Abstract
Background
Magnifying endoscopy (ME) with narrow-band imaging (NBI) has been described as useful in diagnosing colorectal neoplasms. However, there is no standardized simple classification system, and its usefulness in comparison with pit pattern diagnosis by magnifying chromoendoscopy (MC) is unclear. The aim of this study was to investigate the usefulness of evaluations of microvascular architecture (MV) and microsurface structure (MS) by ME with NBI in the diagnosis of colorectal neoplasms.
Methods
A total of 360 colorectal neoplasms were analyzed by retrospective analysis of prospectively collected data. The vessel plus surface (VS) classification system was applied for ME with NBI diagnosis. The main outcome measurement was comparison of the diagnostic performance of ME with NBI and MC.
Results
The sensitivity of ME with NBI and MC for the diagnosis of adenomas was 91.9% and 95.7%, respectively, and their specificity was 79.2% and 79.9%, respectively. The sensitivity of ME with NBI and MC for the diagnosis of cancer was 70.5% and 79.9%, respectively, and the specificity was 95.3% and 95.7%, respectively. The sensitivity of ME with NBI and MC for the diagnosis of cancer with deep submucosal invasion was 50.0% and 88.0%, respectively (P < 0.0001), and their specificity was 100% and 82.8%, respectively (P < 0.0001).
Conclusions
The specificity of evaluation of MV and MS by ME with NBI for the diagnosis of cancer with deep submucosal invasion was much higher than that of pit pattern analysis by MC.
Keywords: Magnifying endoscopy, narrow-band imaging, colorectal neoplasm, cancer
Introduction
Differentiating between adenoma and carcinoma and determining the depth of invasion of colorectal neoplasias are very important in deciding upon the treatment strategies. The development of high-resolution endoscopy with optical magnification enables endoscopists to perform detailed endoscopic examinations of neoplasias, and that has contributed to an improvement in diagnostic performance. Moreover, narrow-band imaging (NBI) [1], an optical method of image-enhanced endoscopy, has been reported to be useful for estimating histological diagnosis of colorectal lesions [2,3] as well as detection colonoscopy [4,5]. NBI provides better visualization of more superficial mucosal structures due to the shorter penetration depth and better optical features of the short-wavelength light delivered within the narrow bandwidth [6]. As the degree of histological atypia increases in colorectal tumor lesions and angiogenesis progresses, the diameter and density of the blood vessels increase [7]. Accordingly, the contrast in the neoplasms is enhanced in comparison with the surrounding mucosa, and the lesions become easier to detect. Moreover, classifications based the morphology of the microvascular architecture [8] or classifications that include the pit pattern observed indirectly [9] have been proposed in order to accurately diagnose colorectal neoplasms by magnifying endoscopy (ME) with NBI. However, these classifications are complicated for endoscopists to use in clinical practice.
We therefore investigated whether the simplified diagnostic system by ME with NBI that Yao et al [6,10-12] has already proposed for the stomach, which has a glandular epithelium similar to the large bowel, would be useful for diagnosing colorectal neoplasms. First, we attempted to identify the characteristics of adenomas, carcinomas, and SM carcinomas by using the vessel plus surface (VS) classification system based on observations made by ME with NBI. Then, we drew up diagnostic criteria for qualitative diagnosis based on the findings, and compared its diagnostic performance with that of pit pattern analysis by magnifying chromoendoscopy (MC).
Methods
Patients
The subjects of this study were prospectively collected consecutive patients who had a colorectal epithelial tumor resected endoscopically or by surgery at Fukuoka University Chikushi Hospital between January 2007 and July 2011 and in which a definite diagnosis was made histopathologically. The inclusion criteria were a colorectal adenoma or early colorectal carcinoma that had been examined by ME with NBI and in which it had been possible to adequately examine the pit pattern by MC with crystal violet staining preoperatively. Nonneoplastic polyps that did not require treatment and in which the microvascular architecture was not visually confirmed [2] were excluded. All patients provided written informed consents for undergoing colonoscopic examinations.
