Abstract
The unprocessed precursor of the neurotrophin nerve growth factor (NGF), proNGF, has been suggested to be a death-inducing ligand for the neurotrophin receptor p75. Whether proNGF is a true pathophysiological ligand that is secreted, binds p75, and activates cell death in vivo, however, has remained unknown. Here, we report that after brain injury, proNGF was induced and secreted in an active form capable of triggering apoptosis in culture. We further demonstrate that proNGF binds p75 in vivo and that disruption of this binding results in complete rescue of injured adult corticospinal neurons. These data together suggest that proNGF binding to p75 is responsible for the death of adult corticospinal neurons after lesion, and they help to establish proNGF as the pathophysiological ligand that activates the cell-death program by means of p75 after brain injury. Interference in the binding of proNGF to p75 may provide a therapeutic approach for the treatment of disorders involving neuronal loss.
Neuronal loss is typically associated with trauma and degenerative or ischemic disorders of the nervous system. The common neurotrophin receptor, p75, has been implicated in such damage-induced death (1, 2), but the identity of the physiological ligand has remained unclear. The precursor of the neurotrophin nerve growth factor (NGF), proNGF, was recently suggested to be such a ligand. proNGF was induced along with p75 after spinal-cord injury (3). In addition, the recombinant, cleavage-resistant proNGF was shown to bind p75 selectively in vitro (4), and proNGF present in the injured spinal-cord lysates induced apoptosis in culture (3). For proNGF to be a true pathophysiological ligand for p75, however, endogenous proNGF must be secreted and then bind and activate p75 in vivo. Furthermore, if proNGF binding to p75 is responsible for cell death after injury, disruption of the interaction between proNGF and p75 may be expected to prevent the death of cells that would otherwise undergo cell death in vivo. Here, these questions were addressed by using adult corticospinal neurons (CSN), for which p75 is implicated in death after axotomy (5).
Methods
Animals and Surgery. Experimental procedures and maintenance of animals were approved by the local Animal Care Committee (Homburg, Saar), according to the German law regulating the experimental use of animals. Male Sprague–Dawley rats, weighing 190–330 g, and p75 (6) and NGF mutant mice of both sexes at 5–9 weeks of age were used. P75 null mutants were back-crossed from a C57BL/6 to a 129Sv genetic background for several generations until homozygous null mutants could again be obtained. NGF null mutant mice were generated by replacement of exon IV of the NGF gene by a lacZ cDNA (K.M. and M.M., unpublished data). They were kept on a mixed C57BL/6–129Sv background. The procedure and stereotaxic coordinates for internal-capsule lesion, fast blue labeling of CSN, and intracortical delivery of solutions in rats have been described (7), as well as the determination of the lesion and the “cell death areas” (8).
Tissue Processing. At the times indicated, animals were killed by an overdose of sodium pentobarbital and chloral hydrate and transcardially perfused with PBS, followed by 4% paraformal-dehyde. The brains were processed as described (8). For biochemical analyses, sensory motor cortices were removed quickly, frozen on dry ice, and stored at -80°C until further processing. For preparation of tissue lysates, the tissues were thawed in the lysis buffer on ice before being homogenized with Dounce homogenizer.
Application of the Antibodies to Lesioned CSN. Alzet 2001 (Alzet, Palo Alto, CA) osmotic minipumps were used to deliver either 20 mM PBS, NGF-neutralizing mouse NGF mAb, 27/21 mAbs (0.5 mg/ml in PBS) (9), rabbit polyclonal proNGF-neutralizing antiserum (anti-proNGF, 1:20 in PBS) (3), protein A column-purified IgG fraction of anti-proNGF (2.4 mg/ml), rabbit serum (1:20 in PBS), protein A column-purified IgG from rabbit serum (2.4 mg/ml), or mouse IgG (0.5 mg/ml in PBS) at a rate of 1 μl/h. All solutions contained 50 units/ml penicillin/streptomycin and were infused continuously over the indicated periods.
