 alone effectively sensitized two CARP2-overexpressing cell lines (the ovarian cancer cell line SkOV3 and the prostate cancer cell line DU-145) to TNF-α-induced cell death (Fig. 6B). Knockdown of CARP2 converted both cell lines from a TNF-α-resistant state to a sensitive one. Furthermore, the lung cancer cell line H460 used in Fig. 4 is predicted by SAGE to contain high CARP2 levels (Fig. 6A), consistent with the ability of CARP2 knockdown to lead to sensitization to apoptotic stimuli. In an attempt to determine whether CARPs provide a potential growth advantage to tumor cells, vectors expressing siRNA hairpins were transfected into tumor cells followed by puromycin selection to assess colony formation (Fig. 6C). Stable knockdown of CARP1 or -2 resulted in a dramatic reduction in colony formation, suggesting that CARP expression is required for cell viability in some cell lines. Hairpin vectors designed against CARP1 or -2 that did not result in down-regulation of CARP expression resembled vector alone and did not suppress colony formation (Fig. 10, which is published as supporting information on the PNAS web site). Lastly, comparison of CARP expression in normal vs. tumor tissue was conducted by cDNA hybridization to total RNA samples to assess the frequency of CARP overexpression in primary tumors (Fig. 6D). The blots were subsequently stripped and reprobed with ubiquitin to serve as a loading control. Comparison of the relative levels of CARP expression in various tissues revealed up-regulation of CARP1 in ovarian (30%) and cervical cancer (40%) and upregulation of CARP2 in breast (50%), uterine (30%), and testicular cancer (20%). These data in cell lines and primary tumors suggest that CARPs may represent novel targets for deregulation in cancer cells to provide resistance to death-receptor-mediated apoptosis.
Fig. 6.
CARP proteins are deregulated in cancer cell lines and primary tumors. (A) SAGE data for CARP1 and -2 on the NCI 60 cell line panel was retrieved from http://cgap.nci.nih.gov. (B) SkOV3 and DU-145 were transfected with siRNA duplexes for 48 h and then pretreated for 1 h with 10 μg/ml cycloheximide followed by a 6-h 10 ng/ml TNF-α treatment before SubG1 analysis. (C) Cells were transfected with siRNA hairpin vectors and selected for 3 days in puromycin. Resulting colonies were replated and grown in 24-well plates followed by Coomassie staining to assess colony formation. (D) Cancer profiling arrays comparing matched normal vs. tumor samples were probed with cDNAs of CARP1, -2, or ubiquitin as a loading control.
Discussion
CARPs represent a family of antiapoptotic proteins that specifically bind to and regulate DED caspases. The necessity of two related CARPs is unclear at the present time but closely mimics c-IAP1 and -2, which are highly homologous and are both recruited to TNF receptors after ligand binding (24). The lack of a clear caspase-10 homolog in mouse combined with the existence of both CARP1 and -2 argues against the idea of DED caspase specificity but rather points toward a potentially diverse set of ubiquitination target proteins and/or differential regulation of the CARPs. These studies suggest two models (see below) in which CARPs ultimately resemble IAP proteins, in function and RING sequence, by inhibiting DED caspases within the cytoplasm. Despite strong evidence that ablation of CARPs results in sensitization to various forms of apoptotic stimuli, it is unclear whether the mechanism involves (i) direct caspase ubiquitination and degradation, as the overexpression data would suggest, or (ii) simply a sequestration model (i.e., ubiquitin ligase independent), as is seen with the IAPs. If CARPs constitutively affected basal DED caspase stability via ubiquitin-mediated proteolysis, knockdown of CARPs would be predicted to result in increased levels of caspases. This has not been observed in these systems, suggesting that caspase stability may be regulated in a signal-dependent manner. In accordance with this idea, the instability and disappearance of caspase-8 subunits after prolonged death ligand signaling has been documented (25), which could be accounted for by caspase-8 interacting E3 ligases such as CARPs or the BAR protein. In addition, the transient and incomplete ablation of a single CARP may not be sufficient to affect overall caspase protein levels while still allowing for an overall increase in the free cellular pool of DED caspases to become activated by death ligands. Although it is obvious that CARPs possess RING domains with intrinsic E3 activity, the contribution of this activity toward their antiapoptotic function is not fully clear. Nevertheless, the silencing of CARPs allows for efficient DED caspase activation in response to the extrinsic pathway, and the importance of the CARPs is best demonstrated by the dramatic loss in cell viability after prolonged CARP ablation, supporting the model that CARPs inhibit DED caspases as an important means for cell survival.