There were 339 patients, and they had a total of 360 lesions. The male-to-female ratio was 214:125, and the mean age (range) of the subjects was 65.4 (26-85) years. The clinicopathological features of the 360 lesions are shown in Table 1. The macroscopic classification was according to the update on the Paris classification [13].
Table 1.
Clinicopathological features of colorectal neoplasms (n=360) Mean size (range) 17.9 (4-80) mm
All endoscopic findings were analyzed retrospectively by two endoscopists (T.H. and K.Y.) who were experienced for more than 15 years in the field of colonoscopy and who had previously assessed more than 500 cases with NBI with ME. Reviewers were blinded to the histopathological diagnosis. The final evaluation of endoscopic findings was decided by agreement of reviewers.
This study was approved by the Medical Ethics Committee of Fukuoka University Chikushi Hospital.
Histopathological investigations
Resected specimens were mounted on a plate and fixed in 20% buffered formalin solution overnight. These specimens were embedded on paraffin and cut into 5 μm-thick tissue sections. Then the sections were subsequently stained with hematoxylin-eosin. Histopathological diagnosis was made by an experienced pathologist (A.I.) who was blind to endoscopic findings. Histopathological diagnosis was made based upon the World Health Organization criteria [14]. The tumors were classified into three groups: 1) adenoma; 2) intramucosal carcinoma to cancer with superficial submucosal invasion (M/ SMs); and 3) cancer with deep submucosal invasion (SMd). The histopathological diagnosis was used as the gold standard. The SMs was defined as “the depth of submucosal invasion is less than 1000 μm” and the SMd was defined as “the depth of submucosal invasion is 1000 μm or more” [15,16]. According to the histopathological diagnoses, 211 lesions (58.6%) were adenomas, 99 lesions (27.5%) were M/SMs, and 50 lesions (13.9%) were SMd.
Endoscopic specification
The endoscopic procedures were performed using an electronic endoscopy system (EVIS-LUCERA SPECTRUM; Olympus, Optical Co. Ltd., Tokyo, Japan) with a magnifying colonoscopy (CF-H260AZI, PCF-Q240ZI; Olympus, Optical Co. Ltd., Tokyo, Japan). We used a soft black hood attachment at the tip of the scope. All of the patients were given 2-3 L of a polyethylene glycol-electrolyte solution starting in the morning of the day of the examination as preparation. When a colorectal epithelial lesion was detected by routine observations, the mucosa on the tumor surface was washed and immediately examined by ME with NBI. Subsequently, pit pattern diagnosis was performed by MC after staining 0.05% crystal violet.
Magnifying endoscopy with Narrow-band imaging diagnosis
The VS classification system [6,10-12] was applied for ME with NBI diagnosis. More specifically, the microvascular (MV) patterns and microsurface (MS) patterns observed by ME with NBI were classified into three types.
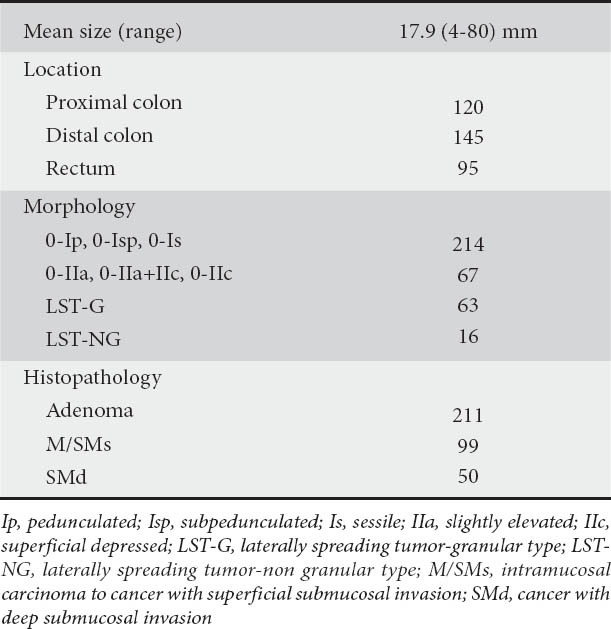
Three types of MV pattern are defined as follows (Fig. 1):
Figure 1.
