Abstract
Systemic administration of adenovirus and adenovirus vectors induces a robust innate and adaptive immune response in a variety of animal models. In tumor necrosis factor (TNF)-/- mice, a diminished immune response to adenovirus (Ad) infection has been attributed to compromised dendritic cell (DC) maturation. In this report, we investigated the mechanisms responsible for Ad-mediated activation and maturation of DC. Ad infection induced high levels of TNF-α expression by murine bone marrow-derived DC, comparable to levels observed with lipopolysaccharide exposure. Ad-induced TNF-α production was necessary for DC maturation and acts in an autocrine manner. Unlike TNF-α production associated with exposure to lipopolysaccharide, Ad induction of TNF-α was not dependent on the MyD88 signaling pathway. In contrast, Ad-induced TNF-α production and DC maturation were dependent on signaling by phosphoinositide-3-OH kinase (PI3K), as determined by wortmannin and LY294002 blocking experiments. The adenovirus capsid protein penton contains a well characterized arginine-glycine-aspartic acid integrin-binding domain that stimulates PI3K in fibroblast cell lines. When this region of the penton was mutated, TNF-α expression and bone marrow-derived DC maturation were attenuated. We propose that integrin-mediated PI3K induction of NF-κB activates an autocrine TNF-α pathway required for DC maturation in response to Ad.
Early inflammatory response to adenoviral vectors is triggered by virus interaction with cells of the innate immune system, and these early activation events establish the framework for subsequent activation of the antiviral adaptive response (reviewed in ref. 1). Macrophages and dendritic cells (DC) contribute to the early immune response to viruses including adenovirus (Ad) (2–5). Both cell types respond to virus through production of cytokines, such as tumor necrosis factor α (TNF-α) (6). Both cell types also serve as antigen-presenting cells, and DC are the most potent APC known (2). Immature DC efficiently take up microbes through specific and nonspecific pathways (7). The recognition and internalization of microbial antigen induce maturation of the DC and migration to the draining lymph nodes, where T cell stimulation occurs (7, 8). Activated DC are critical for presentation of viral antigens and stimulation of antigen specific T lymphocytes and, therefore, play a pivotal role in developing an adaptive immune response to viral infection (9). Previous work has shown that TNF-α is a key cytokine in promoting both the innate and the adaptive immune response against Ad (10–12). In the absence of TNF-α, DC maturation was compromised, resulting in defective T and B cell immune responses to Ad (12).
The mechanism(s) responsible for the induction of Ad-mediated TNF-α production in DC or macrophages is not well understood. In contrast, bacterial products, such as lipopolysaccharide (LPS) activate both macrophages and DC through Toll-like receptor 4 (TLR-4) (13). TLR-4 signals through MyD88-dependent and MyD88-independent pathways (14, 15) that in turn lead to the activation of NF-κB (14, 16). NF-κB is a transcription factor that induces the expression of a number of genes associated with inflammation, including TNF-α, and antiapoptotic pathways (17–19). Given that certain viruses, such as respiratory syncytial virus, activate innate immunity through the TLR-4/MyD88 pathway (20) and Ad infection stimulates NF-κB and TNF-α expression (21), we explored the possibility that Ad may be stimulating a similar pathway when interacting with DC. We find that Ad stimulates DC TNF-α production through a MyD88-independent, phosphoinositide-3-OH kinase (PI3K)-dependent manner and that the integrin-binding domain of penton played a dominant role in Ad mediated activation of these events in DC.
Materials and Methods
Viruses and Peptides. Ad serotype 5 β-galactosidase (Ad5βgal) (22) and Ad5βgalRGD- (23) are first generation, E1-E3- replication-deficient adenoviruses containing a βgal reporter gene and were generously provided by the laboratory of Ronald Crystal (Weill Medical College of Cornell University) and GenVec (Gaithersburg, MD), respectively. Ad5βgalRGD- (23) is a virus that has a deletion in the arginine-glycine-aspartic acid (RGD) motif of penton. This motif has been demonstrated to function as a secondary binding site for adenovirus through αvβ integrin binding. Binding to integrin through this motif has been shown to induce endocytosis of Ad (24). Viruses were grown according to standard protocols and purified through two CsCl gradients. Virus was stored at -20°C in viral storage buffer (50 mM Tris, pH 7.5/100 mM NaCl/40% glycerol). Aliquots were free of LPS as determined by the BioWhittaker Limulus Amebocyte Lysate Assay. Unless otherwise indicated, DC were infected at 5,000 particles per cell in a volume of 100 μl per 106 cells. After incubation for 2 h, cells were diluted by addition of 1 ml of complete RPMI 1640 medium. The penton base peptides were obtained from Biosynthesis (Lewisville, TX). The amino acid sequences are as follows: RGD-positive, EDMNDHAIRGDTFATRAEEK; and RGD-negative, EDMNDHAITFATRAEEK.
