Abstract
Background
Helicobacter pylori (H. pylori), infects gastric mucosa causing gastritis. Treatment failure is mainly due to certain genetic changes in the peptidyltransferase loop of 23S rRNA of the microorganism. The aim of the study was to evaluate genetic changes in gastric biopsies of H. pylori (+) patients that lead to clarithromycin resistance and to correlate them with histology data.
Methods
A total of 150 H. pylori (+) gastric biopsies were studied, taken before and after eradication therapy from 75 dyspeptic patients divided in 2 groups: group A consisted of 25 H. pylori (+) triple-therapy resistant patients and group B consisted of 50 H. pylori (+) successfully treated patients. Histological classification of the H. pylori (+) gastritis was done according to the Sydney criteria. Genetic material was analyzed with the ClariRes™ RT-PCR bi-probe based assay for the determination of point mutations in the 23S rRNA gene and with a Quantitative-RT-PCR (Q-RT-PCR) method for the quantitation of H. pylori.
Results
We showed that in 18/ 25 group A patients certain point mutations of 23S rRNA at sites A2142C, A2142G and A2143G had occurred. Nine of these 18 mutated cases (50%) were characterized as mixed infections. Mixed infections in 2/50 patients of group B were also observed. Using Q-RT-PCR, we found that gastric mucosal density of H. pylori correlates well with bacterial colonization. There was a statistically significant association (P<0.005) between the presence of the detected H. pylori genetic alterations and inflammation, activity and H. pylori density as histologically determined.
Conclusion
Certain point mutations in H. pylori genome that affect susceptibility to clarithromycin correlate with histological features of gastritis.
Keywords: Clarithromycin resistance, ClariRes assay, real-time PCR, quantitative real-time PCR, histology, Sydney classification, Helicobacter pylori infection
Introduction
Helicobacter pylori (H. pylori) is a gram negative bacterium, associated with gastritis and duodenal ulceration as well as mucosa-associated lymphoid tissue (MALT) lymphoma and gastric cancer [1-3]. Among the treatments used for H. pylori infection, the highest eradication rates were achieved with the use of a proton pump inhibitor (PPi) or ranitidine bismuth citrate in combination with two antibiotics, mainly amoxicillin with either clarithromycin or metronidazole. However, there is still a failure rate of 21 to 25% with these combined therapies [4-7]. Primary clarithromycin resistance is increasing worldwide, and it has been regarded as the main factor reducing the efficacy of H. pylori therapy [8-11]. The major cause of macrolide resistance in H. pylori is the lack of drug binding to the 23S rRNA components of the bacterial ribosomes, mainly due to an adenine-to-guanine transition at positions 2142 and 2143 and to adenine-to-cytosine transversion at position 2142, all of which are included in the peptidyltransferase loop of the 23S rRNA [12-15].
In this retrospective analysis in archival material, we applied in formalin-fixed paraffin-embedded (ffpe) biopsies a relative new and sensitive in vitro diagnostic (IVD) Real-Time (RT) PCR-based method that permits direct identification of different mutations in the 23SrRNA. At the same time and in the given material, we tried to evaluate the H. pylori density by employing a Q-RT-PCR technique and correlating it with histological parameters such as those expressed by the Sydney classification.
Material and methods
Patients
Seventy-five H. pylori positive dyspeptic patients were treated with a standard triple therapy based on a PPi plus two antibiotics for 7 days [16]. Patients were divided in 2 groups: Group A consisted of 25 non-responding to triple therapy patients (13 men; mean age 65±12.5 years) and Group B consisted of 50 patients successfully treated (26 men; mean age 61±14.5 years). None of the patients had previously received any kind of antibiotic treatment for H. pylori.
Diagnostic assays and classification of H. pylori gastritis
One hundred and fifty blocks (one before and one after therapy) from groups A+B of ffpe gastric mucosa biopsies, obtained from the antrum and from the middle portion of the great curvature of the corpus, were investigated. Tissue sections (2 μim in thickness) were cut and stained with Hematoxylin & Eosin (H&E) for histological evaluation, May-Grunwald-Giemsa for H. pylori identification and High Iron Diamine (HID) for intestinal metaplasia determination [17]. Semi-quantitative method of scoring was undertaken according to the Updated Sydney Classification System [18-20]. The histological variables (inflammation, activity, atrophy, intestinal metaplasia and colonization of H. pylori) were graded on a scale of 3 (mild=1+, moderate=2+, severe=3+).