Analysis of Cell Survival. The number of surviving CSN was assessed by blinded cell counts of every second section collected for cell counts (i.e., every fourth section of the mice and every 10th section of the rat brains) (8). The criterion for a CSN was a Tracer-filled pyramidal-shaped profile of >4 μm (rats) or >3 μm (mice) in diameter (8). For the quantitative survival data, only the data from the cell-death areas (8) were used. Within the cell death area, percentage of survival is defined as number of fast blue-labeled CSN on the lesion side divided by the number of fast blue-labeled CSN in contralateral to the lesion side × 100%. The total number of cells counted was >280,000 in rats and >120,000 in mice. For statistical analyses, one-way ANOVA, followed by a post hoc Newman–Keuls test, was used.
Analysis of Cerebrospinal Fluid (CSF). Animals were anesthetized as described (8) and the dorsal aspect of the atlanto-occipital membrane was exposed to provide access to the cerebello-medullar cisterna. A glass micropipette connected by Teflon tubing to a 200-μl Hamilton syringe was used to puncture the cerebello-medullar cisterna, and the initial 100 μl of the aspirated CSF was collected for Western blot analyses. We used 10 μl of CSF for Western blot analyses and 30–50 μl of CSF for immunoprecipitation reactions. CSF samples did not contain blood contamination.
Immunoprecipitation and Western Blot Analyses. Cortical tissues were homogenized according to Kim et al. (10). Briefly, tissues were homogenized in a lysis buffer containing 1% Nonidet P-40, 20 mM Tris (pH 8.0) 137 mM NaCl, 0.5 mM EDTA, 10% glycerol, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 1 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM vanadate, and 1 mM phenylmethylsulfonyl fluoride. The procedures for immunodepletion and immunoprecipitation were as described (3). For NGF Western blot analyses, 27/21 mouse mAb (Chemicon), rabbit H-20 and M-20 polyclonal antibodies (Santa Cruz Biotechnology) were used.
Oligodendrocyte Culture and Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL) Assay. For quantification of apoptotic rat oligodendrocytes, cells were fixed after overnight treatment with CSF from the lesioned or the control rats. The fixed cells were processed for TUNEL and immunostained with myelin basic protein as described (3).
Vascular Smooth-Muscle Culture and TUNEL Assay. P75-expressing mouse vascular smooth-muscle cells (11) were plated at 10,000 per well in media containing 0.1% serum, and the temperature was shifted to 39.5°C to induce differentiation. Purified, recombinant cleavage-resistant proNGF (4) or diluent controls were added to cultures in the presence or absence of protein A column-purified IgG from rabbit antiprodomain polyclonal antibody or comparably protein A column-purified IgG from nonimmune rabbit serum. After a 14-h incubation, cells were fixed, processed for TUNEL reaction, and counterstained with 4′,6-diamidino-2-phenylindole. Five fields were analyzed in a blinded manner in each of duplicate wells, and experiments were performed twice.
Results
P75 Is Induced by the Lesion and Required for the Death of Lesioned CSN. Differentiation and survival of CSN are largely governed by neurotrophins. During embryonic development, neurotrophin 3 (NT3) promotes differentiation of cortical precursors into brain-derived neurotrophic factor (BDNF)-dependent neurons in culture (12, 13). When fully differentiated, these neurons adopt the adult phenotype and lose these trophic requirements (5, 14). After axotomy at internal capsule levels, the survival of adult CSN is again regulated by endogenous neurotrophins, with BDNF supporting survival, whereas NT3 promotes death of BDNF-dependent CSN (5, 14). NT3, however, appears to act by means of an indirect mechanism and not by activating its own death program. When NT3 action was neutralized, CSN no longer depended on BDNF for survival, suggesting that NT3 is required for CSN to become dependent on BDNF after the lesion.
