Abstract
A gastrocutaneous fistula (GCF) represents a fistula connecting the stomach with the skin. The aim of the present review is to clarify the entity of a GCF and to discuss the various treating strategies employed. In order to elucidate GCFs as an entity etiology was pointed out and relative pathogenetic mechanisms were explored. Moreover, diagnostic modalities are discussed with a special focus on GCFs following bariatric operations. Finally, the treating strategies currently employed when confronting GCFs are presented, as well as their effectiveness.
Keywords: fistulas, gastrocutaneous, gastrostomy complications, bariatric complications
Introduction
A gastrocutaneous fistula (GCF) represents a fistula connecting the stomach and the skin. By definition, it consists of an internal orifice (gastric outlet), an external orifice (cutaneous outlet) and a tract (usually covered by epithelium). A GCF is a rare and difficult to treat complication accounting for 0.5–3.9% of the normal-weight patients undergoing gastric surgery.
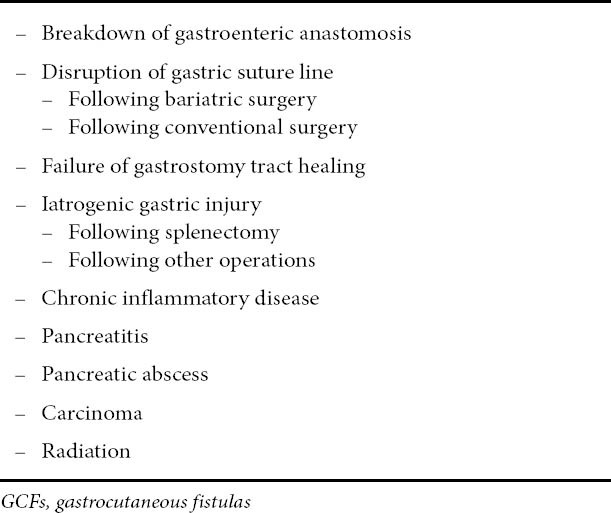
The aim of the present review is to clarify the entity of a GCF and to discuss the various treating strategies employed. Gastrocutaneous fistulas (GCF) mostly occur after iatrogenic gastric injury (particularly after splenectomy), breakdown of a gastroenteric anastomosis, disruption of the gastric suture lines, or failure of healing of a gastrostomy tube tract [1-4]. Although, the external gastric fistula most often has been related to the above mentioned operative complications, other rare causes that include chronic inflammatory disease, carcinoma, pancreatic abscess and radiation therapy should be acknowledged [5-7]. All conditions related to GCF formation are presented in Table 1.
Table 1.
Conditions etiologically related to GCFs
Pathogenesis
The pathogenetic mechanism implied in the formation of GCF is not universal, and is relative to the underlying etiologic factor.
The pathogenesis of the gastric fistulae resulting from iatrogenic gastric injury or disruption of the gastric suture lines seems to be on the basis of vascular necrosis. This was first reported in 1953 by Rutter [8], and in 1956 by Spencer [9]. Studies of blood supply [10-13] of the stomach indicated that either the left gastric or gastroepiploic artery alone is adequate to support the circulation to a gastric stump. Devascularization to produce necrosis of the gastric remnant ordinarily requires division of both gastric arteries, both gastroepiploic arteries and the vasa brevia of the spleen. Even after all vessels are divided, the left inferior phrenic and esophageal arteries frequently provide adequate blood supply to maintain viability of the remnant. Kilgore [12] postulates from studies on dogs that leakage from gastroenteric anastomoses is probably due to compromised blood supply of the gastric remnant insufficient to produce overt gangrene but sufficient to prevent complete healing of a suture line. Other factors associated with iatrogenic GCF are unrecognized operative injury and erosion of the hollow viscus due to the drain placed adjacent to it.
Percutaneous gastrostomy, endoscopic or not, was introduced in order to provide sufficient enteral feeding in patients unable to feed satisfactorily orally. Nowadays, percutaneous endoscopic gastrostomy (PEG) is widely used and constitutes the golden standard. Development of persistent GCF after removal of a PEG tube is a rare but difficult to manage problem. Several factors hamper healing such as delayed gastric emptying, poor wound healing, flow of gastric juice through the fistula, medication and mainly long-term use of the gastrostomy. Nevertheless, the crucial element in GCF formation is epithelialization of the fistula tract. Actually, the only evidence–based statement concerning GCF formation is that chronic gastrostomies (>6 months) are more likely to epithelialize and lead to a persistent fistula [14].