RT-PCR and genetic analysis
The novel, commercially available ClariRes™ assay (Ingenetix, Vienna, Austria), was used for the detection of mutations in the peptidyltransferase region of the H. pylori 23S rRNA [21,22]. ClariRes™ is an IVD assay that contains in the kit all positive and negative controls needed. Originally used for stool analysis, we slightly modified the extraction method to better suit the needs of a Pathology Department. Briefly, DNA was extracted from biopsies using the Nucleospin TissueXS kit (Macherey-Nagel, Duren, Germany) and checked with NanoDrop 2000UV-Vis Spectrophotometer (Thermo Scientific, Wilmington, Del, USA). The 20-μL PCR mixture contained 2 μL of LightCycler™ Fast Start DNA Master SYBR green I (Roche Molecular Biochemicals, Mannheim, Germany), 2.4 μL of 4 mM MgCl2, 12.1 μL of deionized water, 0.5 μL H. pylori ClariRes assay solution (Ingenetix, Vienna, Austria), 1 μL of freshly diluted internal control (Ingenetix) and 2 μL of the DNA extract. Extra positive controls were also used, consisting of microorganisms with known concentration/ volume of culture medium. Detailed description of the original RT-PCR method introduced by Schabereiter-Gutner et al, has been published elsewhere [23]. The data and the melting point curves were analyzed with Roche LightCycler software (version 3.5.3).
Q-RT-PCR and H. pylori density
The primers used for Q-RT-PCR were designated as HP23S1 (5'-GGA GCT GTC TCA ACC AGA GAT TC-3') (nucleotide positions 2071 to 2093) and HP23S2 (5'-CGC ATG ATA TTC CC[AG] TTA GCA G-3') (nucleotide positions 2181 to 2201), and the result was a 132-bp product. The two hybridization probes used were designated as HP23S3 and HP23S4 respectively. HP23S3 (5'-GGA GCT GTC TCA ACC AGA GA[Red640]T TC-3') had the same sequence as primer HP23S1 and was internally labeled with Red640. HP23S4 (5'-GGA ATT TTC ACC TCC ACT ACA ATT TCA CTG[Fluo]-3') (nucleotide positions 2201 to 2230) was 3' labeled with fluorescein and located just downstream of HP23S3 on the other strand. H. pylori DNA was extracted as previously mentioned [23,24]. After DNA extraction, one 10-fold serial dilution of H. pylori DNA was made, with bacterial concentrations ranging from 3×101 to 3×108 bacteria per 5 μL. A series of 10-fold dilutions of H. pylori DNA was included in each amplification run. DNA from 0.25 mL of gastric biopsy homogenates was extracted by using the High Pure PCR template preparation kit (Roche Molecular Biochemicals, Mannheim, Germany). PCR was performed in a final volume of 25 μL with the DNA master hybridization probes kit (Roche Molecular Biochemicals, Mannheim, Germany), 5 and 10 pmol of oligonucleotide primers HP23S1 and HP23S2, respectively, 5 pmol of hybridization probes HP23S3 and HP23S4, and 5 μL of extracted DNA sample. Carryover was prevented by using heat-labile uracil-DNA glycosylase (Roche Molecular Biochemicals, Mannheim, Germany). Amplification was performed for 50 cycles of denaturation (95°C, 10 sec), annealing (55°C, 10 sec), and extension (72°C, 15 sec).
A single fluorescence reading for each sample was taken at the annealing step. Quantitative results were expressed by determination of the threshold of detection, or the crossing point, which marked the cycle when the fluorescence of a given sample significantly exceeded the baseline signal. The bacterial count for a given bacterial sample was calculated by interpolation from standard curve. To compare the densities obtained by PCR to those of histology and culture, the values obtained by Q-RT-PCR were converted into decimal logarithmic values [24].
Statistical analysis
For comparison of the different Sydney criteria histological parameters with genomic alterations and density, a non-parametric Kruskal-Wallis test was done using statistical program SPSS 17. For the evaluation of the correlation between the density of H. pylori genomes and H. pylori's distribution in biopsies, Spearman's rank correlation coefficient was calculated. A P value of <0.05 was considered statistically significant.