Bone Marrow-Derived DC (BMDC) Isolation. BMDC were isolated from C57BL/6 mice or MyD88-/- mice [generously provided by Shizuo Akira (Osaka University, Osaka) through Aihoa Ding (Weill Medical College of Cornell University)] according to the protocol of Inaba et al. (25). Bone marrow was isolated, and the red blood cells were lysed. The bone marrow cells were then placed in 24-well plates at a density of 106 cells per ml of R5 media (5% FCS in RPMI 1640 medium) plus granulocyte–macrophage colony-stimulating factor. Cells were gently washed on days 2 and 4 to remove granulocytes and harvested on day 5. Purity and differentiation of the day 5–6 BMDC populations were determined by flow cytometry (see below). Both immature and mature DC express CD11c, and ≈90% of the BMDC populations used in these studies expressed the CD11c marker.
Analysis of DC Surface Phenotype. DC were stained on ice with mouse monoclonal antibodies for 30 min in PBS containing 1% BSA. Cells were fixed in 1% formalin and analyzed by flow cytometry. The following antibodies were used: phycoerythrin (PE)-conjugated anti-CD11c, PE-conjugated anti-CD14, PE-conjugated anti-CD86, PE-conjugated anti-CD80, and PE-conjugated anti-MHC class II (Becton Dickinson).
TNF-α ELISA. On day 5 after harvest, 106 BMDC were infected with adenovirus at 5,000 particles per cell and maintained at a concentration of 5 × 106 cells per ml under normal culture conditions. At 24 h after infection, cells were pelleted, and media supernatants were used to measure TNF-α secretion by using a TNF ELISA kit (Becton Dickinson) according to the manufacturer's instructions.
PI3K Pharmacological Inhibitor Studies. BMDC were preincubated with 100 nM wortmannin or 10 μM LY294002 (Sigma) in serum-free media containing a 1:50,000 dilution of DMSO at 5 days after harvest. Cells were then infected with Ad5βgal at 5,000 particles per cell.
DNA Preparation and PCR. Intracellular DNA was prepared according to the protocol of Pickles et al. (26). Briefly, at 5 and 24 h after infection 106 DC were washed three times in cold media and once with an acid-salt wash (0.2 N acetic acid/0.5 M NaCl, pH 2.5). Cells were then incubated at 4°C in a 0.5% protease/0.0025% DNase/1 mM MgCl2 solution according to the manufacturer's protocols. After digestion the cells were washed twice in PBS and whole-cell DNA was isolated with a standard salting-out protocol (27).
SYBR Green I Real-Time PCR. Real-time PCR was performed in an Applied Biosystems Prism 7900H Sequence Detection System with SDS 2.1 software by using the following parameters: 1 cycle of 95°C for 15 min; 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min; and 1 cycle of 72°C for 5 min. Fluorescence data were collected during the 72°C step at the end of each cycle. All determinations were performed at least in triplicates to achieve reproducibility. Nontemplate controls run with every assay had consistently no cycle threshold values before 35 cycles of PCR. Amplifications were carried out in a total volume of 20 μl by using a QuantiTect SYBR Green kit (Qiagen, Valenica, CA). Briefly, forward Ad5 (5′-ACCTAACCGGTAGGCCAC-3′) and reverse (5′-TCCGGCCCCTGAATG-3′) primers amplified a 631-bp fragment of the hexon gene. For housekeeping gene level detection, forward (5′-ACCTAACCGGTAGGCCAC-3′) and reverse (5′-TCCGGCCCCTGAATG-3′) primers amplified a 531-bp fragment of the GAPDH gene.
Results
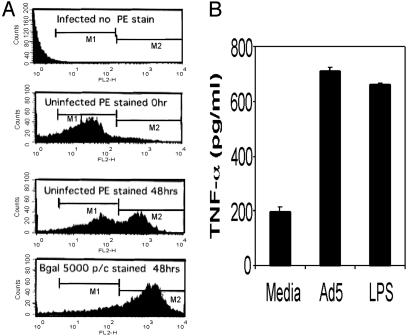
Autocrine Activation of BMDC Through Ad-Induced TNF-α Secretion. In preliminary studies, we examined the transduction efficiency of immature BMDC when exposed to increasing concentrations of Ad5βgal virus. In agreement with several published studies that failed to detect the coxsackie adenovirus receptor (CAR) on murine BMDC (28–31), we found that BMDC were relatively refractory to Ad-mediated gene transduction. When exposed to 5,000 particles per cell, <5% of BMDC were transduced (data not shown). In marked contrast to the low level of Ad5βgal transduction, exposure of BMDC to 5,000 particles of virus per cell resulted in a substantial increase in the expression of the DC maturation marker CD-86 (Fig. 1A). Our results are consistent with studies with psoralen-UV-inactivated recombinant Ad (31, 32), where transcriptionally inactive viral templates were found to induce BMDC maturation as efficiently as unmodified virus. Therefore, BMDC maturation in response to Ad exposure does not depend on efficient gene expression associated with the high affinity uptake pathway known to function with CAR-containing cell lines.
Fig. 1.
(A) Ad-induced up-regulation of DC maturation marker CD86. DC were infected with Ad5βgal at 5,000 particles per cell (p/c) or mock infected with viral storage buffer. Flow cytometry histograms show the expression of CD86 on the surface of uninfected and adenoviral-infected DC (staining as described in Materials and Methods). DC in the CD86 High-M2 peak are characterized as the mature DC or High-DC. (B) TNF ELISA showing Ad-induced TNF secretion from DC. As a positive control, DC were exposed to 20 ng/ml LPS. TNF-α secretion was measured 24 h after infection.
