Abstract
Mice housed in social isolation exhibit a decreased response to γ-aminobutyric acid-mimetic drugs [i.e., pentobarbital (PTB)] associated with a down-regulation of telencephalic allopregnanolone (Allo) levels. In these mice, the PTB-induced loss of righting reflex is greatly reduced. Fluoxetine (FLX) and norfluoxetine (NFLX) stereospecifically reverse the effect of social isolation on the PTB-induced loss of righting reflex and on the decrease of telencephalic Allo content. The S-isomers of FLX and NFLX are 2- and 7-fold more potent, respectively, than their respective R-isomers. The EC50s of FLX and NFLX required to normalize brain Allo content and PTB action are 10–50 times lower than the IC50s required for selective serotonin reuptake inhibitor activity. We conclude that normalization of PTB action elicited by the S-isomers of FLX and NFLX is related to the reversal of the down-regulation of brain Allo content and is independent of selective serotonin reuptake inhibitor activity.
Male mice that have been housed in social isolation (SI) for 15–30 days develop increased anxiety-related behaviors, respond aggressively to an intruder of the same sex (1–3), and display decreased responsiveness to drugs that stimulate γ-aminobutyric acid type A (GABAA) receptors, as shown by the shorter duration of the loss of righting reflex (RRL) induced by pentobarbital (PTB) or muscimol (1, 4).
Several independent lines of evidence suggest that these effects are maintained in part by SI-induced down-regulation of neurosteroid biosynthesis. First, protracted SI decreases the telencephalic levels of allopregnanolone (Allo) (5), a neurosteroid that positively modulates the action of GABA and other GABA receptor ligands (i.e., muscimol or barbiturates) on GABAA receptors (6–9). Thus, reduced levels of Allo could mediate the SI-induced decrease in GABA responsiveness.
Second, administration of SKF 105,111, a potent type I 5α-reductase inhibitor, to nïve mice depletes brain Allo content by 60–80% in ≈1 h and concurrently reduces the duration of PTB- or muscimol-induced RRL (1, 4). In SI or SKF 105,111-treated mice, administration of Allo in doses that per se fail to change gross behavior normalizes the reduced action of muscimol or PTB (1, 4).
Third, electrophysiological studies using the patch-clamp technique to record GABA-evoked Cl- currents from neocortical pyramidal neurons obtained from brain slices of mice in which the concentration of endogenous brain Allo was decreased by ≈80% after the administration of SKF 105,111 showed a reduction in the potency of exogenously applied GABA or muscimol to evoke Cl- currents (10). Furthermore, direct application of Allo to these slices reverted the dose–response curve of GABA toward the control profile of non-SKF 105,111-treated slices (10).
Fourth, the administration of fluoxetine (FLX) to SI mice in doses that normalize brain Allo content reverses the reduced duration of PTB-induced RRL (1). The effect of FLX on brain Allo content is stereospecific; the S-isomers of FLX (S-FLX) and norfluoxetine (NFLX) (S-NFLX) are at least 10 times more potent than their R-isomers (R-FLX and R-NFLX, respectively) (3). Furthermore, this stereospecificity also holds for the reduction of aggressive behaviors in SI mice (3).
These considerations prompted us to study whether the action of FLX and NFLX on the reduced duration of PTB-induced RRL in SI mice is stereospecific and whether this action relates to the down-regulation of Allo biosynthesis. The results suggest that the S-stereoisomers of FLX and NFLX are more potent than their R-stereoisomers in normalizing reduced PTB action and brain Allo content down-regulation in SI mice. These actions were unrelated to FLX or NFLX intrinsic selective serotonin reuptake inhibitor (SSRI) activity (11), which is not stereospecific.
Materials and Methods
Animals and Drug Treatment. Adult male Swiss–Webster mice (Harlan Breeders, Indianapolis), 22- to 25-g body weight, maintained under a 12-h dark/12-h light cycle with food and water available ad libitum, were used for all experiments. Animals were housed either in groups of five to six per cage (24 × 17 × 12 cm) or individually (SI) in a cage of the same size for a time period varying from 4 to 6 weeks preceding our behavioral and biochemical measurements (1, 4). The vivarium temperature was ≈24°C, and the humidity was ≈65%. Group-housed (GH) and SI male mice were subjected to i.p. injections of racemic FLX, R- or S-FLX, R- or S-NFLX, or imipramine. Vehicle or tested drugs were prepared in 1% DMSO solutions and given i.p. as 0.1 ml per 10 g of body weight.
