Abstract
Since the original description of the effectiveness of β-blockers in lowering the portal pressure and therefore the risk of variceal bleeding, more than 500 articles in the English literature on the use of non selective β-blockers (NSBB) in cirrhosis have been published. The use of NSBB in pre-primary prophylaxis of variceal bleeding is currently not indicated. In primary prophylaxis, patients with high risk small varices or large/medium varices should receive primary prophylaxis either with NSBB or with endoscopic band ligation if there are contraindications to NSBB. For secondary prophylaxis the current recommendation is to receive a combination of NSBB and endoscopic variceal ligation. In addition to lowering portal pressure, NSBB can also reduce bacterial translocation, potentially exerting multiple beneficial effects which go beyond the reduction of bleeding risk. Carvedilol is a NSBB with intrinsic anti-α(1)-adrenergic activity, possibly more effective than propranolol in lowering portal hypertension. A potential harmful effect of propranolol in patients with cirrhosis with refractory ascites deserves further confirmation. NSBB remain the cornerstone of therapy in cirrhotic patients with portal hypertension.
Keywords: β-Blockers, portal hypertension, propranolol, nadolol, carvedilol, cirrhosis, HVPG, variceal bleeding
Introduction
In 1981, Lebrec et al performed the first randomized clinical trial involving 74 cirrhotic patients with a history of variceal bleeding. This study documented a significant reduction in rebleeding in patients on propranolol as compared to placebo [1,2]. Since then, there has been a growing interest among hepatologists regarding the role of non-selective β-blockers (NSBB) in decreasing portal hypertension and preventing its complications.
Materials and methods
The information extracted from each of the selected publications included study design details, patient characteristics, treatment regimens and efficacy and tolerability end points. Bibliographic searches were performed in MEDLINE for the following words (all fields): (‘‘Beta blockers” [MeSH] or “propanolol or “nadolol” or “carvedilol”) and (‘‘cirrhosis” [MeSH]) and (‘‘refractory ascites” [MeSH] or ‘‘hemodynamic” or “HVPG”) and (‘‘prophylaxis” [MeSH] and “variceal bleeding”). Other relevant trials were identified by hand searched of the reference lists of the clinical trials identified during electronic. A first review was performed on the abstracts of the articles selected and if the inclusion criteria were satisfied, articles were included in the analysis. Studies published in abstract form only, or in non-English language were excluded.
Hemodynamic effects of NSBB and current guidelines
In patients with cirrhosis and esophageal varices, the incidence of first variceal bleeding is about 12-15% per year [3]. Despite improvements in treating this complication, the mortality rate associated with variceal bleeding is still high (15-20%) [3-5]. Therefore, the prevention of first variceal bleeding has always been considered mandatory. Several randomized trials have confirmed that NSBB represent an effective treatment in primary prophylaxis for variceal bleeding in patients with esophageal varices as confirmed by a meta-analysis [6]. The role of medical treatment in secondary prophylaxis of gastrointestinal bleeding due to varices has also been well established [7]. The effect of NSBB in preventing variceal bleeding is thought to be mediated by several mechanisms acting on the hemodynamic alterations present in patients with cirrhosis [5,8,9]. Patients with portal hypertension have a hyperdynamic circulation characterized by an increased cardiac output and splanchnic blood inflow and a reduced peripheral and splanchnic vascular resistance, associated with an expanded plasma volume. Along with the increase in the intra-hepatic resistance this hyperdynamic circulatory state plays a major role in the pathogenesis of portal hypertension and its complications [10]. The most important hemodynamic effect of NSBB is a decrease in cardiac output via β1 receptors and a splanchnic vasoconstriction through β2 receptors, leading to a reduction in portal inflow [1,11]. Moreover a direct reduction in variceal flow, due to the increase in porto-collateral resistance [12,13] and to a decrease in total effective vascular compliance, seems to contribute to the prevention of variceal bleeding during propranolol treatment [14].
The hepato-venous pressure gradient (HVPG), a surrogate marker of portal pressure [15-17], has been utilized to monitor the hemodynamic response in patients with portal hypertension taking NSBB. Previous studies have shown that those patients who obtain a reduction of HVPG greater than 20% from baseline or to <12 mmHg, are to be considered “hemodynamic responders”, and will benefit from a significant reduction in risk of variceal bleeding [18,19].
