Abstract
Background
The aim of our study was to ascertain factors that favor early discharge and predict mortality in post-percutaneous endoscopic gastrostomy (PEG) patients.
Methods
Successive patients who underwent successful PEG placement during a 10-year period in a single New York City hospital were included in the study. Data was retrospectively extracted from hospital electronic medical records.
Results
Two hundred and eighty-four patients underwent successful PEG placement. Forty-six patients (16%) were discharged within 3 days of PEG placement (early discharge). Two hundred and thirty six patients (84%) remained in hospital from 4 to 244 days (median 13.5) after PEG insertion (late discharge). Twenty-six (9%) patients died in-house after PEG placement. A serum albumin level <2.2 g/dL (P=0.007) and presence of 2 or more co-morbidities (P=0.019) were predictors of late discharge. A dementia indication was twice as likely to result in an early discharge compared to a stroke indication (OR 2.39; 95% CI 1.07-5.36; P=0.033). Female sex, positive urine cultures and low serum albumin levels were independent predictors of in-house mortality.
Conclusion
Clinical and laboratory markers may predict post-PEG mortality as well as early patient discharge.
Keywords: Post-PEG mortality, co-morbidities
Introduction
Since its introduction in 1980 [1,2], percutaneous endoscopic gastrostomy (PEG) has become increasingly popular and has supplanted surgical gastrostomy as the procedure of choice for enteral nutrition in patients unable to partake of adequate oral feeds due to impairment of swallowing function or dementia.
Despite its popularity, the beneficial effect of PEG is uncertain or limited to selected patients [3-8]. The utility of PEG in stroke patients has not been validated. One study [9] indicated that stroke patients with PEGs were more likely to have a higher complication rate and mortality when compared to those without PEGs. Likewise, PEG insertion has not shown to provide survival benefit in patients with dementia [10].
In patients with head and neck cancer early placement of a PEG may enhance the nutritional status and facilitate treatment [3,4]. But even this subset suffers from an over usage of PEGs [11].
Notwithstanding its narrow scope of benefit, PEGs continue to be placed in significant numbers. Over 200,000 PEGs are performed annually in the United States [2].
Driving these numbers is an ethical nuance. Artificial nutrition and hydration (ANH) is ethically and legally accepted as medical treatment [12]. Healthcare providers therefore feel obligated to provide this ‘medical’ treatment and family members are more likely to equate withholding ANH with ‘starving a person to death’ [12,13].
Apart from ethical and medical considerations a logistic factor has been added to the equation making a complex issue even more complicated. Nursing homes are increasingly insisting on PEG placement prior to admission which in turn is prompting hospitals to take an aggressive approach to PEG placement in order to shorten the length of hospital stay. Our study attempts to ascertain factors that predict prolonged stay after PEG insertion that will guide physicians in their timing of PEG placement.
Methods
Study design
This study involved a retrospective review of endoscopy reports and electronic medical records (EMR) of all patients who had undergone successful PEG placement at Elmhurst General Hospital. Patient data extracted from the EMR included the following: age, sex, white cell count, chest X-ray findings, albumin levels, blood culture and urine culture reports, co-morbidities, length of stay in hospital after PEG insertion and mortality.
The presence of the following co-morbidities was documented: hypertension, diabetes mellitus, congestive heart failure, chronic obstructive pulmonary diseases and chronic renal failure.
Patients
All patients who had successful PEG placement from October 1, 1998 to March 31, 2009 were included in the study.
Procedure
PEG tubes were placed using the standard “pull through” technique described by Ponsky and Gauderer [1]. All patients were in-patients at the time of PEG insertion and received a prophylactic dose of cefazolin 1 g intravenously 30 min prior to the procedure. A standard 20-Fr PEG tube kit was used in all cases. The PEG site was examined for evidence of infection 24 h after insertion and tube feeds were subsequently started.
Discharge
Patients were routinely revaluated 24 h after PEG insertion and barring any complication, discharge planning was initiated. The next step involved completion of a Patient Review Instrument (a clinical tool used to assess a patient’s condition and the amount of care required), submitted to a long stay unit in preparation for discharge, a process that usually took 48 h. The usual duration to discharge post PEG placement was 72 h.
The primary endpoint of our study was early discharge (ED) that is discharge within 3 days of PEG placement. Based on this time frame, patients were divided into two groups: early discharge (ED, <3 days post PEG placement) and late discharge (LD, >3 days).