Racemic FLX, R-FLX, S-FLX, R-NFLX, and S-NFLX were a generous gift of Eli Lilly. Imipramine was provided by Sigma. Heptafluorobutyric acid anhydride (HFBA) was purchased from Pierce. Unless otherwise specified, all organic solvents were of HPLC grade and were purchased from Fisher Scientific.
Measurement of PTB-Induced RRL. The duration of the PTB-induced RRL in GH and SI male mice was measured as previously reported (1) after i.p. injections of PTB sodium (50 mg/kg; 0.5 mg/0.1 ml).
Analysis of PTB Brain Content. Extraction and HPLC quantification of PTB were performed as previously reported (1, 12). In brief, cerebral cortex samples (≈200 mg) were homogenized in 10 vol of 0.45 M perchloric acid in 150 mM NaCl containing 20 nmol of secobarbital sodium [5-(methylbutyl)-5-(2-propenyl)-2,4,6 (1H, 3H, 5H) pyrimidinetrione-monosodium salt]. The homogenate was extracted in chloroform. The organic layer was then extracted with 1 vol of 1 M NaOH in saline and subsequently with 1.5 vol of 1 M HCl in diethyl ether, collected, dried, taken up in distilled water, and injected into a reverse-phase Bio-Sil ODS-5S column (250 × 4 mm, Bio-Rad). The HPLC column was equilibrated with 1% trifluoroacetic acid (TFA) in HPLC-grade distilled water and developed with a gradient of 0.1% TFA in acetonitrile. PTB and the internal standard were eluted from the column with 35% and 33% acetonitrile, respectively, and were detected by UV absorbance at 220 nm.
Brain Neurosteroid Content. Extraction, derivatization, and GC MS analyses of neurosteroids were performed with minor modifications as previously described (3). (i) Olfactory bulbs (OBs; this structure expresses the highest Allo levels in rodent brains) (4) or frontal cortices were homogenized in 10 vol of distilled water containing 2–5 fmol/ml [3H]Allo (New England Nuclear) to monitor the HPLC retention profile, and 1 pmol deuterium-labeled Allo (Allo-17,21,21,21-D4) (Cambridge Isotope Laboratories, Andover, MA) was used as internal standard. The supernatants were extracted with ethyl acetate and after lyophilization were purified with HPLC, as previously described (1, 4). (ii) The HPLC fractions containing Allo were derivatized with heptafluorobutyric acid anhydride (HFBA) and subjected to GC mass fragmentographic analysis.
Mass fragmentographic analysis of derivatized Allo was performed in the standard electron impact mode (13, 14). The detection limit for Allo was ≈10 fmol; the standard curve was linear between 5 and 105 fmol. For Allo quantification, the m/z ion-monitoring mode was 496 for HFBA-Allo and 500 for HFBA-deuterated-Allo.
Serotonin (5-HT) Uptake ex Vivo. Ex vivo inhibition of [14C]5-HT uptake was measured after administration of equimolar doses of R- and S-NFLX to SI mice by using a modification of the method of Shaskan and Snyder (15). Thirty minutes after treatment with each compound, mice were decapitated and their brains were immediately excised and cut into cubic slices (0.3 × 0.3 mm, ≈6 mg of protein). After a first washing step with Locke's solution (154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 2.3 mM CaCl2, 10 mM glucose, 1 mM MgSO4, 1 mM Hepes, 10 μM pargyline, 1 mM ascorbic acid, pH 7.4), brain slices were incubated at 37°C for 5 min in the presence of 50 nM [14C]5-HT (60 mCi/mmol; 1 Ci = 37 GBq).
The uptake was terminated by filtration through GF/B glass fiber filters. The uptake of [14C]5-HT detected in the presence of 1 μM FLX was considered to be nonspecific (because of the uptake of [14C]5-HT by other monoaminergic uptake systems) (15) and was considered a background value, which was subtracted from the total uptake of [14C]5-HT.
Statistical Analysis. Data are given as the mean ± SEM unless otherwise indicated. Comparisons between the control group and each of the treatment groups were performed by one-way ANOVA followed by Dunnett's test.
The IC50 was calculated from dose–response curves analyzed by the “quantal dose–response: probits test” as described by Tallarida and Murray (16) equipped with a statistical package. Statistical comparisons among the different IC50 values were performed by using the cohort package (CoHort Software, Monterey, CA). Differences were considered significant at P < 0.05.