The most commonly used guidelines for the management of portal hypertension are the ones agreed at the last international consensus conference on portal hypertension (Baveno V), which include recommendations for pre-primary, primary and secondary prophylaxis of variceal bleeding with NSBB [20].
Pre-primary prophylaxis is aimed at preventing the development of esophageal varices [20]. While some pathophysiological experimental studies have suggested a role of NSBB in preventing the development of the collateral circulation [21,22], only few studies were performed to test this hypothesis in humans. A multicenter study randomized 213 cirrhotics without varices and HVPG >6 mmHg to be treated with NSBB or placebo. This study showed that NSBB did not prevent the formation of varices and were even associated with an increased number of adverse events requiring discontinuation of treatment [25]. As a result, the use of NSBB in pre-primary prophylaxis of variceal bleeding is currently not indicated [20].
NSBB might also have a role in reducing the rate at which varices increase in size. An analysis of 206 cirrhotics with small or no varices performed by Calès et al reported that an increase in size of varices over a period of one year was more frequent in patients treated with NSBB, as compared to those on placebo [23]. On the other hand, Merkel and coworkers showed a trend towards a decreased progression of varices (from small to large) in patients treated with nadolol as compared to placebo [24].
The main factors predicting risk of variceal bleeding are the size of varices, the presence of red wale marks and the severity of liver disease as expressed by the Child Pugh score [3,26]. Therefore, based on these epidemiological data, patients at high risk are those with medium/large varices or those with small varices and red wale marks or Child Pugh class B/C. Patients with large/medium varices should receive primary prophylaxis either with NSBB or with endoscopic band ligation, whereas in high risk patients with small varices the main treatment is represented by NSBB (Fig. 1). Regarding low risk patients with small varices, these may be treated with NSBB to prevent progression of varices and bleeding [20].
Figure 1.
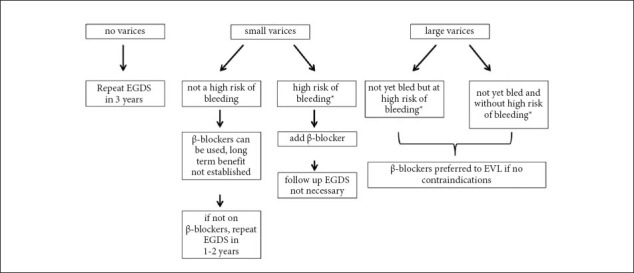
β-Blockers in primary prophylaxis for variceal bleeding
*Child B/C cirrhosis and/or red wale marks on endoscopy
EVL, endoscopic variceal ligation; EGDS, esophago-gastroduodenoscopy
The risk of rebleeding in patients who survive after a first bleeding episode is high (median 60%) and this event is associated with a 30% mortality. Secondary prophylaxis with NSBB has been shown to be effective in decreasing both the risk of recurrent bleeding and mortality [7,27]. The current recommendation for these patients is to receive a combination of NSBB and endoscopic variceal ligation, as this appears to be superior to either treatment alone [28].
Carvedilol: a new agent in the pipeline
In addition to propranolol and nadolol, carvedilol has been investigated as a promising NSBB with the additional property of vasodilatation due to its intrinsic anti-α1 adrenergic activity and its capacity to enhance the release of nitric oxide [29]. Thus, carvedilol reduces portal pressure not only by decreasing portal-collateral blood flow (as all other NSBB) but also by diminishing the functional component of hepatic vascular resistance which is increased in cirrhosis. Due to these effects, this drug may cause a higher risk of arterial hypotension leading to discontinuation of treatment. Indeed, a reduction in mean arterial pressure has been documented in patients on carvedilol, proportionally to the dosage [30]. Other promising effects of carvedilol are those of scavenging and suppressing reactive oxygen species [31-33], leading to a possible cytoprotective and anti-oxidant effect [34]. Amelioration of oxidative stress with carvedilol might even lead to antifibrotic effects [35].
