Abstract
Rhythmicity of the rat suprachiasmatic nucleus (SCN), a site of the circadian clock, develops prenatally. A molecular clockwork responsible for the rhythmicity consists of clock genes and their negative and positive transcriptional–translational feedback loops. The aim of the present study was to discover the development of the clockwork during ontogenesis. Daily profiles of Per1, Per2, Cry1, Bmal1, and Clock mRNA in the SCN of fetuses at the embryonic day (E)19 and of newborn rats at the postnatal day (P)3 and P10 were assessed by the in situ hybridization method. In addition, daily profiles of PER1, PER2, and CRY1 proteins at E19 were assessed by immunohistochemistry. As early as at E19, all the studied clock genes were already expressed in the SCN. However, no SCN rhythm in their expression was detected; Per1, Cry1, and Clock mRNA levels were low, whereas Bmal1 mRNA levels were high and Per2 mRNA levels were medium. Moreover, no rhythms of PER1, PER2, and CRY1 were detectable, as no immunoreactive cells were present at E19. At P3, rhythms in Per1, Per2, Cry1, and Bmal1, but not in Clock mRNA, were expressed in the SCN. The rhythm matured gradually; at P10, the amplitude of Per1, Per2, and Bmal1 mRNA rhythms was more pronounced than at P3. Altogether, the data show a gradual development of both the positive and negative elements of the molecular clockwork, from no detectable rhythmicity at E19 to highly developed rhythms at P10.
All mammals exhibit an array of daily behavioral, physiological, hormonal, biochemical, and molecular rhythms. The circadian rhythms persist even in a nonperiodic environment with a period close, but not equal to, 24 h (1). Under natural conditions, the rhythms are entrained to the 24-h day by the light–dark (LD) cycle, mostly by the light period of the day. The circadian rhythms are controlled by a pacemaker located in two suprachiasmatic nuclei (SCN) of the hypothalamus (2). The SCN themselves exhibit rhythms in the uptake of 2-deoxyglucose, a marker of metabolic activity (3), in electrical activity (4), in spontaneous as well as light-induced expression of immediate early genes, namely c-fos, a marker of neural activity (5–9), in the production of many peptides, e.g., of arginine vasopressin (10, 11), and other rhythms.
The SCN rhythmicity is due to the SCN molecular clockwork (for review see refs. 12 and 13). Eight mammalian, mostly mouse (m) clock genes cloned so far, namely three period genes (mPer1, -2, and -3), two cryptochrome genes (mCry1 and -2), Clock, Bmal1, and casein kinase 1 epsilon (CK1ε), are thought to be involved in the clockwork by forming interacting transcriptional–translational feedback loops. Briefly, the protein products CLOCK and BMAL1 heterodimers positively activate rhythmic expression of Per and Cry genes. In the cytoplasm, the PER and CRY proteins form complexes important for nuclear translocation of both proteins; the phosphorylation state of PER protein monomers by CK1ε may also regulate their cellular location and stability. The nuclear localized PER·CRY complexes directly interact with the CLOCK·BMAL1 heterodimer to inhibit the CLOCK·BMAL1-mediated transcription due to chromatin remodeling (14). PER1 may operate via posttranscriptional control, probably by affecting rhythmicity through interaction with other circadian regulatory proteins. Moreover, BMAL1 is negatively autoregulated, and CRY1, CRY2, and PER2 proteins positively activate the Bmal1 transcription (15–17). The regulation of Bmal1 transcription is likely mediated by REV-ERBα, a protein product of gene Rev-erbα that is controlled by CLOCK·BMAL1 (18). Expression of Per1 and Per2, but not of other clock genes, is activated by light at night (13).
The mammalian SCN and its rhythmicity develops through more phases (19). In the rat, the SCN is formed from the embryonic day (E)14 through E17 from the specialized zone of the ventral diencephalic germinal epithelium as a component of the periventricular cell groups. Synaptogenesis in the SCN progresses slowly in the late prenatal and early postnatal periods and then markedly increases from postnatal day (P)4 to P10. Intrinsic SCN rhythmicity is already present in the late embryonic stage: a clear day–night oscillation of metabolic activity monitored by 2-deoxyglucose uptake was detected in the fetal rat SCN already from E19 through E21 (20), of arginine vasopressin mRNA level at E21 (21), and of the firing rate of the SCN neurons at E22 (22). Measurement of the expression of two clock genes, namely Per1 and Per2, revealed also intrinsic prenatal rhythms. In the fetal mouse SCN, a daily rhythm of Per1, but not of Per2, mRNA was detected already at E17. The rhythm of Per2 expression was not present even at P3, and it appeared only at P6 (23). In the rat SCN, rhythms of both Per1 and Per2 mRNAs were detected at E20 (24, 25).