Because Ad induced BMDC maturation and we have previously observed that TNF-α promotes this phenomenon (12), we next determined whether BMDC exposed to Ad resulted in secretion of TNF-α. Immature BMDC were exposed to buffer alone, Ad vector (Ad5βgal) at 5,000 particles per cell or to 20 ng/ml of LPS as a positive control. Twenty-four hours after infection, supernatants were harvested and TNF-α secretion was measured by ELISA (Fig. 1B). Exposure of immature BMDC to Ad5βgal resulted in elevation of soluble TNF-α to levels comparable with those induced by LPS, indicating an association between Ad-mediated BMDC maturation and TNF-α expression.
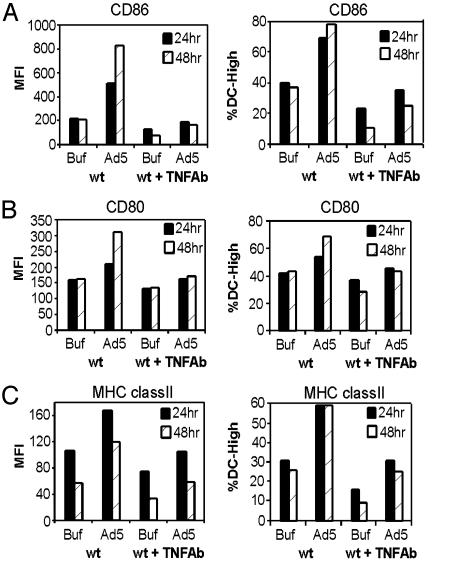
To determine the role of Ad-induced TNF-α in BMDC maturation, BMDC cultures were infected with Ad5βgal at 5,000 particles per cell in the presence of an anti-TNF-α antibody. Twenty-four and 48 h after infection, Ad induction of DC maturation [indicated by increased expression of the three maturation markers, CD86, CD80, and MHC class II (Fig. 2 A, B, and C, respectively)] was inhibited by anti-TNF-α blocking antibody. The control anti-IFN-γ antibody did not inhibit Ad-induced expression of the DC maturation markers (data not shown). Taken together, these data demonstrate that low levels of Ad are sufficient to induce an autocrine TNF-α response, which promotes BMDC maturation.
Fig. 2.
TNF antibody blocks Ad-induced DC maturation. DC were incubated with anti-TNF antibody and infected with Ad5βgal (Ad5) at 5,000 particles per cell or mock-infected with viral storage buffer (Buf). Twenty-four and 48 h after infection, cells were incubated with antibodies to DC maturation markers. Flow cytometry was used to assess cell surface expression of CD86 (A), CD80 (B), and MHC class II (C). The data shown are representative of three independent experiments. MFI, mean fluorescence intensity.
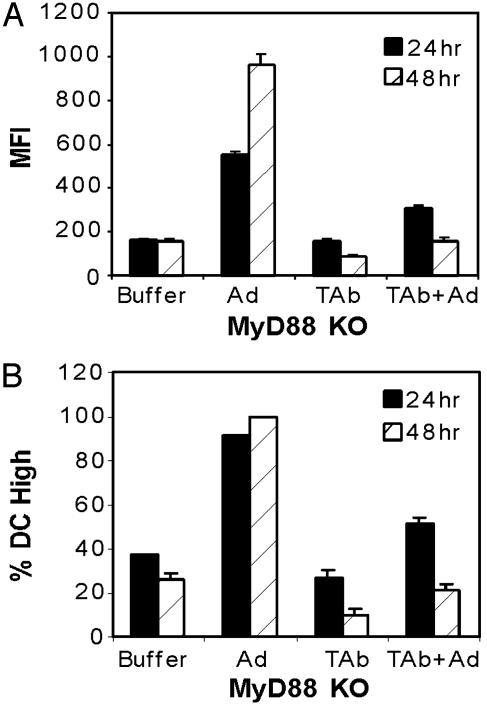
Ad-Induced DC Activation Is MyD88-Independent. Host recognition of many microbial pathogens is initially mediated by TLR that are expressed on antigen presenting cells, such as DC (13). MyD88 is an adaptor protein that is activated by various TLR (including TLR-4) during pathogen stimulation (14). To determine whether MyD88 is involved in adenoviral-induced DC maturation, MyD88 knockout (KO) DC were infected with Ad5βgal in the presence and absence of anti-TNF-α antibody. Twenty-four and 48 h after infection, the DC were stained with anti-CD86 PE-conjugated antibody, then analyzed by flow cytometry. At both time points, CD86 expression clearly increased after addition of adenovirus in contrast to buffer alone (Fig. 3). In addition, Ad-induced activation of MyD88 KO DC was inhibited by the anti-TNF-α antibody, similar to WT DC. These results indicate that Ad-induced DC activation can occur through a MyD88-independent pathway and that this pathway depends on an autocrine production of TNF-α.
Fig. 3.
Ad-induced DC maturation is MyD88-independent. DC were incubated with or without anti-TNF antibody and infected with Ad5βgal at 5,000 particles per cell or mock-infected with viral storage buffer. Twenty-four and 48 h after infection, cells were incubated with anti-CD86 antibody. Flow cytometry was used to assess cell surface expression of CD86 as a measure of mean fluorescence intensity (A) or percentage of total DC population in a mature state (B).