Results
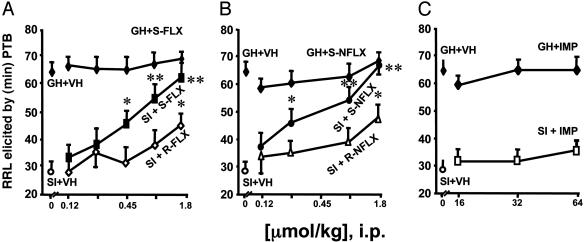
In the GH control mice, administration of PTB (50 mg/kg, i.p.) induced RRL that lasted for ≈1 h. The duration of PTB-induced RRL in SI mice was decreased by ≈50% relative to that of GH mice (Fig. 1). Administration of low doses (less than micromolar/kilogram) of S-FLX and S-NFLX 30 min before PTB treatment reversed the effect of SI on PTB-induced RRL, but it did not alter the duration of PTB-induced RRL in GH mice (Fig. 1 A and B). The EC50 of the S-FLX isomer was 0.7 μmol/kg and was three to four times smaller than the EC50 of the R-FLX isomer (Table 1 and Fig. 1 A). S-NFLX (EC50, ≈0.25 μmol/kg) was at least 2-fold more potent than S-FLX and 7-fold more potent than R-NFLX in normalizing the PTB action in SI mice (Table 1 and Fig. 1B).
Fig. 1.
FLX and NFLX, but not imipramine (IMP), potentiate in a dose-related manner the duration of PTB-elicited RRL in SI mice. Each value is the mean ± SEM of six to eight mice. R- and S-FLX (A) and R- and S-NFLX (B) were given 30 min before and imipramine (C) was given 60 min before PTB (50 mg/kg i.p.). P values are from the comparison of FLX- or NFLX-treated SI mice with vehicle (VH)-treated SI mice. *, P < 0.05; **, P < 0.01.
Table 1. FLX and NFLX stereoisomers' normalization of PTB-induced RRL and decrease of OB Allo levels in SI mice is not linked to their inhibition of 5-HT uptake.
| Drug | Allo (EC50, μmol/kg) | PTB RRL (EC50, μmol/kg) | 5-HT, uptake (IC50, μmol/kg) |
|---|---|---|---|
| S-FLX | 0.80 ± 0.07* | 0.70 ± 0.2* | 10.5 ± 2.4 |
| R-FLX | >1.80 | >1.80 | 13.7 ± 3.2 |
| S-NFLX | 0.15 ± 0.03** | 0.25 ± 0.1** | 8.3 ± 3.1 |
| R-NFLX | >0.90 | 1.70 ± 0.3 | 10.1 ± 3.8 |
| Imipramine | >60.00 | >60.00 | >10.0 |
Drugs were administered 30 min (R- and S-FLX and S- and R-NFLX) and 60 min (imipramine) before ex vivo [14C]5-HT uptake measurements. Data represent the mean ± SEM of four to six SI mice. *, P < 0.01 when S-FLX is compared with R-FLX. **, P<0.001 when S-NFLX is compared with R-NFLX and S-FLX.
In contrast to the SSRIs, imipramine, in doses that block 5-HT uptake (Table 1), failed to reverse the decreased duration of PTB-induced RRL (Fig. 1C) or to normalize the decrease of brain Allo content in SI mice (data not shown).
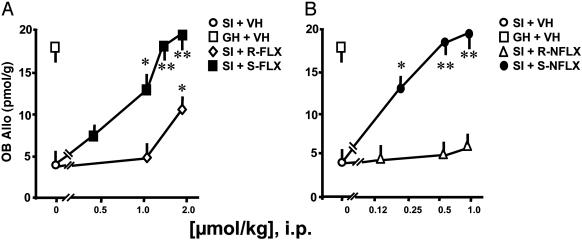
S-FLX or S-NFLX dose ranges that normalized PTB action also normalized brain Allo content (Fig. 2 and Table 1) and were severalfold lower than the doses of R-FLX or R-NFLX that were required to obtain similar results. However, these doses of S-FLX, R-FLX, and S-NFLX or R-NFLX failed to change cortical Allo levels in GH mice (data not shown).
Fig. 2.
Increase of Allo levels in the OB of SI male mice after treatment with FLX and NFLX stereoisomers. Each value is the mean ± SEM of six mice. R- and S-FLX (A) and R- and S-NFLX (B) were given 30 min before PTB (50 mg/kg i.p.). Allo determination was conducted in mice killed immediately after termination of the PTB-induced RRL test. P values are from the comparison of R- and S-FLX-treated and R- and S-NFLX-treated SI mice with vehicle (VH)-treated SI mice. *, P < 0.05; **, P < 0.01.