The aim of the present work was to ascertain the pre- and postnatal development of both negative and positive elements of the molecular clockwork feedback loops. Circadian profiles of five clock gene mRNAs, namely of Per1, Per2, Cry1, Bmal1, and Clock, were studied in the rat SCN at E19, P3, and P10. In addition, circadian profiles of clock proteins PER1, PER2, and CRY1 were also studied in the fetal and adult SCN.
Materials and Methods
Animals. Female Wistar rats (Bio Test, Konarovice, Czech Republic) were housed in a temperature of 23 ± 2°C with free access to food and water. They were maintained under an LD cycle with 12 h of light and 12 h of darkness per day (LD12:12) for at least 2 months. Light was provided by overhead 40-W fluorescent tubes, and illumination was between 50 and 200 lux, depending on cage position in the animal room. Day 0 of gestation, when the rats were found to be sperm-positive, was designated the embryonic day 0 (E0); day of delivery was designated the postnatal day 0 (P0). For prenatal studies at day E19, the morning light was not turned on and mothers were released into constant darkness. Starting 1 h after expected lights-on, mothers were decapitated in 2- to 3-h intervals during the circadian cycle, and 12 fetuses per each time point were sampled. For postnatal studies at P3 or at P10, 24 mothers with their pups were released into constant darkness, i.e., the morning light was not turned on. Thereafter, four pups per each time point were sampled every 2 h throughout the whole circadian cycle in complete darkness. The time of the expected lights-on was designated circadian time 0 (CT0); the time of the expected lights-off was designated as CT12.
Fetuses and pups were killed by rapid decapitation. The brains were removed, immediately frozen on dry ice, and stored under -80°C. Pup brains were sectioned into five series of 12-μm-thick slices in alternating order throughout the whole rostrocaudal extent of the SCN and processed for in situ hybridization to determine levels of Per1, Per2, Cry1, Bmal1, and Clock gene mRNAs. Eight fetal brains per time point were cut into three series per each brain, and four brains were used for one clock gene mRNA. Moreover, four fetal brains per time point were processed for immunocytochemistry to determine levels of PER1, PER2, and CRY1 proteins in the SCN.
All experiments were conducted under license A5228-01 with the U.S. National Institutes of Health and in accordance with the Animal Protection Law of the Czech Republic (license 1020/491/A/00).
In Situ Hybridization Histochemistry. The cDNA fragments of rat rPer1 (980 bp; corresponds to nucleotides 581–1561 of the sequence in GenBank accession no. AB002108), rat rPer2 (1,512 bp; corresponds to nucleotides 369–1,881 of the sequence in GenBank accession no. NM031678), rat rBmal1 (841 bp; identical to nucleotides 257–1,098 of the sequence in GenBank accession no. AB012600), rat rClock (1,158 bp; identical to nucleotides 167–1325 of the sequence in GenBank accession no. AB019258), and mouse mCry1 (719 bp; corresponds to nucleotides 1,074–1,793 of the sequence in GenBank accession no. NM007771) were used as templates for in vitro transcription of complementary RNA probes. The rPer1, rPer2, and mCry1 fragment-containing vectors were generously donated by H. Okamura (Kobe University School of Medicine, Kobe, Japan), and rBmal1 and rClock were cloned in our laboratory. Briefly, cDNA fragments of Bmal1 and Clock were yielded from the rat hypothalamic mRNA. After reverse transcription, cDNA was amplified by standard PCR and ligated into vector pGem-T-Easy and pBluescript, respectively. The cloned inserts were sequenced to verify the amplified products.
The probes were labeled by using α-[35S]thio-UTP, and the in situ hybridization was performed as described previously (26). Briefly, the sections were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 40 min and transferred through 0.2 M HCl for 20 min and 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min, and dehydrated in 70% and 96% ethanol for 5 min. The sections were then incubated with a hybridization buffer [50% formamide/10% dextran sulfate/5× SSPE (0.9 M NaCl/50 mM NaH2PO4/5 mM EDTA)/2× Denhardt's solution/500 μg/ml yeast tRNA/500 μg/ml salmon sperm DNA/0.1% SDS/5% NaPPi/50 mM DTT in 0.1% diethylpyrocarbonate/H2O] containing purified radioactive labeled probes and hybridized for 20 h at 60°C (Per1, Per2, and Clock), 58°C (Bmal1), or 55°C (Cry1). After a posthybridization wash, the sections were dehydrated in ethanol and dried in air. Finally, the slides were exposed to Hyperfilm-beta-max (Amersham Pharmacia) for 8–10 days and developed by using the developer Fomatol LQN and fixer FOMAFIX (FOMA, Hradec Králové, Czech Republic).