Several clinical trials reported the efficacy of carvedilol in lowering HVPG [36,37]. Carvedilol succeeded in reducing HVPG more than propranolol in some randomized clinical trials [30,38,39], while De and colleagues found the two to be equivalent [40]. Lo and co-workers recently evaluated the use of carvedilol vs nadolol plus isosorbide-mononitrate in preventing variceal rebleeding. These authors concluded that carvedilol was as effective as nadolol plus isosorbidemononitrate with fewer severe adverse events and a similar rate of survival [41]. This trial should be considered with caution due to the high rebleeding rates (60% in both groups) probably caused by the fact that these patients did not receive endoscopic treatment together with drug therapy [42]. Moreover, β-blockage was not reflected by a decrease in heart rate and HVPG measurements were not performed.
Only one randomized non-blinded trial compared the role of carvedilol for primary prophylaxis of variceal bleeding with endoscopic band ligation (EBL), demonstrating a significantly lower bleeding rate in patients on carvedilol as compared to those treated with EBL, but without any differences in mortality between the two groups [43]. The study however did not include hemodynamic measurements. A recent study also showed that carvedilol achieved a hemodynamic response in 56% of hemodynamic non responders to propranolol [44].
It is advised that carvedilol should be started at low doses (6.25 mg/day). If tolerated, the dose is increased stepwise up to a maximum of 25 mg b.i.d. (50 mg in patients weighing >85 kg). Titration should be done slowly, increasing the dose at intervals of 1-2 weeks. The dose should not be increased in patients developing symptoms or with a systolic blood pressure <90 mmHg or a heart rate <50 beats per min.
Further studies are required to confirm these results and to analyze the role of carvedilol in secondary prophylaxis and its effects in certain subgroups, such as patients with decompensated cirrhosis or patients not responding to propranolol.
NSBB: non-hemodynamic mechanisms
The use of NSBB might also be beneficial for other outcomes, such as ascites, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome (HRS), hepatic encephalopathy and overall survival. A landmark study by Abraldes et al documented a positive effect of NSBB in the prevention of the development of ascites, SBP and hepatic encephalopathy [45]. Likewise, Hernandez-Gea and colleagues demonstrated that in patients with compensated cirrhosis and large varices treated with NSBB, even an HVPG decrease >10% was able to significantly reduce the risk of developing ascites and other complications such as refractory ascites and HRS [46]. These findings may suggest that NSBB, in addition to their protective role for variceal bleeding, might also be beneficial for other complications of portal hypertension.
During recent years there has been increasing attention towards the role of infections in cirrhotic patients [47-49]. Episodes of infection represent a frequent and severe burden in this group. Up to 40% of hospitalized patients with cirrhosis have infections and this is associated with longer hospital stay and a higher risk of death. Infection related mortality is reported to be between 15% and 19% [50,51]. Bacterial infections have also been found to be associated with upper gastrointestinal bleeding [52,53], failure to control variceal bleeding [54] and early variceal rebleeding [55]. Moreover, the occurrence of bacterial infections marks an important point in the natural history of patients with cirrhosis, carrying a 30% mortality at one month and a 60% mortality at one year [56].
A substantial number of infections in cirrhotic patients is likely to be linked to bacterial translocation (BT), defined as the migration of microorganisms or microbial products from the intestinal lumen to the mesenteric lymph nodes or other extra-intestinal sites [57,58]. Mechanisms contributing to this phenomenon are small intestinal bacterial overgrowth (SIBO) and structural and functional alterations of the intestinal mucosa, together with an impaired immunity [58,59].
The advanced stages of cirrhosis are characterized by an elevated activity of the sympathetic nervous system whose fibers terminate in blood vessels, gut-associated lymphatic tissue and in the intestinal mucosa. The increased level of norepinephrine promotes the development of SIBO by decreasing the intestinal transit time, impairing the mucosal barrier function and inhibiting local chemotaxis and phagocytosis [60].
Two in-vitro studies also suggest that in the intestinal lumen, norepinephrine is absorbed by Escherichia coli and other gram-negative gut bacteria resulting in an increase in growth, virulence and ability to adhere to the gut mucosa [61,62].
Portal hypertension leads to an increase in intestinal permeability by reducing velocity of mucosal blood flow, causing phlebectasia and congestion of sub-mucous capillaries and veins [63]. Thus, while there is an increase in total splanchnic blood flow due to progressive vasodilatation [64], there is also an irregular distribution of the intestinal microcirculation, with a reduction in effective mucosal blood flow leading to hyperemia, edema, ischemia, and potentially erosions [63].