Inhibitors of PI3K Block Ad-Induced DC Maturation. Ad infection occurs through a well characterized uptake process (33–35). Virus attaches to the cell surface through the high-affinity binding of fiber to CAR, followed by penton binding to αvβ integrins to stimulate endocytosis. Given that BMDC contain relatively low levels of CAR and are refractory to transduction at the levels of virus used to characterize Ad internalization, the fiber/CAR-binding pathway was unlikely to be a primary mediator of DC activation. However DC contain a variety of integrin membrane proteins, including αvβ1,3,5 integrins known to bind Ad (24, 36, 37). Integrin stimulation by Ad has been shown in several cell lines to activate phosphoinositide-3-OH kinase (PI3K) (38, 39), but this pathway has not been characterized in DC. Recent reports have also indicated that PI3K activation plays a prominent role in DC stimulation through MyD88-independent pathways (13).
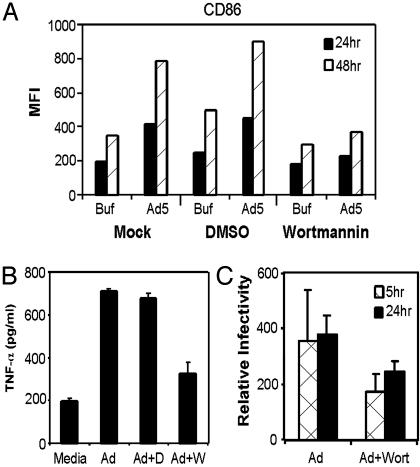
Wortmannin and LY294002, two well known inhibitors of PI3K (40, 41), were used to block PI3K in DC before exposure to Ad5βgal. Wortmannin (100 nM) efficiently blocked CD86 expression on DC 24 and 48 h after infection (Fig. 4A). Similar results were obtained with 10 μM LY294002 (data not shown). These data suggest that Ad-mediated DC activation involves a PI3K-dependent pathway. Because we previously demonstrated that Ad-induced BMDC maturation depended on the induction of TNF-α secretion, we predicted that the PI3K pathway was required for TNF-α secretion. To test this hypothesis, DC were incubated with wortmannin or in media alone and then infected with Ad5βgal. Wortmannin pretreatment inhibited Ad-induced TNF-α secretion to almost background levels (Fig. 4B), supporting the notion that Ad-induced TNF secretion and DC maturation occurs through a PI3K-dependent pathway.
Fig. 4.
PI3K inhibitors compromise expression of DC maturation markers in presence of Ad. DC were incubated with either the PI3K inhibitor wortmannin or vehicle (DMSO). Then cells were infected with Ad5βgal at 5,000 particles per cell or mock-infected with viral storage buffer. (A) Twenty-four and 48 h after infection, cells were incubated with anti-CD86 antibody and flow cytometry was used to assess cell surface expression of CD86. The data shown are representative of three independent experiments. (B) TNF ELISA showing Ad-induced TNF secretion from DC. DC were incubated with the PI3K inhibitor wortmannin, vehicle (DMSO), or media alone and then infected with Ad5βgal at 5,000 particles per cell. TNF-α secretion was measured 24 h after infection. (C) Ad uptake by DC was analyzed by using hexon real-time PCR. Whole-cell DNA was isolated from infected DC and used as template for real-time PCR. Amplified hexon DNA products were normalized to GAPDH cellular controls.
Ad infection at 5 × 103 particles per cell does not mediate an efficient transduction of DC, yet the entire population of DC matured in response to exposure to virus. To assess the amount of virus in stable association with DC, we used a real-time PCR assay to quantify persistent viral DNA 5 and 24 h after infection. Because wortmannin inhibits PI3K and because PI3K activation is required for Ad internalization (38), we also characterized the influence of wortmannin on stable virus association with DC. DC were incubated with wortmannin or buffer and then infected with virus as previously described. Five and 24 h after infection, the cells were acid washed and subjected to protease and DNase digestion to remove free and bound adenovirus. Total DNA was harvested and aliquots were used in a real-time PCR assay. Five and 24 h after infection, ≈350 ± 200 copies of viral DNA were present per cell (Fig. 4C). In DC pretreated with wortmannin, ≈200 ± 50 copies of viral DNA were detected per cell. Thus, in the absence of an efficient fiber-mediated transduction pathway, even in the presence of the PI3K inhibitor, several hundred copies of viral DNA are taken up by each BMDC.
Penton Base RGD Motif Is Required for Ad-Induced DC Maturation. The data presented demonstrate that Ad-induced TNF-α expression stimulates BMDC maturation, and this TNF-α induction is occurring through a MyD88-independent but PI3K-dependent pathway. These observations strongly suggest that this early event is associated with adenovirus binding to the BMDC. In other cell types, PI3K is activated through the binding of the adenoviral penton base to cellular αvβ integrins (38).
