Abstract
Background
The aim of the study was to evaluate the clinical presentation, and to investigate the effectiveness of continuous catheter drainage in comparison to needle aspiration in the treatment of liver abscesses.
Methods
This is a prospective randomized comparative study of 60 patients, presented in outpatient and emergency department at the hospital, randomized equally into two groups, percutaneous needle aspiration and pigtail catheter drainage. The effectiveness of either treatment was measured in terms of duration of hospital stay, days to achieve clinical improvement, 50% reduction in abscess cavity size and total/near total resolution of abscess cavity. Independent t-test was used to analyze these parameters.
Results
The success rate was significantly better in catheter drainage group (P=0.006). The patients in pigtail catheter drainage group showed earlier clinical improvement (P=0.039) and 50% decrease in abscess cavity volume (P=0.000) as compared to those who underwent percutaneous needle aspiration.
Conclusion
Percutaneous catheter drainage is a better modality as compared to percutaneous needle aspiration especially in larger abscesses which are partially liquefied or with thick pus.
Keywords: Liver abscess, catheter drainage, needle aspiration
Introduction
A liver abscess is a suppurative cavity in the liver resulting from the invasion and multiplication of microorganisms, entering directly from an injury through the blood vessels or by the way of the biliary ductal system. Liver abscesses are most commonly due to pyogenic, amebic or mixed infections. Less commonly these may be fungal in origin.
Liver abscess has been recognized since Hippocrates (circa 400 B.C.) who speculated that the prognoses of the patients were related to the type of fluid within the abscess cavity [1]. Although amebic liver abscess occurs more commonly on a worldwide basis, the pyogenic liver abscess predominates in the United States.
Liver abscess is found more commonly in men between 20 and 40 years of age, but can occur at any age. Approximately 60% are solitary and mainly located in the right lobe of the liver, as a result of the streaming of portal blood flow secondary to the fact that the right lobe is predominantly supplied by the superior mesenteric vein, and because most of the hepatic volume is in the right lobe. When multiple abscesses are present, pyogenic or mixed is the most probable type. Patients usually present with a constant dull pain in the right upper quadrant of the abdomen which may be referred to the scapular region or the right shoulder. These patients usually have fever of between 38oC and 40oC.
Liver abscesses, both amebic and pyogenic, continue to be an important cause of morbidity and mortality in tropical countries. However, recent advances in interventional radiology, intensive care, progress in antibiotic therapy, and liberal use of sonography and computerized tomography scanning of the abdomen have led to early diagnosis and treatment of patients with liver abscess, thus improving the patient outcome. Percutaneous drainage of liver abscess has been an important advancement in the treatment of pyogenic liver abscesses.
The primary mode of treatment of amebic liver abscess is medical; however as many as 15% of amebic abscesses may be refractory to medical therapy [2]. Also, secondary bacterial infection may complicate 20% of amebic liver abscesses [3]. In such patients and in patients with pyogenic liver abscesses, surgical drainage has been the traditional mode of treatment [4]. However, operative drainage is associated with significant (10-47%) morbidity and mortality [5].
In recent years, image-guided percutaneous drainage has been increasingly used to treat liver abscesses with reported success rates ranging from 70-100% [6-8]. Although percutaneous placement of an indwelling catheter is the method most widely preferred to drain liver abscesses [9], recent studies have claimed needle aspiration to be a simpler, less costly, and equally effective mode of treatment [10,11].
Materials and methods
Study design
This was a single-center prospective randomized comparative study conducted by the departments of Surgery and Radiology. A total of 60 patients were included in the study, randomized into two groups; percutaneous needle aspiration (PNA) (n=30) and pigtail catheter drainage (PCD) (n=30). The patients were studied from November 2011 till March 2012. The protocol was approved by the ethical review board of the institute. According to the principles of the declaration of Helsinki 1975, written, informed consent was obtained from all participants.
Participants
The patients were selected from those attending the outpatient department and emergency department at the hospital. The age of patients varied from 16 to 58 years with most of the patients falling within the range from 31-40 years. All the patients diagnosed to have liver abscess clinically and radiologically [on ultrasonography (USG) and/or CT scan] were included in the study. Exclusion criteria were: all abscess cavities smaller than 5 cm in their greatest dimension; prior intervention; ruptured liver abscess; uncertain diagnosis; concomitant biliary tract malignancy; and uncorrectable coagulopathy. 72 patients were enrolled, and after assessment 5 patients were excluded due to prior PNA attempt, and 7 patients were excluded owing to a ruptured abscess.