The measurements of Allo reported in Fig. 2 refer to the extracts from OB, an area of the brain where Allo is highly expressed. However, similar results were obtained in samples from the frontal cortex [Allo levels, means ± SEM of five mice: vehicle, 2.9 ± 0.2 pmol/g; R-FLX at 1.5 μmol/kg, 3.8 ± 0.3, nonsignificant when compared with vehicle; S-FLX at 1.5 μmol/kg, 7.2 ± 0.2 pmol/g, P < 0.05 when compared with vehicle].
To study whether the FLX-induced normalization of the action of PTB in SI mice is due to a change in the PTB degradation rate, we measured the brain content of PTB 30 min after an injection of 2.9 μmol/kg racemic FLX. At the time when vehicle-treated SI mice were reacquiring the righting reflex, the brain content of PTB (mean ± SEM, 197 ± 12 nmol/g; n = 5) was virtually identical to that of racemic FLX-treated SI mice (mean ± SEM, 210 ± 18 nmol/g; n = 5), in which the RRL induced by PTB was still present.
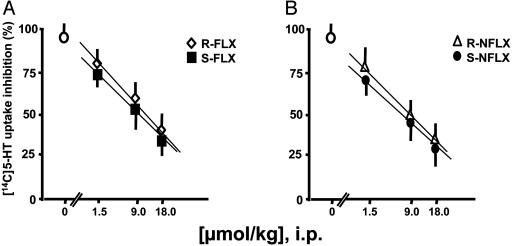
FLX and NFLX are known to be potent SSRIs (11). Thus, it was important to establish whether the difference between the S- and R-stereoisomers in eliciting a normalization of PTB action in SI mice was related to differences in 5-HT reuptake inhibition potency. The SSRI potency of FLX and NFLX measured ex vivo was 1–2 orders of magnitude higher than the EC50 doses that normalized the duration of PTB-induced RRL in SI mice (Table 1). Moreover, FLX and NFLX stereoisomers were equipotent in inhibiting 5-HT reuptake (Fig. 3), indicating a lack of stereospecificity for this action.
Fig. 3.
Ex vivo inhibition of 5-HT uptake in brain cortical slices of SI mice treated with FLX (A) or NFLX (B) stereoisomers. Drugs were administered 30 min before ex vivo [14C]5-HT uptake measurements. Each value represents the mean ± SEM of four mice.
Discussion
In previous reports we have shown that racemic FLX normalizes the reduction of PTB-induced sedation in SI mice, as measured by RRL, and at the same time increases brain levels of Allo (1). The present study shows that normalization of PTB-induced sedation in SI mice is stereospecific for both FLX and NFLX and parallels the ability of FLX and NFLX to stereospecifically increase brain Allo levels. The studies on brain Allo levels confirm and extend our previous report showing that FLX and NFLX at similar dose ranges normalize brain Allo content in a stereospecific manner and have a parallel anticonflict effect in SI mice (3).
The potency of the S-FLX or S-NFLX isomers to normalize the duration of PTB-induced RRL was 2- to 7-fold higher, respectively, than that of their respective R-isomers and appeared not to be caused by an alteration in the pharmacokinetic properties of PTB. Furthermore, the EC50 of S-FLX and S-NFLX required to reverse the reduced PTB-elicited RRL in SI mice was virtually identical to the EC50 required to normalize the reduced brain (OB or frontal cortex) Allo levels in such mice.
The present study further demonstrates that S-FLX and S-NFLX actions on neither PTB-induced RRL nor brain Allo content are related to their intrinsic SSRI activity. In fact, we show that the PTB and Allo effects of FLX and NFLX are stereospecific, whereas their SSRI activity lacks stereospecificity (Table 1 and Fig. 3). Additionally, the EC50 of S-FLX or S-NFLX required to normalize the PTB-induced RRL and brain Allo levels in SI mice is at least 1 order of magnitude lower than the IC50 required to inhibit 5-HT reuptake (Table 1). Indirect evidence that SSRI activity is not part of the mechanism of action of the S-FLX and S-NFLX on PTB and Allo was obtained in experiments with imipramine. In doses that block 5-HT reuptake (Table 1), imipramine fails to correct the reduced PTB action or to normalize brain Allo level down-regulation in SI mice. These data are consistent with a previous report (1) showing that p-chlorophenylalanine, in doses that reduce brain levels of 5-HT by >80%, does not prevent FLX action on PTB-induced sedation and on the increase of brain Allo levels in SI mice (1).
