Abstract
In this review we summarize latest data on the role of endoscopic ultrasonography (EUS) in the diagnosis and management of gastric carcinoma. Since its initial introduction in clinical practice, EUS has been considered a valuable tool for the diagnosis and locoregional staging of gastric cancer and a method of inarguable value for the assessment of gastric wall involvement and presence of infiltrated paragastric lymph nodes. Moreover, another application of EUS, i.e. its role in the assessment of early gastric cancer has come into focus, especially nowadays in the era of endoscopic mucosal resection and endoscopic submucosal dissection. These topics, together with other aspects of EUS in gastric cancer are discussed. On the other hand, despite its indisputable value, EUS for gastric cancer evaluation is “threatened” nowadays by other modern cross-sectional imaging methods (including trans-abdominal ultrasound, CT, MRI and PET), whose quality has lately improved. A brief comparison between the available imaging methods, attempts to show that their role ismore complementary than competitive.
Keywords: endoscopic ultrasonography, EUS, gastric cancer, locoregional staging, TNM
Introduction
Despite its decreasing incidence, gastric adenocarcinoma remains one of the leading causes of cancer-related deaths worldwide. Therapeutic options for gastric cancer rely on timely diagnosis and accurate staging of the tumor, whereas patients’ prognosis is directly associated with the lesion’s extent, as well as lymph node involvement and possible extension beyond the gastric wall layers [1,2]. Endoscopic ultrasonography (EUS) was introduced into clinical practice in the late1970’s and due to its unique ability to examine tumors from within the gastrointestinal (GI) lumen with extremely close proximity, it has evolved into an important and widely accepted diagnostic tool for the diagnosis and staging of various GI-lesions. Various studies highlight the value of EUS, especially in the diagnosis and staging of gastric diseases. Since the years of its initial development, EUS has been considered a valuable tool for the diagnosis and staging of tumors of the GI-tract and especially for gastric cancer. Accurate locoregional cancer staging provides necessary information to differentiate patients who will benefit from surgical resection from those who are better candidates for a multimodal approach (e.g. radiochemotherapy with or without surgery).
EUS: Examination technique in the stomach
Modern EUS utilizes a wide spectrum of echoendoscopes and probes, which precludes any detailed analysis of instrument types and specifications currently in use. However, basic aspects of EUS-instrumentarium have been reviewed [3]. In principal, EUS-imaging is currently performed with radial or linear echoendoscopes. In their modern version, these scopes are video-endoscopes coupled to electronic ultrasound processors for generation of electronic EUS-images, endowed with special aspects including Doppler, contrast, harmonic imaging and others; standard EUS usually utilizes high ultrasound frequencies, which vary between 5 and 20 MHz (7.5 MHz being the ultrasound frequency that is usually used). They generate a high-resolution image in the near field with limited penetration depth, ranging from 1-2 to 5-6 cm, depending on the ultrasound frequency used [4]. Gastric EUS is performed with the patient in the left lateral position, usually under conscious sedation mostly with benzodiazepines, sometimes in conjunction with a central analgesic and recently with propofol and it is associated with very low complication rates [5]. The transducer in most radial echoendoscopes generates radial images of 360°, oriented perpendicular to the shaft axis of the instrument. On the other hand, linear echoendoscopes produce images directed parallel to the shaft axis of the endoscope, thus allowing for an effective and safe performance of EUS-guided fine-needle aspiration puncture (EUS-FNA) when needed. Although it has been supported that both types of scanning principles -radial and linear- fare, more or less, equally well, in the authors’ personal experience this mainly applies to pancreatobiliary imaging, whereas complete gastric and perigastric scanning appears to be more difficult with linear instruments; here, radial imaging offers a better overview of the gastrointestinal wall and paramural structures [6-8].
Acoustic coupling of the ultrasonic transducer to the GI wall requires application of fluid as interface between the transducer and the wall: This is done using either a water-filled balloon around the tip of the instrument, or by instillation of water in the lumen. When performing gastric EUS, the following scanning principles should be adhered to, in order to avoid artifacts and misinterpretation:
Scanning of targets should be perpendicular; oblique scanning may lead to broadening and blurring of structures (giving rise to erroneous diagnoses or overstaging).