As a control, in situ hybridization was performed in parallel with sense probes (apart from Per2) on sections containing the SCN. All sections hybridized with the same probe were processed simultaneously under identical conditions.
Autoradiographs of sections were analyzed by using an image analysis system (ImagePro, Olympus, New Hyde Park, NY) to detect the relative OD of the specific hybridization signal. In each animal, the mRNA level was quantified bilaterally at the midcaudal SCN section containing the strongest hybridization signal. Each measurement was corrected for a nonspecific background by subtracting the OD values from neighboring areas expected to be free of the specific signal, thus serving as their own internal standard. At the end, slides were counter-stained with cresyl violet to check the presence and position of the SCN in each section. In no case did in situ hybridization with a sense probe yield any specific signal.
Four animals were used for each time point; occasionally, one sample was omitted. Data were expressed as means of OD ± SEM; OD for each animal was calculated as a mean of the left and right SCN values.
Immunohistochemistry. Coronal 12-μm-thick sections of fetal brains were cut, mounted on slides, fixed in 4% paraformaldehyde in PBS, and processed for immunocytochemistry by using the standard avidin–biotin method with diaminobenzidine as the chromogen (Vector Laboratories) as described elsewhere (27). As controls of the immunohistochemical procedure in the fetal brain, coronal sections of adult rat brains were treated simultaneously in each assay as well. The PER1 polyclonal primary antiserum was synthesized at the Massachusetts General Hospital Biopolymer Core Facility. It was generated against amino acids 6–21 of the peptide sequence of mPER1 and characterized elsewhere (28–30). The PER2 and CRY1 polyclonal antisera were obtained from ADI (Greenwich, CT). Specificity of the staining of the rat tissue was checked in adult brain sections with and without blocking peptide (ADI, PER21-P, and CRY11-P, respectively; data not shown). Labeled cell nuclei in the whole SCN were counted irrespective of the intensity of the staining by an independent observer using the aforementioned image analysis system. Counting was performed on one representative section per brain, which contained the highest number of labeled cells at the level of the midcaudal SCN. The position of the SCN was checked in alternate sections stained with cresyl violet. Four brains were used for each time point, and data were expressed as means of the number of immunoreactive cells ± SEM. Number of immunoreactive cells for each fetus was calculated as a mean of the left and right SCN values.
Statistical Analysis. Data were analyzed by two-way ANOVA for group and time differences and by one-way ANOVA for time differences and the subsequent Student–Newman–Keuls multiple range test, with P < 0.05 required for significance. In addition, data were also analyzed by Student's t test. The difference between groups in the rhythm amplitudes, measured by differences between two maximum and two minimum values, was tested by the ANOVA linear contrasts method.
Results
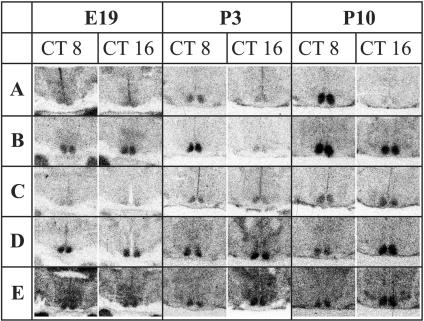
Daily Profiles of Clock Gene RNAs. Fig. 1 shows representative in situ hybridization studies of Per1, Per2, Cry1, Bmal1, and Clock mRNA in the SCN of 19-day fetuses and 3- and 10-day-old rats at CT8, i.e., during the day, and at CT16, i.e., during the night. From these and other similar autoradiographs, relative OD levels, i.e., relative mRNA amounts of the above-mentioned clock genes, were estimated.
Fig. 1.
Per1 (A), Per2 (B), Cry1 (C), Bmal1 (D), and Clock (E) mRNA levels in 19-day fetuses (E19) and in 3-(P3) and 10-day-old (P10) rats maintained in LD12:12 and sampled in darkness either at CT8 or at CT16. Representative autoradiographs of coronal brain sections at the level of the SCN are depicted. The sections were examined by in situ hybridization using complementary RNA probes. Note that in postnatal rats Per1 and Per2 mRNAs are in antiphase with Bmal1 mRNA.
On E19, there was no clear circadian rhythm of any of the clock gene mRNA, although the one-way ANOVA revealed some variation throughout the day (Fig. 2 a, c, e, g, and i). For Per1 (Fig. 2a) and Cry1 (Fig. 2e) mRNA, there were no significant differences among time points. Per2 mRNA at CT14 was significantly higher than that at CT22 (P < 0.01) (Fig. 2c), Bmal1 mRNA at CT22 was significantly lower than values at any other time except at CT10 (P < 0.05) (Fig. 2g), and Clock mRNA at CT22 was significantly higher than that at CT8, CT12, and CT14 (Fig. 2i).
