Summary
Commensal flora plays an important role in the development of the mucosal immune system and in maintaining intestinal homeostasis. However, the mechanisms involved in regulation of host-microbiota interaction are still not completely understood. In this study, we examined how microbiota and intestinal inflammatory conditions regulate host microRNA expression and observed lower microRNA-107 (miR-107) expression in the inflamed intestines of colitic mice, compared with that in normal control mice. miR-107 was predominantly reduced in epithelial cells and CD11c+ myeloid cells including dendritic cells and macrophages in the inflamed intestines. We demonstrate that IL-6, IFN-γ and TNF-α downregulated, whereas TGF-β promoted, miR-107 expression. In addition, miR-107 expression was higher in the intestines of germ-free mice than in mice housed under specific pathogen-free conditions, and the presence of microbiota downregulated miR-107 expression in DCs and macrophages in a MyD88- and NF-κB-dependent manner. We determined that the ectopic expression of miR-107 specifically repressed the expression of IL-23p19, a key molecule in innate immune responses to commensal bacteria. We concluded that regulation of miR-107 by intestinal microbiota and pro-inflammatory cytokine serve as an important pathway for maintaining intestinal homeostasis.
Keywords: IL-23p19, Inflammatory bowel disease (IBD), microRNAs, miR-107, Toll-like Receptor (TLR), colitis, DC, Microbiota
Introduction
The intestines represent a complex ecosystem composed of a massive and diverse microbiota, with the most biodiversity in the large intestine and colon [1]. The gut microbiota is both physiologically and pathologically important to intestinal homeostasis and the pathogenesis of inflammatory bowel diseases (IBD), among other diseases. The intestinal microbiota benefits the host by stimulating intestinal epithelium renewal [2], breaking down indigestible food, regulating host fat storage [3], and instructing the development of the immune system [4]. However, perturbation of the microbiota has also been shown to be involved in a variety of diseases, by extensively modulating the expression of host genes involved in fundamental physiological functions [5, 6]. The most compelling evidence for a role of microbiota in the pathogenesis of IBD is provided by the observation that there is an absence of immune activation and colitis in the absence of luminal bacteria in different animal models. Genetically susceptible animals raised in a germ-free environment failed to develop colitis, yet littermates colonized with normal enteric bacteria exhibited macrophage and T-cell activation, leading to the onset of chronic colitis [7, 8]. However, it is still unclear as to how the interaction between host and commensal is regulated.
microRNAs (miRNAs) are a small, non-coding RNA species of 19–25 nucleotides involved in biological processes at multiple levels [9]. microRNAs regulate the expression of genes essential for cell differentiation and function through mRNA degradation or translational inhibition by binding with the “seed sequence” located in the 3′UTR of target genes. miRNAs are highly conserved among species and play critical roles in regulation of immune responses and the pathogenesis of human disease, including IBD [10–14]. The intestinal microbiota has been shown to be able to positively and negatively regulate host miRNA expression [15, 16]. In germ-free (GF) mice that are colonized with microbiota from specific pathogen-free (SPF) mice, the expression of certain miRNAs, including miR-128, miR-298 and miR-342-5p is upregulated, whereas the expression of miR-465c-5p, miR-466d-3p, miR-665 and miR-683, is downregulated [15]. Treatment with microbiota downregulates dendritic cell (DC) miR-10a expression, which targets IL-12/IL-23p40 [16]. It has been shown recently that LPS downregulates macrophage expression of miR-107 [17], which targets CDK6, Dicer1 and HIF-1α [17–19], molecules important in cell cycle, cellular adhesion, miRNA expression and T-cell differentiation. In the current study, we demonstrate that microbiota, as well as pro-inflammatory cytokines, downregulates CD11c+ myeloid cell expression of miR-107. The intestinal expression level of miR-107 in colitic mice was lower than that of normal mice. Microbiota downregulated intestinal miR-107 expression, in that GF mice expressed higher level of miR-107 in the intestines than intestines from SPF mice. Furthermore, when mouse bone marrow-derived dendritic cells (BMDCs) and bone marrow-derived macrophages (BMMs) were stimulated with commensal bacteria and their TLR ligands, as well as pro-inflammatory cytokines, miR-107 was significantly downregulated. MyD88 was involved in microbiota downregulation of miR-107 expression in a NFκB dependent manner. Further studies showed that IL-23p19, a molecule important in IBD, was a target gene of miR-107.
Results
Inflamed intestines of mice with colitis have lower levels of miR-107 expression
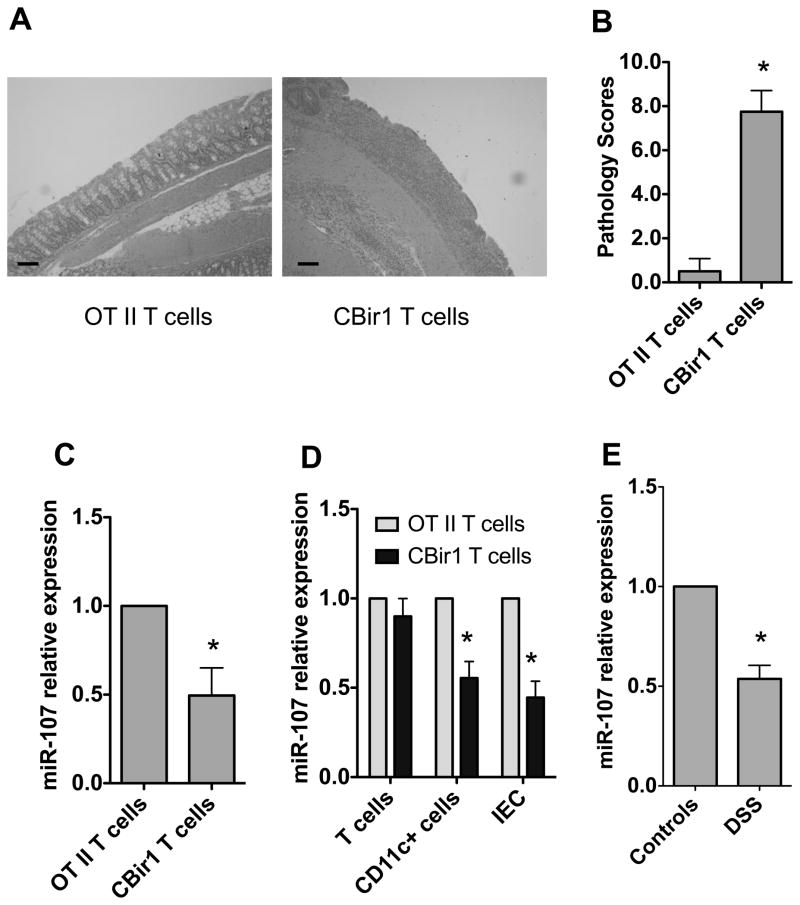
We have previously established that adoptive transfer of CD4+ T cells from CBir1 transgenic (Tg) mice, which are specific for an immunodominant microbiota antigen CBir1 flagellin in the intestinal lumen, but not from OT II mice, which are specific for a model antigen ovalbumin (OVA), induced colitis in RAG−/− mice [20, 21]. To determine whether miR-107 was differential expressed in colitic mice, we transferred CBir1 Tg CD4+ T cells into RAG−/− mice. We also transferred OT II CD4+ T cells as negative controls. As described previously [20], the RAG−/− recipient mice of CBir1 T cells but not OT II T cells developed severe colitis 6 weeks later (Fig. 1A and 1B). RNA was isolated from the inflamed intestines from the colitic mice which received CBir1 T cells, as well as in control RAG−/− mice which received OT II CD4+ T cells, and their miR-107 expression was determined by real-time PCR. Intestinal miR-107 expression was decreased in colitic CBir1 T cells-reconstituted RAG−/− mice compared with that in control RAG−/− mice which received OT II CD4+ T cells (Fig. 1C).