Microvascular pattern according to VS classification by ME with NBI for colorectal lesions. (A) Regular MV pattern. (B) Irregular MV pattern. (C) Absent MV pattern
VS, vessel plus surface; ME, magnifying endoscopy; NBI, narrow-band imaging; MV, microvascular pattern
-
1)
Regular MV pattern: The mucosal capillaries have a uniform shape that can be closed-loop or open-loop. They have a consistent size and their arrangement and distribution are regular and symmetrical.
-
2)
Irregular MV pattern: The vessels differ in shape, being closed-loop, open-loop, tortuous, branched and bizarrely shaped, with or without a network. The size of the vessels also varies and their arrangement and distribution are irregular and asymmetrical.
-
3)
Absent MV pattern: No subepithelial microvascular pattern is visible.
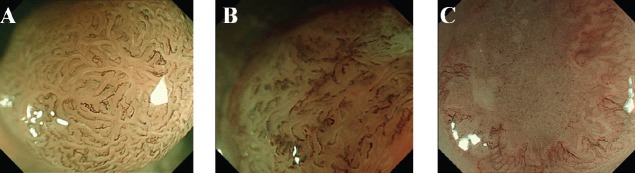
Three types of MS pattern are defined as follow (Fig. 2).
Figure 2.
Microsurface pattern according to VS classification by ME with NBI for colorectal lesions. (A) Regular MS pattern. (B) Irregular MS pattern. (C) Absent MS pattern
VS, vessel plus surface; ME, magnifying endoscopy; NBI, narrow-band imaging; MS, microsurface pattern
-
1)
Regular MS pattern: The individual morphology of marginal crypt epithelium shows a uniform round/oval/tubular/linear/curved/papillary structure. The width and length of the marginal crypt epithelium are constant. The arrangement and distribution are regular and symmetrical.
-
2)
Irregular MS pattern: The individual morphology of marginal crypt epithelium shows an irregular tubular/ linear/curved/papillary/villous structure. The width and length of the marginal crypt epithelium vary. The arrangement and distribution are irregular and asymmetrical.
-
3)
Absent MS pattern: No epithelial structure is visible by magnifying endoscopy.
Pit pattern analysis by magnifying chromoendoscopy
The endoscopic evaluation focused on the pit pattern was categorized according to Kudo’s classification (type I·II·III·IV·VI·VN) [17,18]. The type III, and IV pit patterns were considered indicators of adenoma, and the type V pit pattern was considered an indicator of cancer. In addition, the type V pit pattern was divided into a VI-low irregularity pattern and a VI-high irregularity pattern [19-21]. The type VI-high irregularity pit pattern and type VN pit pattern were considered indicators of deep invasion of the SM.
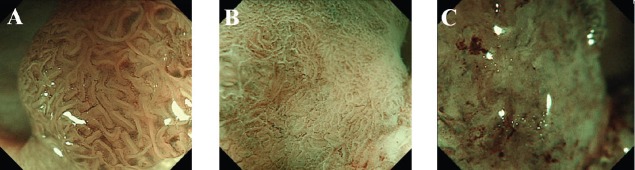
These indicators were used as the basis for comparing the sensitivity, specificity, and diagnostic accuracy of ME with NBI and MC (Fig. 3).
Figure 3.