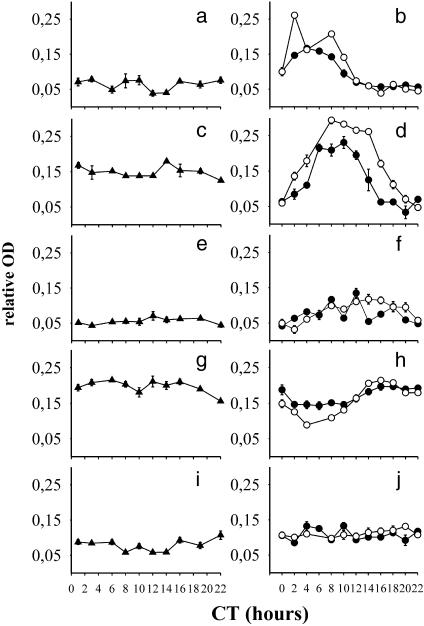
Fig. 2.
Daily profiles of Per1 (a), Per2 (c), Cry1 (e), Bmal1 (g), and Clock (i) mRNA in the SCN of 19-day fetuses and of Per1 (b), Per2 (d), Cry1 (f), Bmal1 (h), and Clock (j) in the SCN of 3-(•) and 10-day-old (○) rats. Rats maintained in LD12:12 were released into darkness at the time of the expected dark–light transition (CT0) and assayed for mRNA by in situ hybridization using complementary RNA probes during the first cycle in darkness. Data are expressed as means ± SEM from four animals.
Whereas no clear rhythms were detectable in the embryonic stage, all of the studied clock genes (Fig. 2 b, d, f, and h) except Clock (Fig. 2j) were expressed in a cyclic manner postnatally, as the one-way ANOVA revealed significant daily rhythms in their mRNAs (P < 0.01). In 3-day-old as well as in 10-day-old rats, Per1 mRNA started to rise after CT22, and at CT2 it was already significantly higher than at CT0 (P < 0.01); a significant decline from high daytime values occurred between CT8 and 10 at both ages (P < 0.01, Fig. 2b). Per2 mRNA began to rise after CT0 on P3 as well as on P10, and a significant increase above the CT0 value occurred at CT2 in 10-day-old rats, but only at CT6 in 3-day-old rats (P < 0.01, Fig. 2d). A significant decline from high daytime values occurred at CT14 (vs. CT12, P < 0.001) in 3-day-old rats, but only at CT16 (vs. CT14, P < 0.01) in 10-day-old animals. Due to a slightly delayed rise and a markedly advanced decline of Per2 mRNA in 3-day-old rats as compared with the rise and decline in 10-day-old animals, the time interval of high Per2 expression was longer on P10 than on P3. Cry1 mRNA began to rise after CT0 in 3-day-old rats and after CT2 in 10-day-old rats, but in both age groups the increase was significant only at CT8 (vs. CT0 and CT2, respectively; P < 0.01). A significant decline from higher values occurred at CT22 (vs. CT18 on P3, P < 0.05 and CT16 on P10, P < 0.01; Fig. 2f). Bmal1 mRNA began to rise around CT10, and the increase was significant at CT16 (vs. CT6, P < 0.05) on P3, whereas on P10 the rise began after CT4 and was already significant at CT10 (vs. CT4, P < 0.05, Fig. 2h). Similar to that in adult animals, even in 3- and 10-day-old rats, the Bmal1 circadian profile was roughly in an opposite phase to the rhythm of Per1 mRNA (Figs. 1 and 2 b and h).
Amplitude of Rhythms in Clock Gene mRNAs. For the rhythms of Per1, Per2, and Bmal1 mRNA, but not for the Cry1 mRNA rhythm, the two-way ANOVA revealed a significant main effect of age (F = 22.7, 86.6, and 12.7, respectively, P < 0.01) as well as a significant main effect of time (F = 89.3, 58.6, and 25.4, respectively, P < 0.01) and a significant interaction effect (F = 11.7, 4.7, and 4.3, respectively, P < 0.01). Per1 mRNA was significantly higher on P10 than on P3 at CT2, -8, and -10 (P < 0.001), whereas Per2 mRNA was higher on P10 than on P3 at CT4 (P < 0.05), -12, -14, and -16 (P < 0.01). Bmal1 mRNA was significantly lower on P10 than on P3 at CT4 (P < 0.01) and -8 (P < 0.05).