FIGURE 1. Decreased miR-107 expression in the inflamed intestine of mice with colitis.
1×106 CD4+ T cells from CBir1 Tg mice or OT II mice were transferred into groups of 4 RAG−/− mice. Six weeks after transfer, the mice were sacrificed and the severity of intestinal inflammation was assessed by histological analysis and miR-107 expression was measured by real-time PCR. (A) Colonic histopathology of the recipient mice after HE staining is shown (bars, 100 μm). (B) Pathological scores are analyzed as previously described [22]. Data are shown as mean ± SD of eight mice pooled from two experiments *p<0.05, unpaired, two tailed Student’s t test. (C) RNA was isolated from the intestines of the recipient mice, and miR-107 expression in individual mice was determined by real-time PCR. Gapdh expression was used as a control. The miR-107 expression of RAG−/− mice receiving OT II T cells was arbitrarily set to 1.0, and used as the basis for calculating the relative changes in miR-107 expression. Data are shown as mean ± SD of four mice from one of two experiments performed. *p<0.05, unpaired, two tailed Student’s t test. (D) IEC, CD11C+ myeloid cells and T cells were isolated from the intestines of recipient mice and miR-107 expression was analyzed by real-time PCR. Gapdh was used as a house-keeping gene control. Data are shown as mean ± SD of four samples from one of two experiments performed. *p<0.05 compared with RAG−/− mice receiving OT II T cells, unpaired, two tailed Student’s t test. (E). Four C57BL/6 mice were fed with 2% DSS for 7 days and then water for 3 days. Control mice were fed with water only. miR-107 expression in the intestines was determined 10 days later by real-time PCR. Data are shown as mean ± SD of four samples from one of two experiments performed. *p<0.05, compared with control C57BL/6 mice, unpaired, two tailed Student’s t test.
We then investigated which types of cells are sensitive to the reduction of miR-107 in the inflamed intestines. We isolated intestinal epithelial cells (IEC), lamina propria CD11c+ myeloid cells, and T cells, and analyzed their miR-107 expression. As shown in Figure 1D, miR-107 expression was decreased in CD11c+ cells and IECs in colitic mice compared with that in normal mice, whereas T cell expression of miR-107 was unaffected.
To investigate whether miR-107 expression was also decreased in other models of experimental colitis, we induced colitis in C57BL/6 mice by feeding them with DSS as previously described [22]. Consistently, miR-107 expression was decreased in the intestines of the colitic C57BL/6 mice which were fed with DSS compared with that in control mice (Fig. 1E).
IFN-γ, IL-6 and TNF-α downregulate DC and macrophage miR-107 expression while TGF-β promotes it
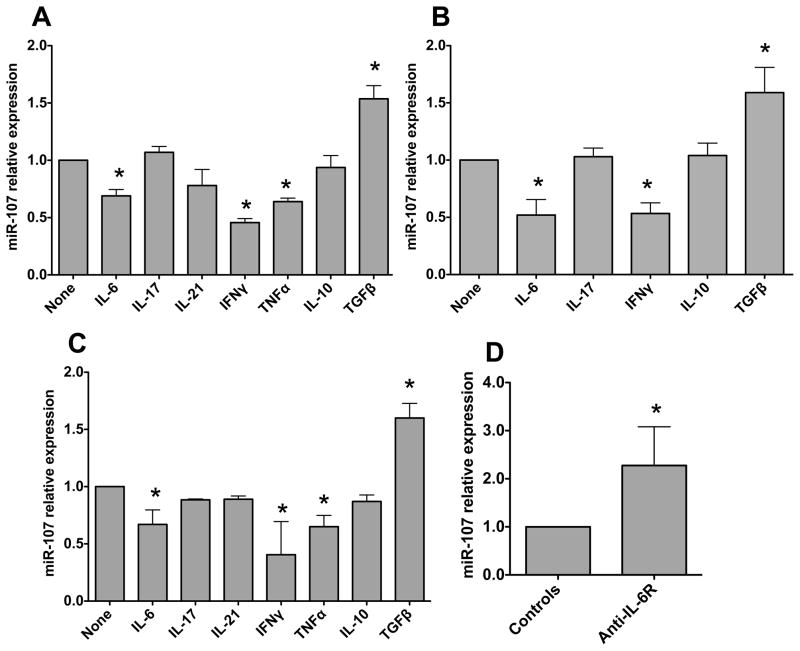
Among many factors that could contribute to the decreased miR-107 expression in the inflamed intestines of colitis mice, pro-inflammatory cytokines are increased in the inflamed tissues, and there is also an increased microbiota translocation across the intestinal epithelium in colitic mice. We then reasoned that microbiota and pro-inflammatory cytokines could regulate intestinal miR-107 expression. To determine whether pro-cytokines downregulate DC miR-107 expression, bone marrow-derived DC (BMDC) were generated from C57BL/6 mice and stimulated with IFNγ, IL-17, IL-21, IL-6 and TNF-α. miR-107 expression was determined 24 h later. IFN-γ, IL-6 and TNF-α inhibited miR-107 expression at different levels, whereas IL-17 and IL-21 did not affect miR-107 expression (Fig. 2A). We also treated BMDC with anti-inflammatory cytokines IL-10 and TGF-β, which inhibit intestinal inflammation. Interestingly, while IL-10 had no effect on miR-107 expression, TGF-β promoted