Methods
All the subjects satisfying the inclusion criteria were carefully worked up in terms of a detailed history and clinical examination. Lab and imaging investigations included complete hemogram; liver function tests; prothrombin time; international normalized ratio; activated partial thromboplastin time; blood culture; amebic serology; imaging-CXR; abdominal USG with or without CT scan of the abdomen; and other investigations as per specific indications in different patients.
An informed consent was obtained from the participating patients and all the consenting patients were started on medical treatment as per our protocol.
All the patients empirically received injection Metronidazole 750 mg IV every t.i.d., injection Cefazolin 1 g IV b.i.d., injection Gentamicin 80 mg IV b.i.d., and chloroquine 600 mg for 2 days (600 mg is total dose for a day which is given in 2 divided doses and not 600 mg q.i.d.) followed by 300 mg for 19 days (given in 2 divided doses). The empirical treatment was revised based on the culture and sensitivity report. However, patients in whom pus culture was sterile continued on the same treatment. The antibiotics and metronidazole were given for duration of 10 and 14 days respectively.
Randomization was done using computer software, according to a standardized previously reported protocol [12]. The two sets of random numbers generated were assigned to the two intervention groups and sealed numbered envelopes were made with the serial number mentioned on the outside and intervention mentioned inside by a non-participating individual. Once a participating subject gave valid consent the pre-determined intervention was carried out as follows: The percutaneous procedures were carried out under local anesthesia (2% lignocaine) with IV analgesia and sedation if required. The procedures were carried out under continuous real-time USG guidance using Philips HD11 ultrasound machine.
PNA
The patient was subjected to USG of the abdomen and the characteristics of the abscess cavity(ies) were recorded. Local anesthesia was infiltrated at the proposed puncture site using a 23 G needle. Under real-time USG guidance and using 18 G BD spinal needle (Becton Dickinson S.A. s. Agustin del Guadalix Madrid, Spain) the abscess cavity was entered and pus was aspirated till no more pus could be aspirated further. A sample of pus was sent for Gram stain, culture, sensitivity and wet mount for Entamoeba histolytica trophozoites. A dressing was applied at the needle puncture site.
PCD
The PCD was accomplished by placing a 12-Fr pigtail catheter in the abscess cavity under USG guidance using the Seldinger technique. The patient was subjected to USG of the abdomen and the characteristics of the abscess cavity(ies) were recorded. Local anesthetic was infiltrated in the proposed area of puncture. Using a No. 11 blade a small stab was made on the anesthetized skin. A percutaneous nephrostomy pigtail catheter set (Devon Innovations Private limited, Bangalore, India) with a 12 Fr catheter was used for drainage. Under real time sonographic guidance the initial puncture needle (18 G, 21 cm long with three parts) was inserted through the skin stab and guided to the center of the abscess cavity. The stylet was taken out and pus was aspirated to reconfirm the position and the aspirated pus was sent to the lab for testing. A 0.038” straight tip guide wire was inserted through the needle and the needle was taken out without displacing the guide wire. The tract was dilated with plastic dilators serially up to 12 Fr size. The 12-Fr pigtail catheter was then passed over the guide wire which was taken out, and the catheter was fixed to the skin using 1-0 Silk suture. The catheter was attached to a collecting bag via the supplied connector.
Evaluation of the response to intervention
The clinical response (temperature) and laboratory parameters [total leukocyte count (TLC), liver function test (LFT), etc.] were recorded on a daily basis. In the patients undergoing PNA, USG was repeated after a gap of two days and aspiration repeated if the cavity size was still found to be greater than 5 cm. The same procedure was repeated after a gap of another two days and aspiration repeated if needed.
The failure of clinical improvement in terms of fever, abdominal pain and tenderness and leukocytosis or decrease in size of the abscess cavity after a third attempt of aspiration was taken as failure of needle aspiration. These patients underwent PCD but were not added to the PCD group.