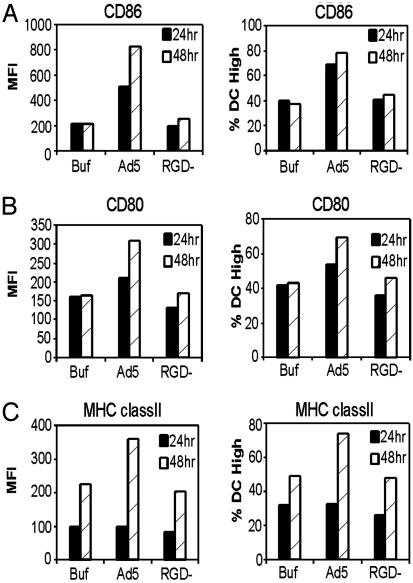
To determine whether the interaction between penton base and integrins contribute to BMDC activation, an RGD motif-deleted vector (Ad5βgalRGD-) (23) was used. BMDC were infected with Ad5βgal, Ad5βgalRGD-, or mock infected with viral storage buffer. Twenty-four and 48 h after infection, the DC were assessed for expression of DC maturation markers CD86, CD80, and MHC class II. BMDC exposed to adenovirus demonstrated increased mean fluorescence and an increased percentage of cells expressing each of three maturation markers, CD86, CD80, and MHC class II (Fig. 5 A, B, and C, respectively) compared with buffer control. In contrast to Ad5βgal, the Ad5βgalRGD- virus did not induce DC maturation above background levels. The expression of CD86, CD80, and MHC class II proteins in cells exposed to Ad5βgalRGD- was the same as in cells exposed to the viral buffer, as shown in Fig. 5. These data suggest that the RGD motif of penton base is required for adenoviral induction of DC maturation.
Fig. 5.
RGD motif is required for Ad-induced expression of BMDC maturation markers. DC were infected with Ad5βgal or Ad5βgalRGD- at 5,000 particles per cell or mock-infected with viral storage buffer. Twenty-four and 48 h after infection, cells were incubated with antibodies to DC maturation markers. Flow cytometry was used to assess cell surface expression of CD86 (A), CD80 (B), and MHC class II (C). The data shown are representative of three independent experiments.
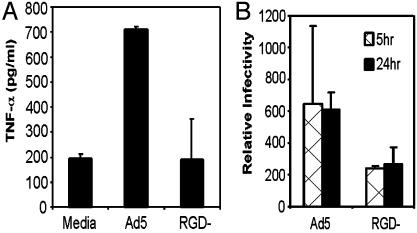
To examine whether the lack of DC maturation mediated by infection with Ad5βgalRGD- was explained by a lack of TNF activation, BMDC were infected with Ad5βgal or Ad5βgalRGD- at 5,000 particles per cell or mock infected. In contrast to the high level of TNF-α secreted by Ad5βgal-infected DC, the Ad5βgalRGD- virus did not induce TNF-α secretion above background levels (Fig. 6A). Thus, Ad-induced TNF-α secretion occurs through an RGD-dependent pathway in DC. Cells were also harvested for Ad5βgal and Ad5βgalRGD- viral DNA in stable association with BMDC 5 and 24 h after infection (Fig. 6B). The viral copy number per cell was consistent with the results found in the previous experiment with wortmannin inhibition of PI3K, where ≈200 copies of viral DNA were detected within each BMDC.
Fig. 6.
(A) TNF ELISA showing Ad-induced TNF secretion from DC. DC were infected with Ad5βgal or Ad5βgalRGD- at 5,000 particles per cell or mock-infected with viral storage buffer. TNF-α secretion was measured 24 h after infection. (B) Real-time PCR analysis of Ad5βgal- and Ad5βgalRGD--infected DC. Whole-cell DNA was isolated from infected DC and used as template for real-time PCR. Amplified hexon DNA products were normalized to GAPDH cellular controls.
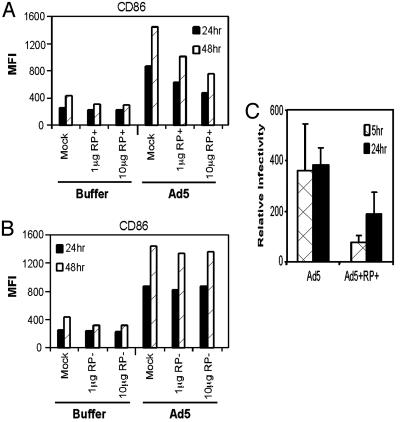
To determine whether a simple RGD motif-containing peptide is sufficient to initiate DC maturation, two peptides were designed. Both peptides contain similar sequences corresponding to the RGD region of penton base, except that one has the RGD motif deleted. DC were incubated with either peptide and then infected with Ad5βgal or viral buffer. Treatment of DC with either the RGD-containing peptide or the ΔRGD-peptide shows that addition of up to 10 μg of peptide did not induce expression of CD86 (Fig. 7A and B). The lack of peptide activation is consistent with studies by Chiu et al. (42), which have suggested that integrin signaling requires a specific pentameric arrangement of RGD in the virion capsid.
Fig. 7.
RGD peptide inhibits Ad-induced expression of DC maturation markers. DC were incubated with peptide containing the RGD motif (RP+) (A) or with the RGD motif deleted (RP-)(B) and then infected with Ad5βgal at 5,000 particles per cell or mock-infected with viral storage buffer. Twenty-four and 48 h after infection, cells were incubated with anti-CD86 antibody, and flow cytometry was used to assess cell surface expression of CD86. The data shown are representative of three independent experiments. (C) Real-time PCR analysis showing Ad5βgal uptake in presence of peptide. Whole-cell DNA was isolated from infected DC and used as template for real-time PCR. Amplified hexon DNA products were normalized to GAPDH cellular controls.