The secondary enpoint of our study was in-house mortality.
Statistical analysis
Descriptive statistics with regards to age are expressed in terms of range, mean and standard deviation. Unpaired t-test and X² contingency table were used to compare continuous variables. Univariate analysis was performed using Kaplan Meier Survival Analysis (Log rank test). Multivariate analysis was done using Cox regression analysis or a multiple logistic regression model. P value was considered significant at a value <0.05.
Results
Patients
Two hundred and eighty-four patients underwent successful PEG placement during the study period. All patients were in-patients at the time of PEG placement. There were 157 males and 127 females in the study group. The age range was 19 to 100 years (median 74, mean (SD) 70.5±16.4).
The indication for PEG placement was as follows: stroke 85 (30%); dementia 81 (29%); neurosurgical causes 62 (21%); oropharyngeal tumors 18 (6%); esophageal tumors 7 (3%); respiratory failure 22 (8%); and failure to thrive 9 (3%).
The mean age in years (SD) for three main indication sub-groups, i.e., stroke, neurosurgical causes and dementia, were: 71.3 (±15.7), 59.8 (±17.3) and 80 (±10.4), respectively. These differences in mean age were statistically significant (P= <0.001).
PEG to discharge
Post-PEG placement length of stay in hospital ranged from 1 to 244 days (median 11 days). One hundred and twelve patients (40%) were discharged in 7 days, 169 (60%) within 14 days, 202 (72%) within 21 days and 224 (79%) within 31 days (Fig. 1).
Figure 1.
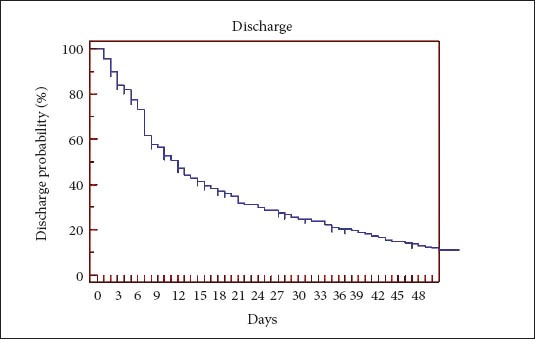
Kaplan Meier curve-Discharge
Two patients died within 3 days post-PEG placement. Forty-six patients (16%) were discharged from the hospital within 3 days of PEG placement (ED). Two hundred and thirty six patients (84%) remained in hospital from 4 to 244 days (median 13.5 days) post-PEG placement (LD). Of these 236 patients, 24 died in-house and the remaining 212 were discharged from the hospital.
Mortality
Twenty-six (9%) of patients died in-house post-PEG placement. Eight patients (3%) died within 14 days and 17 (6%) within 31 days of PEG placement (Fig. 2). Nine patients died 31 to 185 days post-PEG placement. The median survival in this group was 27 days. None of the deaths were related directly to complications from PEG insertion.
Figure 2.
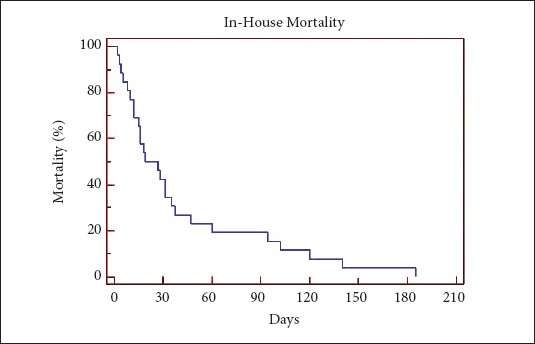
Kaplan Meier curve-Mortality
Predictor variables: ED vs. LD
The following variables were analyzed to determine their impact on time of discharge: age, sex, indication, ventilator dependency, serum albumin levels, co-morbidities, positive blood cultures, positive urine cultures and Clostridium dif-ficile infection (Table 1).
Table 1.