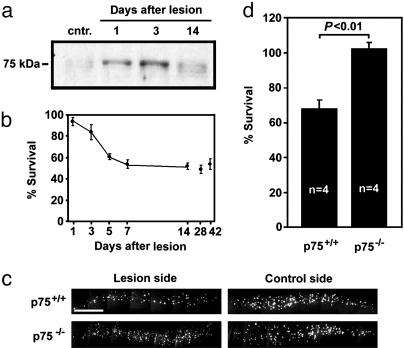
Consistent with their responses to BDNF and NT3, CSN express TrkB and TrkC, but not TrkA (7, 14). Of the neurotrophin receptors, p75 is the only receptor whose mRNA expression is dramatically induced in lesioned CSN, whereas Trk receptor expression remains unchanged (5, 14). In agreement with in situ hybridization data (5), p75 protein was undetectable in the unlesioned cortex, but beginning 1 d after internal-capsule lesion, it was present in the lesioned cortex and reached highest levels by 3 d after lesion (Fig. 1a). This peak coincides with the peak of cell death of axotomized CSN (Fig. 1b). The p75 protein levels decreased by 14 d after lesion, the time when CSN no longer underwent cell death (Fig. 1 a and b).
Fig. 1.
P75 is induced by cortical axotomy and is required for axotomy-induced death of CSN. (a) p75 is induced in the sensory motor cortex after axotomy. We analyzed 30 μg of cortical lysates for each time point by Western blot analysis with anti-p75 antibody (Covance, Berkeley, CA). (b) CSN undergo cell death after internal-capsule lesion in a temporally coordinated manner to p75 induction. Some of the time points were published in ref. 7. (c and d) Axotomy-induced death of CSN is prevented in p75-/- animals. (c) Fast blue-labeled CSN in sections of representative animals. (Scale bar, 0.5 mm.) (d) Quantification of CSN survival. Data are given as mean ± SEM.
In rats, infusion of p75-blocking antibody during week 1 after lesion prevented axotomy-induced death of CSN, suggesting that neurotrophin binding to p75 plays a role in inducing death among the lesioned CSN (5). We, therefore, investigated whether genetic depletion of p75 would similarly protect CSN from axotomy-induced death. In p75 null mutant mice, death of CSN was circumvented completely, whereas the survival of CSN in the wild-type controls remained at 68% (Fig. 1 c and d). Together, these data demonstrate that CSN up-regulate p75 expression after internal-capsule lesion and that p75 is a critical factor for the death of lesioned CSN.
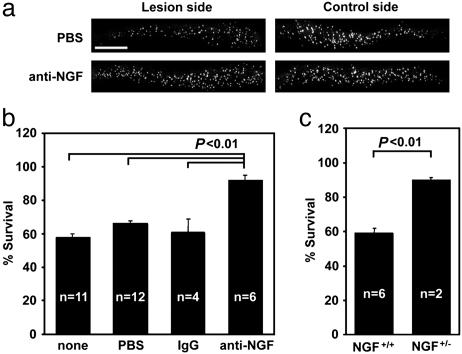
NGF Gene Product Is Required for the Death of Lesioned CSN. We next asked whether NGF contributes to the activation of p75 after axotomy because the effects of BDNF and NT3 on CSN-survival are mediated primarily by TrkB/C (5). Continuous intracortical infusion of an NGF-neutralizing antibody, 27/21 mAb, for the entire 7 d after lesion period resulted in 92% of adult-rat CSN surviving after the lesion, whereas vehicle or mouse IgG infusion resulted in only 61–66% survival (Fig. 2 a and b). This result suggests that an NGF gene product is involved in inducing the death of CSN. As predicted from 27/21 mAb infusion data, 87% of CSN survived axotomy in NGF heterozygous mice, whereas the survival remained at 60% in the wild-type mice (Fig. 2c). Together, these data implicate an NGF gene product as a death-inducing ligand for the lesioned CSN.
Fig. 2.