In patients who underwent PCD, besides recording the clinical and laboratory parameters of the patient every day, daily output of the catheter was measured and the catheter was flushed with 20 cc of normal saline (this volume was deducted from the total drainage). A decision to remove the pigtail catheter was made when the total drainage from the catheter decreased to less than 10 mL/24 h for two consecutive days. The patient was administered Tab. Diloxanide Furoate 500 mg p.o. b.i.d. for 10 days at the time of discharge.
Follow up
The patients were followed up weekly for a month, monthly for three months and at the end of six months, for clinical evaluation and USG assessment of abscess cavity until complete resolution of the abscesses was achieved. Data was collected and recorded in the printed proforma by the investigator.
Statistical analysis
The effectiveness of either treatment was measured in terms of: duration of hospital stay; days to achieve clinical improvement; days to achieve 50% reduction in abscess cavity size; and days to achieve total/near total resolution of abscess cavity. Independent t-test was used to analyze these parameters. The level of significance was set at P<0.05.Volume of abscess cavity and duration of drainage (applicable to PCD group only) were also analyzed and range and mean values were calculated for both the parameters.
Results
A total of 60 patients randomized into two groups of 30 each were included in the study. The following observations were made:
General characteristics
The age of the patients varied from 16 years to 58 years with most of the patients falling within the age range from 31-40 years (21 patients). The second most common age group was 21-30 years (19 patients) and the number of patients was less in extremes of age. There were 53 male and 7 female patients with liver abscess involved in the study. The male to female ratio was 7:1.
Symptoms and signs
It was observed that pain in the right upper quadrant of the abdomen was the most common symptom, found in 93% of the cases. Weakness (90%) and fever (88%) were other frequently presenting symptoms. Approximately half of the patients had symptoms of anorexia, weight loss and night sweats. Pain in the right shoulder region and cough were present in 22% and 27% of the patients respectively. Only 10% of the patients gave a history of diarrhea prior to illness (Table 1). In this study, hepatomegaly was found to be present in 48 of 60 patients (80%) whereas pleural effusion was found in 5 of 60 patients (8%).
Table 1.
Symptoms in order of decreasing frequency

Laboratory data
It was observed that 44 of 60 patients (73%) had leukocytosis. Elevation of serum alkaline phosphatase was also observed in 75% of the patients. Amebic serology positivity (>0.90, EIA) was found in 65% of the patients (Fig. 1).
Figure 1.

Laboratory data
TLC, total leukocyte count; S. bilirubin, serum bilirubin; ALP, alkaline phosphatase
Pus culture
Pus aspirated from all abscesses was sent for culture and sensitivity. Cultures were found to be positive in 18 of 60 (30%) of the cases. The rest were sterile.
Microbiology
Among the pus culture positive cases Escherichia coli was isolated most frequently i.e. 8 of 18 culture positive patients. It was closely followed by Klebsiella spp. which was isolated in 6 cases. Pseudomonas spp. and Staphyloccocus aureus were isolated in 2 patients each (Table 2).
Table 2.
Microbiology (Aerobic cultures were considered negative after 48 h of incubation)

Type of abscess
Amebic liver abscesses were encountered more frequently (58%) compared to pyogenic (23%), amebic abscesses with secondary bacterial infection (7%) and abscesses of indeterminate etiology (12%) (Tables 3A, 3B).
Table 3A.
Type of abscess (showing amebic serology and pus culture)

Table 3B.
Type of abscesses in each group

Location of the abscess
The majority (about 80%) of the abscesses were located in the right lobe of liver, 15% in the left and 7% in both lobes (Fig. 2).
Figure 2.

Location of abscess
Number of abscess
Three quarters of the cases studied were found to have solitary liver abscess cavity, whereas the rest of the patients had multiple abscesses.
Volume of the abscess
It was observed that the volume of the abscess cavities was mostly between 150-350 mL (Table 4).
Table 4.