Although the RGD-containing peptide did not stimulate DC maturation, it could antagonize Ad-mediated activation of BMDC through binding to integrin. To test this idea, BMDC were incubated with increasing concentrations of the RGD-containing peptide followed by Ad5βgal infection. Consistent with an RGD-containing peptide functioning as an antagonist to Ad-mediated DC maturation, increasing concentrations of peptide caused a reduction in Ad-mediated DC activation (Fig. 7A), whereas increasing concentrations of the RGD-negative peptide had no effect on Ad-mediated DC maturation (Fig. 7B). Blocking Ad binding to integrin by RGD-peptide was shown to result in a 2- to 3-fold reduction in viral DNA 5 and 24 h after infection (Fig. 7C). These results complement those obtained by using the Ad5βgalRGD- virus and indicate that within the context of the intact Ad virion, the cell surface interaction of penton base with the αv integrins is a critical step in Ad-mediated induction of DC maturation.
Discussion
DC are referred to as sentinels of the immune system because of their ability to sense microbial antigen. Precisely how DC recognize Ad is uncertain. Immature DC lack CAR, but they are endowed with a variety of integrins and antigen uptake mechanisms that include receptor mediated endocytosis, macropinocytosis, and phagocytosis (reviewed in ref. 7). Here we report that DC “sense” the presence of Ad through integrin binding of the penton base RGD motif to αvβ integrins. This binding interaction led to DC activation, TNF-α secretion, and DC maturation promoted by autocrine TNF-α stimulation.
The cytokine TNF-α is one of the prime “danger signals” (43) activated after exposure to bacterial or virus infections, including systemic Ad. By using either TNF-/- KO mice or an Ad expressing a soluble inhibitor of TNF-α (11, 12), we previously documented the importance of TNF-α in promoting both early inflammation and the adaptive immune response to Ad. The TNF-α-mediated effects are multiple and complex. Impaired recruitment of inflammatory cells to the site of infection (10) and impaired maturation (but not migration) of DC in TNF-α-deficient mice have been demonstrated in vivo (12). Here, we show that, in DC, TNF-α induction is downstream of the integrin–PI3K activation described above. Furthermore, TNF-α activated DC in an autocrine manner, as evidenced by the antibody neutralization experiments.
A pivotal pathway linking upstream signaling with TNF-α expression is the transcription factor NF-κB. NF-κB is activated by Ad in HeLa cells (44) and is implicated in Ad-induced DC maturation (21) and during DC-like monocyte differentiation when induced by either LPS or adenovirus (45, 46). Given that multiple foreign antigens recognized by TLR or autocrine pathways, such as IL-1, activate NF-κB through the adapter protein MyD88 (13), we investigated whether this pathway linked Ad infection with TNF-α expression. MyD88-deficient cells were fully competent to produce TNF-α and DC activation markers. Therefore, we conclude that MyD88-dependent pathways are not implicated in Ad induction of TNF-α in murine DC.
It has been shown in HeLa cells and human fibroblasts that PI3K promotes endocytosis and macropinocytosis of Ad following binding to integrins (34, 38). Here we report that, in addition to promoting the internalization of Ad, the PI3K pathway is necessary for induction of TNF-α expression. In addition, we observed that RGD-containing monomeric peptides did not induce activation of DC but inhibited Ad-mediated maturation of DC. These data support a model in which penton-mediated integrin clustering (42) is required for PI3K activation in DC.
In a complementary study, Liu et al. (39) demonstrated that, in normal mice, there was no overall difference in the acute inflammatory response to WT Ad vector versus an RGD-negative Ad vector. However, when mice were depleted of Kupffer cells, the RGD-negative virus induced a lower level of inflammation than an RGD-positive virus (39). This finding suggests that in the absence of macrophages, the RGD motif of adenovirus has an impact on the antiviral innate immune response. In their study, Liu identified endothelial cells as a cell type that has an altered inflammatory response to the RGD-negative virus. In our studies, we have shown that DC also respond to the RGD motif of penton. Because DC maturation is important for activation of T cells, this finding is highly relevant to the secondary (adaptive) phase of the immune response to Ad.
In contrast to our results, Molinier-Frenkel et al. (28) found that bacterially produced Ad5 fiber knob was sufficient to induce murine DC maturation, whereas purified hexon or penton base monomeric subunits were not. The threshold for DC activation required at least 5 × 106 molecules of fiber monomer per DC. At higher concentrations of knob, the authors found both TNF-α and IL-12 were secreted. Given that DC express very little CAR (28, 47), the influence of fiber is unlikely to occur through high-affinity binding pathways. A major difference between our study and that of Molinier-Frenkel was our use of relatively low concentrations of virus as the DC activating agent. We can envision that, in addition to the very potent RGD activation pathway characterized in this study, additional synergistic signals, such as fiber, can further facilitate DC activation.