miR-107 expression (Fig. 2A). To determine whether the cytokines have the similar effects on human DC miR-107 expression, we generated human monocyte-derived DC and treated them with various cytokines. miR-107 expression was measured 24 h later. Among cytokines tested, IL-6 and IFN-γ inhibited, whereas TGF-β promoted, human DC miR-107 expression. IL-10 and IL-17 had not effects (Fig. 2B). As most intestinal macrophages also express CD11c, especially during inflammation, we then investigated how cytokines regulate macrophage expression of miR-107. We generated BMMs by culturing mouse bone marrow cells with M-CSF and stimulated the BMMs with various cytokines. Similar to the effects on DC, IFN-γ, IL-6 and TNF-α inhibited, whereas TGF-β enhanced, BMM miR-107 expression. In contrast, IL-10, IL-17 and IL-21 did not affect BMM miR-107 expression (Fig. 2C). Collectively, these data indicated that pro-inflammatory cytokines downregulate, whereas TGFβ up-regulates, DC and macrophage miR-107 expression. To determine whether inhibition of pro-inflammatory signaling promotes intestinal miR-107 expression in colitic mice, we treated RAG−/− mice that received CBir1 T cells with anti-IL-6R or control antibodies from the day of cell transfer, as we have shown that treatment with anti-IL-6R inhibited intestinal inflammation and decreased local pro-inflammatory cytokine production [20]. As shown in Figure 2D, treatment with anti-IL-6R antibody enhanced intestinal miR-107 expression.
FIGURE 2. Cytokines differentially regulate DC and macrophage miR-107 expression.
(A) BMDCs were generated from C57BL/6 mice and treated with the indicated cytokines for 24 h. miR-107 expression was then analyzed by quantitative PCR, and Gapdh was used as a house-keeping gene control. miR-107 expression in BMDCs was considered baseline and arbitrarily set to 1.0, and the relative changes were calculated based on the baseline expression. (B) Human monocyte-derived DCs (MoDC) were generated from PBMCs and treated with the indicated cytokines for 24 h. miR-107 expression was analyzed by quantitative PCR with Gapdh as a house-keeping gene control. (C) BMMs were generated from C57BL/6 mice and treated with the indicated cytokines for 24 h. miR-107 expression was analyzed by quantitative PCR with Gapdh as a house-keeping gene control. Data are shown as mean ± SD of two samples from one of two experiments performed. *p<0.05 compared with controls, unpaired, two tailed Student’s t test. (D) CD4+ T cells from CBir1 Tg mice were transferred into groups of 4 RAG−/− mice and treated with anti-IL-6R antibody or control antibody from the day of cell transfer. Six weeks after transfer, the mice were sacrificed and intestinal RNA was isolated. miR-107 expression in individual mouse was determined by real-time PCR. Gapdh was used as a control. Data are shown as mean ± SD of four samples from one of two experiments performed. *p<0.05, compared with controls, unpaired, two tailed Student’s t test.
Commensal bacteria downregulate miR-107 expression through the interaction of TLR-TLR ligands
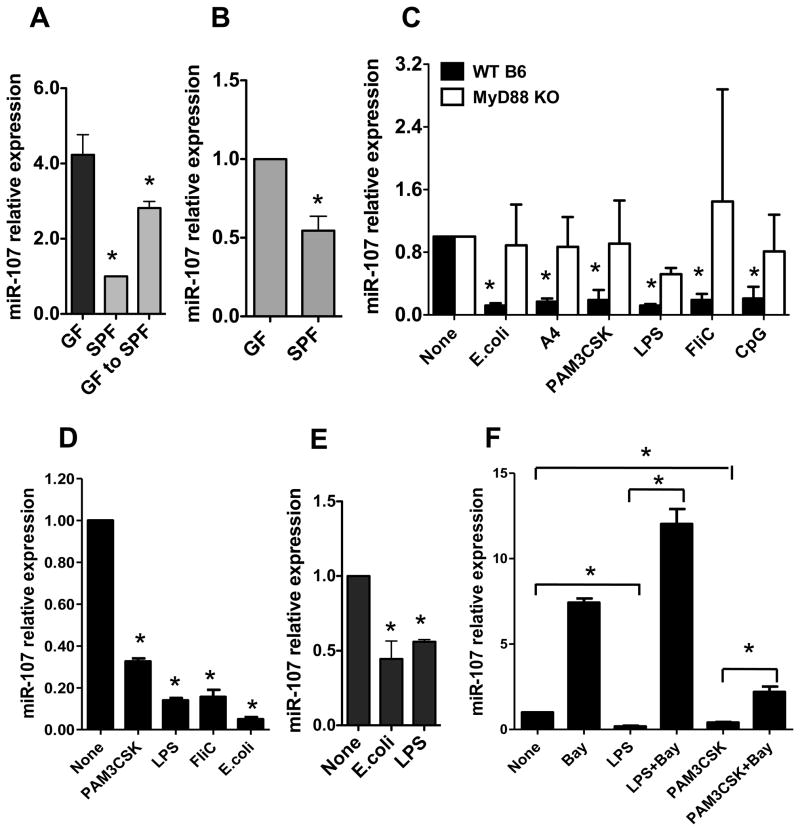
It has been shown that LPS downregulated macrophage expression of miR-107 [17]. To determine whether commensal bacterial stimulation inhibited miR-107 expression, we first compared intestinal miR-107 expression between GF mice and SPF mice. The intestinal miR-107 expression was significantly higher in GF mice than that in SPF mice (Fig. 3A). Recolonization of GF mice with normal flora led to a decreased intestinal miR-107 expression. Further, intestinal CD11c+ myeloid cell expression of miR-107 was decreased in SPF mice compared with that in GF mice (Fig. 3B), indicating that microbiota downregulates CD11c+ myeloid cell expression of miR-107.