Discharge variables
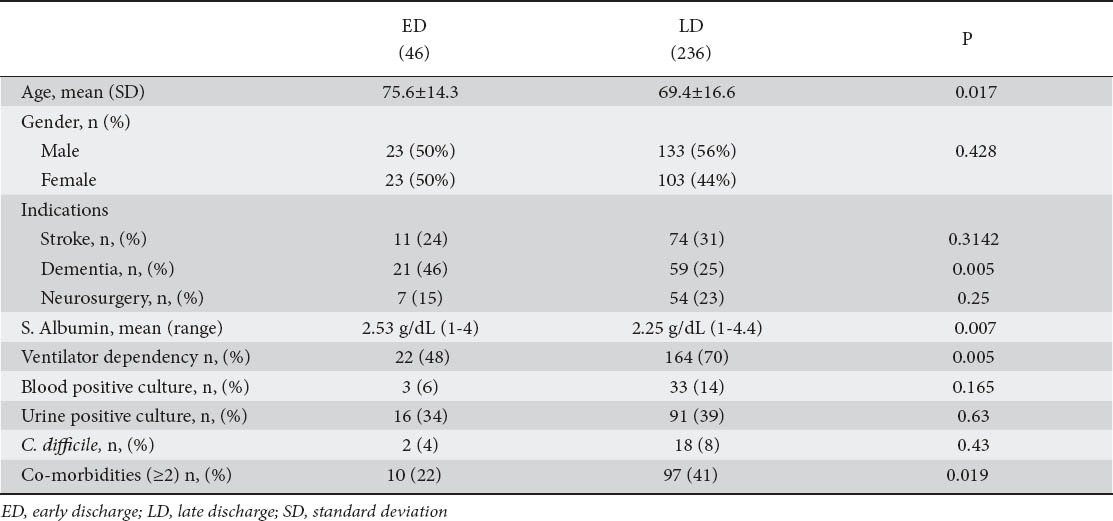
Univariate analysis revealed a statistically significant difference between ED and LD with regards to age, ventilator dependency, co-morbidities and serum albumin levels. But on multivariate scrutiny, only age, presence of 2 or more co-morbidities and low serum albumin levels proved to be independent predictors of LD (Table 2).
Table 2.
Discharge variables. Multivariate analysis
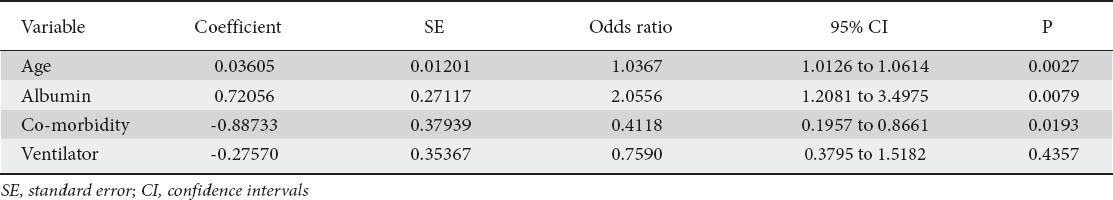
The serum albumin levels in the ED group ranged from 1-4 g/dL (mean (SD) 2.53±0.69 median 2.55). In the LD group the range was 1-4.4 g/dL (mean (SD) 2.25±0.64, median 2.20). A serum albumin value of <2.2 g/dL had a positive predictive value of 87% for a late discharge. Sensitivity was 44% and specificity 65%.
A dementia indication was twice as likely to result in an early discharge compared to a stroke indication (OR 2.39; 95% CI 1.07-5.36; P=0.033). There was no statistically significant difference between the other indications and discharge outcome.
Of the 46 patients in the ED group 13 had no co-morbidities; 23 had 1 and 10 had 2. In the LD group of 236 patients 51 had no co-morbidities; 88 had 1; 65 had 2; 31 had 3 and 1 had 4 co-morbid conditions. Ninety-seven patients (41%) in the LD group had 2 or more co-morbidities compared to 10 patients (22%) in the ED group. These differences were statistically significant (Chi-square=5.49; DF=1; P=0.019).
Predictor variables: mortality
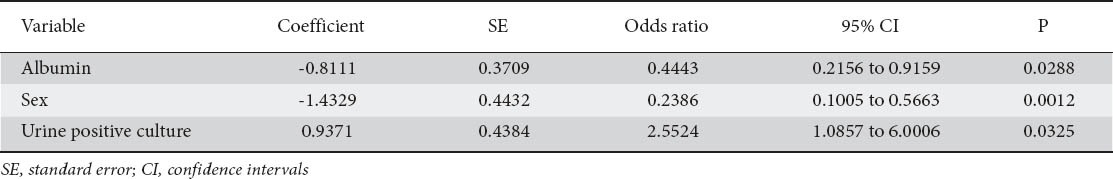
Female gender, low serum albumin levels and positive urine cultures proved to be predictors of mortality on univariate analysis (Table 3), a correlation that was confirmed by the multivariate model (Table 4).