The differences between P3 and P10 suggest a difference of the mRNA rhythm amplitudes between 3- and 10-day-old rats. Indeed, the ANOVA linear contrasts method using differences between maximum and minimum levels revealed a significant difference between P3 and P10 in the rhythm amplitudes. The difference between the maximum and minimum for the Per1 and Bmal1 mRNA rhythms was significantly larger in 10- than in 3-day-old rats (P < 0.01, Fig. 2 b and h). The difference between the maximum and minimum for the Per2 mRNA rhythm also appeared to be larger in 10- than in 3-day-old rats; however, it was just at the border of significance (Fig. 2d).
Level of Clock Gene mRNAs in the Fetal SCN. As already mentioned, no clear rhythm of Per1, Per2, Cry1, Bmal1, or Clock mRNA was detectable on E19. To compare the levels of clock gene mRNAs in the fetal SCN with that in the early postnatal SCN, the mean embryonic level calculated from all values sampled at all measured times was compared to either the maximum or the minimum of mRNA on P3. The maximum and the minimum represented the highest and lowest mean values, respectively, which did not differ significantly between themselves, but which differed significantly from mean values at other times. The mean fetal Per1, Cry1, and Clock mRNAs were lower than the maximum of corresponding mRNAs on P3 (P < 0.01) but did not differ significantly from the Per1, Cry1, and Clock mRNA minimum. Consequently, Per1, Cry1, and Clock mRNA levels on E19 were roughly equal to the minimum levels on P3 (Fig. 2 a, b, e, f, i, and j). The mean fetal Per2 mRNA level was significantly lower than the maximum level and higher than the minimum level on P3 (P < 0.01). Hence, the Per2 mRNA level on E19 was approximately medium, i.e., between the maximum and the minimum of 3-day-old rats (Fig. 2 c and d). Bmal1 mRNA on E19 was higher than the Bmal1 mRNA minimum on P3 (P < 0.01) and did not differ significantly from the maximum. Consequently, the fetal Bmal1 mRNA was high and roughly equal to the maximum Bmal1 mRNA level in 3-day-old rats (Fig. 2 g and h).
Clock Gene Products in the Fetal SCN. In adult rats, immunocyto-chemical analysis with PER1, PER2, and CRY1 protein antibodies revealed immunoreactive cells in their SCN (Fig. 3). No such cells were, however, revealed in the SCN of 19-day fetuses at any measured time of day, although the same antibodies in the same assay were used for the adult and for the fetal tissue (Fig. 3).
Fig. 3.
Daily profiles in the number of PER1-, PER2-, and CRY1-immunoreactive (-ir) cells in the SCN of 19-day fetuses. Pregnant rats maintained in LD12:12 were released into darkness at the time of the expected dark–light transition (CT0), and the fetal SCN were assayed for immunoreactivity during the first cycle in darkness. Data are means from four fetuses. Representative photomicrographs of coronal sections of SCN of 19-day fetuses assayed at CT6 (A, D, and G) and CT16 (B, E, and H) and of adult rats assayed at the expected time of the immunoreactivity maximum (C, F, and I) show PER1 (A–C), PER2 (D–F), and CRY1 (G–I) immunopositive cells.
Discussion
As early as at E19, all studied clock genes were already expressed. However, no endogenous SCN rhythm in their expression was detected. The mean level of Per1, Cry1, and Clock mRNA corresponded to the minimum level of these mRNAs at P3, whereas the mean level of Bmal1 mRNA corresponded to the maximum of the mRNA at P3. The level of Per2 mRNA was between the maximum and the minimum of the P3 level. The lack of any of these rhythms at E19 was not expected. E19 was chosen for our studies as a representative embryonic day when the fetal SCN was already formed (19) and a rhythm in metabolic activity was present (20). Moreover, Ohta et al. (24, 25) recently reported on rhythms of Per1 and Per2 mRNA in the fetal rat SCN at E20. A plausible explanation for the lack of rhythms in molecular clockwork at E19 may be that at E19 the SCN rhythmicity just starts (20), and amplitude of rhythms in clock gene expression might be too low to be detected. Another explanation may be that rhythms in clock gene expression were already present in individual SCN neurons but were not yet mutually synchronized because of insufficient synapses at E19 (19). However, such a possibility might concern only Per2 expression, because Per2 mRNA at E19 was just between the maximum and minimum P3 levels, but not Per1, Cry1, Clock, and Bmal1 expression, as their mRNAs corresponded either to the maximum or minimum of P3 values. Still there is a possibility that another mechanism besides the molecular clockwork might drive the SCN rhythmicity during the late embryonic development. For example, maternal cues, such as dopamine or melatonin, might trigger directly the fetal SCN rhythm in metabolic activity (31, 32). The discrepancy between our data and those of Ohta et al. (24, 25) might be explained not only by an age difference between E19 and E20 but also by maintenance of pregnant rats: whereas in our study rats were released into constant darkness before sampling, in the reported studies rats were killed during an LD cycle. However, such a difference could hardly account for the discrepancy, as the fetal clock is supposed to be entrained by the mother's cue and probably does not use an LD cycle for synchronization (31–33).