Concerning the rare causes responsible for GCF, the etiopathologic mechanism should be considered case-by-case: In chronic inflammatory disease a GCF can be precipitated by inflammatory erosion of the gastric wall, creation of an abscess and finally fistula formation. When radiation therapy is applied, it influences the gastric mucosa and induces gastric ulcerations. Seldom these micro-ulcerations can perforate and lead to abscess and fistula formation [5]. When GCFs are associated with pancreatitis and pancreatic abscesses they have as common denominator the ongoing active pancreatic inflammation. Vascular thrombosis is well known within areas affected by severe pancreatitis, perhaps due to the action of liberated enzymes on blood vessels, and may have caused ischemic necrosis of the gastric wall [7, 15]. Finally, carcinomas may guide to an undeviating GCF formation due to destruction of the gastric wall together with direct adhesion of the tumor to the abdominal wall. This process could lead to the GCF formation.
Diagnostic and pre-treatment workout
In order to manage a GCF, its proper recognition is necessary. Both internal and external orifices should be appropriately identified, as well as the topographic relations and the trajectory of the fistula tractus. The most cost-effective examination in order to acquire the above-mentioned data is a radiologic investigation with a water-soluble contrast agent. Normally, plain x-rays with a contrast media should be more than sufficient, however more sophisticated imaging studies could be in need, like CT scanning or MRI fistulography. It is of major importance, when trying to identify a GCF following operation, to know the exact operation performed. This is particularly true concerning bariatric operations. Figures 1 and 2 depict the various common bariatric operations and the most probable sites of GCF formation.
Figure 1.
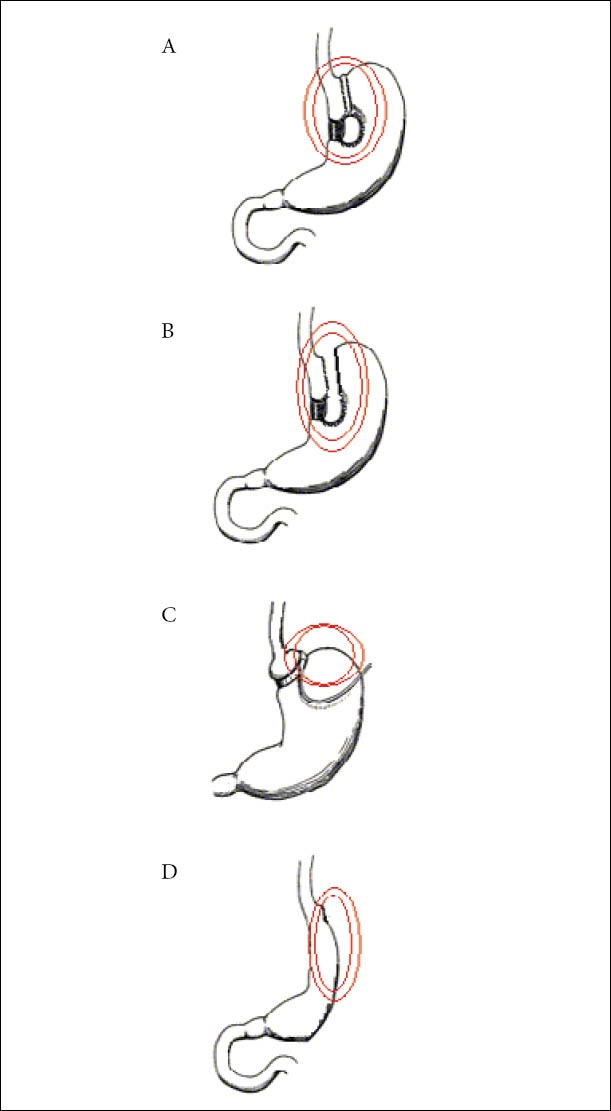
Common restrictive bariatric operations and sites of GCF formation (Double circle). (A) Vertical banded of gastroplasty (Mason Operation). (B) Modified VBG (McLean operation). (C) Gastric banding. (D) Sleeve gastrectomy
Figure 2.