Table 3.
Mortality variables
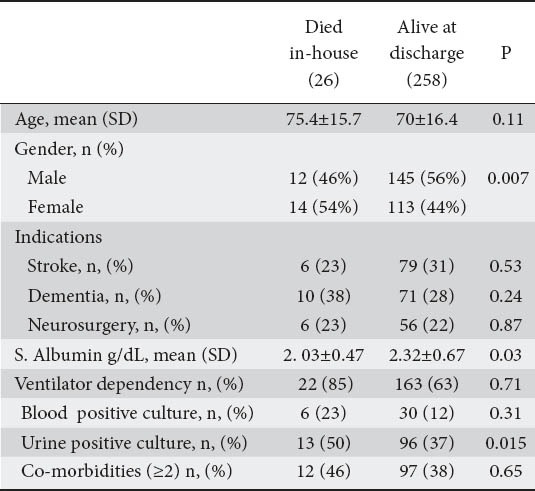
Table 4.
Mortality variables. Multivariate analysis
Complications
Eight patients (2.8%) had PEG related complications. Bleeding occurred at the PEG site in 4 (1.4%) patients. One patient required surgical sutures to control the bleeding while in the remaining 3 patients, local measures like a pressure bandage and the discontinuation of low-dose anticoagulation helped control the bleeding.
Peristomal infection was noted in 4 patients (2.8%). Two patients had a definite purulent discharge at the site of PEG placement; one patient had erythema and fever while 1 patient had erythema and a minimal discharge. All peristomal infections resolved with local wound care and antibiotic therapy.
Discussion
Rates for PEG insertion vary widely in the United States with rates ranging from 0 to 38.9 per 100 hospitalizations of nursing home patients [14]. Rates appear to be affected by hospital characteristics (for-profit vs. government and size) as well as location. One study [15] reported a national average of 53.6 per 1000 elderly nursing home residents with advanced dementia, with rates ranging from 2.1 (Utah) to 100.5 (Alabama).
This wide range points to the lack of a standardized approach with no uniform guidelines to regulate the process. It is also a reflection of the subjective factors that play a role in PEG insertion like patient and family preference and provider ‘threshold’ for placing a PEG.
Percutaneous endoscopic gastrostomy is usually performed in patients with serious disease conditions who are usually elderly and closer towards the end of their life span. The median age of patients undergoing PEG in our study was 74 years. Several studies [16-19] have reported similar findings with the mean age ranging from 66 to 80 years.
In our study, stroke was the commonest indications for PEG placement accounting for 30% of patients. Other studies [18,20] have also noted stroke to be the commonest indication with figures ranging from 42-49%. Dementia is another important indication [18-20]. In one study [18] dementia was the dominant indication making up 59% of hospitalized patients and 70% of nursing home patients undergoing PEG placement. Twenty nine percent of our patients had dementia. Our cohort of patients was unique in that we had 21% of patients with neurosurgical causes like head trauma as the indication.
Peristomal infection was observed in 4 (2.8%) of our patients similar to previous studies [21].
The in-house mortality post-PEG placement was 9% in our study. Two studies [16,17] reported slightly higher rates of 11%. Another study [22] indicated that in-house mortality was possibly as high as 15%.
Sixty-five percent of in house mortalities in our study occurred within 31 days. Smith et al [16] reported that 40 (50%) of 80 patients who died prior to discharge, died within one week of PEG placement.
When discharged patients are included in the analysis the mortality rate is even more concerning. While we were unable to address this issue in our study because of insufficient data on discharged patients, reports from other studies [16,18,20,23] indicate an overall 30-day mortality rate (inclusive of discharged patients) ranging from 22-26%. The combined 90-day mortality rate exceeds 40% [18,20] and the cumulative one-year mortality rates may be as high as 61-67% [20,24,25].