Volume of the abscesses

Interventions and their results (Table 5)
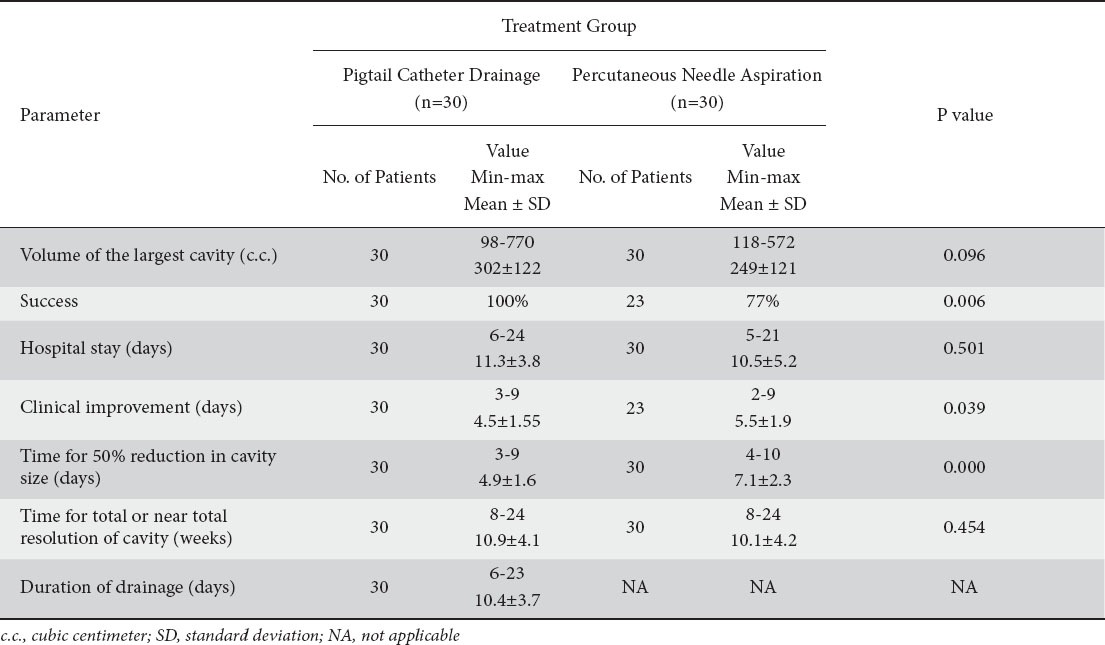
A total of 60 patients underwent either of the two percutaneous procedures randomly and their response to treatment was recorded and analyzed (Table 5). Pigtail percutaneous drainage was successful in all the 30 cases. On the other hand, image-guided needle aspiration was successful only in 23 of 30 patients (P=0.006). Out of these 23 patients successfully treated, 9 patients required only one aspiration, 10 required two aspirations, and 4 required three aspirations. The 7 patients who did not show clinical improvement and / or decrease in cavity size despite 3 aspirations were taken as failures. In the PNA group, on comparing the cavity volumes the mean cavity volume in those who were successfully treated was 201.4 cc which was significantly less than those failing treatment; the mean volume being 403.6 cc (P<0.011). The patients in PCD group showed earlier clinical improvement (P=0.039) and 50% decrease in abscess cavity volume (P=0.000) as compared to those who underwent PNA. However, there was no significant difference between the duration of hospital stay or the time required for total or near-total resolution of cavity.
Table 5.
Intervention and their results
Discussion
Liver abscess is a major tropical disease of the gastrointestinal system [13,14]. The liver abscess is mainly classified into amebic and pyogenic. Pyogenic liver abscess which used to be mainly tropical in location is now more common due to increased biliary interventions, stenting, A total of 60 patients underwent either of the two cholecystitis, cholangitis etc. Liver abscess is 3 to 10 times percutaneous procedures randomly and their response more common in men [15]. In our study we found the male to female ratio to be 7: 1. The most frequently affected age group was in the third and fourth decade.
The clinical presentation of the patients studied in our series was similar to the descriptions in previous reports [16]. The common symptoms and signs of liver abscess in our study were fever (88%), right upper quadrant pain and tenderness (93%) and hepatomegaly (80%). These clinical manifestations are similar to those described in previous studies [17,18].
In our study, 78% of the abscesses were located in the right lobe of liver, similar to previous studies [16,19], and 77% of our patients had solitary abscesses, similar to a previous report [20]. We encountered multiple liver abscesses in 23% of the patients, similar to the 20-25% incidence of multiple liver abscesses reported by Sharma et al [21].
The type of abscess was determined on the basis of amebic serology and pus culture reports [22]. In our study we found 58% of the abscesses to be amebic in etiology, 23% to be pyogenic, 12% to be indeterminate and 7% to be amebic with secondary bacterial infection (or mixed liver abscess, MLA). Khan et al in their series reported 68% amebic, 21% pyogenic, 8% indeterminate, and 3% MLA [22]. The use of serological testing for diagnosis of amebic liver abscesses can occasionally lead to either false negative results early in the course of the disease, due to delay in rise of antibody titer, or to false positives due to background subclinical amebic infections. Consideration of high titers for diagnosis may help exclude these false positives [23].