An adequate focal distance (0.5–1.0 cm, depending on the ultrasonic frequencies) should be kept.
Use of higher frequencies may help achieve better visualization of structures and lesions close to the EUS transducer.
The technique for proper EUS-scanning of the stomach generally follows conventional upper GI-endoscopy, which is performed in order to assess the macroscopic appearance of the stomach as well as the morphology and location (if possible) of the lesion(s) in question. The echoendoscope is then introduced and positioned at the lesion. Once the lesion is identified on EUS, the echoendoscope is only moved slightly and slowly backward and forward, with fine movements of the instrument tip. This will help depict the full extent of the lesion and its relation to neighboring organs and structures. Gastric EUS also includes evaluation of the stomach wall-layers, analyses of mucosal or submucosal lesions and imaging of perigastric structures. The water-filling method is the most frequently used technique to evaluate the gastric wall. The stomach is initially collapsed by aspiration, followed by instillation of 200-400 mL water into the lumen up to the fundus. The examination is done from the antrum, while the instrument is slowly withdrawn and all parts of the gastric circumference are visualized as far as possible with perpendicular scanning. However, EUS of the stomach can sometimes be rather difficult, especially in the prepyloric region and the gastric angle: maintaining the water level and the probe scanning perpendicular to the wall can sometimes be hard to achieve. In these cases rotating the patient might help to keep the water level constant, whereas pushing the scope in, pulling it out and then rotating it might help to achieve a perpendicular position [9]. The GI wall (including the gastric wall) normally consists of 5 distinct layers [Fig. 1]. The 2 inner layers (echo-rich and echo-poor) represent the interface/ superficial mucosa and deep mucosa/muscularis mucosa. The 3rd (echo-rich) layer corresponds to the submucosa, the 4th (echo-poor) to the muscularis propria, and the 5th (echorich) to the serosa, which is usually not easily distinguishable from the surrounding echo-rich tissue. Surrounding organs, vessels and other structures are important for orientation and for other diagnostic purposes (e.g. tumor infiltration depth). These consist of various organs including the pancreatic body and tail, parts of the liver (especially the left lobe) and parts of the left kidney and spleen, as well as vessels such as the aorta, the vena cava (proximal stomach), the celiac trunk and the splenic and left renal veins [Fig. 2]. In everyday practice, both the water-filling and the balloon-inflation method can be combined for better imaging. There are no established values for the normal gastrointestinal wall, but a figure of 2–4 mm is usually considered to be the normal range, as well as a 1:1:1 relation between the mucosa, submucosa, and muscularis propria [9,10].
Figure 1.
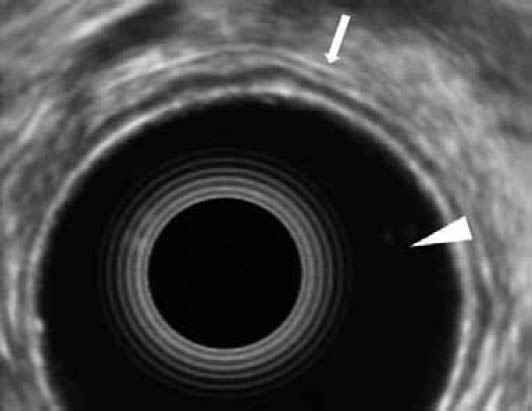
Electronic radial EUS-image of the gastric wall. Note the normal 5 distinct layers (arrow) and the echoendoscope's balloon, which is filled with water (arrowhead)
Figure 2.

Electronic radial EUS-image of the gastric wall with its surrounding organs, including the pancreatic body (white arrowhead) and the splenic vein (black arrowhead). Note the normal 5 distinct layers of the gastric wall (white arrow) and the gastric folds (black arrow)
EUS in gastric cancer – general considerations
The role of EUS in locoregional staging of gastric cancer remains pivotal, although other modern, non-invasive cross-sectional imaging modalities (including transabdominal ultrasound, CT, MRI and lately PET) emerge as possible alternatives, especially when it comes to assessment of distant lymph node involvement or other distant metastases, where they are deemed methods of choice [11-13]. Despite the indisputable value of EUS in the assessment of gastric wall involvement and presence of infiltrated paragastric lymph nodes of a (histologically proven) gastric cancer, its effectiveness in primary diagnosis or as a screening method is limited [14]. Despite the favorable performance of EUS in TNM staging, its impact in the management of patients with advanced gastric cancer appears to be small [15]. Another controversial subject is the role of EUS (performed either with standard or high frequency probes) in the era of endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) of early gastric cancer; here, the previously indisputable significance of EUS prior to endoscopic resection of early lesions seems lately to be under discussion. These subjects, as well as other aspects of EUS in gastric cancer will be further discussed.