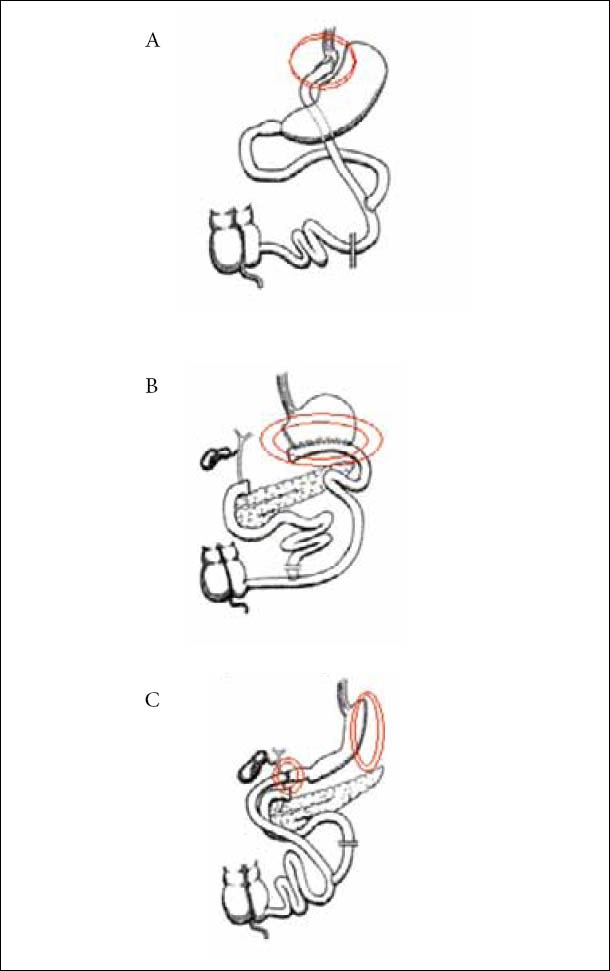
Common malabsorptive bariatric operations and sites of GCF formation (Double circle). (A) Roux-en-Y. (B) Biliopancreatic diversion without duodenal switch (Scopinaro operation). (C) Biliopancreatic diversion with duodenal switch (Marceau operation)
Treating strategies
GCFs close spontaneously in only 6% of cases, and the mortality rate is about 35% among patients with normal body weight who underwent recent gastric surgery [16]. The mortality rates are higher in patients undergoing bariatric surgery [2]. Previous reports emphasize the benign nature of gastrocutaneous fistulas and the probability of spontaneous healing without surgical intervention [1,17-19], however persistent GCFs as well as those that introduce septic conditions require courageous actions.
Once gastric leakage (fistula) has occurred, the management varies according to the gravity of the presentation of the complication. When the patient presents with abdominal tenderness, sepsis signs and/or hemodynamic instability, then laparotomy or repeat laparoscopy is inevitable. The choice of laparoscopic or open approach is made according to the surgeon’s expertise and the clinical situation. The aim of re-exploration of the abdominal cavity is dual: on the one hand control of the septic condition, and on the other management of the leakage [2]. The tenets of surgery include washout of the peritoneal cavity, appropriate debridement and repair of the anastomotic leak, followed by drainage of this region [20]. Various techniques are reported in performing the repair of the anastomotic leak ranging from the “minimal” direct suture to the “maximal” total gastrectomy. The simple direct suture of the defect shows no evidence of its effectiveness in treating the fistula, however, according to Casella et al, it managed to convert the acute abdomen into a chronic gastrocutaneous fistula without sepsis that was then susceptible to a more conservative management [21]. In our opinion the most effective solution in confronting GCFs that endanger the life of the patient is the intraluminal placement of a T-tube at the leak site.
When the patient with the GCF does not present with hemodynamic instability or a septic condition, a conservative approach can be tried [21-23]. Conservative treatment of GCFs has to have multiple aims traditionally starting with excluding the leak site, then draining the existing debris and finally occluding the fistulas’ tract.
Upon the establishment of the diagnosis of a GCF, exclusion of the leak site by employing parenteral nutrition or post-GCF enteral nutrition will allow the leak to heal spontaneously most of the time [24,25]. At the same time blood and electrolyte imbalance restoration, optimal nutrition, and infection management must be the absolute priority; then, gastric acid secretion suppression by PPI, as well as inhibition of total gastrointestinal secretion by somatostatin, must be applied [2,4,22]. When the GCF is the result of a chronic inflammatory disease, then in the general approach we should include the etiologic treatment of the disease.
Infection management includes both maintaining the outer (cutaneous) orifice of the fistula in good condition and drainage of the possible intra-abdominal collections [23, 24]. Drainage modalities range from percutaneous radiographically-guided drainage (either by using CT or echography), open surgical drainage, or intraluminal T-tube placement at the leak site [4, 22].