Representative images of adenoma, M/SMs and SMd. Adenoma. (A) VS classification by ME with NBI. MV and MS both regular. (B) Pit pattern analysis by MC. Type IIIL pit pattern. (2) M/SMs. (C) VS classification by ME with NBI. MV and MS both irregular. (D) Pit pattern analysis by MC. Type VI-low irregularity pit pattern. (3) SMd. (E) VS classification by ME with NBI. MV and MS both irregular plus both absent. (F) Pit pattern analysis by MC. Type VN pit pattern
M/SMs, intramucosal carcinoma to cancer with superficial submucosal invasion; SMd, cancer sith deep submucosal invasion; VS, vessel plus surface; ME, magnifying endoscopy; NBI, narrow-band imaging; MV, microvascular pattern; MS, microsurface pattern; MC, magnifying chromoendoscopy
Statistical analysis
Data are presented as 95% confidence intervals. The mean values were compared by Student's t-test. Differences at P < 0.05 were considered to be significant. The κ statistics was used for inter-observer variation. SPSS 11.5J (SPSS Inc, Chicago, IL) was used for the statistical processing.
Results
The results of the 360 cases based on the VS classification system are shown in Table 2. In the adenoma cases, the most common MV pattern was regular (88.6%), and the most common MS pattern was regular (95.3%). In the M/SMs cases, the most common MV pattern was irregular (59.6%), and the most common MS pattern was irregular (50.5%). In the SMd cases, the most common MV pattern was irregular plus absent (62.0%), and the most common MS pattern was irregular plus absent (56.0%).
Table 2.
ME with NBI findings according to VS classification system (n=360)
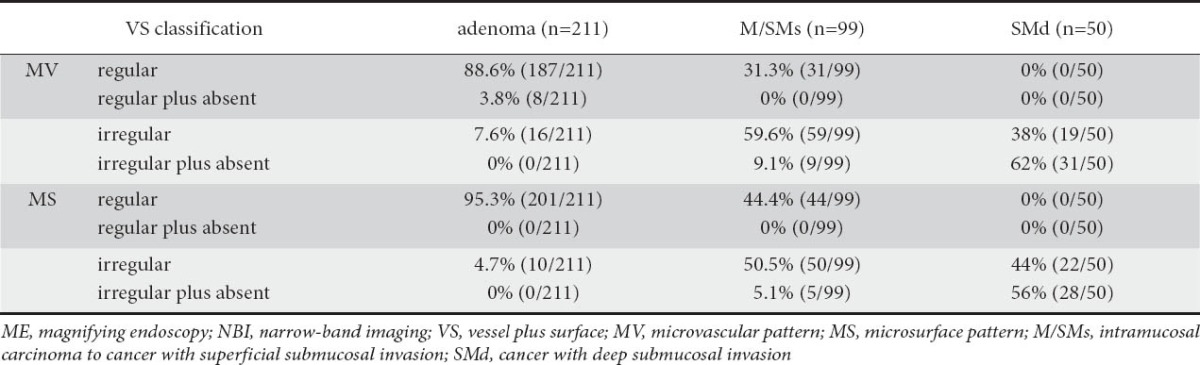
Based on these findings, the presence of MV pattern and MS pattern both regular (regardless of whether an absent area was present or not) was considered an indicator of adenoma, the presence of MV pattern and MS pattern both irregular (regardless of whether an absent area was present or not) was considered an indicator of cancer, and the presence of MV pattern and MS pattern both irregular plus absent was considered an indicator of SMd.