An NGF gene product is required for the induction of cell death of axotomized CSN. (a and b) Treatment with the 27/21 NGF mAb prevents axotomy-induced death of CSN. (a) Fast blue-labeled CSN in representative sections of a control animal (PBS) and an antibody-treated animal. (Scale bar, 1 mm.) (b) Quantification of survival after 27/21 mAb and control infusions. Data are given as mean ± SEM. (c) Death of axotomized CSN in NGF heterozygous mice is ameliorated. Data are given as mean ± SEM.
proNGF Is Induced by CSN Lesion and Secreted in an Active Form. It has been shown that the p75 level increased among oligodendrocytes after spinal-cord injury (3). In the same study, it was suggested that activation of p75 among oligodendrocytes is likely to be mediated by proNGF in vivo: proNGF expression was induced by the injury, and proNGF derived from the injured spinal-cord lysates induced apoptosis of cultured oligodendrocytes in a p75-dependent manner. Although these results strongly suggest that proNGF was responsible (3), a question still remained whether the apoptotic action of proNGF in the lysates was due to proNGF that was secreted or proNGF that was synthesized and remained inside the cell. Activation of p75 has been mediated predominantly by soluble ligands that bind p75 on the cell surface. proNGF was shown (15) to be secreted only in vitro.
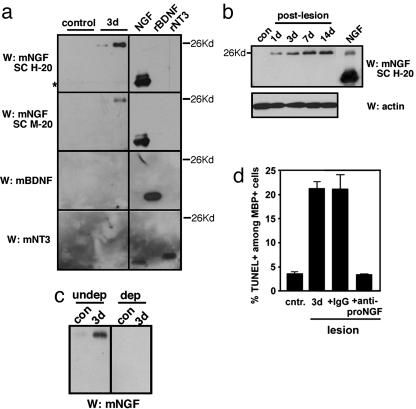
To assess whether proNGF is produced and secreted in vivo, we collected CSF after the lesion because CSF is known to contain most molecules secreted from brain parenchyma (16). A 26- to 28-kDa product, immunoreactive for NGF, was detected from CSF at 3 d after lesion by using two different NGF antibodies, but not by BDNF or NT3 antibodies (Fig. 3A). The size of the NGF-reactive band indicates that the product is proNGF, as we have observed after spinal-cord injury (3). When the cortical lysates were examined, 26- to 28-kDa immunoreactive bands for NGF were detected also in the lesioned cortex, and its expression increased over time (Fig. 3b). NGF was not detected in unlesioned cortex. It should be noted that we detected both BDNF and NT3 reactive bands in the tissue lysates, but the levels did not change after the lesion (data not shown). This result is similar to what we have reported by using spinal-cord injury as a model, in which BDNF and NT3 levels did not change with the injury, whereas NGF level increased dramatically (3). These data are consistent also with our earlier reports in which BDNF (14, 17) and NT3 (data not shown) message levels did not change after the CSN lesion. These results strongly suggest that proNGF, but not proBDNF or proNT-3, is secreted into the CSF.
Fig. 3.
proNGF is induced after internal-capsule lesion and released as a biologically active form into CSF. (a) proNGF, but not BDNF or NT3, is secreted into CSF. (Left) Each lane in Western blots represents 1/10th of the total 100 μl of CSF collected from individual animals that were either unlesioned (control) or received an internal-capsule lesion 3 d before the analysis. Two NGF polyclonal antibodies, H-20 and M-20 (Santa Cruz Biotechnology), detect a 26- to 28-kDa band in CSF samples 3 d after lesion and not in the control. *, nonspecific bands. Note that there is no BDNF or NT3 in the CSF, either in mature or proform, with or without the lesion. BDNF and NT3 antibodies were obtained from Promega. (Right) Each lane contains 100 ng of purified mature NGF, recombinant BDNF and NT3. (b) proNGF is induced in the cortex after the lesion. Western blot analyses of 100 μg of cortical lysates with anti-mNGF antibody (Santa Cruz Biotechnology, H-20) from unlesioned and lesioned animals are shown at the indicated times. Purified mature NGF (Harlan Bioproducts, Indianapolis) is also included (100 ng). Note that purified mature NGF contains proNGF. Actin Western blotting is shown as a control. (c) The proNGF antibody immunoprecipitates the 26- to 28-kDa band from the lesioned CSF sample (undep). The resulting supernatant (dep) lacks the band, suggesting that the 26- to 28-kDa band is proNGF. (d) proNGF secreted to CSF is active in inducing death in culture. We added 0.2 μl of CSF to oligodendrocyte cultures for 16–18 h, and processed them for TUNEL and double staining with myelin basic protein. For quantification, 450–550 cells were counted in duplicates for a total of 900–1,100 counted cells. From lesioned animals, 0.2 μl of CSF contains ≈10 ng of proNGF, which yields 0.35 nM in estimated concentration in 1 ml of media.