The pus cultures were negative in 42 of 60 patients. Aerobic cultures were declared negative after 48 h of incubation. There were 8 patients (12%) in whom the amebic serology as well as pus cultures were negative. As several of our patients prior to reporting to our hospital had been given antibiotics as well as antiamebic drugs, this might explain the finding of 12% cases with indeterminate etiology. Similar experience has been reported by other researchers as well [22].
The most frequently isolated bacteria on pus culture was Escherichia coli (44%) closely followed by Klebsiella species (33%). Escherichia coli has been reported to be the organism most frequently grown in western series [8,24]. However, Asian series have reported Klebsiella to be the most frequently isolated bacteria [25-27].
We performed image-guided percutaneous intervention in 60 patients with uncomplicated liver abscess and obtained good results. There was no mortality or any major complication requiring any treatment. Several researchers have employed both the modalities, i.e. PNA as well as PCD with varying degrees of success. Several groups have documented that significant number of patients can be managed with a combination of systemic antibiotics and percutaneous drainage with excellent results [20,28-30].
Many solitary and some carefully selected macroscopic multiple abscesses are amenable to percutaneous abscess drainage. Surgical drainage is usually reserved for patients who have failed percutaneous drainage, those who require surgery for management of underlying problems, and some patients with multiple macroscopic abscesses [31].
Several groups have reported reasonably good results with PNA along with systemic antibiotics [11,29]. Giorgio et al performed on an average 2.2 aspirations in 115 patients and reported resolution of symptoms and hepatic lesions in 98% of the patients. In our study we treated 30 patients with PNA along with systemic antibiotics. Of these 30 patients, 23 were successfully treated with 9 requiring only one aspiration, 10 requiring a second aspiration and 4 patients requiring a third aspiration as well. 7 of these 30 patients failed to improve clinically and did not show significant decrease in abscess cavity even after 3 aspirations. Thus, 23 patients who were successfully treated with aspiration required an average of 1.8 aspirations. The mean duration of time taken for clinical improvement was 5.5±1.9 days in this modality of treatment. Rajak et al [32] reported a success rate of 60% with needle aspiration. However, in their study only two attempts of aspiration were made and failure to attain clinical, hematological and radiological improvement was taken as failure of therapy.
Of 7 patients who did not respond to PNA, 6 improved on PCD and 1 was lost to follow up. But these patients were not included in the PCD group as success. Of these 7 patients, 2 were amebic, 3 were pyogenic and 2 were secondary infection; of 3 pyogenic abscesses, Escherichia coli was seen in 1 and Klebsiella in 2 patients.
It can be said that in recent years image-guided percutaneous treatment (needle aspiration or catheter drainage) has replaced surgical intervention as the primary treatment for liver abscess [6-9,33,34].
The major advantages of PNA over PCD are: 1) it is less invasive and less expensive; 2) avoids problems related to catheter care; and 3) multiple abscess cavities can be aspirated easier in the same setting [10,11]. However, in our study we had a success rate which was significantly lower than with catheter drainage (77% versus 100%, P=0.006). There are some problems with catheter drainage like nuisance to the patient, pain, cellulites at the insertion site and sometimes catheter dislodgement.
The success rate of PNA in the literature varies from 79-100% [10,35]. The success rate in our study after single aspiration was 30%, after second aspiration 63% and after third aspiration it was 77%. Although, needle aspiration is a much simpler procedure when compared to catheter drainage repeated procedures are quite unpleasant and traumatic for the patients and may not be acceptable to many. Even after repeated aspirations the success rate was far from being 100%. Therefore, those patients who failed after a third aspiration attempt were offered catheter drainage.
The average size of abscess in our study was 302±122 mL and 249±121 mL for the PCD and PNA group respectively, comparable to the study reported by Rajak et al (335 mL and 221 mL respectively) [32]. The success rate achieved by Rajak et al was 60%, comparable to the success rate after the second aspiration in our study, i.e. 63%. Subsequent aspirations seem to improve the success rate of therapy.