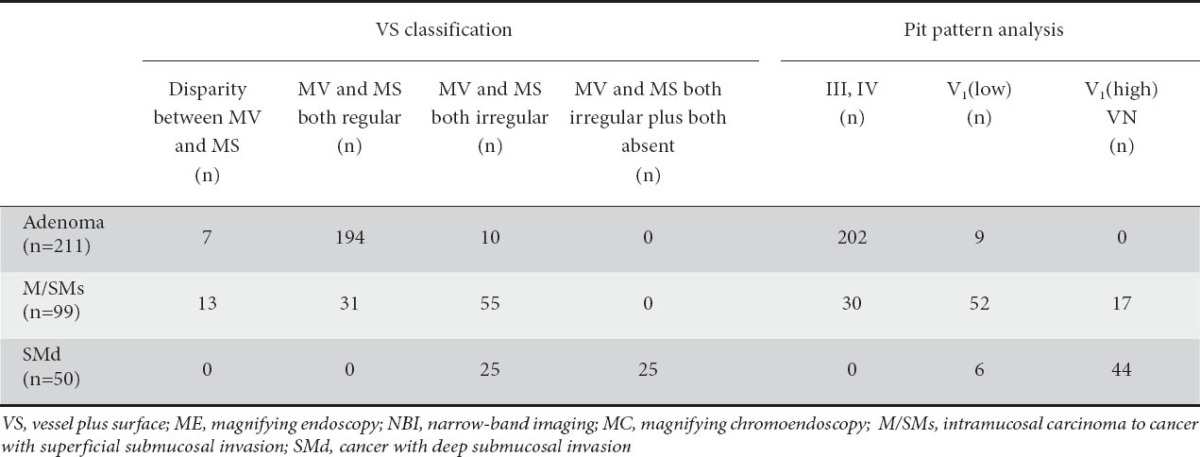
In 194 of the 211 adenoma cases, MV and MS patterns were both regular, in 105 of the 149 cancer cases (M/SMs and SMd), MV and MS patterns were both irregular, and in 25 of the 50 SMd cases, MV and MS patterns were both irregular plus absent. In 7 of the 211 adenoma cases and in 13 of the 99 M/SMs cases, there was a disparity between MV and MS (e.g. MV: regular, MS: irregular).
According to the pit pattern analysis, 202 of the 211 adenomas showed a type III, or IV pit pattern, 119 of the 149 cancers showed a type V pit pattern, and 44 of the 50 SMd cases showed a type VI-high irregularity pit pattern and type VN pit pattern (Table 3).
Table 3.
Relationship between VS classification by ME with NBI and pit pattern analysis by MC
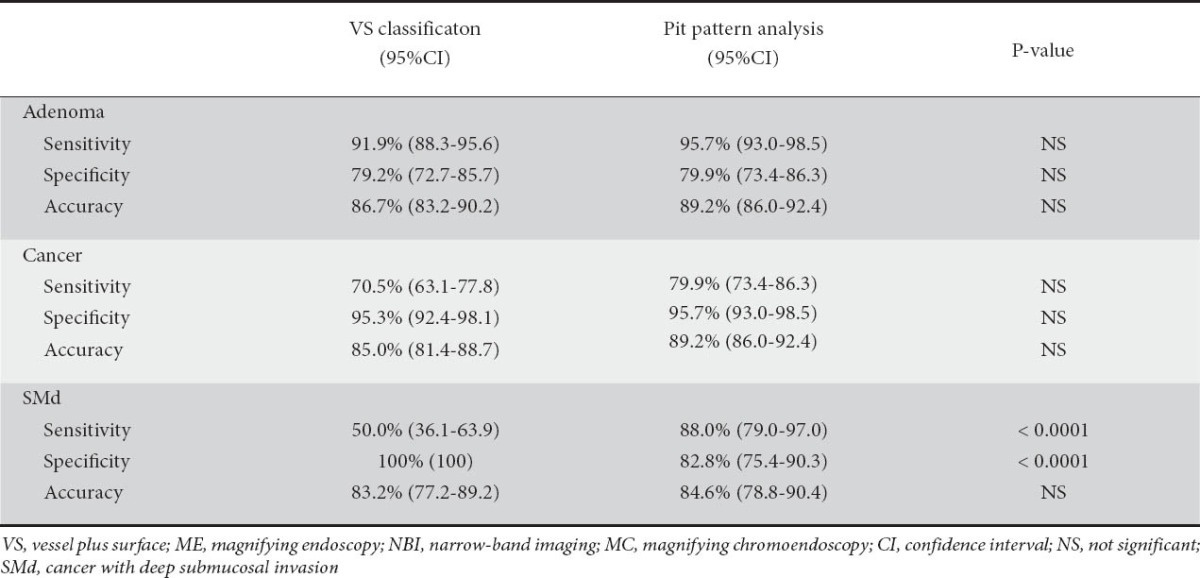
The diagnostic performance of the VS classification by ME with NBI and pit pattern analysis by MC is compared in Table 4. There was no significant difference between the sensitivity, specificity, or accuracy of VS classification and pit pattern analysis for the diagnosis of adenoma and cancer. The sensitivity of pit pattern analysis (88.0%) for the diagnosis of SMd was significantly higher than that of VS classification (50%) (P <0.0001). On the other hand, the specificity of VS classification (100%) was significantly higher than that of pit pattern analysis (82.8%) (P <0.0001).