FLX and NFLX have been reported to increase GABA action at most GABAA receptor subtypes, presumably via an allosteric modulatory mechanism (17). This effect occurs for FLX and NFLX doses in the high micromolar range. Based on pharmacokinetic studies (18), it can be expected that the content of FLX or NFLX present in brain 30 min after the systemic administration of micromolar/kilogram doses of these compounds is only in the low nanomolar range. Thus, it is likely that at these concentrations S-FLX and S-NFLX do not have a direct action on GABAA receptors. Instead they may exert an indirect positive GABAA receptor modulatory action by increasing brain Allo levels.
We have recently demonstrated that variations in the physiological (nanomolar) concentrations of brain Allo play a significant role in regulating the responses of GABAA receptors to GABA or GABAmimetic drugs (4, 10). Moreover, administration of Allo in doses that per se fail to change the gross behavior of GH mice corrects the reduced response to PTB and muscimol observed in SI and SKF 105,111-treated mice, which express reduced levels of telencephalic Allo (1, 4).
Thus, these data provide important pharmacological evidence suggesting that the action of S-FLX and S-NFLX on PTB-induced RRL in SI mice is indirectly mediated at GABAA receptors by the ability of S-FLX and S-NFLX to increase brain Allo content. They also encourage an in-depth study of the mechanisms of action of S-FLX and S-NFLX on neurosteroid biosynthesis in normal and SI mice.
In mouse neurons, Allo is synthesized from progesterone (pyramidal neurons in the cortex and the hippocampus, mitral cells in OBs, and Purkinje cells in the cerebellum) (unpublished data) via the action of 5α-reductase type I, which transforms progesterone into 5α-dihydroprogesterone (5α-DHP), and 3α-hydroxysteroidoxydo-reductase (3α-HSOR), which reduces 5α-DHP into Allo (5, 19, 20). Because the decrease of brain Allo content in SI mice is associated with a decrease of 5α-reductase type I expression (5) and not with a decrease of 3α-HSOR, an attractive potential mechanism to explain the normalization of brain Allo content by S-FLX or S-NFLX may be through a direct action on 5α-reductase activity. In a previous study, we have shown that racemic FLX facilitates the conversion of 5α-DHP into Allo (21) in rat cortical brain slices. This effect has not been studied in brain slices from SI mice. Thus, whether the effect of FLX occurs at the levels of 3α-HSOR in SI mice, either by accelerating the reduction of 5α-DHP into Allo (22) or by inhibiting the oxidation of Allo into 5α-DHP, remains to be elucidated.
In conclusion, based on the data reported in this study, FLX could be considered a prototypic molecule for a new class of anxiolytic and antiaggressive drugs. Although acting with high potency and stereoselectivity on neurosteroid brain biosynthesis, this new drug class would not require significant action on brain 5-HT reuptake mechanisms.
FLX analogs that are weak inhibitors or fail to inhibit 5-HT transporter are available (23). Identification of derivatives of FLX or NFLX with weak or no SSRI activity that stimulate neurosteroidogenesis may ameliorate signs of anxiety and the response of GABAA receptors to GABAA receptor agonists in SI mice. These drugs may represent new pharmacological tools that could be beneficial in the treatment of anxiety, impulsive behavior, and premenstrual dysphoria in the absence of an inhibitory action on 5-HT uptake.
Acknowledgments
We thank Drs. Giulia Puia (Department of Pharmaceutical Sciences, University of Modena, Modena, Italy) and David H. Farb (Department of Pharmacology, School of Medicine, Boston University, Boston) for constructive criticism and suggestions in the preparation of the manuscript, and Ulana Liskevych for excellent technical assistance. This study was supported by National Institute of Mental Health Grants MH 49486 and MH 56890 (to A.G.).
Abbreviations: SI, social isolation; GABA, γ-aminobutyric acid; GABAA, GABA type A; RRL, loss of righting reflex; PTB, pentobarbital; Allo, allopregnanolone; FLX, fluoxetine; NFLX, norfluoxetine; SSRI, selective serotonin reuptake inhibitor; GH, group-housed; OB, olfactory bulb; 5-HT, serotonin.