To test whether the immunoreactive band is indeed proNGF, we subjected the CSF samples to immunoprecipitation with the rabbit proNGF-specific polyclonal antibody, and then the precipitated proteins were probed by Western blot analyses using the NGF mAb, 27/21. Although 27/21 is less specific to NGF in Western blot analyses than rabbit H-20/M-20 polyclonal antibody (data not shown), its use was necessary in Western blot analyses after immunoprecipitation with rabbit polyclonal antibodies because H-20/M-20 detect the rabbit light chain of 25–26 kDa from the immunoprecipitates, obscuring the signal from proNGF. It should also be pointed out that there is no BDNF and NT3 secreted into the CSF (Fig. 3a), and the proNGF antibody used in immunoprecipitation reactions detects only proNGF and not mature NGF or proBDNF (3), providing the specificity.
At 3 d after lesion, a substantial amount of proNGF was detected, but very little in the control CSF (Fig. 3c Left). The resulting supernatant from the immunoprecipitation reaction with proNGF antibody did not contain the 26- to 28-kDa band, indicating that the 26- to 28-kDa band on NGF Western blotting is proNGF (Fig. 3c Right). Together, these results suggest that proNGF is induced by internal-capsule lesion and secreted into the CSF. When CSF samples from unlesioned and lesioned animals were added to primary oligodendrocytes, the 3-d lesion CSF induced apoptosis, whereas the nonlesion CSF did not (Fig. 3d). The apoptotic activity of the 3-d lesion CSF was blocked by proNGF antibody and not by control IgG, suggesting that the proNGF present in CSF is responsible for inducing the death of cultured oligodendrocytes. We, therefore, conclude that active, functional proNGF is induced and secreted into the CSF after cortical axotomy.
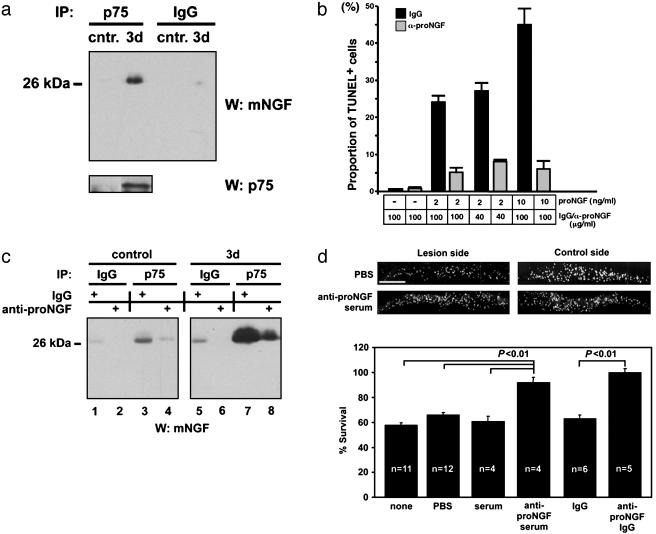
proNGF Binds p75 in Vivo, and Inhibition of proNGF Binding to p75 in Vivo Rescues CSN. Because proNGF is secreted in a functional form, we next asked whether proNGF binds p75 in vivo. For this binding, cortical lysates were subjected to immunoprecipitation with p75 antibody, and the presence or absence of proNGF was assessed by using the NGF mAb in Western blot analyses. proNGF was present in p75 immune complexes from the lysates of lesioned cortices, but not in control immunoprecipitates or in nonlesion samples (Fig. 4a). These data suggest that proNGF binds p75 in vivo after cortical axotomy.