Visualization and staging of gastric cancer with EUS: Pitfalls and difficulties
As previously mentioned, EUS is considered an extremely valuable tool in the locoregional staging of gastric cancer; its accuracy in T-staging varies between 60% and 90%, whereas N-staging accuracy ranges between 50% and 80%. In more recent reports these aforementioned accuracies (especially for the T stage) seem to show a trend towards a slight (though statistically non-significant) decline [12,16-18]. However, as previously demonstrated, various factors might also influence accuracy in the everyday life setting, leading to discrepancies between results reported in studies and routine practice [17].
GI-tumors (including gastric cancer) generally appear as echo-poor, inhomogeneous wall thickenings, localized or diffuse, involving deeper layers, growing outside the wall, and eventually infiltrating into other structures, depending on the tumor stage. Stage T1 is characterized by involvement of the inner two layers (T1 mucosa) or the 3 inner layers (T1 submucosa, or T1sm); subclassification of T1 tumors into T1a for those involving the lamina propria or the muscularis mucosae and T1b for tumors involving the submucosa were proposed in 2009, in the 7th edition of the TNM staging system. The term muscularis mucosa is used to distinguish the layer between the mucosal and submucosal layers; subsequently the indications between EMR and ESD (see chapter 6 as well) [19]. Flat lesions may not be visible by EUS. The balloon may compress discrete ulcers or elevations so water filling and the use of higher frequencies is therefore recommended in such a situation. Microscopic infiltration can be missed (understaging), and peritumoral inflammation can lead to overstaging,especially when dealing with ulcerated cancers [20,21]. Cancer stages that are more advanced, involve all of the wall layers, with a layer structure not being visible; smooth outer margins are seen in stage T2 (involvement of the muscularis propria, however without it being “broken”). Stage T3 on the other hand, is characterized by irregular outer margins [Fig. 3]. In stage T4, the boundary between the tumor and other organs (e.g., the liver or pancreas) or large vessels (e.g., aorta) cannot be recognized. The absence of relative movements between organs (e.g., between a gastric cancer and the liver) on respiration can be used as an indirect sign of tumor infiltration. Lymph-node metastases are seen on EUS as roundish, echo-poor lesions, para- or perigastrically (N+) [Fig. 4]. Although EUS is not reliable in differentiating between benign and malignant lesions in individual nodes, the likelihood of lymph-node metastases increases with their size and the concomitant T stage (i.e., > 80% likelihood in stage T3 versus < 5% in stage T1m).
Figure 3.
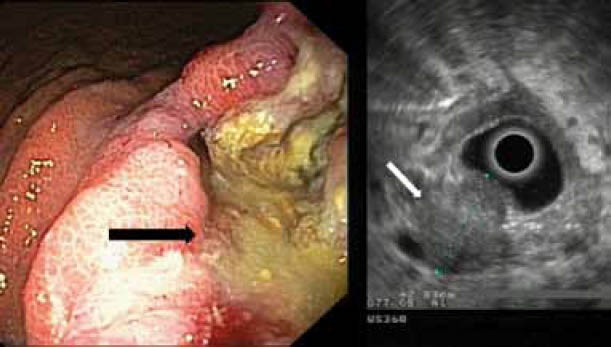
A case of gastric cancer. Left: endoscopic image. Note the tumor that appears as a deep ulcer (black arrow). Right: EUS image of the same tumor (white arrow). Note the echo-poor, inhomogeneous appearance of the tumor, which spreads throughout the whole wall and its irregular outer margin (stage T3)
Figure 4.