In our study, not only the rhythms of Per1, Per2, and Cry1 mRNA but also the rhythms of PER1, PER2, and CRY1 proteins were not detectable at E19. At any CT, no immunore-active cells were detected in the fetal SCN, although in the same assay an abundance of immunoreactive cells was observed in adult rat SCN. Whereas levels of Per1 and Cry1 mRNA at E19 might be too low to be translated into gene products, Per2 mRNA attained already a medium level between the P3 maximum and minimum. Hence, either Per2 mRNA was not yet translated into PER2 protein or the protein was rapidly degraded. The rapid degradation might be due to the lack of a sufficient level of CRY1 protein that would prevent ubiquitination and subsequent degradation of PER2 protein (34). The absence of a detectable level of CRY1 protein excludes the possibility that the low levels of Per1 and Cry1 mRNA at E19 were caused by suppression of Per1 and Cry1 gene transcription by CRY1 (13). Rather, other mechanisms prohibiting expression of these genes might operate at E19 and the mechanisms enabling the rhythmic expression of clock genes may not yet be maturated.
Early postnatally at P3, significant rhythms in Per1, Per2, Cry1, and Bmal1 mRNA, but not in Clock mRNA, were expressed in the SCN. At that developmental stage, the dorsomedial, but not yet the ventrolateral, SCN displays a clear rhythmicity (Z.B., A.S., and H.I., unpublished results). Occurrence of rhythms in clock gene expression at P3 might thus be in agreement with reports on high levels of Per1 and Per2 transcripts in the dorsomedial rather than in the ventrolateral rat SCN (30, 35–37). The rhythms in clock gene expression matured only gradually; at P10, the amplitude of Per1, Per2, and Bmal1 mRNA rhythms was more pronounced than at P3. Different mechanisms are responsible for the amplitude increase during maturation. In Per1, Cry1, and Clock mRNA rhythms, the rhythm maximum increased, whereas in Bmal1 mRNA rhythm, the rhythm minimum decreased. Maturation of Per2 mRNA rhythm proceeded by both mechanisms, i.e., the maximum increased and the minimum decreased. Maturation of overt rhythms controlled by the SCN may proceed in a similar way. For example, during the postnatal increase of the pineal arylalkylamine-N-acetyltransferase rhythm amplitude, the maximum nighttime activity increases, and simultaneously the minimum daytime activity decreases (38). In the early postnatal stage, the rhythm in Bmal1 expression was already in an opposite phase to those in the Per1 and Per2 mRNA expression, similarly as in the case of the adult SCN (13). No clear rhythm in Clock mRNA was detected either at P3 or at P10. The finding is in agreement with studies reporting no rhythm in Clock expression in the adult mammalian SCN (12, 13). Nevertheless, under conditions of a short but not a long photoperiod, a mild Clock mRNA rhythm was recently reported in the rat SCN (39). Although at P3 and even at P10 intrinsic SCN rhythmicity (unpublished results) as well as overt rhythms controlled by the SCN (40) are not yet fully entrained by the photoperiod and resemble rather rhythms under a short photo-period, no rhythm in Clock mRNA expression appeared early postnatally.
Our data indicate that in the rat SCN, four of the studied clock genes, namely Per1, Per2, Cry1, and Bmal1, were expressed in a cyclic manner already at P3. Such a situation may not, however, happen in peripheral oscillators. A recent study on ontogeny of circadian rhythmicity in the rat heart shows that although the rhythmic expression of Per1 and Bmal1 started between P2 and P5, Per2 mRNA did not show rhythmicity until P14 (41). In the heart, rhythmic expression of Per2 mRNA was apparently not essential for the generation of circadian rhythmicity at the early postnatal age.
Our study shows the gradual pre- and postnatal development of both positive and negative feedback loops in the mammalian SCN. Rhythms of Per1, Per2, Cry1, and Bmal1 expression were not yet detectable in the fetal rat SCN at E19 by in situ hybridization; however, they were clearly present at P3 postnatally and thereafter slowly matured. To find out the mechanism that actually triggers the cycling of clock gene expression, a still more detailed analysis of the molecular clockwork development is needed. This would include not just studies on clock gene expression and levels of their products, but also on the development of posttranslational modulation of these products and on the dynamism of their nucleocytoplasmic shuttling in the SCN cells (34, 42, 43).