Results
Histology
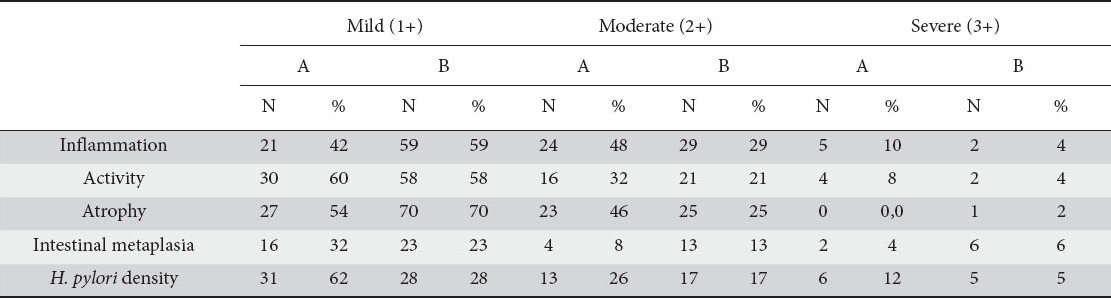
The histological data of this study (concerning groups A and B) are summarized in Table 1.
Table 1.
Updated Sydney classification of H. pylori gastritis cases (groups A and B)
Direct detection of mutations conferring clarithromycin resistance
The ClariRes™ RT-PCR bi-probe IVD assay to detect point mutations in the 23S rRNA gene was applied to ffpe material to determine clarithromycin susceptibilities on DNA from 150 gastric biopsy samples taken before and after H. pylori eradication therapy. In 2 paraffin blocks of group A, no amplification product was obtained mainly because of inadequate or fragmented DNA. 23S rRNA PCR and further analysis with DNA extracts of H. pylori control strains produced melting curves with melting temperatures of 61-63°C for the wild type, 58°C for the A2142C mutant and 52-54°C for the A2142G and A2143G mutants. Of the 25 H. pylori(+), clarithromycin-resistant patients comprising group A, 18 were mutated (76%). In 9 of these 18 cases (50%), RT-PCR results suggest the presence of both wild-type and mutant (mixed) genotypes. All these cases were resistant to clarithromycin. Two of 50 group B cases also exhibited a mixed genotype, although all group B patients responded to eradication therapy. Further analysis of DNA from 18 mutated patients (17 from group A and 1 from group B) generated melting peaks indicative of a clarithromycin-resistant phenotype with an A→G mutation in positions 2142 or 2143. The remaining 2 mutated biopsy samples (1 from group A and 1 from group B), produced a melting peak indicative of clarithromycin resistance due to an A→C mutation in position 2142 (Table 2). Fig. 1 shows the double melting peaks obtained from DNA extract of a patient with mixed (wild type and mutated) H. pylori genotype and Fig. 2 shows several melting peaks obtained from DNA extract of three patients infected with clarithromycin-resistant microbes with mutant genotypes.
Table 2.
Mutations in the 23S rRNA gene of H. pylori in 150 bioptic materials from 75 H. pylori-infected patients. Values in parentheses are number of biopsies in which both wild type and mutant 23S RNA genes were simultaneously detected (mixed infections)
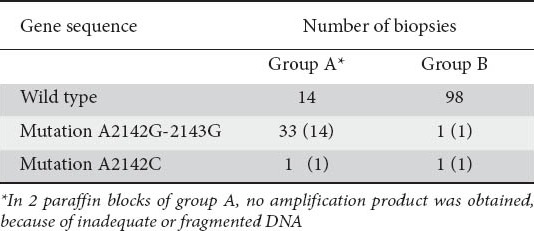
Figure 1.
Melting peaks obtained from formalin-fixed paraffin-embedded DNA extracts of an Helicobacter pylori-infected patient with mixed infection. Sensitive genotypes showed a melting temperature of 61°C and resistant genotypes with an A-to-G transition showed a melting point of 52.5°C
Figure 2.