EUS image of a gastric cancer (white arrow) with a lymph node (black arrow). The lymph node appears as an oval, paragastric echo-poor lesion
Some parts of the stomach (including the lesser curvature —especially at the angular fold— and the subcardial region) are more difficult to cover by EUS; therefore, lesions in these areas may be more difficult to visualize and stage [10]. Diffuse gastric cancers (linitis plastica, scirrhous carcinoma) are visualized endosonographically as a diffuse wall thickening. In these cases, some of the “normal” five-layered structure can often still be recognized (although with thickened and distorted layers), whereas its outer margin is irregular, but relatively well preserved. A similar picture can be seen with advanced gastric lymphoma and other (benign) conditions.
Another difficult site for accurate EUS-staging in gastric cancer is the gastroesophageal junction; it seems that the accuracy of EUS-based TNM staging is lower when the cardia is affected, as the anatomy of the gastroesophageal junction can lead to oblique EUS-scanning through the gastric wall, which eventually results in artefactual misrepresentation of the true penetration of the wall by the tumor [Fig. 5] [22].
Figure 5.
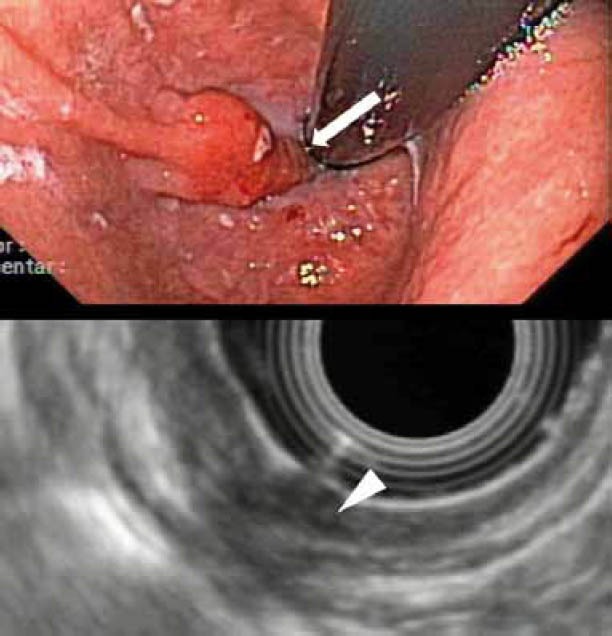
EUS-staging of a T1 cancer in the gastroesophageal junction: Up: Endoscopic image. Note the tumor in retroflex imaging (arrow). Down: EUS-imaging of the tumor. Note the involvement of the 3 inner layers (T1sm), as well as the artefacts which make interpretation of the wall penetration by the tumor difficult
Finally, it should be stressed that in advanced gastric cancer, EUS has been reported to be more sensitive than CT for the presence of peritoneal carcinomatosis and local ascites [22]. In a study of 402 patients with gastric adenocarcinoma and negative CT exam, clinically significant peritoneal carcinomatosis and ascites was identified in 36 of 56 patients using a 12-MHz high-frequency ultrasound miniprobe [23].
Influence on patient management
In spite of the fact that EUS is a well-established diagnostic method in the preoperative staging and restaging of GI-tumors [5], its influence on patient management has been recently analyzed, with controversial results. It seems that EUS, although being very accurate for TNM staging of early-stage gastric cancer, does not substantially influence the management of patients with advanced gastric cancer [15]. However, such analyses are definitely methodologically hard to perform, as the impact of a certain test on management should not be assessed by the person performing the test himself. On the other hand, although recent studies on other GI-cancers (i.e. esophageal) supported that management changes were more often due to EUS than PET or CT, the type of management changes were different: EUS mainly switched decisions towards neoadjuvant therapy, whereas PET and CT would find distant metastases, thus preventing primary or secondary surgery [25]. In another study setting, a retrospective selection of cases was made and a detailed history – with and without EUS – was presented to 4 surgeons, who would decide for the best treatment under both scenarios; although inter-observer agreement was poor, management was changed in a third of cases with EUS, mostly (85%) switching patients from surgery to palliation [26]. Finally, implementation of EUSFNA and tissue sampling in preoperative strategies might also influence treatment decisions, by helping to discriminate malignant lymph nodes. Although this has been shown to occur in other GI-malignancies (e.g. esophageal cancer), the role of EUS-FNA with regard to lymph nodes remains somewhat ill-defined [5]. However, recent data support that EUS-FNA could be integrated as a routine procedure in the preoperative staging algorithm of gastric cancer [27]. In this study concerning 234 patients with gastric carcinoma, 81 consecutive patients with lymph nodes or lesions considered to be distant metastases underwent EUS-FNA; distant spread to mediastinal lymph nodes was confirmed by EUS-FNA in 27/81 patients and treatment was changed in 15%. On the other hand, EUS-FNA has its own limitations; it was recently shown in an ex vivo assessment in 13 patients with esophageal cancer that the working channel of echoendoscopes can be contaminated during EUS-FNA by malignant cells, resulting in false-positive cytology results [28]. As treatment decisions in gastric cancer hinge on metastasis staging, it is crucial to avoid such contamination by flushing the echoendoscope between punctures. Another novel application deriving from EUS-FNA is the ability to accurately