Researchers have attempted to delineate markers that would predict early mortality in order to stratify patients referred for PEG placement. Light et al [23] found old age (>75 years), urinary tract infection and previous aspiration to be associated with a high 30-day mortality. Stroke patients with PEGs did poorly when compared to other indication groups [25]. In one study [20] although the 30-day mortality between the indication groups was not significantly different, the one year mortality after PEG was 67% in stroke patients compared to 58% in dementia patients. Mechanical ventilation is also associated with a poor prognosis [16]. Co-morbidities like diabetes mellitus and chronic obstructive pulmonary disease also contribute to early mortality [19,26].
Several studies have consistently shown a low serum albumin level to be a marker of early mortality [19,27,28]. Prealbumin is a reliable indicator of in-house mortality in elderly hospitalized patients and could be a better indicator than albumin because of its short half-life (2 days) in patients requiring PEGs [29]. So far there are no studies that have evaluated the role of prealbumin in patients undergoing PEG placement.
In addition to positive urine cultures and low serum albumin levels which have shown to be independent predictors of mortality in other studies [19,23,27,28] we also found female sex to be an independent predictor as well. Other studies have reported a male predisposition [26] or no difference at all [16,19,30]. Co-morbidities did not prove to be an independent predictor of mortality in our study, similar to that found by Blomberg et al [28].
The majority of patients, 84% in our study, had a prolonged stay in the hospital post-PEG placement. Only 16% of patients were discharged from the hospital within 3 days of PEG insertion (Fig. 1).
Therefore to aid in better patient selection our study attempted to determine whether markers of early mortality after PEG insertion could also be used to predict the length of hospital stay post-PEG placement.
Univariate analysis identified mechanical ventilation, low albumin levels and presence of 2 or more co-morbidities as predictors of late discharge, but only two of these low albumin levels and presence of 2 or more co-morbidities proved to be valid on multivariate analysis. A diagnosis of dementia was more likely to result in an early discharge when compared to the other categories.
In our study interestingly, the LD patients had a mean age that was lower than the ED patients which was statistically significant. We attribute this to the significantly higher proportion of dementia patients in the ED category who had a higher mean age.
In view of the significant post-PEG mortality, patient selection must be prudent to avoid PEG placement in futile cases [30]. Some researchers [7,30] have proposed a ‘cooling’ period or a wait period as a strategy to prevent early deaths.
One study [7] compared mortality rates after PEG insertion in two hospitals, one which had a one week waiting list policy and one which did not. The hospital with a one-week waiting policy inserted fewer PEGs and had a lower one-month post-PEG mortality when compared to the hospital with no waiting policy. In the hospital with a one-week waiting policy there were nine deaths after referral for a PEG but prior to PEG insertion indicating that a significant number of patients will die prior to PEG insertion after a referral is made.
In our study 17 patients died in-house after PEG insertion within 31 days and at least 2 patients died after discharge within the same period (complete data on discharged patients was not available). None of these deaths were related to the procedure and were the consequence of the patient overall medical condition. A hypothetical one-month waiting period would have resulted in at least 6% less PEGs being inserted. During this waiting period the patient maybe fed with other means like a nasogastric tube as some researchers have suggested [30]. The FOOD trial [5] indicated that in the first 2-3 weeks after an acute stroke, a better outcome is reached by nasogastric tube feeding as opposed to a PEG.
Approximately 200,000 PEGs are placed in the United States every year. A waiting period of 30 days would result in nearly 20,000 less PEGs being inserted if we use a conservative 10% rate for the 30-day mortality post-PEG placement. Some studies have indicated the overall 30-day mortality rate (inclusive of deaths post discharge) to be as high as 22-26% [16,18,20,23].
One study [31] indicated that the cost of a PEG placement is $1870. Using this figure, the amount of savings netted by a wait and watch strategy would be of the tune of 37.4 million dollars, a significant amount in these cost-cutting days.
A waiting period also allows adequate time for recovery of swallowing function after a stroke or to assess any signs of improvement. Studies indicate that 37% of patients with dysphagia after a stroke recover swallowing function within 8 days and 87% maybe swallowing normally by day 14 [32].
Patient selection and timing of PEG placement must be guided by prognostic markers of mortality as well early discharge. Additionally a ‘cooling’ period may decrease post-PEG mortality, aid in spontaneous recovery of swallowing function in some patient and result in significant cost saving.
Summary Box.