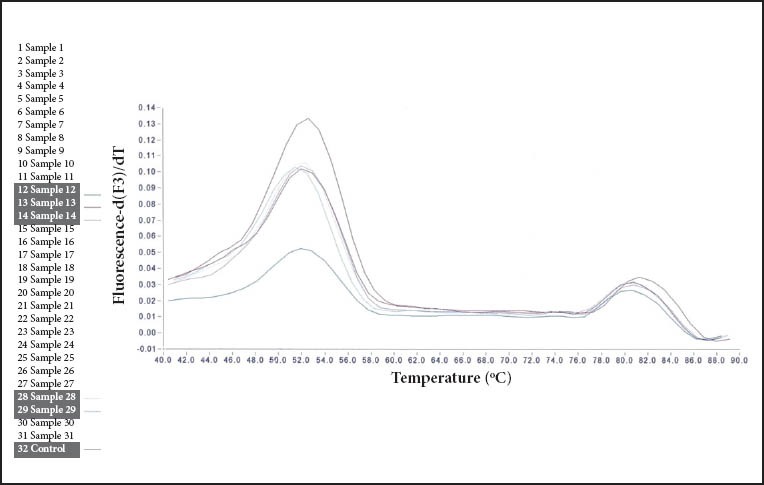
Melting peaks obtained from formalin-fixed paraffin-embedded DNA extracts of three patients infected with a clarithromycin resistant (mutant) Helicobacter pylori genotype. Mutations at sites A2142G and A2143G are displayed. Melting point at 52.5°C
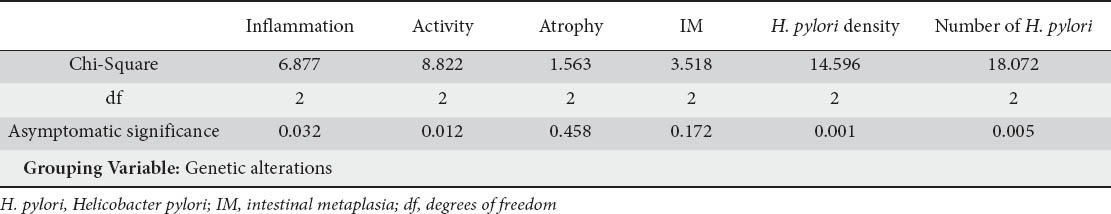
Comparison of genetic alterations and histology data
A statistically significant association between almost all Sydney classification parameters and genetic alterations in 23S rRNA of H. pylori were noticed, besides atrophy and intestinal metaplasia (results are summarized in Table 3). More specifically, 17 mutated cases correlated with mild, 14 with moderate and 5 with marked inflammation. Twenty one mutated cases correlated with mild, 11 with moderate and 4 with marked activity. H. pylori density correlated well with mutated genotype, especially when mild and moderate colonization occurred (22 and 12 cases respectively). Interestingly, only 2 markedly colonized cases expressed mixed genotype, in contrast to mild and moderate colonized cases which expressed 9 mixed genotypes in both groups of patients.
Table 3.
Kruskal-Wallis non-parametric test results from comparison of genetic with histological data
Quantitation of the mucosal bacterial density
Mucosal bacterial density quantitation was performed by histology and we found that in our material it was greater in the antrum than in the corpus. Bacterial density by Q-RT-PCR was evaluated for 173 of the 175 PCR-positive samples and ranged from 30 to 67,000 bacteria in the DNA sample. There was significant correlation between the grade of bacterial density estimated by Q-RT-PCR and histology, especially between grades 1 and 3 (P<0.001). The mean density of H. pylori genomes was higher in grade 1 (P<0.04), grade 2 (P<0.001) and grade 3 (P<0.001) with no significant differences between adjacent grades from 1 to 3. Fig. 3 shows the density of H. pylori genomes in tissue samples plotted against the histological grade as defined by the Sydney system. As shown in this figure, H. pylori genome even in small quantities was detected in 30% 30% of samples where no microorganism was histologically detected.
Figure 3.
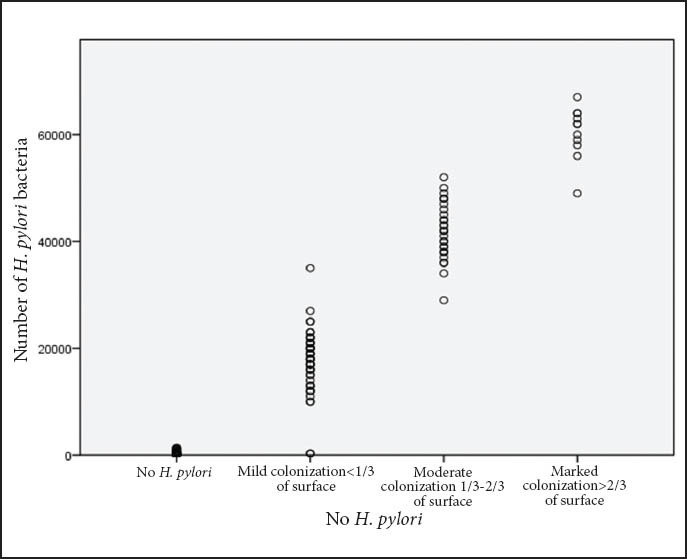
Graphic plot showing density of Helicobacter pylori (H. pylori) genomes by quantitative RT-PCR in tissue samples and histological grade by the Sydney system
Discussion
In this study we attempted to correlate H. pylori genetic alterations that resulted in clarithromycin resistance, with histological changes as classified by the Sydney criteria in archival material from gastric biopsies. The Sydney system-based grading scale applied in our study provided an objective histological evaluation of H. pylori gastritis and was of great value in estimating treatment efficacy. Furthermore, by using a Q-RT-PCR assay in the same biopsy specimens, it was possible to quantitate as few as 30 bacteria (approximately 60 copies of the H. pylori gene) directly from gastric biopsy specimens.