mark lymph nodes for possible surgery and radiotherapy (EUS-FNM). This was prospectively shown in 25 patients with suspected or confirmed upper GI-malignancies using 5mm silver pins inserted by EUS into or near lymph nodes; 23 nodes were marked and 18/19 surgically isolated; in 89 % of these cases, the marked lymph node corresponded to the location described by EUS [29]. Standardized extensive (D2) lymphadenectomy, followed by postoperative adjuvant chemotherapy or chemoradiotherapy is currently recommended as the standard of care for the management of gastric cancer [30,31]. However, for the level II lymph nodes this technique cannot be used. There are also other factors that limit the clinical utility of EUS-FNM, including the fact that lymph nodes even smaller than 1cm may be involved, which have micrometastatic disease, or contain isolated tumor cells.
Restaging in GI cancers
Tumor restaging in its traditional sense, using the TNM classification system, has been shown to be of low accuracy, as an irregular outer margin (primarily indicating stage T3) is also maintained after (radio)chemotherapy and thus the tumor stage remains the same. This has been repeatedly demonstrated mostly in tumors located in other parts of the GI-tract (e.g. esophageal tumors) [32]. It seems that in this setting, 3D reconstructions of tumor volume changes could possibly be better suited. Here, preliminary studies based on in vitro data showed that 3D-EUS probes allowed accurate volume measurements of small pseudotumors in porcine stomach models [33]. Similar results were also demonstrated in studies that combined experimental and clinical data including 4 gastric (as well as 6 esophageal and 1 colonic) cancer cases [34]. The basic limitation of 3D-EUS technology is penetration of the ultrasound waves when evaluating large tumors, as the transducer is of high frequency and, therefore, has a limited penetration. Whether this will prove to be a true difficulty and most importantly, whether the potential advantage of 3D-EUS will have some true clinical impact on patient management in tumor restaging after neo-adjuvant treatment has not been systematically analyzed and appropriate trials with 3D imaging (assessing tumor volume reduction) must still be performed.
Pre-EMR staging of early gastric cancer – should we still be performing EUS?
Due to the increased rates of lymph node metastases in cancers with deeper infiltration, only tumors limited to the mucosa can be curatively resected by endoscopic techniques such as EMR or ESD, provided that histology shows well or moderately differentiated cancers. Although previous experience in these techniques, especially ESD, was mostly confined to Japanese centers, it seems that recently expertise has been gained in Europe as well, resulting in 78% en-bloc and 77% Ro- resections (with 13% major complications) [35]. Distinguishing between mucosal and submucosal infiltration in stage T1 is clinically relevant when endoscopic resection is being considered [36-38]; cases with absence of any visible endosonographic abnormality in an area in which a flat endoscopic lesion has been seen and confirmed histologically is a sign of a superficial (T1m) cancer. EUS has been advocated prior to EMR to differentiate between mucosal and submucosal infiltration and is routine in many centres dealing with mucosal resection techniques; however, data published (mostly from studies on esophageal cancer) do not fully support this, as recent studies in early Barrett and squamous esophageal cancers have demonstrated poor sensitivities/specificities and/or accuracies of EUS (with or without miniprobes) in differentiating mucosal from submucosal infiltration [39-41]. The few published data on early gastric cancer seem to support the poor performance of EUS. According to a recent study, EUS was significantly prone to understage poorly differentiated early gastric cancers and to overstage lesions located in the mid 1/3 of the stomach larger than 3cm [42]. In a recent study including 955 patients from a single institution, the accuracy of EUS using miniprobe or radial EUS in early gastric cancer staging, either operated (n=586),or undergoing ESD (n=369) was 67.4 % (644 / 955) whereas that of conventional endoscopy was 73.7 % (704/955) (P < 0.001). The accuracy of miniprobe EUS was significantly higher than that of radial EUS (79.5 % versus 59.6 %, P<0.001), but did not differ significantly from that of conventional endoscopy (79.0 %), thus supporting that EUS does not substantially impact on pretreatment T-staging of patients with early gastric cancer compared with conventional endoscopy [43].