Table 4.
Diagnostic accuracy of VS classsification by ME with NBI versus pit pattern analysis by MC
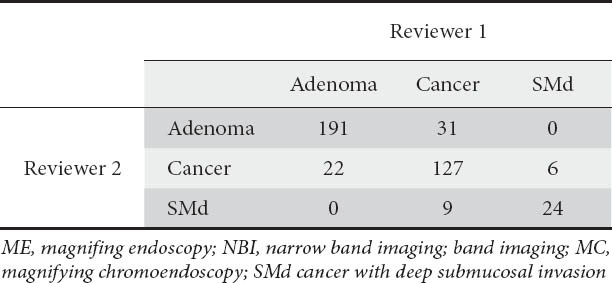
The κ values of inter-observer variation for ME with NBI diagnosis was 0.703 (Table 5) (moderate agreement [22]).
Table 5.
Inter-observer variation fo ME with NBI analysis by MC analysis by MC
Discussion
In this study, we applied the VS classification system by ME with NBI for use in colorectal neoplasms, a simplified diagnostic system that has already been proposed by Yao et al [6,10-12]. This study suggests that the VS classification system can be applied to the colorectum and can identify the characteristics of the colorectal neoplasms. In particular, the specificity of VS classification for the diagnosis of SMd was significantly higher than that of pit pattern analysis.
The number of reports claiming that NBI is useful for making a histological diagnosis of colorectal lesions has been increasing in recent years. Sano et al classified microvascular architecture into 3 patterns based on its morphology in observations of lesions with an NBI system and proposed a capillary pattern (CP) classification [2,8,23]. Wada et al [18,24] conducted a study in which they classified vascular patterns into 6 categories. However, since microsurface structure patterns as well as microvascular patterns are visualized in actual NBI observations, studies that employed both patterns have also been conducted. As described in the report by East et al [3], the morphology of the crypt openings of small diminutive colonic polyps was clearly depicted in NBI observations. Based on such findings, an attempt has even been made to devise an NBI classification that includes the morphology of the pit patterns that can be observed. Kanao et al [9] devised a classification into type A, B, and C (C1, C2, and C3) based on both the pit appearance and morphology of the microvascular architecture that could be observed by NBI.
Pit pattern analysis by MC has long been known to be a useful diagnostic method for histopathological diagnosis [17,18,25-27]. It is very useful if the pit pattern can also be observed simultaneously during NBI observations. However, when the crypt openings are small or the microsurface structure of cancers has been distorted, it is sometimes difficult to observe the morphology of the crypt openings by NBI.
For these reasons, it is impossible to consistently observe the pit pattern by NBI alone. Furthermore, there are many categories in each of these classifications, and they are too complicated to use by endoscopists in clinical practice. We therefore applied the VS classification system, a simple diagnostic system that has already been proposed by Yao et al [6,10-12] for use in the stomach. Briefly, we simply classified the morphology of the microvascular pattern and microsurface pattern into regular, irregular or absent. Then we integrated both the microvascular pattern and microsurface pattern. This VS classification system is a simple but comprehensive diagnostic system that can be commonly applied throughout the GI tract [28]. This study demonstrates that the diagnostic performance between the VS classification and pit pattern analysis of adenoma and cancer was almost the same, except that the sensitivity of pit pattern analysis for the diagnosis of adenoma was significantly higher than that of VS classification. We concluded that the VS classification system can also be applied to the diagnosis of colorectal neoplasms. Certainly, this diagnostic test results (sensitivity, specificity, accuracy) were not representative of those in clinical practice, because we recruited 360 lesions, 50 of which were SMd and 99 of which were M/SMs at a single hospital. Further longitudinal studies are warranted in order to clarify the potential of the VS classification system.