H. pylori is eradicated by a triple or quadruple therapy regimen, containing a PPi and antibiotics, mainly clarithromycin and amoxicillin [8-11]. Unfortunately, primary clarithromycin resistance is increasing worldwide, and it has been regarded as a main factor for H. pylori eradication therapy failure [25-27]. Clarithromycin acts by binding to the peptidyltransferase region of 23S rRNA and inhibits protein synthesis [28].
In our study, the most frequent point mutation encountered (90%) was an A→G transition (2142G-2143G). This mutation predominates in H. pylori strains in Europe (more than 85%) [29]. Reasons to explain that 7/25 cases of group A showed no genetic alterations were that other mutations (such as A2115G, G2141A, T2147G etc.) might also be associated with clarithromycin resistance, or different mechanisms not related to the 23S rRNA gene sequence (such as the presence of an efflux pump), may also play an important role in this resistance [30]. Another question that emerged from our experiments was: does the same strain persist after treatment and, if a new strain appears, is this a phenomenon linked to clarithromycin resistance? Genotyping experiments performed suggest that when the strain is susceptible to previous treatment, eradication failures are associated either with persistence of the strain or with the emergence of a new strain [31,32].
The ClariRes™ RT-PCR method in combination with melting curve analysis described in this study, has proven to be very sensitive in the detection of mixed populations, since it was able to detect the presence of a mutant among wild-type strains at a level of less than 10% (data not shown). In fact, 11 of the 20 genetic altered cases in our study revealed a mixed genotype, comprising of mutated and wild type strains. In the study of Cambau et al, more than 30% of the H. pylori strains present in gastric biopsies were mixtures of several genotypes [33]. In another study, Schabereiter-Gutner et al, also reported mixed infections in 4.4% of the patients examined, using an RT-PCR assay in feces [24]. These authors suggested either that different strains coexisting in the gastric mucosa, or mutated and wild type alleles are present in the same strain [34,35]. In two of our cases with susceptibility to clarithromycin, the ClariRes™ RT-PCR method was also able to detect the presence of both the wild type and A→C, A→G mutants. This discrepant result may be explained by the fact that either the resistant population was present at a low concentration or that the two genotypes may correspond to different 23S ribosomal DNA alleles in a single strain. Similar findings were observed in the study by Oleastro et al [36].
In comparing the genetic and histological data, we noticed a significant statistical correlation between genetic alterations and histological characteristics of the gastritis such as the severity of inflammation, the activity, and the bacterial density (P<0.005). As our experiments showed, an accumulation of mutations and/or mixed genotype distributed mainly among cases with mild or moderate inflammation and activity were observed.
In this report we also applied a Q-RT-PCR method for the quantification of H. pylori microorganisms. The method can reliably measure as low as 30 bacteria in ffpe specimens. The PCR-determined bacterial density was found to be statistically related to semi-quantitative densities determined by histology especially between grades 1 and 3 but not between grades 1-2, and 2-3. This is in agreement with other published reports [37-40]. It seems that measurement of H. pylori density may be useful where the severity of infection has clinical or pathological meaning. In the study of Yilmaz et al [41], using the Fluorescent In Situ Hybridization (FISH) method for the determination of clarithromycin susceptibility and for the evaluation of H. pylori strains in biopsies, the authors concluded that FISH results correlated well with H. pylori infection, also showing a relationship between resistance and density of microorganisms.
The most striking result of our examination was that almost all eradicated cases that were histologically negative for H. pylori, when analyzed with Q-RT-PCR contained variable amounts of H. pylori DNA. We speculated that we may detect a form of H. pylori unrecognized in H&E and/or Giemsa stains. It is known that H. pylori exists in two forms, an active dividing spiral form and a coccoid form [42]. Growing evidence supports the concept that the coccoid form is not simply a degenerated morphological manifestation invisible during microscopic examination, but is alive and may be metabolically active [43-45]. In this case, maybe the antibiotic concentration needs to be high and sustained longer as it is not only needed to kill the extracellular microorganisms but it must also be capable of penetrating the epithelial cells to kill the intracellular H. pylori. Another explanation for our findings is that there were no viable bacteria in the stomach biopsy samples but only dead organisms or chromosomal DNA left over after cell death [46,47].
In summary, H. pylori genome is characterized by genetic variability that permits the survival of the bacterium in a quite hostile environment. This genetic diversity affects the response of H. pylori in the followed treatment strategies, and it correlates with some of histopathological features of gastritis.