In contrast to some of the earlier reports that show that the initial size of the abscess cavity did not affect the ultimate outcome [11,29], larger abscesses are more difficult to evacuate completely in one attempt, necessitating subsequent aspirations [32]. The average volume of the 7 patients in whom PNA failed was significantly larger than the average volume of the patients who could be successfully treated with PNA (403.6 mL and 201.4 mL respectively, P=0.011).
Another important reason for failure of needle aspiration is the inability to completely evacuate the thick viscous pus that may be present in some of the abscesses. Rapid re-accumulation of pus in the abscess is another reason described for failure of needle aspiration [35].
Placement of an indwelling drainage catheter addresses all three of these issues as it provides continuous drainage, drains thick pus because of wider caliber catheter, and prevents re-accumulation. This explains the higher success rates (100%) observed in our study and several previous studies [10,32,33,36].
The only reasons for failure of PCD as reported in some of the earlier series [37,38] have been either thick pus not amenable to percutaneous drainage (this can be overcome by placement of a wider bore catheter) or premature removal of drainage catheter. No recurrence occurred in any of our cases during the follow up period. However, both treatment modalities resulted in rapid clinical relief with most patients showing resolution of signs and symptoms within the first 3 days of the procedure.
The time required for 50% reduction in the cavity size was significantly less in the PCD compared to PNA group (4.9 days and 7.1 days respectively, P=0.000). However, time required for total or near-total resolution of the abscess cavity did not show any significant difference in the two groups (PCD=10.9 weeks, PNA=10.1 weeks, P=0.454). It can be concluded that the abscess cavities showed faster collapse during the initial period in the PCD group but it did not have an advantage as far as total or near-total resolution of cavity is concerned. Similar observations were recorded by other investigators as well [9,32].
Complications such as hemorrhage, pleural effusion/empyema, persistent bile drainage, catheter displacement, sepsis etc., have been reported with both PNA (4% in series of Baek et al) [10] and PCD (12% in the series of Lambiase et al) [30]. Baek et al and Giorgio et al described the much lower incidence of complications with PNA than with PCD as one of the major advantages of needle aspiration over catheter drainage. However, in our study and some recent studies (Rajak et al 1998 [32], Yu et al 2004 [39]), both the procedures were found to be safe if performed properly with minimal complications. There was no mortality in either of the study groups.
Singh and Kashyap, 1989 [9] reported a 15% incidence of secondary bacterial contamination after repeated needle aspirations; however, others (Baek SY et al [10], Giorgio et al [11], Rajak et al [32]) have not encountered this problem. Although secondary bacterial infection remains a possibility with indwelling drainage catheters this complication has been rarely reported in liver abscess [7].
One limitation of our study is that the etiology of abscess was not uniform and formed a heterogeneous group with abscesses of both amebic and pyogenic etiology existing in both groups. Also, about 12% of the abscesses were of indeterminate etiology. Anaerobic culture was not performed and no studies to detect fungus were done.
Summary Box.
What is already known:
Image-guided percutaneous treatment (aspiration or catheter drainage) has replaced surgical intervention as the procedure of choice
If performed carefully, both the procedures are safe with minimal complications
What the new findings are:
Percutaneous catheter drainage is a better modality as compared to percutaneous needle aspiration
Each repeated aspiration improved the success of treatment by percutaneous needle aspiration