Fig. 4.
proNGF binds p75 in vivo, and its binding to p75 is responsible for axotomy-induced death of CSN in vivo.(a) proNGF binds p75 in vivo. Cortical lysates (500 μg) from unlesioned (cntr) or animals 3 d after lesioning were subjected to immunoprecipitation by using anti-p75 antibody or the control anti-IgG, followed by Western blot analyses with 27/21 (Chemicon). (b) proNGF antibody rescues the death of vascular smooth-muscle cells in a dose-dependent manner. Purified, recombinant proNGF, or diluent controls were added at 2 or 10 ng/ml to cultures, in the presence or absence of IgG purified from rabbit antiprodomain antibody or comparably purified nonimmune rabbit IgG. The amount of the antibodies was 40 or 100 μg/ml, respectively. After a 14-h incubation, cells were fixed, processed for TUNEL reaction, and counterstained with 4′,6-diamidino-2-phenylindole. Five independent fields were analyzed in a blinded manner in each of duplicate wells, and experiments were performed twice. (c) The proNGF antibody disrupts the binding between proNGF and p75. Cortical lysates (500 μg) from proNGF antibody-treated or the control antibody-treated animals were subjected to immunoprecipitation with the control IgG or p75 antibodies. The resulting immunoprecipitates were analyzed by Western blot analysis with 27/21 (Chemicon). Note that there is a significant reduction in the amount of proNGF immunoprecipitated with p75 after proNGF antibody infusion (compare lanes 7 and 8). (d) Infusion of proNGF antibody at the same concentration rescues axotomized CSN. (Upper) Photographs of fast blue-labeled CSN from representative animals of the indicated experimental groups. (Scale bar, 1 mm.) (Lower) Quantification of CSN survival. Data are given as mean ± SEM.
We then investigated whether disrupting the binding between p75 and proNGF could result in a rescue of the lesioned CSN. To disrupt the binding between p75 and proNGF, we chose to infuse the proNGF antibody intracortically at the time of lesion. This antibody, derived from the prodomain of NGF, was shown (3) to be proNGF-specific and not to deplete mature NGF. In addition, the proNGF antibody was able to attenuate in a dose-dependent manner the proNGF-mediated apoptosis in p75+ vascular smooth-muscle cells, whereas comparable concentration of control IgG was not (Fig. 4b). This bioassay was used because this cell line exhibits highly reproducible and dose-dependent responsivity to proNGF (4, 18).
To test whether the infused proNGF antibody disrupts the binding between p75 and proNGF, we subjected the cortical lysates from rats that had been infused with proNGF antibody or the control IgG in immunoprecipitation reactions. For immunoprecipitation, we used anti-p75 or control IgG, which was followed by Western blotting with NGF antibody. Little proNGF was immunoprecipitated with the control IgG in the lesion lysates (Fig. 4c, lanes 5 and 6) or with the IgG or p75 antibody in the nonlesion lysates (Fig. 4c, lanes 1–4). In the lysates from lesioned rats that had been infused with IgG, a significant amount of proNGF was found in complex with p75 after immunoprecipitation with p75 antibody at 3 d after lesion, (Fig. 4c, lane 7). In contrast, in the lysates from the lesioned rats that had been infused with proNGF antibody, the amount of proNGF that immunoprecipitated with p75 was greatly reduced (Fig. 4c, compare lanes 7 and 8). These data suggest that infusion of proNGF antibody was effective in attenuating the proNGF binding to p75 after the lesion.
We then asked whether infusion of the proNGF antibody could rescue CSN from axotomy-induced death. Infusion with vehicle, preimmune serum, or control IgG resulted in the survival of 61–66% of CSN, whereas proNGF serum (92% survival) or the purified IgG fraction of proNGF antibody (100% survival) prevented axotomy-induced death of CSN (Fig. 4d). Infusion of proNGF antibody at the same dose resulted in significant attenuation of proNGF binding to p75 (Fig. 4c). Therefore, these results together indicate that proNGF binding to p75 is responsible for initiating a death-inducing signal in vivo.
Discussion
In this article, we present data establishing that proNGF is the physiological ligand for p75 under pathological conditions. proNGF was induced after axotomy on a time course similar to the time course of p75 and the subsequent death of CSN. More importantly, endogenous proNGF was secreted in a functional form that is capable of binding and activating p75, thereby inducing neuronal death in vivo. Consistent with this conclusion, disruption of the binding between endogenous proNGF and p75 resulted in complete rescue of the lesioned CSN. These data establish proNGF as a bona fide ligand for p75.