References
- 1.Matsumoto, K., Uzunova, V., Pinna, G., Taki, K., Uzunov, D. P., Watanabe, H., Mienville, J. M., Guidotti, A. & Costa, E. (1999) Neuropharmacology 38, 955-963. [DOI] [PubMed] [Google Scholar]
- 2.Guidotti, A., Dong, E., Matsumoto, K., Pinna, G., Rasmusson, A. M. & Costa, E. (2001) Brain Res. Rev. 37, 110-115. [DOI] [PubMed] [Google Scholar]
- 3.Pinna, G., Dong, E., Matsumoto, K., Costa, E. & Guidotti, A. (2003) Proc. Natl. Acad. Sci. USA 100, 2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinna, G., Uzunova, V., Matsumoto, K., Puia, G., Mienville, J. M., Costa, E. & Guidotti, A. (2000) Neuropharmacology 39, 440-448. [DOI] [PubMed] [Google Scholar]
- 5.Dong, E., Matsumoto, K., Uzunova, V., Sugaya, I., Costa, E. & Guidotti, A. (2001) Proc. Natl. Acad. Sci. USA 98, 2849-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puia, G., Santi, M. R., Vicini, S., Pritchett, D. B., Purdy, R. H., Paul, S. M., Seeburg, P. H. & Costa, E. (1990) Neuron 4, 759-765. [DOI] [PubMed] [Google Scholar]
- 7.Belelli, D., Casula, A., Ling, A. & Lambert, J. J. (2002) Neuropharmacology 43, 651-661. [DOI] [PubMed] [Google Scholar]
- 8.Lambert, J. J., Belelli, D., Peden, D. R., Vardy, A. W. & Peters, J. A. (2003) Prog. Neurobiol. 71, 67-80. [DOI] [PubMed] [Google Scholar]
- 9.Brussaard, A. B., Devay, P., Leyting-Vermeulen, J. L. & Kits, K. S. (1999) J. Physiol. (London) 516, 513-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puia, G., Mienville, J.-M., Matsumoto, K., Takahata, H., Watanabe, H., Costa, E. & Guidotti, A. (2002) Neuropharmacology 44, 49-55. [DOI] [PubMed] [Google Scholar]
- 11.Wong, D. T., Bymaster, F. P., Reid, L. R., Mayle, D. A., Krushinski, J. H. & Robertson, D. W. (1993) Neuropsychopharmacology 8, 337-344. [DOI] [PubMed] [Google Scholar]
- 12.Shiu, G. K. & Nemoto, E. M. (1982) J. Chromatogr. 227, 207-212. [DOI] [PubMed] [Google Scholar]
- 13.Cheney, D. L., Uzunov, D., Costa, E. & Guidotti, A. (1995) J. Neurosci. 15, 4641-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uzunova, V., Sheline, Y., Davis, J. M., Rasmusson, A., Uzunov, D. P., Costa, E. & Guidotti, A. (1998) Proc. Natl. Acad. Sci. USA 95, 3239-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaskan, E. G. & Snyder, S. H. (1970) J. Pharmacol. Exp. Ther. 175, 404-418. [PubMed] [Google Scholar]
- 16.Tallarida, R. J. & Murray, R. B. (1987) Manual of Pharmacologic Calculations with Computer Programs (Springer, New York), 2nd Ed.
- 17.Robinson, R. T., Drafts, B. C. & Fisher, J. L. (2003) J. Pharmacol. Exp. Ther. 304, 978-984. [DOI] [PubMed] [Google Scholar]
- 18.Potts, B. D. & Parli, C. J. (1992) J. Liq. Chromatogr. 15, 665-681. [Google Scholar]
- 19.Costa, E., Auta, J., Guidotti, A., Korneyev, A. & Romeo, E. (1994) J. Steroid Biochem. Mol. Biol. 49, 385-389. [DOI] [PubMed] [Google Scholar]
- 20.Karavolas, H. J. & Hodges, D. R. (1991) in Neurosteroids and Brain Function, eds. Costa, E. & Paul, S. M. (Thieme, New York), pp. 135-145.
- 21.Uzunov, D., Cooper, T. B., Costa, E. & Guidotti, A. (1996) Proc. Natl. Acad. Sci. USA 93, 12599-12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin, L. D. & Mellon, S. H. (1998) Proc. Natl. Acad. Sci. USA 23, 13512-13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong, D. T., Bymaster, F. P. & Engleman, E. A. (1995) Life Sci. 57, 411-441. [DOI] [PubMed] [Google Scholar]