NSBB, through the decrease in portal hypertension and also through their sympathicolythic action, may exert a beneficial effect by improving the intestinal congestion and edema and by normalizing the intestinal transit. Both these mechanisms may play a role in the prevention of BT. In experimental animal models of portal hypertension, propranolol increases bowel motility and reduces the overgrowth of enteric bacterial flora, the migration of microbiota into the systemic circulation and the development of SBP [65]. However, direct evidence of the effect of NSBB on BT in cirrhotic patients is still lacking and the only available data is based on post-hoc analysis evaluating the effect of NSBB in development of SBP [66-69]. These studies point towards a decreased incidence of SBP in patients taking NSBB. A recent meta-analysis also confirmed a protective effect of NSBB on the development of SBP [70].
It is important to underline that none of these studies analyzed the modifications in intestinal permeability and in BT related to NSBB. The effects of propranolol on gastroduodenal/intestinal permeability (assessed by sucrose-lactulosemannitol test) and BT (assessed by determination of levels of lipopolysaccharide-binding protein, interleukin-6 and malondialdehyde) have been investigated in a recent study [71]. The authors concluded that NSBB are able to ameliorate intestinal permeability and BT in cirrhotic patients [71]. Importantly, this protective effect appeared to be partially independent from their hemodynamic effect on portal pressure, supporting the involvement of non-hemodynamic mechanisms.
The presence of beneficial effects of NSBB unrelated to their hemodynamic mechanism is also suggested by the fact that patients, even when “hemodynamic non responders”, may have a reduced risk of bleeding while receiving NSBB [72]. Another study found a reduction in the incidence of SBP with a reduction in HVPG of 11% [67], which is less than that required for the definition of hemodynamic response. It is certainly possible that some of these beneficial effects are not related at all to reductions in portal pressure.
NSBB in patients with refractory ascites
While a beneficial role of NSBB on several outcomes in cirrhotic patients is well established [45,46], the effect in patients with refractory ascites is still unclear, and a possible harmful effect is under debate.
The effects of NSBB in reducing cardiac output and splanchnic blood flow, while potentially beneficial in reducing splanchnic inflow and portal pressure, might be harmful in decompensated cirrhotics and particularly in those with refractory ascites. The cardiac function of patients with advanced cirrhosis may already be impaired by the so called cirrhotic cardiomyopathy. This consists in systolic incompetence under situation of stress, diastolic dysfunction related to altered diastolic relaxation, and electrophysiological abnormalities [73-75]. The underlying pathogenetic mechanisms include abnormalities in the β-adrenergic signaling pathway, the presence of substances with a negative inotropic effect (such as nitric oxide and carbon monoxide), the excessive sodium and volume retention leading to cardiomyocyte hypertrophy, and possible ion channel defects [76,77]. Clinically, systolic incompetence is most evident when the extent of peripheral arterial vasodilatation demands an increased cardiac output as in the case of bacterial infections or procedures such as TIPS placement or large-volume paracentesis [78-80]. In this scenario, the use of a drug such as NSBB may theoretically further worsen the hemodynamic status.
Didier Lebrec and co-workers, who originally described the effectiveness of propranolol in reducing the risk of variceal bleeding [2], analyzed the hemodynamic effects and the impact on survival of NSBB in patients with refractory ascites [81]. Of the 151 enrolled patients, 51% had esophageal varices and were taking propranolol (it is not specified whether for primary or secondary prophylaxis), whereas only 4 patients without varices were taking this drug. The authors reported a median survival of 5 months in patients on propranolol versus 20 months in those not receiving this drug. They concluded that the use of NSBB in cirrhotic patients with refractory ascites might be deleterious. The results of this study were extensively criticized. Indeed, this study was not a randomized trial but a prospective observational analysis and patients taking propranolol seemed to have more severe liver disease, besides obviously having a much higher prevalence of varices. HVPG measurement was only performed in a small number of patients, and therefore a significant difference in HVPG (a major prognostic variable) cannot be excluded. Indeed a difference in HVPG would be expected given the difference in prevalence of varices. Another criticism concerns the causes of death: among 97 patients, 50 died of sepsis, 13 of progression of hepatocellular carcinoma and 25 of unknown causes while 9 patients were unaccounted for. This is in contrast to previous findings suggesting a protective effect of NSBB for infections. The mortality rate was also much higher than in other comparable studies [41,70,82].