After establishing all the above mentioned measures the tendency for closure has to be evaluated by daily reporting of fistula output for 1 week. In cases where there is no sign of a tendency to output elimination, the endoscopist should proceed to endoscopic exploration of the anastomotic area. Our opinion on the procedures following the endoscopic approach is expressed in an article in 2008 [4] and is as follows: After the identification of an opening, fibrin glue should be applied. Attempts at glue sealing of the inner orifice of the GCF should be repeated in case of no success (with 2–3-day intervals between them). The point at which gluing should be stopped is guided mainly by two parameters: (i) the tendency to closure; and (ii) the experience of the endoscopist in this technique. More experienced endoscopists have the tendency to persist in the gluing repetition. Alternatively, endoscopic clips can be applied. Endoscopic clip application is considered successful when no leakage occurs for a minimum of 3 days. Otherwise, a combination of clips and glue is proposed. Stent placement would be another option [26-28]; however, experience in managing leaks after isolated sleeve gastrectomy or duodenal switch has been limited, with reported cure rates of 60–83% [29-31]. The use of stents can improve and simplify management, since stents immediately seal the leak from additional contamination of the peritoneal cavity and the patient can generally start oral feeding soon after stent placement, allowing oral nutrition and eliminating the need for costly and high-risk total parenteral nutrition [32]. However, stents have two drawbacks: (i) they have to be removed within 3 months after their placement, and (ii) their migration rate is extremely high. Most migrations are just of a few centimeters, but this is enough to uncover the leak. Migration can be confronted with replacement or repositioning of the stent, but can result in major morbidity since surgery may be required for stent removal from the small bowel. The rates of migration seem to be similar with all types of stents employed [27]. Recently Maluf-Filho et al proposed the occlusion of the fistula tract with endoscopic application of SurgSIS. This biomaterial is widely used in perianal fistulas with success, but its value in GCF treatment remains to be proved [33]. Finally, in cases where all the above-mentioned ‘conservative’ techniques fail, surgery remains the ultimate option.
Biography
AHEPA University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
Footnotes
Conflict of interest: None
References
- 1.Harrison BF, Glanges E, Sparkman RS. Gastric fistula following splenectomy: its cause and prevention. Ann Surg. 1977;185:210–13. doi: 10.1097/00000658-197702000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papavramidis ST, Eleftheriadis EE, Papavramidis TS, et al. Endoscopic management of gastrocutaneous fistulas after bariatric surgery by using a fibrin sealant. Gastrointest Endosc. 2004;59:296–300. doi: 10.1016/s0016-5107(03)02545-8. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui AA, Kowalski T, Cohen S. Closure of a non-healing gastrocutaneous fistula using an endoscopic clip. South Med J. 2007;100:75–76. doi: 10.1097/SMJ.0b013e31802f86a2. [DOI] [PubMed] [Google Scholar]
- 4.Papavramidis TS, Kotzampassi K, Kotidis E, et al. Endoscopic fibrin sealing of gastrocutaneous fistulas after sleeve gastrectomy and biliopancreatic diversion with duodenal switch. J Gastroenterol Hepatol. 2008;23:1802–1805. doi: 10.1111/j.1440-1746.2008.05545.x. [DOI] [PubMed] [Google Scholar]
- 5.Hothem AL, Newsome JF. Gastrocutaneous Fistula Following Radiation Therapy for Seminoma of the Testis. Ann Surg. 1974;180:323–328. doi: 10.1097/00000658-197409000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maingot R. 6th ed. New York: Appleton-Century Crofts; 1974. Abdominal Operations; p. 153. [Google Scholar]
- 7.Warshaw AL, Moncure AC, Rattner DW. Gastrocutaneous Fistulas Associated with Pancreatic Abscesses. An Aggressive Entity. Ann Surg. 1989;210:603–607. doi: 10.1097/00000658-198911000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter AG. Ischemic Necrosis of the Stomach Following Subtotal Gastrectomy. Lancet. 1953;11:1021. doi: 10.1016/s0140-6736(53)91310-5. [DOI] [PubMed] [Google Scholar]
- 9.Spencer FC. Ischemic Necrosis of the Remaining Stomach Following Subtotal Gastrectomy. Arch Surg. 1956;73:844. doi: 10.1001/archsurg.1956.01280050112021. [DOI] [PubMed] [Google Scholar]