A potential, although unproven, role for CARPs at the plasma membrane is implicated by its partial localization there as well as a functional phospholipid-binding domain. Interestingly, elimination of CARP1 or -2 has a more dramatic effect on cell death in response to TNF-α (compared to Fas or TRAIL). Recent experiments with TNF-α suggest that TNFR1 does not form a membrane associated DISC containing FADD and caspase-8 as is seen with Fas and TRAIL (26, 27). However, subsequent activation of caspase-8 within the cytoplasm is required for TNF-α-induced cell death. Because elimination of CARPs does not appear to alter Fas DISC formation but does enhance Fas and TNF-α-mediated caspase-8 activation and cell death, CARP proteins may act downstream of the membrane-bound DISC on DED caspases at a point in the cytoplasm directly engaged by the TNF pathway. Future experiments may be aimed at identifying the level within the death receptor pathways at which CARPs exert their effects. Finally, the identification of cell lines and primary tumors with overexpressed CARPs supports the hypothesis that CARP deregulation provides a mechanism of apoptotic resistance. This is exemplified by the ability of CARP knockdown to convert cells from a death ligand-resistant state to a sensitive one. Thus, CARPs represent a previously unrecognized layer of regulation imposed on the death receptor pathway impacting caspase activation and ultimately cell death.
Supplementary Material
Acknowledgments
W.S.E.-D. is an Assistant Investigator of the Howard Hughes Medical Institute. This work was supported by the Howard Hughes Medical Institute and by a grant from the National Institutes of Health (CA097100).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: IAP, inhibitor of apoptosis proteins; DED, death effector domain; CARP, caspases-8- and -10-associated RING protein; DISC, death-inducing signaling complex; siRNA, small interfering RNA; SAGE, serial analysis of gene expression; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; PARP, poly ADP-ribose polymerase; c-FLIP, cellular FLICE-like inhibitory protein.
References
- 1.Thornberry, N. A. & Lazebnik, Y. (1998) Science 281, 1312-1316. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, A. (2002) Nat. Rev. Cancer 2, 420-430. [DOI] [PubMed] [Google Scholar]
- 3.Igney, F. H. & Krammer, P. H. (2002) Nat. Rev. Cancer 2, 277-288. [DOI] [PubMed] [Google Scholar]
- 4.Hengartner, M. O. (2000) Nature 407, 770-776. [DOI] [PubMed] [Google Scholar]
- 5.Verhagen, A. M., Coulson, E. J. & Vaux, D. L. (2001) Genome Biol. 2, REVIEWS3009, Epub 2001 Jul 05. [DOI] [PMC free article] [PubMed]
- 6.Deveraux, Q. L. & Reed, J. C. (1999) Genes Dev. 13, 239-252. [DOI] [PubMed] [Google Scholar]
- 7.Richardson, H. & Kumar, S. (2002) J. Immunol. Methods 265, 21-38. [DOI] [PubMed] [Google Scholar]