Summary Box.
What is already known:
Among the treatments used for H. pylori infection, the highest eradication rates are achieved with the use of a PPi or ranitidine bismuth citrate in combination with two antibiotics
Primary clarithromycin resistance is regarded as the main factor reducing the efficacy of H. pylori therapy
The major cause of macrolide resistance in H. pylori is the lack of drug binding to the 23S rRNA components of the bacterial ribosomes, mainly due to an adenine-to-guanine transition at positions 2142 and 2143 and to adenine-to-cytosine transversion at position 2142, all of which are included in the peptidyltransferase loop of the 23S rRNA
It has been shown that some molecular assays can be applied directly on archival material without the need for in vitro cultures of the microorganism
What the new findings are:
A significant statistical correlation between mutations in 23SrRNA of the H. pylori and histological parameters of gastritis such as the severity of inflammation, the activity of gastritis, and the bacterial density was noticed
It seems that the density of microorganisms correlates with an accumulation of genetic events that lead to clarithromycin resistance
An accumulation of mutations and/or mixed genotype were observed, distributed mainly among cases with mild or moderate inflammation and activity. These data suggest that the existence of both clarithromycin resistant and susceptible H. pylori strains may be also a reason of therapy failure
Almost all cases histologically negative for H. pylori after eradication therapy, when analyzed with Q-RT-PCR contained variable amounts of microbial DNA
Acknowledgements
Authors are highly indebted to Mrs. Panagiota Tzoumakari, Mr. George Vilaras and Mr. George Apergis, PhD, for their excellent technical assistance, design of experiments, modifications and helpful discussions
Biography
National Organization for Medicines (EOF), Athens, Greece; NIMTS Hospital, Athens, Greece; Private Clinic, Nicosia, Cyprus; Henry Dunant Hospital, Athens, Greece; University of Ioannina, Greece
Footnotes
Conflict of Interest: None
References
- 1.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983–1991. doi: 10.1016/s0016-5085(97)70019-2. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;35(Suppl 12):90–97. [PubMed] [Google Scholar]
- 3.Crump M, Gospodarowicz M, Shepherd FA. Lymphoma of the gastrointestinal tract. Semin Oncol. 1999;26:324–337. [PubMed] [Google Scholar]
- 4.Graham DY, Shiotani A. Which Therapy for Helicobacter pylori Infection? Gastroenterology. 2012;143:10–12. doi: 10.1053/j.gastro.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Rimbara E, Thirumurthi S, et al. Detection of clarithromycin resistance in Helicobacter pylori following noncryogenic storage of rapid urease tests for 30 days. J Dig Dis. 2012;13:54–59. doi: 10.1111/j.1751-2980.2011.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilaichone RK, Mahachai V, Graham DY. Helicobacter pylori diagnosis and management. Gastroenterol Clin North Am. 2006;35:229–247. doi: 10.1016/j.gtc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Elitsur Y, Lawrence Z, Russmann H, Koletzko S. Primary clarithromycin resistance to Helicobacter pylori and therapy failure in children: the experience in West Virginia. J Pediatr Gastroenterol Nutr. 2006;42:327–328. doi: 10.1097/01.mpg.0000214157.52822.40. [DOI] [PubMed] [Google Scholar]
- 9.Fiorini G, Zullo A, Gatta L, et al. Newer agents for Helicobacter pylori eradication. Clin Exp Gastroenterol. 2012;5:109–112. doi: 10.2147/CEG.S25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horiki N, Omata F, Uemura M, et al. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009;14:86–90. doi: 10.1111/j.1523-5378.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 11.Koletzko S, Richy F, Bontems P, et al. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut. 2006;55:1711–1716. doi: 10.1136/gut.2006.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agudo S, Alarcon T, Urruzuno P, Martinez MJ, Lopez-Brea M. Detection of Helicobacter pylori and clarithromycin resistance in gastric biopsies of pediatric patients by using a commercially available real-time polymerase chain reaction after NucliSens semiautomated DNA extraction. Diagn Microbiol Infect Dis. 2010;67:213–219. doi: 10.1016/j.diagmicrobio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 13.van Doorn LJ, Debets-Ossenkopp YJ, Marais A, et al. Rapid detection, by PCR and reverse hybridization, of mutations in the Helicobacter pylori 23S rRNA gene, associated with macrolide resistance. Antimicrob Agents Chemother. 