The use of Ad as a vector for gene therapy is limited because of the inflammatory response to virus and the subsequent adaptive response that is responsible for T cell mediated clearance of infected cells and a neutralizing humoral immune response. Our identification of viral capsid interactions that mediate immune cell specific activation cascades in DC provides an opportunity to develop viral vectors that avoid or minimally stimulate these pathways. In addition, use of Ad vectors for anti-cancer applications and vaccine vehicles will also benefit from the insight provided by these studies. Vectors can be designed to take advantage of immune activation cascades, such as RGD stimulation of PI3K. Enhancing these pathways may lead to more potent anti-cancer Ad vectors and more effective vehicles for vaccine applications.
Acknowledgments
We thank T. Wickham and GenVec for providing access to Ad5BgalRGD- vector, the Cornell Gene Therapy Core Facility for vector stocks, Dr. Shizuo Akira for permission to use the MyD88-/- mice, Drs. Aihao Ding and Carl Nathan for providing MyD88-/- mice and critical review of the manuscript, and Jose M. Treuejo for insights and critical discussions that helped formulate these studies. This work was supported by National Institutes of Health Grants P50 HL51746 (to E.F.-P. and K.E.) and KO1 HL70438-01 (to M.N.). GenVec has licensing agreements with Cornell Research Foundation for technology developed in collaboration with E.F.-P.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TNF, tumor necrosis factor; Ad, adenovirus; DC, dendritic cell; BMDC, bone marrow-derived DC; LPS, lipopolysaccharide; TLR, Toll-like receptor; βgal, β-galactosidase; RGD, arginine-glycine-aspartic acid; CAR, coxsackie adenovirus receptor; PE, phycoerythrin; KO, knockout; MFI, mean fluorescence intensity; PI3K, phosphoinositide-3-OH kinase.
References
- 1.Liu, Q. & Muruve, D. A. (2003) Gene Ther. 10, 935-940. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245-252. [DOI] [PubMed] [Google Scholar]
- 3.Jooss, K., Yang, Y., Fisher, K. J. & Wilson, J. M. (1998) J. Virol. 72, 4212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieber, A., He, C. Y., Meuse, L., Schowalter, D., Kirillova, I., Winther, B. & Kay, M. A. (1997) J. Virol. 71, 8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worgall, S., Wolff, G., Falck-Pedersen, E. & Crystal, R. G. (1997) Hum. Gene Ther. 8, 37-44. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, Y., Chirmule, N., Gao, G. P., Qian, R., Croyle, M., Joshi, B., Tazelaar, J. & Wilson, J. M. (2001) Mol. Ther. 3, 697-707. [DOI] [PubMed] [Google Scholar]
- 7.Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. (2002) Annu. Rev. Immunol. 20, 621-667. [DOI] [PubMed] [Google Scholar]
- 8.Moll, H. (2003) Cell Microbiol. 5, 493-500. [DOI] [PubMed] [Google Scholar]
- 9.Palucka, K. & Banchereau, J. (2002) Curr. Opin. Immunol. 14, 420-431. [DOI] [PubMed] [Google Scholar]
- 10.Elkon, K. B., Liu, C. C., Gall, J. G., Trevejo, J., Marino, M. W., Abrahamsen, K. A., Song, X., Zhou, J. L., Old, L. J., Crystal, R. G. & Falck-Pedersen, E. (1997) Proc. Natl. Acad. Sci. USA 94, 9814-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng, Y., Trevejo, J., Zhou, J., Marino, M. W., Crystal, R. G., Falck-Pedersen, E. & Elkon, K. B. (1999) J. Virol. 73, 5098-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevejo, J. M., Marino, M. W., Philpott, N., Josien, R., Richards, E. C., Elkon, K. B. & Falck-Pedersen, E. (2001) Proc. Natl. Acad. Sci. USA 98, 12162-12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akira, S., Takeda, K. & Kaisho, T. (2001) Nat. Immunol. 2, 675-680. [DOI] [PubMed] [Google Scholar]
- 14.Kaisho, T. & Akira, S. (2001) Trends Immunol. 22, 78-83. [DOI] [PubMed] [Google Scholar]
- 15.Kaisho, T., Hoshino, K., Iwabe, T., Takeuchi, O., Yasui, T. & Akira, S. (2002) Int. Immunol. 14, 695-700. [DOI] [PubMed] [Google Scholar]
- 16.Matsushima, A., Kaisho, T., Rennert, P. D., Nakano, H., Kurosawa, K., Uchida, D., Takeda, K., Akira, S. & Matsumoto, M. (2001) J. Exp. Med. 193, 631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus, J., Borner, C., Giannini, E. & Hollt, V. (2003) Mol. Pharmacol. 64, 876-884. [DOI] [PubMed] [Google Scholar]
- 18.Wong, B. R., Josien, R., Lee, S. Y., Vologodskaia, M., Steinman, R. M. & Choi, Y. (1998) J. Biol. Chem. 273, 28355-28359. [DOI] [PubMed] [Google Scholar]