When a colorectal lesion was detected by conventional endoscopy, ME with NBI was immediately performed. Since not a single SMd case was included when MV and MS were both regular by ME with NBI, endoscopic resection might be simply indicated. Since lesions that ranged from adenomas to deeply invasive SM cancers were included when MV and MS were both irregular, it appeared preferable to make the diagnosis based on a combination of examinations, such as pit pattern analysis by MC and endoscopic ultrasonography, and to decide upon the approach to treatment. Although MV and MS both irregular plus both absent had poor sensitivity as an indicator of SMd, the combination had remarkably high specificity (100%). With regard to the pit pattern and analysis of these 25 lesions, 4 of the 25 lesions showed the type VI-high irregularity pit pattern, while 21 of the 25 lesions demonstrated the type VN pit pattern (data not shown in the results). The probability of lymph node metastasis is known to be low when the depth of SM invasion is less than 1000 µm [15]. Determining whether there is scanty submucosal invasion or submucosal deep invasion by an endoscopic examination before treatment is also very important in order to decide upon the therapeutic strategies. Furthermore, since there are 20 cases in which it is difficult differentiate between regular and irregular pit patterns and in which there is a disparity between MV and MS, it is necessary to perform pit pattern analysis by MC.
We assumed that the simple VS classification system that has been proposed for the stomach might also be applied to the differentiation of neoplastic from non-neoplastic colorectal lesions in combination with pit patterns until a wider remit of lesions is investigated. Further investigations are necessary to elucidate whether this classification may provide better clinical decision making in the management of colorectal neoplasms.
Summary Box.
What is already known:
ME with NBI is useful for making a histological diagnosis of colorectal lesions
The VS classification system is a simple but comprehensive diagnostic system that has been proposed for the stomach
What the new findings are:
The VS classification system can be applied to clorectal lesions
MV and MS both regular was consedered an indecator of adenoma. MV and MS both irregular was considered an incicator of cancer
MV and MS both irregular plus both absent was significant indicator of SMd
Biography
Fukuoka University Chikushi Hospital, Fukuoka, Japan
Footnotes
Conflict of Interest: None
References
- 1.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 2.Sano Y, Ikematsu H, Fu KI, et al. Meshed capillary vessels by use of narrow-band imaging for differenial diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278–283. doi: 10.1016/j.gie.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 3.East JE, Suzuki N, Bassett P, et al. Narrow band imaging with magnification for the characterization of small and diminutive colonic polyps: pit pattern and vascular pattern intensity. Endoscopy. 2008;40:811–817. doi: 10.1055/s-2008-1077586. [DOI] [PubMed] [Google Scholar]
- 4.Uraoka T, Saito Y, Matsuda T, et al. Detectability of colorectal neoplastic lesions using a narrow-band imaging system: a pilot study. J Gastroenterol Hepatol. 2008;23:1810–1815. doi: 10.1111/j.1440-1746.2008.05635.x. [DOI] [PubMed] [Google Scholar]
- 5.Sikka S, Ringold DA, Jonnalagadda S, Jonnalagadda S, Banerjee B. Comparison of white light and narrow band high definition images in predicting colon polyp histology, using standard colonoscopies without optical magnification. Endoscopy. 2008;40:818–822. doi: 10.1055/s-2008-1077437. [DOI] [PubMed] [Google Scholar]
- 6.Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462–467. doi: 10.1055/s-0029-1214594. [DOI] [PubMed] [Google Scholar]