1999;43:1779–1782. doi: 10.1128/aac.43.7.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Doorn LJ, Glupczynski Y, Kusters JG, et al. Accurate prediction of macrolide resistance in Helicobacter pylori by a PCR line probe assay for detection of mutations in the 23S rRNA gene: multicenter validation study. Antimicrob Agents Chemother. 2001;45:1500–1504. doi: 10.1128/AAC.45.5.1500-1504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YJ, Yang JC, Jeng YM, Chang MH, Ni YH. Prevalence and rapid identification of clarithromycin-resistant Helicobacter pylori isolates in children. Pediatr Infect Dis J. 2001;20:662–666. doi: 10.1097/00006454-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gasparetto M, Pescarin M, Guariso G. Helicobacter pylori eradication therapy: current availabilities. ISRN Gastroenterol 2012. 2012 doi: 10.5402/2012/186734. 186734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JL, Dixon MF. Is subtyping of intestinal metaplasia in the upper gastrointestinal tract a worthwhile exercise? An evaluation of current mucin histochemical stains. Br J Biomed Sci. 2003;60:180–186. doi: 10.1080/09674845.2003.11783696. [DOI] [PubMed] [Google Scholar]
- 18.Turkay C, Erbayrak M, Bavbek N, Yenidunya S, Eraslan E, Kasapoglu B. Helicobacter pylori and histopathological findings in patients with dyspepsia. Turk J Gastroenterol. 2011;22:122–127. doi: 10.4318/tjg.2011.0179. [DOI] [PubMed] [Google Scholar]
- 19.Manxhuka-Kerliu S, Telaku S, Devolli-Disha E, et al. Helicobacter pylori gastritis updated Sydney classification applied in our material. Prilozi. 2009;30:45–60. [PubMed] [Google Scholar]
- 20.Guarner J, Herrera-Goepfert R, Mohar A, et al. Interobserver variability in application of the revised Sydney classification for gastritis. Hum Pathol. 1999;30:1431–1434. doi: 10.1016/s0046-8177(99)90164-8. [DOI] [PubMed] [Google Scholar]
- 21.Scaletsky IC, Aranda KR, Garcia GT, et al. Application of realtime PCR stool assay for Helicobacter pylori detection and clarithromycin susceptibility testing in Brazilian children. Helicobacter. 2011;16:311–315. doi: 10.1111/j.1523-5378.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 22.Lottspeich C, Schwarzer A, Panthel K, Koletzko S, Russmann H. Evaluation of the novel Helicobacter pylori ClariRes real-time PCR assay for detection and clarithromycin susceptibility testing of H. pylori in stool specimens from symptomatic children. J Clin Microbiol. 2007;45:1718–1722. doi: 10.1128/JCM.00103-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schabereiter-Gurtner C, Hirschl AM, Dragosics B, et al. Novel real-time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing of stool and biopsy specimens. J Clin Microbiol. 2004;42:4512–4518. doi: 10.1128/JCM.42.10.4512-4518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lascols C, Lamarque D, Costa JM, et al. Fast and accurate quantitative detection of Helicobacter pylori and identification of clarithromycin resistance mutations in H. pylori isolates from gastric biopsy specimens by real-time PCR. J Clin Microbiol. 2003;41:4573–4577. doi: 10.1128/JCM.41.10.4573-4577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sereni G, Azzolini F, Camellini L, et al. Efficacy of a therapeutic strategy for eradication of Helicobacter pylori infection. World J Gastroenterol. 2012;18:4542–4548. doi: 10.3748/wjg.v18.i33.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gisbert JP. Rescue therapy for Helicobacter pylori infection 2012. Gastroenterol Res Pract 2012. 2012 doi: 10.1155/2012/974594. 974594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosme A, Montes M, Martos M, et al. Usefulness of antimicrobial susceptibility in the eradication of Helicobacter pylori. Clin Microbiol Infect. 2012 doi: 10.1111/j.1469-0691.2012.03844.x. [DOI] [PubMed] [Google Scholar]
- 28.Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621–2628. doi: 10.1128/aac.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De FV, Zullo A, Ierardi E, Vaira D. Minimal inhibitory concentration (MIC) values and different point mutations in the 23S rRNA gene for clarithromycin resistance in Helicobacter pylori. Dig Liver Dis. 2009;41:610–611. doi: 10.1016/j.dld.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Levy SB. Active efflux mechanisms for antimicrobial resistance. Antimicrob Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tankovic J, Lamarque D, Lascols C, Soussy CJ, Delchier JC. Clarithromycin resistance of Helicobacter pylori has a major impact on the efficacy of the omeprazole-amoxicillin-clarithromycin therapy. Pathol Biol (Paris) 2001;49:528–533. doi: 10.1016/s0369-8114(01)00209-7. [DOI] [PubMed] [Google Scholar]