- 19.Hofbauer, L. C. & Heufelder, A. E. (2001) J. Mol. Med. 79, 243-253. [DOI] [PubMed] [Google Scholar]
- 20.Kurt-Jones, E. A., Popova, L., Kwinn, L., Haynes, L. M., Jones, L. P., Tripp, R. A., Walsh, E. E., Freeman, M. W., Golenbock, D. T., Anderson, L. J. & Finberg, R. W. (2000) Nat. Immunol. 1, 398-401. [DOI] [PubMed] [Google Scholar]
- 21.Morelli, A. E., Larregina, A. T., Ganster, R. W., Zahorchak, A. F., Plowey, J. M., Takayama, T., Logar, A. J., Robbins, P. D., Falo, L. D. & Thomson, A. W. (2000) J. Virol. 74, 9617-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersh, J., Crystal, R. G. & Bewig, B. (1995) Gene Ther. 2, 124-131. [PubMed] [Google Scholar]
- 23.Wickham, T. J., Carrion, M. E. & Kovesdi, I. (1995) Gene Ther. 2, 750-756. [PubMed] [Google Scholar]
- 24.Li, E., Brown, S. L., Stupack, D. G., Puente, X. S., Cheresh, D. A. & Nemerow, G. R. (2001) J. Virol. 75, 5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba, K., Inaba, M., Romani, N., Aya, H., Deguchi, M., Ikehara, S., Muramatsu, S. & Steinman, R. M. (1992) J. Exp. Med. 176, 1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickles, R. J., McCarty, D., Matsui, H., Hart, P. J., Randell, S. H. & Boucher, R. C. (1998) J. Virol. 72, 6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, S. A., Dykes, D. D. & Polesky, H. F. (1988) Nucleic Acids Res. 16, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molinier-Frenkel, V., Prevost-Blondel, A., Hong, S. S., Lengagne, R., Boudaly, S., Magnusson, M. K., Boulanger, P. & Guillet, J. G. (2003) J. Biol. Chem. 39, 37175-37182. [DOI] [PubMed] [Google Scholar]
- 29.Okada, N., Tsukada, Y., Nakagawa, S., Mizuguchi, H., Mori, K., Saito, T., Fujita, T., Yamamoto, A., Hayakawa, T. & Mayumi, T. (2001) Biochem. Biophys. Res. Commun. 282, 173-179. [DOI] [PubMed] [Google Scholar]
- 30.Song, W., Kong, H. L., Carpenter, H., Torii, H., Granstein, R., Rafii, S., Moore, M. A. & Crystal, R. G. (1997) J. Exp. Med. 186, 1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirschowitz, E. A., Weaver, J. D., Hidalgo, G. E. & Doherty, D. E. (2000) Gene Ther. 7, 1112-1120. [DOI] [PubMed] [Google Scholar]
- 32.Miller, G., Lahrs, S., Shah, A. B. & DeMatteo, R. P. (2003) Cancer Immunol. Immunother 52, 347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemerow, G. R. & Stewart, P. L. (1999) Microbiol. Mol. Biol. Rev. 63, 725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemerow, G. R. (2000) Virology 274, 1-4. [DOI] [PubMed] [Google Scholar]
- 35.Meier, O. & Greber, U. F. (2003) J. Gene Med. 5, 451-462. [DOI] [PubMed] [Google Scholar]
- 36.Wickham, T. J., Mathias, P., Cheresh, D. A. & Nemerow, G. R. (1993) Cell 73, 309-319. [DOI] [PubMed] [Google Scholar]
- 37.Huang, S., Kamata, T., Takada, Y., Ruggeri, Z. M. & Nemerow, G. R. (1996) J. Virol. 70, 4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, E., Stupack, D., Klemke, R., Cheresh, D. A. & Nemerow, G. R. (1998) J. Virol. 72, 2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, Q., Zaiss, A. K., Colarusso, P., Patel, K., Haljan, G., Wickham, T. J. & Muruve, D. A. (2003) Hum. Gene Ther. 14, 627-643. [DOI] [PubMed] [Google Scholar]
- 40.Ui, M., Okada, T., Hazeki, K. & Hazeki, O. (1995) Trends Biochem. Sci. 20, 303-307. [DOI] [PubMed] [Google Scholar]
- 41.Vlahos, C. J., Matter, W. F., Hui, K. Y. & Brown, R. F. (1994) J. Biol. Chem. 269, 5241-5248. [PubMed] [Google Scholar]
- 42.Chiu, C. Y., Mathias, P., Nemerow, G. R. & Stewart, P. L. (1999) J. Virol. 73, 6759-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matzinger, P. (2002) Science 296, 301-305. [DOI] [PubMed] [Google Scholar]
- 44.Bowen, G. P., Borgland, S. L., Lam, M., Libermann, T. A., Wong, N. C. & Muruve, D. A. (2002) Hum. Gene Ther. 13, 367-379. [DOI] [PubMed] [Google Scholar]
- 45.Lyakh, L. A., Koski, G. K., Telford, W., Gress, R. E., Cohen, P. A. & Rice, N. R. (2000) J. Immunol. 165, 3647-3655. [DOI] [PubMed] [Google Scholar]
- 46.Lyakh, L. A., Koski, G. K., Young, H. A., Spence, S. E., Cohen, P. A. & Rice, N. R. (2002) Blood 99, 600-608. [DOI] [PubMed] [Google Scholar]
- 47.Rea, D., Schagen, F. H., Hoeben, R. C., Mehtali, M., Havenga, M. J., Toes, R. E., Melief, C. J. & Offringa, R. (1999) J. Virol. 73, 10245-10253. [DOI] [PMC free article] [PubMed] [Google Scholar]