FIGURE 3. Commensal bacteria downregulate miR-107.
RNA was isolated from (A) the intestines or (B) intestinal CD11c+ cells of C57BL/6 mice housed under SPF and GF conditions, or GF C57BL/6 mice that had been recolonized with normal flora by housing under SPF conditions for 1 month. miR-107 expression was analyzed by real-time PCR. miR-107 expression in SPF mice was considered baseline and arbitrarily set to 1.0, and the relative changes were analyzed based on the baseline expression. Data are shown as mean ± SD of two samples from one of two experiments performed. *p<0.05 compared with GF mice, unpaired, two tailed Student’s t test. (C) BMDCs were generated from WT or MyD88 KO mice and treated with commensal E. coli, flagellated A4 bacteria or various TLR ligands for 24 h. miR-107 expression was analyzed by real-time PCR. The miR-107 expression in BMDCs was considered baseline and arbitrarily set to 1.0, and the relative changes were analyzed compared with the baseline expression. Data are shown as mean ± SD of two samples from one of three experiments performed. (D) Human monocyte-derived DCs were stimulated with TLR ligands and miR-107 expression was analyzed by real-time PCR. Data are shown as mean ± SD of two samples from one of three experiments performed. (E) BMMs were stimulated with E. coli or LPS, and miR-107 expression was analyzed by real-time PCR. Data are shown as mean ± SD of two samples from one of two experiments performed. (F) BMDCs were treated with TLR ligands with or without NF-κB inhibitor (Bay 11–7082) for 24 h. miR-107 expression was analyzed by quantitative PCR. Data are shown as mean ± SD of two samples from one of three experiments performed. (C–F) *p<0.05 compared with controls, unpaired, two tailed Student’s t test.
To further determine the effects of microbiota on DC expression of miR-107, we stimulated BMDC with lysates of E. coli isolated from the intestinal lumen of the mice and flagellated A4 commensal bacteria which produce immunodominant commensal antigen CBir1 flagellin [23]. BMDC miR-107 expression was downregulated by stimulation with E. coli and A4 bacteria (Fig. 3C).
It has been shown that commensal bacteria express pathogen-associated molecular patterns (PAMPs) which bind pattern recognition receptors (PRR), such as Toll-like receptors (TLRs), which are expressed on host cells and are crucial to the host immune response against microbiota [24, 25]. To investigate whether commensal bacteria regulate miR-107 expression through interaction of TLR-TLR ligand, BMDCs were stimulated with TLR ligands PAM3 CSK (for TLR1/2), LPS (TLR4), FliC (TLR5) and CpG ODN (TLR9). As shown in Figure 3C, miR-107 expression was downregulated by TLR ligands. Collectively, these data demonstrated that microbiota-derived TLR ligands inhibited miR-107 expression in DC.
To determine whether microbiota and their TLR ligands affect human DC miR-107 expression, we treated human monocyte-derived DC with commensal bacteria and various TLR ligands. As shown in Figure 3D, human DC miR-107 expression was inhibited by treatment with commensal E. coli and TLR ligands. In addition to LPS, commensal E. coli and A4 flagellated bacteria also inhibited bone-marrow derived macrophage miR-107 expression (Fig. 3E).
MyD88 is a conserved adaptor molecule that mediates TLR-TLR ligand interaction except TLR3. TLRs can act through MyD88-dependent or independent signaling pathways [26]. To determine if microbiota and their TLR ligands inhibit miR-107 expression through MyD88, we generated BMDCs from wild-type and MyD88 KO mice and treated them with various TLR ligands. As shown in Figure 3C, downregulation of miR-107 by TLR ligands was significantly impaired in MyD88−/− BMDC. As a master transcription factor, NF-κB is involved in many signaling pathways, including MyD88, by regulating target genes. In order to determine whether NF-κB is involved in TLR ligand-downregulation of miR-107, BMDCs were treated with LPS and PAM3CSK in the presence or absence of NF-κB inhibitor, Bay 11–7082. Downregulation of miR-107 by LPS and PAM3CSK was abrogated by addition of NF-κB inhibitor, which notably, also increased the baseline expression of miR-107 (Fig. 3F). Collectively, these data demonstrated that commensal bacteria negatively regulated DC and macrophage miR-107 expression through interaction of TLR-TLR ligands in a MyD88- and NF-κB-dependent manner.