What about lymph nodes? Can EUS at least detect them in early gastric cancer? There is very little published evidence on this issue; older studies on a series of early gastric cancer showed poor results: 2/58 cases had lymph node metastases, which were both missed on EUS (sensitivity 0); EUS was correctly negative in 52 of the 56 remaining cases (specificity of 93%, as high as the negative likelihood for lymph nodes in this setting) [44]. Furthermore, recent data deriving from a meta-analysis seem to support that the overall pooled accuracy of EUS in nodal staging of gastric cancers is not high; moreover, the estimates of EUS are higher when dealing with N2- than for N1-tumors (i.e. higher for advanced than for early disease). It is not clear if using all the endosonographic criteria for malignant nodal involvement (round/echopoor nodes in the vicinity of the tumor, larger than 1cm) together or in combination improves its diagnostic accuracy. On the other hand, the role that EUS-FNA might play in this setting needs also to be clarified, as there is a lack of a substantial number of studies with the data. Tissue acquisition however (especially for histological evaluation), is expected to improve accuracy of EUS-FNA, although more studies are needed before this can be definitely concluded [45]. Finally, although ultrasound transducers of high frequency (20 to 30MHz) for EUS catheter probe have been tested and early data have 14 I.S. Papanikolaou et al
suggested that they could be helpful in the future of EUS-directed EMR, they have not been widely implemented in clinical practice [46]. Therefore, it seems that EUS is not mandatory prior to endoscopic resection, provided that the endoscopist performing EMR/ESD has sufficient experience with the endoscopic assessment of early lesions [47]. EUS (in its current form) is not always accurate enough to assess infiltration of the submucosa and does not seem to definitely detect or exclude lymph node metastases in early lesions with sufficient accuracy. It may be used in doubtful cases to confirm the endoscopic impression of local endoscopic irresectability.
Conclusions
EUS is a valuable method for diagnosis, locoregional staging and presence of infiltrated paragastric lymph nodes in gastric cancer.
The accuracy of EUS-guided T-staging ranges in various studies between 60% and 90%; N-staging accuracies are somewhat lower.
The influence of EUS on patient management remains controversial; it seems to have a greater impact on management of early stages rather than in advanced gastric cancer.
Current data support that EUS-FNA could be integrated as a routine procedure in the preoperative staging algorithm of gastric cancer.
TNM-restaging with EUS demonstrates a low accuracy; it seems that in this scenario, 3D EUS-reconstructions of tumor volume changes could possibly be better suited.
In early gastric cancer, EUS for differentiation of mucosal from submucosal infiltration prior to endoscopic resection is not mandatory, provided the endoscopist has sufficient experience with the macroscopic assessment of early lesions. EUS could possibly detect lymph nodes in early gastric cancer; the role of EUS-FNA in this setting needs to be further clarified.