We have shown (5) that endogenous NT3 also promotes the death of BDNF-dependent CSN; neutralizing endogenous NT3 with the 12 mAb or blocking NT3 binding to TrkC with TC89 antibody rescued lesioned CSN. These data and the data presented in this article suggest that the death of lesioned CSN can result from two different mechanisms. One mechanism is to induce death by activating a proapoptotic receptor, p75; the other mechanism is to create a dependency on a survival factor that is present in a limiting amount, BDNF. Indeed, lesioned CSN do not require endogenous BDNF for survival if NT3 is neutralized simultaneously (5), suggesting that NT3 signaling by TrkC is required to maintain or to cause the initial dependency of CSN on BDNF for survival. This role of NT3 would resemble its role during development, in which NT3 promotes neuronal differentiation of cortical progenitors and their dependency on BDNF as they mature in culture (12, 13).
We have reported also that blockade of p75 with Rex antibody rescued the survival of lesioned CSN (5). We concluded at the time that Rex antibody most likely interfered with p75 binding to NT3 rather than NGF (5) because infusion of sheep anti-NGF-serum did not rescue lesioned CSN (S. Röhrig, M.M., K.M.G., unpublished data). The lack of a rescue when using sheep anti-NGF serum might have been due to a selective interaction of this serum with mature NGF or its insufficient neutralization of endogenous proNGF in vivo. Here, we clearly demonstrated that a NGF gene product is the responsible ligand activating p75: lesion-induced death of CSN was attenuated significantly not only in NGF heterozygotes, but also after infusion with 27/21 mAb, better antibodies for blocking NGF binding to p75 in vivo (19). We would also like to emphasize that our biochemical data complement our in vivo functional-blocking experiments: proNGF is induced selectively by the lesion, and interference in proNGF binding to p75 resulted in rescue of the lesioned CSN.
We demonstrated that proNGF is the predominant form of NGF present in the CSF. To our surprise, proNGF remained in an active state for an extended period (unpublished data). Because secreted proNGF can potentially target p75+ cells at a distance after CNS injury, the extent of cell death may increase over time, affecting regions of the brain that were not damaged initially. P75 is expressed in the adult among Purkinje neurons in the cerebellum and among cholinergic neurons of the basal forebrain, a neuronal population damaged in Alzheimer's disease. Because proNGF can induce apoptosis of cultured SCG neurons (4) that express TrkA, such as cholinergic neurons, the secondary damage can potentially have a pervasive impact. Matrix metalloproteinase 3 and plasmin were identified as the proteinases that can cleave proNGF in vitro (4), but it is not known whether the same proteinases cleave proNGF in vivo. It should be noted that the proNGF level has been shown to increase in Alzheimer's disease (20), whereas the plasmin level was reduced in Alzheimer's disease brain (21). Identification of proteinases that can cleave proNGF in vivo could aid the development of a therapeutic strategy to inactivate the apoptotic proNGF.
In this study, intracortical infusion of proNGF-specific antibody resulted in complete rescue of the lesioned CSN because of the antibody interfering with the binding of proNGF to p75. These data reveal a potential target for pharmacological intervention in diseases in which neuronal death is a pathogenetic factor. When the specific binding sequences are identified, drugs may be designed to impair the interaction between p75 and proNGF specifically to prevent or reduce the rate of cell death in such diseases. Alternatively, identification of critical down-stream players that mediate p75/proNGF signals would provide additional routes for potential therapeutic intervention.
Acknowledgments
We thank Y.-A. Barde for the generous gift of 27/21 mAb. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 530 C6 (to K.M.G.) and ME1121/2-1 (to M.M.), National Institutes of Health Grants R01 NS39472 and ACS (to S.O.Y.) and R01 NS30687 (to B.L.H.), German–Israeli Foundation Grant 483/96 (to M.M.), and the Max Planck Institute of Neurobiology (K.M. and M.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BDNF, brain-derived neurotrophic factor; CSN, corticospinal neurons; NGF, nerve growth factor; NT3, neurotrophin 3; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; CSF, cerebrospinal fluid.