4. miR-107 inhibits DC IL-23p19 expression stimulated by TLR ligands
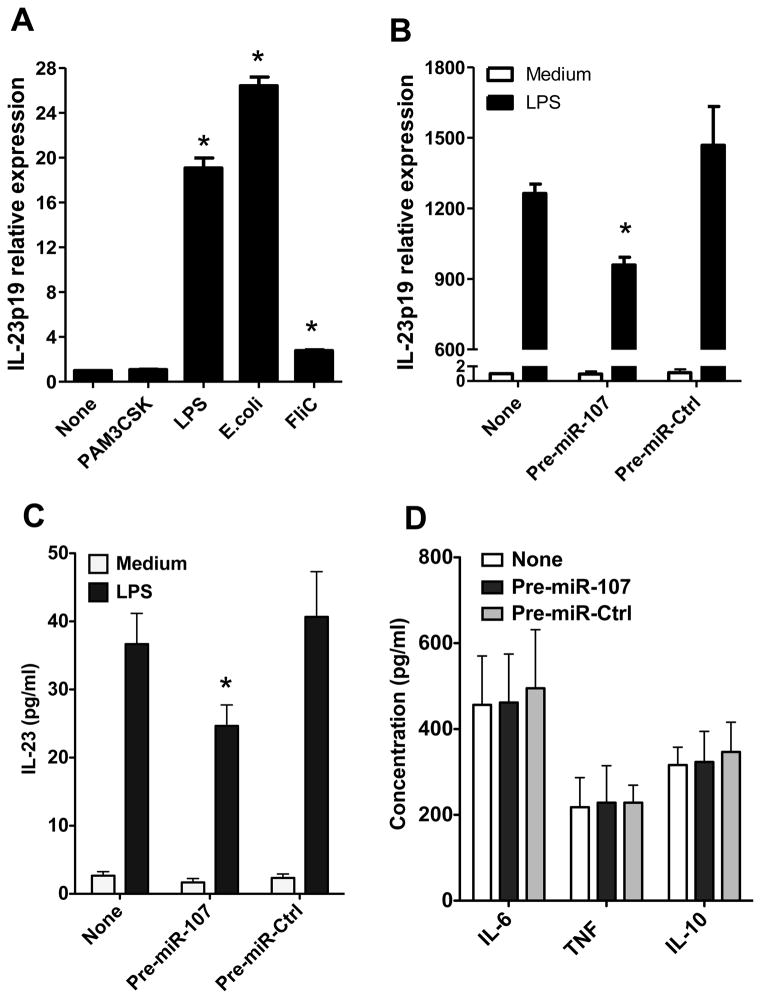
miRNAs repress target gene transcription or translation by binding with the seed sequence located in the 3′-UTR of these genes [27]. To determine the potential target genes of miR-107, miRNA target prediction algorithms were used to predict candidate target genes of miR-107. IL-23p19, an important gene in intestinal immune responses, was predicted as the candidate target gene. To determine the role of miR-107 in DC expression of IL-23p19 in response to the stimulation of TLR ligands, we treated BMDCs with commensal bacteria and various TLR ligands. Commensal E. coli, PAM3CSK, LPS and FliC upregulated IL-23p19 expression (Fig. 4A). We then investigated whether TLR ligands stimulated IL-23p19 through downregulation of miR-107. miR-107 precursor was used to transfect BMDCs to obtain ectopic expression of miR-107. A scrambled miRNA precursor was used as negative control. Four h later, BMDCs were treated with LPS, and the expression of IL-23p19 was analyzed 24 h later. IL-23 production was also measured by ELISA in the culture supernatants. LPS stimulated IL-23p19 expression and IL-23 production, but this effect was repressed by ectopic overexpression of miR-107 (Fig. 4B and 4C). However, ectopic overexpression of miR-107 did not affect production of IL-6, TNF-α and IL-10 (Fig. 4D). These data indicated that TLR ligand stimulation of DC expression of IL-23p19, but not IL-6, TNF-α and IL-10, was mediated by the downregulation of miR-107.
FIGURE 4. miR-107 inhibits IL-23p19 and IL-23 production in BMDCs.
(A) BMDCs were treated with the indicated TLR ligands for 24 h and expression of IL-23p19 was analyzed by quantitative PCR. Data are shown as mean ± SD of two samples from one of three experiments performed. *p<0.01 compared with control BMDCs, unpaired, two tailed Student’s t test. (B–D) BMDCs were transfected with miR-107 precursor (pre-miR-107) and cultured with or without LPS stimulation for 24 h. A scrambled microRNA precursor (pre-miR-Ctrl) was used as negative control. (B) Expression of IL-23p19 was analyzed by quantitative PCR. (C, D) Production of (C) IL-23 and (D) other cytokines in supernatants following 24 h culture was determined by ELISA. (B–D) Data are shown as mean ± SD of two samples from one of three experiments performed. *p<0.05 compared with control BMDCs, unpaired, two tailed Student’s t test.
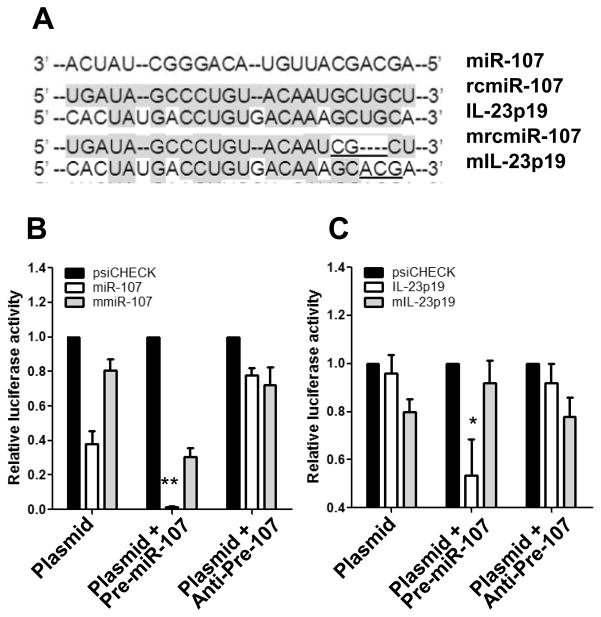
To confirm that miR-107 is directly targeting 3′-UTR of IL-23p19, dual-luciferase reporter vectors were constructed containing the predicted seed sequences in the 3′-UTR of IL-23p19 (Fig 5A). The empty vector psiCHECK-2 and the vector containing reverse complementary miR-107 (rcmiR-107) sequence were used as negative and positive controls, respectively. These vectors were used to transfect the murine RAW264.7 macrophage cell line alone or co-transfect with miR-107 precursor or inhibitor. Luciferase activity was then assessed 24 h later, and the Renilla luciferase (Rluc) was normalized to firefly luciferase (Fluc). As shown in Figures 5B and 5C, co-transfection of the miR-107 precursor significantly repressed, whereas co-transfection of inhibitor up-regulated, the activity of Rluc containing the seed sequences in the 3′-UTR of IL-23p19. To establish whether the miR-107 interaction to IL-23p19 is direct, we abrogated the miR-107-binding sites by introducing mutations/deletions into the seed sequence. Neither precursor nor inhibitor had effects on the activity of Renilla luciferase containing the mutant sequences (Fig. 5B and 5C). Collectively, these data demonstrated that miR-107 specifically downregulated IL-23p19 expression.
FIGURE 5. IL-23p19 is a target gene of miR-107.