Biography
aAttikon University General Hospital, Greece
bUniversity Hospital Hamburg Eppendorf, Hamburg, Germany
Footnotes
Conflict of interest: None
References
- 1.Nakamura K, Ueyama T, Yao T, et al. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030–1037. doi: 10.1002/1097-0142(19920901)70:5<1030::aid-cncr2820700504>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am. 2005;15:13–31. doi: 10.1016/j.giec.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Papanikolaou IS, Delicha EM, Adler A, et al. Prospective, randomized comparison of mechanical and electronic endoscopic ultrasound systems: Assessment of performance parameters and image quality. Scand J Gastroenterol. 2009;44:93–99. doi: 10.1080/00365520802400859. [DOI] [PubMed] [Google Scholar]
- 5.Papanikolaou IS, Fockens P, Hawes R, et al. Update on endoscopic ultrasound: how much for imaging, needling, or therapy? Scand J Gastroenterol. 2008;43:1416–1424. doi: 10.1080/00365520701737252. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MA, Scheiman JM. Initial experience with an electronic radial array echoendoscope: randomized comparison with a mechanical sector scanning echoendoscope in humans. Gastrointest Endosc. 2002;56:573–577. doi: 10.1067/mge.2002.127761. [DOI] [PubMed] [Google Scholar]
- 7.Noh KW, Woodward TA, Raimondo M, et al. Changing trends in endosonography: linear imaging and tissue are increasingly the issue. Dig Dis Sci. 2007;52:1014–1018. doi: 10.1007/s10620-006-9491-8. [DOI] [PubMed] [Google Scholar]
- 8.Rösch T. The radial echoendoscope: here to stay or gone tomorrow? Gastrointest Endosc. 2009;69(Suppl):S159–162. doi: 10.1016/j.gie.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Sabbagh LC. The gut: esophagus, stomach, and rectum. Gastrointest Endosc. 2009;69(Suppl):S90–92. doi: 10.1016/j.gie.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Rösch T, Classen M. New York: Thieme Stuttgart; 1992. Gastroenterologic Endosonograpy. [Google Scholar]
- 11.Wakelin SJ, Deans C, Crofts TJ, et al. Comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol. 2002;41:161–167. doi: 10.1016/s0720-048x(01)00418-1. [DOI] [PubMed] [Google Scholar]
- 12.van Vliet EP, Eijkemans MJ, Kuipers EJ, et al. Publication bias does not play a role in the reporting of the results of endoscopic ultrasound staging of upper gastrointestinal cancers. Endoscopy. 2007;39:325–332. doi: 10.1055/s-2007-966233. [DOI] [PubMed] [Google Scholar]
- 13.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 14.Kida M. EUS in gastric cancer. In: Hawes RH, Fockens P, editors. Endosonography. Saunders Elsevier; 2006. pp. 111–126. [Google Scholar]
- 15.Maluf-Filho F, Dotti CM, Halwan B, et al. An evidence-based consensus statement on the role and application of endosonography in clinical practice. Endoscopy. 2009;41:979–987. doi: 10.1055/s-0029-1215192. [DOI] [PubMed] [Google Scholar]
- 16.Kuntz C, Herfarth C. Imaging diagnosis for staging of gastric cancer. Semin Surg Oncol. 1999;17:96–102. doi: 10.1002/(sici)1098-2388(199909)17:2<96::aid-ssu3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Meining A, Dittler HJ, Wolf A. You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut. 2002;50:599–603. doi: 10.1136/gut.50.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polkowski M. Endosonographic staging of upper intestinal malignancy. Best Pract Res Clin Gastroenterol. 2009;23:649–661. doi: 10.1016/j.bpg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Rausei S, Dionigi G, Boni L, Rovera F, Dionigi R. How Does the 7th TNM Edition Fit in Gastric Cancer Management? Ann Surg Oncol. 2010 Sep 29; doi: 10.1245/s10434-010-1346-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Massari M, Cioffi U, De Simone M, et al. Endoscopic ultrasonography for preoperative staging of gastric carcinoma. Hepatogastroenterology. 1996;43:542–546. [PubMed] [Google Scholar]
- 21.Mouri R, Yoshida S, Tanaka S, et al. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318–322. doi: 10.1097/MCG.0b013e3181775966. [DOI] [PubMed] [Google Scholar]
- 22.Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534–539. doi: 10.1136/gut.49.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YT, Ng EK, Hung LC, et al. Accuracy of endoscopic ultrasonography in diagnosing ascites and predicting peritoneal metastases in gastric cancer patients. Gut. 2005;54:1541–1545. doi: 10.1136/gut.2004.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu KM, Kwok KF, Law S, et al. A prospective evaluation of catheter probe EUS for the detection of ascites in patients with gastric carcinoma. Gastrointest Endosc. 2004;59:471–474. doi: 10.1016/s0016-5107(03)02873-6. [DOI] [PubMed] [Google Scholar]