What is already known:
Beneficial effects of percutaneous endoscopic gastrostomy (PEG) are uncertain; nevertheless over 200,000 PEGs placed annually in the United States
There is a significant mortality post-PEG placement
Markers of mortality in post-PEG placement patients like old age, co-morbidities and serum albumin levels are well known
What the new findings are:
The study identifies markers that predict early discharge in post-PEG patients who survive
We suggest that these markers should be taken into account in decision making and timing of PEG-placement
The high mortality post-PEG placement is reemphasized
The study raises an important question of a waiting period to preclude unnecessary PEGs in patients likely to die in the immediate post-PEG placement period
Biography
City Hospital Center at Elmhurst, New York and the Mount Sinai School of Medicine of the City University of New York, USA
Footnotes
Conflict of Interest: None
References
- 1.Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc. 1981;27:9–11. doi: 10.1016/s0016-5107(81)73133-x. [DOI] [PubMed] [Google Scholar]
- 2.Gauderer MW. Percutaneous endoscopic gastrostomy-20 years later: a historical perspective. J Pediatr Surg. 2001;36:217–219. doi: 10.1053/jpsu.2001.20058. [DOI] [PubMed] [Google Scholar]
- 3.Rutter CE, Yovino S, Taylor R, Wolf J, et al. Impact of early percutaneous endoscopic gastrostomy tube placement on nutritional status and hospitalization in patients with head and neck cancer receiving definitive chemoradiation therapy. Head Neck. 2011;33:1441–1447. doi: 10.1002/hed.21624. [DOI] [PubMed] [Google Scholar]
- 4.Assenat E, Thezenas S, Flori N, et al. Prophylactic percutaneous endoscopic gastrostomy in patients with advanced head and neck tumors treated by combined chemoradiotherapy, J Pain Symptom Manage. 2011;42:548–556. doi: 10.1016/j.jpainsymman.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Dennis M, Lewis S, Cranswick G, et al. FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol Assess. 2006;10:1–120. doi: 10.3310/hta10020. [DOI] [PubMed] [Google Scholar]
- 6.Dharmarajan TS, Unnikrishnan D, Pitchumoni CS. Percutaneous endoscopic gastrostomy and outcome in dementia. Am J Gastroenterol. 2001;96:2556–2563. doi: 10.1111/j.1572-0241.2001.04099.x. [DOI] [PubMed] [Google Scholar]
- 7.Callahan CM, Haag KM, Weinberger M, et al. Outcomes of percutaneous endoscopic gastrostomy among older adults in a community setting. J Am Geriatr Soc. 2000;48:1048–1054. doi: 10.1111/j.1532-5415.2000.tb04779.x. [DOI] [PubMed] [Google Scholar]
- 8.Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95:1472–1475. doi: 10.1111/j.1572-0241.2000.02079.x. [DOI] [PubMed] [Google Scholar]
- 9.Iizuka M, Reding M. Use of percutaneous endoscopic gastrostomy feeding tubes and functional recovery in stroke rehabilitation: a case-matched controlled study. Arch Phys Med Rehabil. 2005;86:1049–1052. doi: 10.1016/j.apmr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Garrow D, Pride P, Moran W, et al. Feeding Alternatives in patients with Dementia: Examing the evidence. Clin Gastroenterol Hepatol. 2007;5:1372–1378. doi: 10.1016/j.cgh.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Madhoun MF, Blankenship MM, Blankenship DM, et al. Prophylactic PEG placement in head and neck cancer: how many feeding tubes are unused (and unnecessary)? World J Gastroenterol. 2011;17:1004–1008. doi: 10.3748/wjg.v17.i8.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angus F, Burakoff R. The percutaneous endoscopic gastrostomy tube: Medical and Ethical Issues in Placement. Am J Gastroenterol. 2003;98:272–277. doi: 10.1111/j.1572-0241.2003.07267.x. [DOI] [PubMed] [Google Scholar]
- 13.Ladas SD, Triantafyllou K, Liappas I, et al. Percutaneous endoscopic gastrostomy: adequacy and quality of information given to decision makers. Dig Dis. 2002;20:289–292. doi: 10.1159/000067667. [DOI] [PubMed] [Google Scholar]