Acknowledgments
We thank Lucie Čížková and Lucie Drábková for their excellent technical assistance; Prof. Hitoshi Okamura (Kobe University School of Medicine, Kobe, Japan) for his generous gift of the plasmid templates used for the synthesis of rPer1, rPer2, and mCry1 riboprobes; and Prof. Steven M. Reppert (University of Massachusetts Medical School, Worcester) for his generous gift of mPER1 antiserum and the blocking peptide. This work was supported by Grant Agency of the Czech Republic Grant 309021241 and by Research Project AVOZ 5011922.
Abbreviations: SCN, suprachiasmatic nucleus; LD, light–dark; En, embryonic day n; Pn, postnatal day n; CTn, circadian time n.
References
- 1.Pittendrigh, C. L. (1981) in Biological Rhythms: Handbook of Behavioral Neurology, ed. Aschoff, J. (Plenum, New York), Vol. 4, pp. 95-124. [Google Scholar]
- 2.Klein, D. C., Moore, R. Y. & Reppert, S. M., eds. (1991) Suprachiasmatic Nucleus: The Mind's Clock (Oxford Univ. Press, New York).
- 3.Schwartz, W. J. (1991) in Suprachiasmatic Nucleus: The Mind's Clock, eds. Klein, D. C., Moore, R. Y. & Reppert, S. M. (Oxford Univ. Press, New York), pp. 144-156.
- 4.Gillette, M. U. (1991) in Suprachiasmatic Nucleus: The Mind's Clock, eds. Klein, D. C., Moore, R. Y. & Reppert, S. M. (Oxford Univ. Press, New York), pp. 125-143.
- 5.Guido, M. E., deGuido, L. B., Goguen, D., Robertson, H. A. & Rusak, B. (1999) J. Biol. Rhythms 14, 275-280. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz, W. J., Caprio, A., Jr., de la Iglesia, H. O., Baler, R., Klein, D. C., Nakabeppu, Y. & Aronin, A. (2000) Neuroscience 98, 535-547. [DOI] [PubMed] [Google Scholar]
- 7.Sumová, A., Trávníčková, Z., Mikkelsen, J. D. & Illnerová, H. (1998) Brain Res. 801, 254-258. [DOI] [PubMed] [Google Scholar]
- 8.Kornhauser, J. M., Mayo, K. E. & Takahashi, J. S. (1993) in Molecular Genetics of Biochemical Rhythms, ed. Young, M. W. (Dekker, New York), pp. 271-307.
- 9.Schwartz, W. J., Aronin, A., Takeuchi, J., Bennet, M. R. & Peters, R. J. (1995) Semin. Neurol. 7, 53-60. [Google Scholar]
- 10.Van den Pol, A. N. (1991) in Suprachiasmatic Nucleus: The Mind's Clock, eds. Klein, D. C., Moore R. Y. & Reppert, S. M. (Oxford Univ. Press, New York), pp. 17-50.
- 11.Majzoub, J. A., Robinson, B. C. & Emanuel, R. L. (1991) in Suprachiasmatic Nucleus: The Mind's Clock, eds. Klein, D. C., Moore, R. Y. & Reppert, S. M. (Oxford Univ. Press, New York), pp. 177-190.
- 12.King, D. P. & Takahashi, J. S. (2000) Annu. Rev. Neurosci. 23, 713-742. [DOI] [PubMed] [Google Scholar]
- 13.Reppert, S. M. & Weaver, D. R. (2001) Annu. Rev. Physiol. 63, 647-676. [DOI] [PubMed] [Google Scholar]
- 14.Etchegaray, J.-P., Lee, C., Wade, P. A. & Reppert, S. M. (2003) Nature 421, 177-181. [DOI] [PubMed] [Google Scholar]
- 15.Bae, K., Jin, X., Maywood, E. S., Hastings, M. H., Reppert, S. M. & Weaver, D. R. (2001) Neuron 30, 525-536. [DOI] [PubMed] [Google Scholar]
- 16.Zheng, B., Albrecht, U., Kaasil, K., Sage, M., Lu, W., Vaishnav, S., Li, Q., Sun, Z. S., Eichele, G., Bradley, A., et al. (2001) Cell 105, 683-694. [DOI] [PubMed] [Google Scholar]
- 17.Yu, W., Nomura, M. & Ikeda, M. (2002) Biochem. Biophys. Res. Commun. 290, 933-941. [DOI] [PubMed] [Google Scholar]
- 18.Preitner, N., Damiola, F., Lopez-Molina, L., Zakany, J., Duboule, D., Albrecht, U. & Schibler, U. (2002) Cell 110, 251-260. [DOI] [PubMed] [Google Scholar]
- 19.Moore, R. Y. (1991) in Suprachiasmatic Nucleus: The Mind's Clock, eds. Klein, D. C., Moore, R. Y. & Reppert, S. M. (Oxford Univ. Press, New York), pp. 197-216.