Significantly earlier clinical improvement (P=0.039) and less time for 50% reduction in abscess cavity (P=0.000) in the percutaneous catheter drainage group
The chances of failure of percutaneous needle aspiration increased with the increase in size of abscess cavity to be aspirated (P=0.011)
There was no significant advantage of catheter drainage over needle aspiration in terms of duration of hospital stay and time needed for total or near total resolution of abscess cavity
Biography
Dr Ram Manohar Lohia Hospital (Dr RMLH and PGIMER), New Delhi, India
Footnotes
Conflict of Interest: None
References
- 1.New York: William Wood & Co; 1886. Hippocrates. 1886: The Genuine Works of Hippocrates, vols 1 & 2. Transl [from the Greek with a preliminary discourse and annotations] 57,58,266,267. [Google Scholar]
- 2.Thompson JE, Jr, Forlenza S, Verma R. Amebic liver abscess: a therapeutic approach. Rev Infect Dis. 1985;7:171–179. doi: 10.1093/clinids/7.2.171. [DOI] [PubMed] [Google Scholar]
- 3.Sherlock S, Dooley J. 9th ed. Oxford: Blackwell Sci Pub; 1993. Diseases of the liver and billiary system; pp. 471–502. [Google Scholar]
- 4.Theron P. Surgical aspects of amoebiasis. Br Med J. 1947;2:123–126. doi: 10.1136/bmj.2.4516.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satiani B, Davidson ED. Hepatic abscesses: improvement in mortality with early diagnosis and treatment. Am J Surg. 1978;135:647–650. doi: 10.1016/0002-9610(78)90128-9. [DOI] [PubMed] [Google Scholar]
- 6.Gerzof SG, Johnson WC, Robbins AH, Nabseth DC. Intrahepatic pyogenic abscesses: treatment by percutaneous drainage. Am J Surg. 1985;149:487–494. doi: 10.1016/s0002-9610(85)80045-3. [DOI] [PubMed] [Google Scholar]
- 7.Attar B, Levendoglu H, Cuasay NS. CT-guided percutaneous aspiration and catheter drainage of pyogenic liver abscesses. Am J Gastroenterol. 1986;81:550–555. [PubMed] [Google Scholar]
- 8.Seeto RK, Rockey DC. Pyogenic liver abscess. Changes in etiology, management, and outcome. Medicine (Baltimore) 1996;75:99–113. doi: 10.1097/00005792-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Kashyap A. A comparative evaluation of percutaneous catheter drainage for resistant amebic liver abscesses. Am J Surg. 1989;158:58–62. doi: 10.1016/0002-9610(89)90316-4. [DOI] [PubMed] [Google Scholar]
- 10.Baek SY, Lee MG, Cho KS, Lee SC, Sung KB, Auh YH. Therapeutic percutaneous aspiration of hepatic abscesses: effectiveness in 25 patients. AJR. 1993;160:799–802. doi: 10.2214/ajr.160.4.8456667. [DOI] [PubMed] [Google Scholar]
- 11.Giorgio A, Tarantino L, Mariniello N, et al. Pyogenic liver abscesses: 13 years of experience in percutaneous needle aspiration with US guidance. Radiology. 1995;195:122–124. doi: 10.1148/radiology.195.1.7892451. [DOI] [PubMed] [Google Scholar]
- 12.Urbaniak GC, Plous S. Research randomizer (version 3.0) [Computer software] [Retrieved on November 7, 2011]. from http://www.randomizer.org .
- 13.Cook GC. Gastroenterological emergencies in the tropics. Baillieres Clin Gastroenterol. 1991;5:861–886. doi: 10.1016/0950-3528(91)90024-u. [DOI] [PubMed] [Google Scholar]
- 14.Reeder MM. Tropical diseases of the liver and bile ducts. Semin Roentgenol. 1975;10:229–243. doi: 10.1016/0037-198x(75)90066-8. [DOI] [PubMed] [Google Scholar]
- 15.Sepulveda B, Manzo NTG. Clinical manifestations and diagnosis of amebiasis. In: Martinez-Palomo A, editor. Amebiasis: Human Parasitic Diseases. No. 2. Amsterdam: Elsevier; 1986. pp. 169–187. [Google Scholar]
- 16.Hughes MA, Petri WA., Jr Amebic liver abscess. Infect Dis Clin North Am. 2000;14:565–582. doi: 10.1016/s0891-5520(05)70121-5. viii. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CT, Lin DY, Wu CS, Chang-Chien CS, Sheen IS, Liaw YF. A clinical study on pyogenic liver abscess. Taiwan Yi Xue Hui Za Zhi. 1987;86:405–412. [PubMed] [Google Scholar]