- 10.Cate WR, Dawson RE. The Viability of Proximal Gastric Remnants Following Radical Subtotal Gastrectomy and Gastroduodenostomy. Surgery. 1957;41:401. [PubMed] [Google Scholar]
- 11.Grieg HW, Anson BJ, Coleman SJ. Inferior Phrenic Artery. Quart Bull N W Univ Med Sch. 1951;28:345. [PMC free article] [PubMed] [Google Scholar]
- 12.Kilgore TL, Turner DM, Hardy JD. Clinical and Experimental Ischemia of Gastric Remnant. Surg Gynec Obstet. 1964;118:1312. [PubMed] [Google Scholar]
- 13.Swigert LL, Siekert RG, Hamley WE, Anson BJ. The Esophageal Arteries. Surg Gynecol Obstet. 1950;90:234. [PubMed] [Google Scholar]
- 14.Shellito PC, Malt RA. Tube gastrostomy. Techniques and complications. Ann Surg. 1985;201:180–185. doi: 10.1097/00000658-198502000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholefield JH, Goodman AJ, Morgan WP. Abdominal wall and gastric infarction in acute pancreatitis. Pancreas. 1988;3:494–496. doi: 10.1097/00006676-198808000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Meguid MM, Campos ACL. Preface: surgical management of gastrointestinal fistulas. Surg Clin North Am. 1996;76:1035–1080. doi: 10.1016/s0039-6109(05)70497-7. [DOI] [PubMed] [Google Scholar]
- 17.Graves HA, Nelson A, Byrd B. Gastrocutaneous fistula as a postoperative complication. Ann Surg. 1970;171:656–660. doi: 10.1097/00000658-197005000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen CP, Lang C, Christensen A, et al. Gastrocolic fistulas. Acta Chir Scand. 1988;154:287–289. [PubMed] [Google Scholar]
- 19.Pearlstein C, Jones CE, Polk HC. Gastrocutaneous fistula: Etiology and treatment. Ann Surg. 1978;187:223–226. doi: 10.1097/00000658-197802000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez R, Sarr MG, Smith CD, et al. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007;204:47–55. doi: 10.1016/j.jamcollsurg.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Casella G, Soricelli E, Rizzello M, et al. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821–826. doi: 10.1007/s11695-009-9840-8. [DOI] [PubMed] [Google Scholar]
- 22.Dudrick SJ, Maharaj AR, McKelvey AA. Artificial nutritional support in patients with gastrointestinal fistulas. World J Surg. 1999;23:570–576. doi: 10.1007/pl00012349. [DOI] [PubMed] [Google Scholar]
- 23.Rose D, Yarborough MF, Canizaro PC, Lowry SF. One hundred and fourteen fistulas of the gastrointestinal tract treated with total parenteral nutrition. Surg Gynecol Obstet. 1986;163:345–350. [PubMed] [Google Scholar]
- 24.Owen ERTC, Abraham R, Kark AE. Gastroplasty for morbid obesity: Technique, complications and results in 60 cases. Br J Surg. 1989;76:131–135. doi: 10.1002/bjs.1800760209. [DOI] [PubMed] [Google Scholar]
- 25.Papavramidis ST, Milias C. Complications after vertical gastroplasty with artificial pseudopylorus in the treatment of morbid obesity: a 7-year experience. Obes Surg. 1999;9:535–538. doi: 10.1381/096089299765552620. [DOI] [PubMed] [Google Scholar]
- 26.Babor R, Talbot M, Tyndal A. Treatment of upper gastrointestinal leaks with a removable, covered, self-expanding metallic stent. Surg Laparosc Endosc Percutan Tech. 2009;19:1–4. doi: 10.1097/SLE.0b013e318196c706. [DOI] [PubMed] [Google Scholar]
- 27.Eubanks S, Edwards CA, Fearing NM, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935–938. doi: 10.1016/j.jamcollsurg.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc. 2009;23:1526–1530. doi: 10.1007/s00464-009-0432-4. [DOI] [PubMed] [Google Scholar]
- 29.Serra C, Baltasar A, Andreo L, et al. Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg. 2007;17:866 –872. doi: 10.1007/s11695-007-9161-8. [DOI] [PubMed] [Google Scholar]
- 30.Tan JT, Kariyawasam S, Wijeratne T, et al. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20:403–409. doi: 10.1007/s11695-009-0020-7. [DOI] [PubMed] [Google Scholar]
- 31.Eisendrath P, Cremer M, Himpens J, Chandratna HS. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625–630. doi: 10.1055/s-2007-966533. [DOI] [PubMed] [Google Scholar]
- 32.Edwards CA, Bui PT, Astudillo JA, et al. Management of anastomotic leaks after Roux-en-Y bypass using self-expanding polyester stents. Surg Obes Relat Dis. 2008;4:594–600. doi: 10.1016/j.soard.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Maluf-Filho F, Hondo F, Halwan B, de Lima MS, Giordano-Nappi JH, Sakai P. Endoscopic treatment of Roux-en-Y gastric bypass-related gastrocutaneous fistulas using a novel biomaterial. Surg Endosc. 2009;23:1541–1545. doi: 10.1007/s00464-009-0440-4. [DOI] [PubMed] [Google Scholar]