- 32.Tankovic J, Lamarque D, Lascols C, Soussy CJ, Delchier JC. Impact of Helicobacter pylori resistance to clarithromycin on the efficacy of the omeprazole-amoxicillin-clarithromycin therapy. Aliment Pharmacol Ther. 2001;15:707–713. doi: 10.1046/j.1365-2036.2001.00971.x. [DOI] [PubMed] [Google Scholar]
- 33.Cambau E, Allerheiligen V, Coulon C, et al. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. J Clin Microbiol. 2009;47:3600–3607. doi: 10.1128/JCM.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguchi N, Rimbara E, Kato A, et al. Detection of mixed clarithromycin-resistant and -susceptible Helicobacter pylori using nested PCR and direct sequencing of DNA extracted from faeces. J Med Microbiol. 2007;56:1174–1180. doi: 10.1099/jmm.0.47302-0. [DOI] [PubMed] [Google Scholar]
- 35.van der Ende A, van Doorn LJ, Rooijakkers S, Feller M, Tytgat GN, Dankert J. Clarithromycin-susceptible and -resistant Helicobacter pylori isolates with identical randomly amplified polymorphic DNA-PCR genotypes cultured from single gastric biopsy specimens prior to antibiotic therapy. J Clin Microbiol. 2001;39:2648–2651. doi: 10.1128/JCM.39.7.2648-2651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oleastro M, Menard A, Santos A, et al. Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol. 2003;41:397–402. doi: 10.1128/JCM.41.1.397-402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi D, Eishi Y, Ohkusa T, et al. Gastric mucosal density of Helicobacter pylori estimated by real-time PCR compared with results of urea breath test and histological grading. J Med Microbiol. 2002;51:305–311. doi: 10.1099/0022-1317-51-4-305. [DOI] [PubMed] [Google Scholar]
- 38.He Q, Wang JP, Osato M, Lachman LB. Real-time quantitative PCR for detection of Helicobacter pylori. J Clin Microbiol. 2002;40:3720–3728. doi: 10.1128/JCM.40.10.3720-3728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikula M, Dzwonek A, Jagusztyn-Krynicka K, Ostrowski J. Quantitative detection for low levels of Helicobacter pylori infection in experimentally infected mice by real-time PCR. J Microbiol Methods. 2003;55:351–359. doi: 10.1016/s0167-7012(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 40.Weiss J, Tsang TK, Meng X, et al. Detection of Helicobacter pylori gastritis by PCR: correlation with inflammation scores and immunohistochemical and CLOtest findings. Am J Clin Pathol. 2008;129:89–96. doi: 10.1309/APMPEP54G7PN958G. [DOI] [PubMed] [Google Scholar]
- 41.Yilmaz O, Demiray E, Tumer S, et al. Detection of Helicobacter pylori and determination of clarithromycin susceptibility using formalin-fixed, paraffin-embedded gastric biopsy specimens by fluorescence in situ hybridization. Helicobacter. 2007;12:136–141. doi: 10.1111/j.1523-5378.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 42.Cellini L, Allocati N, Di CE, Dainelli B. Helicobacter pylori: a fickle germ. Microbiol Immunol. 1994;38:25–30. doi: 10.1111/j.1348-0421.1994.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 43.Li N, Han L, Chen J, Lin X, Chen H, She F. Proliferative and apoptotic effects of gastric epithelial cells induced by coccoid Helicobacter pylori. J Basic Microbiol. 2012 doi: 10.1002/jobm.201100370. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal N, Snyder P, Owens SR. Unusual Helicobacter pylori in gastric resection specimens: an old friend with a new look. Int J Surg Pathol. 2011;19:297–302. doi: 10.1177/1066896911398654. [DOI] [PubMed] [Google Scholar]
- 45.Andersen LP, Rasmussen L. Helicobacter pylori-coccoid forms and biofilm formation. FEMS Immunol Med Microbiol. 2009;56:112–115. doi: 10.1111/j.1574-695X.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro ML, Vitiello L, Miranda MC, et al. Mutations in the 23S rRNA gene are associated with clarithromycin resistance in Helicobacter pylori isolates in Brazil. Ann Clin Microbiol Antimicrob. 2003;2:11. doi: 10.1186/1476-0711-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribeiro ML, Gerrits MM, Benvengo YH, et al. Detection of highlevel tetracycline resistance in clinical isolates of Helicobacter pylori using PCR-RFLP. FEMS Immunol Med Microbiol. 2004;40:57–61. doi: 10.1016/S0928-8244(03)00277-3. [DOI] [PubMed] [Google Scholar]