- 18.Barnes PF, De Cock KM, Reynolds TN, Ralls PW. A comparison of amebic and pyogenic abscess of the liver. Medicine (Baltimore) 1987;66:472–483. doi: 10.1097/00005792-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hoffner RJ, Kilaghbian T, Esekogwu VI, Henderson SO. Common presentations of amebic liver abscess. Ann Emerg Med. 1999;34:351–355. doi: 10.1016/s0196-0644(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 20.Branum GD, Tyson GS, Branum MA, Meyers WC. Hepatic abscess. Changes in etiology, diagnosis, and management. Ann Surg. 1990;212:655–662. doi: 10.1097/00000658-199012000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma MP, Kumar A. Liver abscess in children. Indian J Pediatr. 2006;73:813–817. doi: 10.1007/BF02790392. [DOI] [PubMed] [Google Scholar]
- 22.Khan R, Hamid S, Abid S, et al. Predictive factors for early aspiration in liver abscess. World J Gastroenterol. 2008;14:2089–2093. doi: 10.3748/wjg.14.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson M, Healy GR, Shabot JM. Serologic testing for amoebiasis. Gastroenterology. 1980;78:136–141. [PubMed] [Google Scholar]
- 24.Alvarez Pérez JA, González JJ, Baldonedo RF, et al. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181:177–186. doi: 10.1016/s0002-9610(00)00564-x. [DOI] [PubMed] [Google Scholar]
- 25.Chou FF, Sheen-Chen SM, Chen YS, Chen MC. Single and multiple pyogenic liver abscesses: clinical course, etiology, and results of treatment. World J Surg. 1997;21:384–388. doi: 10.1007/pl00012258. discussion 388-389. [DOI] [PubMed] [Google Scholar]
- 26.Wong W-M, Wong BCY, Hui CK, et al. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol. 2002;17:1001–1007. doi: 10.1046/j.1440-1746.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26:1434–1438. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 28.Donovan AJ, Yellin AE, Ralls PW. Hepatic abscess. World J Surg. 1991;15:162–169. doi: 10.1007/BF01659049. [DOI] [PubMed] [Google Scholar]
- 29.Stain SC, Yellin AE, Donovan AJ, Brien HW. Pyogenic liver abscess. Modern treatment. Arch Surg. 1991;126:991–996. doi: 10.1001/archsurg.1991.01410320077010. [DOI] [PubMed] [Google Scholar]
- 30.Do H, Lambiase RE, Deyoe L, Cronan JJ, Dorfman GS. Percutaneous drainage of hepatic abscesses: comparison of results in abscesses with and without intrahepatic biliary communication. AJR. 1991;157:1209–1212. doi: 10.2214/ajr.157.6.1719787. [DOI] [PubMed] [Google Scholar]
- 31.Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223:600–607. doi: 10.1097/00000658-199605000-00016. discussion 607-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajak CL, Gupta S, Jain S, Chawla Y, Gulati M, Suri S. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR. 1998;170:1035–1039. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- 33.Saraswat VA, Agarwal DK, Baijal SS, et al. Percutaneous catheter drainage of amoebic liver abscess. Clin Radiol. 1992;45:187–189. doi: 10.1016/s0009-9260(05)80639-7. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal DK, Baijal SS, Roy S, Mittal BR, Gupta R, Choudhuri G. Percutaneous catheter drainage of amebic liver abscesses with and without intrahepatic biliary communication: a comparative study. Eur J Radiol. 1995;20:61–64. doi: 10.1016/0720-048x(95)00603-n. [DOI] [PubMed] [Google Scholar]
- 35.Dietrick RB. Experience with liver abscess. Am J Surg. 1984;147:288–291. doi: 10.1016/0002-9610(84)90109-0. [DOI] [PubMed] [Google Scholar]
- 36.Gupta SS, Singh O, Sabharwal G, Hastir A. Catheter drainage versus needle aspiration in management of large (>10 cm diameter) amoebic liver abscesses. ANZ J Surg. 2011;81:547–551. doi: 10.1111/j.1445-2197.2010.05494.x. [DOI] [PubMed] [Google Scholar]
- 37.Bertel CK, van Heerden JA, Sheedy PF., 2nd Treatment of pyogenic hepatic abscesses. Surgical vs percutaneous drainage. Arch Surg. 1986;121:554–558. doi: 10.1001/archsurg.1986.01400050072009. [DOI] [PubMed] [Google Scholar]
- 38.Van Sonnenberg E, Ferrucci JT, Jr, Mueller PR, Wittenberg J, Simeone JF. Percutaneous drainage of abscesses and fluid collections: technique, results, and applications. Radiology. 1982;142:1–10. doi: 10.1148/radiology.142.1.7053517. [DOI] [PubMed] [Google Scholar]
- 39.Yu SCH, Ho SSM, Lau WY, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932–938. doi: 10.1002/hep.20133. [DOI] [PubMed] [Google Scholar]