(A) Sequence alignment of miR-107 with reverse complementary miR-107 (rcmiR-107), IL-23p19, mutant rcmiR-107 (mrcmiR-107), and mutant IL-23p19 (mIL-23p19). Mutant nucleotides are underlined. (B, C) Dual-luciferase reporter assays using vectors constructed with (B) rcmiR-107/mrcmiR-107 and (C) IL-23p19-3′-UTR/mIL-23p19-3′-UTR alone, or in the presence of miR-107 precursor or inhibitor were performed. Vectors constructed with miR-107 or mmiR-107 was used as a positive control. Decreases in Renilla luciferase (RLuc) were measured and normalized to Firefly luciferase (FLuc) activity. The recombinant vector was normalized to an empty psiCHECK-2 vector. (B, C) The relative luciferase activity is shown as mean ± SD of two samples from one of three experiments performed. *p<0.05; **p<0.01 compared with plasmid alone group, unpaired, two tailed Student’s t test.
5. Inflamed intestinal tissues in mice with colitis express high levels of IL-23p19
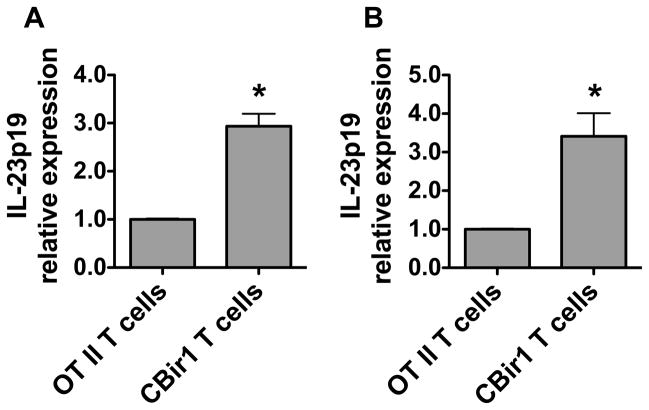
Because miR-107 expression was decreased in colitic RAG−/− mice which received CBir1 Tg T cells compared to control RAG−/− mice which received OT II T cells and did not develop severe colitis (Fig. 1), we then investigated whether IL-23p19 expression and IL-23 production were differentially expressed in colitic mice. We assessed the intestinal CD11c+ myeloid cell expression of IL-23p19 and IL-23 production. CD11c+ myeloid cells of inflamed intestines expressed higher levels of IL-23p19 and produced more IL-23 compared with those of control mice (Fig. 6A and 6B).
FIGURE 6. Expression of IL-23p19 and production of IL-23 by intestinal CD11c+ myeloid cell are increased in colitic mice.
1×106 CD4+ T cells from CBir1 Tg mice or OT II mice were transferred into groups of 4 RAG−/− mice for 6 weeks. (A) RNA was isolated from the intestinal CD11c+ myeloid cells and expression of IL-23p19 was determined in individual mice by quantitative PCR. (B) Intestinal CD11c+ myeloid cells were stimulated with LPS for 24 h and IL-23 production in the supernatants was determined by ELISA. Data are shown as mean ± SD of four samples from one of three experiments performed. *p<0.05 compared with control RAG mice that received OT II T cells, unpaired, two tailed Student’s t test.
Discussion
There is an extensive dialogue between host and commensal microbiota. Microbiota not only contribute to the maintenance of intestinal homeostasis, but also regulate intestinal inflammation, as colitis can be induced in mice housed under conventional conditions, but not under GF conditions [28, 29]. In particular, the endogenous commensal microbiota modulate the gene expression profiles in the host immune system [30, 31]. However, the impact of the endogenous microbiota on host gene expression signature may have been underestimated due to a lack of studies linking microbiota to epigenetic changes in gene expression, in particular, via miRNAs. In the present study, we found that microbiota and pro-inflammatory cytokines downregulated intestinal CD11c+ myeloid cell miR-107 expression. Further, microbiota downregulation of miR-107 was mediated by TLR–TLR ligand interaction in a MyD88- and NFκB-dependent manner. The decrease in CD11c+ myeloid cell miR-107 expression led to an increase in the expression of IL-23p19 and production of IL-23, suggesting that microbiota regulation of miR-107 expression could contribute to the host immune response to microbiota.
miR-107 has been implicated in the regulation of progression and metastasis of several types of cancers [32–33]. A recent study showed that breast cancer patients with higher miR-107 levels displayed a significantly lower probability of metastasis-free survival [34]. Our data indicated that CD11c+ myeloid cell expression of miR-107 was downregulated in inflamed intestinal tissues, it thus could play a role in the regulation of host response to a sizeable microbiota challenge. Use of the dual-luciferase reporter assay showed that one of the target genes of miR-107 was IL-23p19, a subunit of IL-23, which is produced by DCs and macrophages upon microbiota stimulation and has emerged as a key molecule in the regulation of both innate and adaptive immune responses [35]. Ectopic expression of miR-107 inhibited TLR ligand-stimulated IL-23p19 expression and IL-23 production but did not affect production of IL-6, TNF-α and IL-10, indicating that miR-107 specifically targets IL-23p19. Interestingly, we have previously demonstrated that IL-23p40, the other subunit of IL-23, was the target gene of miR-10a, which was predominantly expressed in intestines and downregulated by microbiota [16]. Thus, miR-10a and miR-107 could coordinately mediate microbiota stimulation of host innate responses to negatively regulate IL-23. In additional to its well-appreciated role in the pathogenesis of IBD, IL-23 has also been implicated as a crucial molecule in the maintenance of intestinal homeostasis [36, 37]. Together with IL-6 and TGF-β, IL-23 promotes the development and expansion of Th17 cells [35]. Our recent data demonstrated that Th17 cells stimulate intestinal IgA production through the induction of intestinal epithelial cell expression of pIgR, thus contribute to intestinal homeostasis to microbiota [22]. IL-23 also stimulates ILC cells to produce IL-17 and IL-22, which regulate host responses to intestinal infection, such as citrobacter [38, 39]. Under certain conditions, however, IL-23 has also been implicated in the pathogenesis of colitis. Knockout mice deficient in IL-23 pathways develop mild symptoms of colitis compared with WT mice, highlighting the importance of IL-23 in inflammatory pathways [36, 40]. Our data showed that miR-107 expression was decreased, whereas IL-23 production was increased in the intestines of colitic mice, compared with that in normal mice, demonstrating a possible relationship between miR-107 regulation IL-23 and development of colitis. Thus, microbiota and pro-inflammatory cytokine downregulation of miR-107, allowing for IL-23 production, could set up a basic inflammatory environment and contribute to progression of chronic intestinal inflammation.