- 20.Reppert, S. M. & Schwartz W. J. (1984) J. Neurosci. 4, 1677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reppert, S. M. & Uhl, G. R. (1987) Endocrinology 120, 2483-2487. [DOI] [PubMed] [Google Scholar]
- 22.Shibata, S. & Moore, R. Y. (1987) Brain Res. 431, 311-315. [DOI] [PubMed] [Google Scholar]
- 23.Shimomura, H., Moriya, T., Sudo, M., Wakamatsu, H., Akiyama, M., Miyake, Y. & Shibata, S. (2001) Eur. J. Neurosci. 13, 687-693. [DOI] [PubMed] [Google Scholar]
- 24.Ohta, H., Honma, S., Abe, H. & Honma, K. (2002) Eur. J. Neurosci. 15, 1953-1960. [DOI] [PubMed] [Google Scholar]
- 25.Ohta, H., Honma, S., Abe, H. & Honma, K. (2003) Eur. J. Neurosci. 17, 1628-1634. [DOI] [PubMed] [Google Scholar]
- 26.Shearman, L. P., Jin, X., Lee, C., Reppert, S. M. & Weaver, D. R. (2000) Mol. Cell. Biol. 20, 6269-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebling, F. J. P., Maywood, E. S., Staley, F., Humby, T., Hancock, D. C., Waters, C. M., Evan, G. J. & Hastings, M. H. (1991) J. Neuroendocrinol. 3, 641-652. [DOI] [PubMed] [Google Scholar]
- 28.Sun, Z. S., Albrecht, U., Zhuchenko, O., Barley, J., Eichele, G. & Lee, C. C. (1997) Cell 90, 1003-1011. [DOI] [PubMed] [Google Scholar]
- 29.Hastings, M. H., Field, M. D., Maywood, E. S., Weaver, D. R. & Reppert, S. M. (1999) J. Neurosci. 19, 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumová, A., Sládek, M., Jáč, M. & Illnerová, H. (2000) Brain Res. 947, 260-270. [DOI] [PubMed] [Google Scholar]
- 31.Davis, F. C. & Mannion, Y. (1988) Am. J. Physiol. 255, R439-R448. [DOI] [PubMed] [Google Scholar]
- 32.Weaver, D. R. & Reppert, S. M. (1995) Mol. Brain Res. 33, 136-148. [DOI] [PubMed] [Google Scholar]
- 33.Reppert, S. M. & Weaver, D. R. (1991) in Suprachiasmatic Nucleus: The Mind's Clock, eds. Klein, D. C., Moore, R. Y. & Reppert, S. M. (Oxford Univ. Press, New York), pp. 405-418.
- 34.Yagita, K., Tamanini, F., Yasuda, M., Hoeijmakers, J. H. J., van der Horst, G. T. J. & Okamura, H. (2002) EMBO J. 21, 1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan, L., Shigeyoshi, S. & Okamura, H. (1999) Neuroscience 94, 141-150. [DOI] [PubMed] [Google Scholar]
- 36.Yan, L. & Okamura, H. (2002) Eur. J. Neurosci. 15, 1153-1162. [DOI] [PubMed] [Google Scholar]
- 37.Dardente, H., Poirel, V. J., Pevet, P. & Masson-Pevet, M. (2002) Brain Res. 958, 261-271. [DOI] [PubMed] [Google Scholar]
- 38.Illnerová, H. (1975) Physiol. Bohemoslov. 24, 493-500. [PubMed] [Google Scholar]
- 39.Sumová, A., Jáč, M., Sládek, M., Šauman, I. & Illnerová, H. (2003) J. Biol. Rhythms 18, 134-144. [DOI] [PubMed] [Google Scholar]
- 40.Vaněček, J. & Illnerová, H. (1985) Neuroendocrinology 41, 186-191. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto, K., Oishi, K., Nagase, T., Miyazaki, K. & Ishida, A. (2002) NeuroReport 13, 1239-1242. [DOI] [PubMed] [Google Scholar]
- 42.Yagita, K., Yamaguchi, S., Tamanini, F., van der Horst, G. T. J., Hoeijmakers, J. H. J., Yasui, A., Loros, J. J., Dunlap, J. C. & Okamura, H. (2000) Genes Dev. 14, 1353-1363. [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazaki, K., Mesaki, M. & Ishida, A. (2001) Mol. Cell. Biol. 21, 6651-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]