- 7.Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354–1362. doi: 10.1054/bjoc.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sano Y, Horimatsu T, Fu KI, Katagiri A, Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesions using a narrow-band imaging system. Dig Endosc. 2006;18:S44–S51. [Google Scholar]
- 9.Kanao H, Tanaka S, Oka S, Hirata M, Yoshida S, Chayama K. Narrow-band imaging magnification predicts the histology and invasion depth of colorectal tumors. Gastrointest Endosc. 2009;69:631–636. doi: 10.1016/j.gie.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Yao K, Iwashita A, Kikuchi Y, et al. Novel zoom endoscopy technique for visualizing the microvascular architecture in gastric mucosa. Clin Gastroenterol Hepatol. 2005;3:23–26. doi: 10.1016/s1542-3565(05)00255-7. [DOI] [PubMed] [Google Scholar]
- 11.Yao K, Iwashita A, Tanabe H, et al. Novel zoom endoscopy technique for diagnosis of small flat gastric cancer: a prospective, blind study. Clin Gastroenterol Hepatol. 2007;5:869–878. doi: 10.1016/j.cgh.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Ezoe Y, Muto M, Uedo N, et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017–2025. doi: 10.1053/j.gastro.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton SR, Aaltonen LA. Lyon, France: IARC Press; 2000. World Health Organization classification of tumours: pathology and genetics of tumours of the digestive system; pp. 104–119. [Google Scholar]
- 15.Second English Edition. Tokyo: Kanehara; 2009. Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma. [Google Scholar]
- 16.Kitajiam K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma : a Japanese collaborative study. J Gastroenterol. 2004;39:534–543. doi: 10.1007/s00535-004-1339-4. [DOI] [PubMed] [Google Scholar]
- 17.Kudo S, Hirota S, Nakajima T, et al. Colorectal tumors and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367–373. doi: 10.1055/s-2004-826104. [DOI] [PubMed] [Google Scholar]
- 19.Wada Y, Kashida H, Kudo S, Misawa M, Ikehara N, Hamatani S. Diagnostic accuracy of pit pattern and vascular pattern analysis in colorectal lesions. Dig Endosc. 2010;22:192–199. doi: 10.1111/j.1443-1661.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto Y, Watanabe H, Tominaga K, et al. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification: Including comparison with magnifying chromoendoscopy. Dig Dis Sci. 2011;56:532–538. doi: 10.1007/s10620-010-1293-3. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Nagahara A, Terai T, et al. Evaluation of new subclassification of type VI pit pattern for determining the depth and type of invasion of colorectal neoplasm. J Gastroenterol. 2011;46:31–38. doi: 10.1007/s00535-010-0300-y. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 23.Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010;10:33. doi: 10.1186/1471-230X-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada Y, Kudo S, Kashida H, et al. Diagnosis of colorectal lesions with the magnifying narrow-band imaging system. Gastrointest Endosc. 2009;70:522–531. doi: 10.1016/j.gie.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Hurlstone DP, Cross SS, Adam I, et al. Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut. 2004;53:284–290. doi: 10.1136/gut.2003.027623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S, Kaltenbach T, Chayama K, Soetikno R. High-magnification colonoscopy (with videos) Gastrointest Endosc. 2006;64:604–613. doi: 10.1016/j.gie.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Tobaru T, Mitsuyama K, Tsuruta O, Kawano H, Sata M. Subclassification of type VI pit patterns in colorectal tumors : relation to the depth of tumor invasion. Int J Oncol. 2008;33:503–508. [PubMed] [Google Scholar]
- 28.Singh R, Anagnostopoulos GK, Yao K, et al. Narrow-band imaging with magnification in Barrett's esophagus: validation of a simplified grading system of mucosal morphology patterns against histology. Endoscopy. 2008;40:457–463. doi: 10.1055/s-2007-995741. [DOI] [PubMed] [Google Scholar]