- 8.Deveraux, Q. L., Leo, E., Stennicke, H. R., Welsh, K., Salvesen, G. S. & Reed, J. C. (1999) EMBO J. 18, 5242-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clem, R. J., Sheu, T. T., Richter, B. W., He, W. W., Thornberry, N. A., Duckett, C. S. & Hardwick, J. M. (2001) J. Biol. Chem. 276, 7602-7608. [DOI] [PubMed] [Google Scholar]
- 10.Yang, Y., Fang, S., Jensen, J. P., Weissman, A. M. & Ashwell, J. D. (2000) Science 288, 874-877. [DOI] [PubMed] [Google Scholar]
- 11.Salvesen, G. S. & Duckett, C. S. (2002) Nat. Rev. Mol. Cell. Biol. 3, 401-410. [DOI] [PubMed] [Google Scholar]
- 12.MacFarlane, M., Ahmad, M., Srinivasula, S. M., Fernandes-Alnemri, T., Cohen, G. M. & Alnemri, E. S. (1997) J. Biol. Chem. 272, 25417-25420. [DOI] [PubMed] [Google Scholar]
- 13.Burns, T. F., Bernhard, E. J. & El-Deiry, W. S. (2001) Oncogene 20, 4601-4612. [DOI] [PubMed] [Google Scholar]
- 14.Imai, Y., Kimura, T., Murakami, A., Yajima, N., Sakamaki, K. & Yonehara, S. (1999) Nature 398, 777-785. [DOI] [PubMed] [Google Scholar]
- 15.Stenmark, H., Aasland, R. & Driscoll, P. C. (2002) FEBS Lett. 513, 77-84. [DOI] [PubMed] [Google Scholar]
- 16.Sankaran, V. G., Klein, D. E., Sachdeva, M. M. & Lemmon, M. A. (2001) Biochemistry 40, 8581-8587. [DOI] [PubMed] [Google Scholar]
- 17.Joazeiro, C. A. & Weissman, A. M. (2000) Cell 102, 549-552. [DOI] [PubMed] [Google Scholar]
- 18.Huang, H., Joazeiro, C. A., Bonfoco, E., Kamada, S., Leverson, J. D. & Hunter, T. (2000) J. Biol. Chem. 275, 26661-26664. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki, Y., Nakabayashi, Y. & Takahashi, R. (2001) Proc. Natl. Acad. Sci. USA 98, 8662-8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scaffidi, C., Fulda, S., Srinivasan, A., Friesen, C., Li, F., Tomaselli, K. J., Debatin, K. M., Krammer, P. H. & Peter, M. E. (1998) EMBO J. 17, 1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varfolomeev, E. E., Schuchmann, M., Luria, V., Chiannilkulchai, N., Beckmann, J. S., Mett, I. L., Rebrikov, D., Brodianski, V. M., Kemper, O. C., Kollet, O., et al. (1998) Immunity 9, 267-276. [DOI] [PubMed] [Google Scholar]
- 22.Tang, D., Lahti, J. M. & Kidd, V. J. (2000) J. Biol. Chem. 275, 9303-9307. [DOI] [PubMed] [Google Scholar]
- 23.Engels, I. H., Stepczynska, A., Stroh, C., Lauber, K., Berg, C., Schwenzer, R., Wajant, H., Janicke, R. U., Porter, A. G., Belka, C., et al. (2000) Oncogene 19, 4563-4573. [DOI] [PubMed] [Google Scholar]
- 24.Chen, G. & Goeddel, D. V. (2002) Science 296, 1634-1635. [DOI] [PubMed] [Google Scholar]
- 25.Stegh, A. H., Barnhart, B. C., Volkland, J., Algeciras-Schimnich, A., Ke, N., Reed, J. C. & Peter, M. E. (2002) J. Biol. Chem. 277, 4351-4360. [DOI] [PubMed] [Google Scholar]
- 26.Micheau, O. & Tschopp, J. (2003) Cell 114, 181-190. [DOI] [PubMed] [Google Scholar]
- 27.Harper, N., Hughes, M., MacFarlane, M. & Cohen, G. M. (2003) J. Biol. Chem. 278, 25534-25541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.