See Commentary on page 5703.
References
- 1.Dechant, G. & Barde, Y. A. (2002) Nat. Neurosci. 5, 1131-1136. [DOI] [PubMed] [Google Scholar]
- 2.Chao, M. V. (2003) Nat. Rev. Neurosci. 4, 299-309. [DOI] [PubMed] [Google Scholar]
- 3.Beattie, M. S., Harrington, A. W., Lee, R., Kim, J. Y., Boyce, S. L., Longo, F. M., Bresnahan, J. C., Hempstead, B. L. & Yoon, S. O. (2002) Neuron 36, 375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, R., Kermani, P., Teng, K. K. & Hempstead, B. L. (2001) Science 294, 1945-1948. [DOI] [PubMed] [Google Scholar]
- 5.Giehl, K. M., Rohrig, S., Bonatz, H., Gutjahr, M., Leiner, B., Bartke, I., Yan, Q., Reichardt, L. F., Backus, C., Welcher, A. A., et al. (2001) J. Neurosci. 21, 3492-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, K. F., Li, E., Huber, L. J., Landis, S. C., Sharpe, A. H., Chao, M. V. & Jaenisch, R. (1992) Cell 69, 737-749. [DOI] [PubMed] [Google Scholar]
- 7.Giehl, K. M. & Tetzlaff, W. (1996) Eur. J. Neurosci. 8, 1167-1175. [DOI] [PubMed] [Google Scholar]
- 8.Bonatz, H., Rohrig, S., Mestres, P., Meyer, M. & Giehl, K. M. (2000) J. Neurosci. Methods 100, 105-115. [DOI] [PubMed] [Google Scholar]
- 9.Korsching, S. & Thoenen, H. (1987) Methods Enzymol. 147, 167-185. [DOI] [PubMed] [Google Scholar]
- 10.Kim, J. Y., Sun, Q., Oglesbee, M. & Yoon, S. O. (2003) J. Neurosci. 23, 5561-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang, X., Bauer, J. H., Li, Y., Shao, Z., Zetoune, F. S., Cattaneo, E. & Vincenz, C. (2001) J. Biol. Chem. 276, 33812-33820. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh, A., Carnahan, J. & Greenberg, M. E. (1994) Science 263, 1618-1623. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, A. & Greenberg, M. E. (1995) Neuron 15, 89-103. [DOI] [PubMed] [Google Scholar]
- 14.Giehl, K. M., Schutte, A., Mestres, P. & Yan, Q. (1998) J. Neurosci. 18, 7351-7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mowla, S. J., Pareek, S., Farhadi, H. F., Petrecca, K., Fawcett, J. P., Seidah, N. G., Morris, S. J., Sossin, W. S. & Murphy, R. A. (1999) J. Neurosci. 19, 2069-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, S. V. & Forman, D. T. (1994) Clin. Lab. Sci. 7, 32-38. [PubMed] [Google Scholar]
- 17.Schutte, A., Yan, Q., Mestres, P. & Giehl, K. M. (2000) Neurosci. Lett. 290, 185-188. [DOI] [PubMed] [Google Scholar]
- 18.Wang, S., Bray, P., McCaffrey, T., March, K., Hempstead, B. L. & Kraemer, R. (2000) Am. J. Pathol. 157, 1247-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohrer, H., Hofer, M., Hellweg, R., Korsching, S., Stehle, A. D., Saadat, S. & Thoenen, H. (1988) Development (Cambridge, U.K.) 103, 545-552. [DOI] [PubMed] [Google Scholar]
- 20.Fahnestock, M., Michalski, B., Xu, B. & Coughlin, M. D. (2001) Mol. Cell. Neurosci. 18, 210-220. [DOI] [PubMed] [Google Scholar]
- 21.Ledesma, M. D., Da Silva, J. S., Crassaerts, K., Delacourte, A., De Strooper, B. & Dotti, C. G. (2000) EMBO Rep. 1, 530-535. [DOI] [PMC free article] [PubMed] [Google Scholar]