Multiple mechanisms, including high levels of pro-inflammatory cytokines, contribute to the progression and persistence of intestinal inflammation in colitis. Our current data demonstrated that pro-inflammatory cytokines, including IL-6, IFN-γ and TNF-α, downregulated intestinal CD11c+ myeloid cell miR-107 expression, which leads to increased IL-23 production by relieving suppression of IL-23p19 expression by miR-107, thus provides a novel pathway for high levels of IL-23 in the inflamed intestinal tissues, which could contribute to the progression and persistence of intestinal inflammation. We are actively investigating the mechanisms involved in pro-inflammatory cytokine regulation of miR-107 expression, and how much this pathway contributes to the maintenance of colitis.
In summary, our data demonstrated that microbiota and pro-inflammatory cytokines regulate DC and macrophage expression of IL-23p19 by inhibiting miR-107. Through regulation of the innate cell expression of IL-23p19 in response to microbiota stimulation, miR-107 could contribute to the regulation of the host response to microbiota, it thus could be an important mediator in the maintenance of host immune homeostasis, as well as in the pathogenesis of IBD. Manipulation of miR-107 expression could thus provide a new avenue of therapeutics for IBD.
Materials and Methods
Mice
C57BL/6 (C57BL/6) mice and C57BL/6.RAG−/− mice were purchased from The Jackson Laboratory and housed in the animal facilities of the University of Texas Medical Branch (UTMB). C57BL/6.CBir1 Tg mice [41] were housed in the animal facilities of UTMB. As described previously [16], GF C57BL/6 mice were derived by hysterectomy and maintained in Trexler-type isolators, according to standard gnotobiotic techniques. All mice were used at 6–8 weeks of age, and the procedures involving animals were approved by the Animal Care and Use Committees of UTMB and University of Alabama at Birmingham.
Reagents and Materials
RPMI 1640, HEPES, penicillin/streptomycin, β-Mercaptoethanol (β-ME), sodium pyruvate and L-glutamine were purchased from Life Technologies (Carlsbad, CA). GM-CSF, anti-CD11c, anti-CD4, and anti-B220 were from BD Biosciences (San Diego, CA). Restriction endonucleases and T4 DNA ligase were from New England Biolabs (Ipswich, MA). The psiCHECK-2 vector and the dual-luciferase reporter system were from Promega (Madison, WI). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). Collagenase IV was obtained from Sigma-Aldrich (St. Louis, MO). MiR-107 precursors and inhibitors were purchased from Ambion (Carlsbad, CA) and TaqMan® microRNA reverse transcription kit and gene expression assays from Applied Biosystems (Carlsbad, CA). Nucleotides were synthesized by Fisher Scientific (Pittsburgh, PA).
Isolation of commensal E. coli from mouse intestines
As part of a previous study [23], a mouse E. coli strain was isolated. Briefly, mouse cecal contents were used to inoculate LB broth and after 24 hours growth at 37°C the broth was plated at ~300 cpu/100mm plate on LB agar. Colonies were screened by 16S rDNA PCR analysis [42]. The mouse E. coli isolate 16S rDNA was 99% identical to E. coli using NCBI blast tool and the RDP database [43].
Generation of bone marrow-derived DC and macrophage
Bone-marrow cells were isolated as described previously [16]. In brief, bone marrow cells were suspended at 2.5×105/mL in RPMI 1640 media supplemented with 10% heat-inactivated FCS (Atlanta Biologicals, Lawrenceville, GA), 25 mM HEPES, 2 mM sodium pyruvate, 50 mM β-ME, 100 units/mL Penicillin, and 100 μg/mL Streptomycin. For generation of BMDC, BM cells were cultured in the presence of 20 ng/mL of GM-CSF in 6-well plates at 37°C in a CO2 incubator. On day 8, BMDCs were collected and used as described in text. For generation of BMMs, BM cells were cultured in the presence of 20 ng/mL of M-CSF for 10 days.
Preparation of human monocyte-derived DCs
Human monocytes were isolated from PBMCs in compliance with protocol approved by the University of Texas Medical Branch Institutional Review Board as previously described [44]. Briefly, monocytes were purified from PBMC by negative selection using the RoboSep magnetic separation system from StemCell Technologies Inc. (Vancouver, BC, Canada) according to the manufacturer’s instructions. Monocyte-derived DC were generated from the purified CD14+ monocytes by culture in RPMI1640 medium supplemented with 10% FCS, L-glutamine, Hepes, sodium pyruvate, antibiotics, GM-CSF (100 ng/mL), and IL-4 (50 ng/mL). Cytokines were replenished every 3 to 4 days. Non-adherent, immature DCs were obtained at 7 days of culture and were characterized as homogeneous DC populations by high levels of CD11c expression.
Isolation of IECs and lamina propria cells
To isolate intestinal epithelial cells (IECs), the intestines were washed with PBS and cut into small pieces. The latter were then incubated with 5 mM EDTA and 2% FBS in Ca2+- and Mg2+-free Hanks balanced salt solution for 30 min at 37°C with stirring. The liberated cells were collected by passage through a stainless steel sieve. IECs were separated on a 20/75% discontinuous Percoll gradient (Pharmacia). To isolate lamina propria CD11c+ cells and T and B cells, after removal of IECs and intraepithelial lymphocytes, tissues were incubated with RPMI 1640 containing 5% FBS and 0.5 mg/mL collagenase type IV for 30 min at 37°C with stirring. The liberated cells were collected and separated on a 40/75% discontinuous Percoll gradient. The yield was typically about 2×106 lymphocytes/mouse, with 90% cell viability as assessed by FACS analysis with the vital dye. CD11c+ cells and T cells were further isolated by MACS using CD11c beads and CD3 beads purchased from Miltenyi Biotec (Auburn, CA). The cell purity was generally >95% for each cell population.