- 14.Teno JM, Mitchell SL, Gozalo PL, et al. Hospital characteristics associated with feeding tube placement in nursing home residents with advanced cognitive impairment. JAMA. 2010;303:544–550. doi: 10.1001/jama.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo S, Rhodes RL, Mitchell SL, et al. Natural history of feeding-tube use in nursing home residents with advanced dementia. J Am Med Dir Assoc. 2009;10:264–270. doi: 10.1016/j.jamda.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith BM, Perring P, Engoren M, Sferra JJ. Hospital and long term outcomes after percutaneous endoscopic gastrostomy. Surg Endosc. 2008;22:74–80. doi: 10.1007/s00464-007-9372-z. [DOI] [PubMed] [Google Scholar]
- 17.Poteet SJ, Holzman MD, Melvin WV, et al. Inpatient mortality and length of stay comparison of percutaneous endoscopic gastrostomy and percutaneous endoscopic gastrojejunostomy. J Laparoendosc Adv Surg Tech A. 2010;20:587–590. doi: 10.1089/lap.2010.0207. [DOI] [PubMed] [Google Scholar]
- 18.Sanders DS, Anderson AJ, Bardhan KD. Percutaneous endoscopic gastrostomy: an effective strategy for gastrostomy feeding in patients with dementia. Clin Med. 2004;4:235–241. doi: 10.7861/clinmedicine.4-3-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang A, Bardan E, Chowers Y, et al. Risk factors for mortality in patients undergoing percutaneous endoscopic gastrostomy. Endoscopy. 2004;36:522–526. doi: 10.1055/s-2004-814400. [DOI] [PubMed] [Google Scholar]
- 20.Malmgren A, Hede GW, Karlström B, et al. Indications for percutaneous endoscopic gastrostomy and survival in old adults. Food Nutr Res. 2011:55. doi: 10.3402/fnr.v55i0.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jafri NS, Mahid SS, Minor KS, et al. Meta-analysis: antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25:647–656. doi: 10.1111/j.1365-2036.2007.03247.x. [DOI] [PubMed] [Google Scholar]
- 22.Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973–1976. doi: 10.1001/jama.279.24.1973. [DOI] [PubMed] [Google Scholar]
- 23.Light VL, Slezak FA, Porter JA, et al. Predictive factors for early mortality after percutaneous endoscopic gastrostomy. Gastrointest Endosc. 1995;42:330–335. doi: 10.1016/s0016-5107(95)70132-x. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell SL, Tetroe JM. Survival after percutaneous endoscopic gastrostomy placement in older persons. J Gerontol A Biol Sci Med Sci. 2000;55:M735–M739. doi: 10.1093/gerona/55.12.m735. [DOI] [PubMed] [Google Scholar]
- 25.Fishman DN, Levy AR, Gifford DR, Tamblyn R. Survival after percutaneous endoscopic gastrostomy among older residents of Quebec. J Am Geriatr Soc. 1999;47:349–353. doi: 10.1111/j.1532-5415.1999.tb03000.x. [DOI] [PubMed] [Google Scholar]
- 26.Taylor CA, Larson DE, Ballard DJ, et al. Predictors of outcome after percutaneous endoscopic gastrostomy: a community based study. Mayo Clin Proc. 1992;67:1042–1049. doi: 10.1016/s0025-6196(12)61118-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaw M, Sekas G. Long term follow up of consequences of percutaneous endoscopic gastrostomy (PEG) tubes in nursing home patients. Dig Dis Sci. 1994;39:738–743. doi: 10.1007/BF02087416. [DOI] [PubMed] [Google Scholar]
- 28.Blomberg J, Largergren P, Martin L, et al. Albumin and C-reactive protein levels predict short term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest Endosc. 2011;73:29–36. doi: 10.1016/j.gie.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281:2013–2019. doi: 10.1001/jama.281.21.2013. [DOI] [PubMed] [Google Scholar]
- 30.Stathopoulos P, Karamanolis G, Papanikolaou IS, et al. Percutaneous endoscopic gastrostomy: patients’ outcomes, adequacy and quality of information given to decision-makers and procedure acceptance. Ann Gastroenterol. 2011;24:29–34. [PMC free article] [PubMed] [Google Scholar]
- 31.Barkmeier JM, Trerotola SO, Wiebke EA, et al. Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: comparative study and cost analysis. Cardiovasc Intervent Radiol. 1998;21:324–328. doi: 10.1007/s002709900269. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson TJ, Thomas K, MacGregor S, et al. Tolerance of early diet textures as indicators of recovery from dysphagia after stroke. Dysphagia. 2002;17:227–232. doi: 10.1007/s00455-002-0060-9. [DOI] [PubMed] [Google Scholar]