- 25.Pfau PR, Perlman SB, Stanko P, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65:377–384. doi: 10.1016/j.gie.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Mortensen MB, Edwin B, Hünerbein M, et al. Impact of endoscopic ultrasonography (EUS) on surgical decision-making in upper gastrointestinal tract cancer: an international multicenter study. Surg Endosc. 2007;21:431–438. doi: 10.1007/s00464-006-9029-3. [DOI] [PubMed] [Google Scholar]
- 27.Hassan H, Vilmann P, Sharma V. Impact of EUS-guided FNA on management of gastric carcinoma. Gastrointest Endosc. 2010;71:500–504. doi: 10.1016/j.gie.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 28.van Hemel BM, Lamprou AA, Weersma R, Plukker JT, Suurmeijer AJ, van Dullemen HM. Procedure-related, false-positive cytology results during EUS-guided FNA in patients with esophageal cancer. Gastrointest Endosc. 2010;71:1130–1133. doi: 10.1016/j.gie.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 29.Larsen MH, Fristrup CW, Pless T, et al. Endoscopic ultrasound-guided fine-needle marking of lymph nodes. Endoscopy. 2010;42:133–137. doi: 10.1055/s-0029-1215378. [DOI] [PubMed] [Google Scholar]
- 30.Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 31.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Das A, Chak A. Reassessment of patients with esophageal cancer after neoadjuvant therapy. Endoscopy. 2006;38(Suppl 1):S13–17. doi: 10.1055/s-2006-946644. [DOI] [PubMed] [Google Scholar]
- 33.Vegesna A, Raju R, Asfari W, et al. Three-dimensional US volume analysis of gastric pseudotumors in a porcine model. Gastrointest Endosc. 2006;64:635–640. doi: 10.1016/j.gie.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe M, Kida M, Yamada Y, et al. Measuring tumor volume with three-dimensional endoscopic ultrasonography: an experimental and clinical study (including video) Endoscopy. 2004;36:976–981. doi: 10.1055/s-2004-825866. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro-Mourão F, Pimentel-Nunes P, Dinis-Ribeiro M. ESD for gastric lesions: results of a European inquiry. Endoscopy. 2010;42:814–819. doi: 10.1055/s-0030-1255778. [DOI] [PubMed] [Google Scholar]
- 36.Giovannini M, Bernardini D, Moutardier V, et al. Endoscopic mucosal resection (EMR): results and prognostic factors in 21 patients. Endoscopy. 1999;31:698–701. doi: 10.1055/s-1999-79. [DOI] [PubMed] [Google Scholar]
- 37.Waxman I, Saitoh Y. Clinical outcome of endoscopic mucosal resection for superficial GI lesions and the role of high-frequency US probe sonography in an American population. Gastrointest Endosc. 2000;52:322–327. doi: 10.1067/mge.2000.105723. [DOI] [PubMed] [Google Scholar]
- 38.Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol. 2004;31:530–541. doi: 10.1053/j.seminoncol.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 39.May A, Gunter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004;53:634–640. doi: 10.1136/gut.2003.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett's esophagus. Gastrointest Endosc. 2005;62:16–23. doi: 10.1016/s0016-5107(05)00319-6. [DOI] [PubMed] [Google Scholar]
- 41.Chemaly M, Scalone O, Durivage G, et al. Miniprobe EUS in the protherapetic assessment of early esophageal neoplasia. Endoscopy. 2008;40:2–6. doi: 10.1055/s-2007-966958. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Song KS, Youn YH, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66:901–908. doi: 10.1016/j.gie.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705–713. doi: 10.1055/s-0030-1255617. [DOI] [PubMed] [Google Scholar]
- 44.Akahoshi K, Misawa T, Fujishima H, Chijiiwa Y, Nawata H. Regional lymph node metastasis in gastric cancer: evaluation with endoscopic US. Radiology. 1992;182:559–564. doi: 10.1148/radiology.182.2.1732981. [DOI] [PubMed] [Google Scholar]
- 45.Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:4011–4019. doi: 10.3748/wjg.14.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace MB, Hawes RH. Emerging indications for EUS. Gastrointest Endosc. 2000;52(Suppl 6):S55–60. doi: 10.1067/mge.2000.110714. [DOI] [PubMed] [Google Scholar]
- 47.May A. Stop confusing us with EUS prior to endoscopic resection. Endoscopy. 2008;40:71–72. doi: 10.1055/s-2007-995408. [DOI] [PubMed] [Google Scholar]