Real-time PCR
Total RNA was extracted with TriZol reagent and followed by cDNA synthesis with Superscript reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative PCR reactions were performed by using TaqMan® Gene Expression Assays for miR-107, IL-23p19 (Applied biosystems, Union City) on a Bio-Rad iCycler (Bio-Rad, Hercules, CA) and all data were normalized to Gapdh mRNA expression.
Vector construction and luciferase reporter assays
To identify the target genes of miR-107, the reverse complementary sequence of miR-107 (rcmiR-107) was used as a positive control. The dual-luciferase psiCHECK-rcmiR-107 and psiCHECK-IL-23p19 vectors were constructed by synthesizing the theoretical seed sequences located in the 3′-UTR of these genes and cloning the annealing products into the psiCHECK-2 vector (Promega) between Not I (GCGGCCGC) and Xho I (CTCGAG) restriction endonucleases recognition sites. To confirm the direct, specific regulation of these genes by miR-107, the mutant vectors psiCHECK-mrcmiR-107, and psiCHECK-mIL-23p19, which contain three-base pair mutations/deletions in the seed sequences, were constructed as a negative control. All of the vectors were confirmed by enzyme digestion and DNA sequencing.
The murine macrophage RAW264.7 cells were routinely cultured in complete DMEM medium at 37°C and 5% CO2. For luciferase reporter assays, RAW 264.7 macrophages were seeded in 24-well plates to 60–70% confluence and then transiently transfected with 0.8 mg recombinant dual-luciferase vectors alone or vectors plus 30 nM hairpin precursors or inhibitors, by using Lipofectamine 2000 reagent according to the manufacturer’s instructions. Luciferase assays were performed 24 h later by using the Dual-Luciferase reporter system. The renilla and firefly luciferase activity were measured on a Veritas Microplate Luminometer (Promega).
Statistical analysis
All experiments were repeated at least three times, and the data expressed as mean ± SEM and analyzed by Student’s t-test. Differences were considered significant when the p value was less than 0.05.
Acknowledgments
This work was supported by research grants from NIH DK079918, DK098370, John Sealy Memorial Endowment Fund, the National Natural Science Foundation of China (81061120521, 81270470), and the Shanghai Science and Technology Commission (12XD1404000).
Abbreviations
- miR
microRNA
- BMDC
bone marrow-derived DC
- IL
interleukin
- IBD
inflammatory bowel disease
- TLR
Toll-like receptor
- LP
lamina propria
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 10.Biton M, Levin A, Slyper M, Alkalay I, Horwitz E, Mor H, Kredo-Russo S, et al. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat Immunol. 2011;12:239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 11.Paraskevi A, Theodoropoulos G, Papaconstantinou I, Mantzaris G, Nikiteas N, Gazouli M. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Pekow JR, Dougherty U, Mustafi R, Zhu H, Kocherginsky M, Rubin DT, Hanauer, et al. miR-143 and miR-145 are downregulated in ulcerative colitis: putative regulators of inflammation and protooncogenes. Inflamm Bowel Dis. 2011;18:94–100. doi: 10.1002/ibd.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, et al. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. e1624. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 15.Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Ayyadurai S, Sitaraman SV, et al. Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6:e19293. doi: 10.1371/journal.pone.0019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue X, Feng T, Yao S, Wolf KJ, Liu CG, Liu X, Elson CO, et al. Microbiota downregulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. J Immunol. 2011;187:5879–5886. doi: 10.4049/jimmunol.1100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hennessy EJ, Sheedy FJ, Santamaria D, Barbacid M, O’Neill LA. Toll-like receptor-4 (TLR4) downregulates microRNA-107, increasing macrophage adhesion via cyclin-dependent kinase 6. J Biol Chem. 2011;286:25531–25539. doi: 10.1074/jbc.M111.256206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolt KS, Mishra MK, Karar J, Baig MA, Ahmed Z, Pasha MA. cDNA cloning, gene organization and variant specific expression of HIF-1 alpha in high altitude yak (Bos grunniens) Gene. 2007;386:73–80. doi: 10.1016/j.gene.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhang Y, Shi Y, Dong G, Liang J, Han Y, Wang X, et al. MicroRNA-107, an oncogene microRNA that regulates tumour invasion and metastasis by targeting DICER1 in gastric cancer. J Cell Mol Med. 2011;15:1887–1895. doi: 10.1111/j.1582-4934.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duck LW, Walter MR, Novak J, Kelly D, Tomasi M, Cong Y, Elson CO. Isolation of flagellated bacteria implicated in Crohn’s disease. Inflamm Bowel Dis. 2007;13:1191–1201. doi: 10.1002/ibd.20237. [DOI] [PubMed] [Google Scholar]
- 24.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov R. TLR-mediated innate immune recognition. Semin Immunol. 2007;19 :1–2. doi: 10.1016/j.smim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 27.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, et al. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comelli EM, Simmering R, Faure M, Donnicola D, Mansourian R, Rochat F, Corthesy-Theulaz I, et al. Multifaceted transcriptional regulation of the murine intestinal mucus layer by endogenous microbiota. Genomics. 2008;91:70–77. doi: 10.1016/j.ygeno.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury SR, King DE, Willing BP, Band MR, Beever JE, Lane AB, Loor JJ, et al. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genomics. 2007;8:215. doi: 10.1186/1471-2164-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen HY, Lin YM, Chung HC, Lang YD, Lin CJ, Huang J, Wang WC, et al. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012;72:3631–3641. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 33.Inoue T, Iinuma H, Ogawa E, Inaba T, Fukushima R. Clinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancer. Oncol Rep. 2012;27:1759–1764. doi: 10.3892/or.2012.1709. [DOI] [PubMed] [Google Scholar]
- 34.Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 36.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 38.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 44.Kolli D, Bao X, Liu T, Hong C, Wang T, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G inhibits TLR4-dependent signaling in monocyte-derived dendritic cells. J Immunol. 2011;187:47–54. doi: 10.4049/jimmunol.1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]