To further investigate their hypothesis, the same authors performed a cross-over study aimed at evaluating the effect of NSBB on the development of paracentesis-induced circulatory dysfunction (PICD) [83], which is a circulatory dysfunction syndrome occurring after large-volume paracentesis. This is characterized by systemic vasodilatation and decrease in effective arterial blood flow and associated with reduced survival [78,84-86]. In this study, 10 cirrhotic patients with refractory ascites taking NSBB were enrolled and monitored before, immediately after and one week after a large-volume paracentesis. NSBB were then discontinued (after endoscopic variceal treatment) and paracentesis and clinical evaluations were repeated. The incidence of PICD decreased from 80% to 10% after the withdrawal of NSBB, suggesting that NSBB may have a potentially deleterious effect through further compromising the already impaired hemodynamic balance in patients with advanced cirrhosis. However, these results should be validated in randomized trials before any clinical recommendations can be made.
Hemodynamic responders and non-responders: utility of assessing HVPG response
Advantages of NSBB include not only their low cost and ease of administration, but also the fact that further endoscopic follow up is not necessary once treatment has been started. On the other hand, the main inconvenience of NSBB is that 15% of patients may have absolute or relative contraindications to this treatment and another 15% may present side effects requiring dose reduction or discontinuation [87,88].
The need for assessing hemodynamic response to NSBB is not clear. As already mentioned, longitudinal studies in patients treated with NSBB both in primary and secondary prophylaxis have suggested a very low residual risk of bleeding if there is a decrease of HVPG by at least 20% of baseline or to values ≤12 mmHg [19,45,67,89,90]. Patients achieving such a target reduction in portal pressure have been defined hemodynamic responders. However, concerns have been raised in relation to the feasibility, the clinical appropriateness, the risks and the costs of repeated HVPG measurement [91]. Also, a substantial number of patients rebleed before their hemodynamic response can be assessed [91]. This problem could partially be solved by re-measuring the HVPG after a shorter interval (even less than 1 month) [92]. Some studies investigated the role of acute HVPG response to i.v. propranolol in predicting the risk of bleeding and survival [46,93,94]. It is possible that the assessment of such an acute hemodynamic response may have clinical utility, possibly even with a lower threshold (HVPG reduction of 9-12%). However, further studies are needed.
In addition, there are a substantial number of patients who find themselves in what has been termed a grey area, in which clinical benefit from NSBB is not explained by changes in portal pressure. This could be explained by the non hemodynamic mechanisms of NSBB described earlier. The protective effect of β-blockers may not only be due to a reduction in portal pressure but also to a reduction in bacterial infections and, through this, to a reduction in the risk of bleeding [59,72,95]. Indeed, even hemodynamic non responders to NSBB may benefit from some of these beneficial effects [72].
As a result, it currently seems reasonable to aim for the maximum tolerated dose of NSBB in all patients who have no contraindications to this treatment, without the need for routine assessment of hemodynamic response through HVPG measurement.
Concluding remarks
Despite some debate about the relative benefit of NSBB in patients with refractory ascites, they are still considered the “aspirin of hepatologists’’ [96], both due to their hemodynamic and non-hemodynamic effects. The distinction of hemodynamic responders and non-responders does not fully take into account the complexity of the effects of this class of drugs, which represents one of the most frequently used in patients with cirrhosis over the last thirty years.
Biography
Sapienza Univesity of Rome, Rome, Italy; Royal Devon and Exeter Foundation Trust, Exeter, UK
Footnotes
Conflict of Interest: None
References
- 1.Lebrec D, Nouel O, Corbic M, Benhamou JP. Propranolol-a medical treatment for portal hypertension? Lancet. 1980;26:180–182. doi: 10.1016/s0140-6736(80)90063-x. [DOI] [PubMed] [Google Scholar]
- 2.Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371–1374. doi: 10.1056/NEJM198112033052302. [DOI] [PubMed] [Google Scholar]
- 3.The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multi-center study. N Engl J Med. 1988;319:983–989. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 4.Bosch J. Medical treatment of portal hypertension. Digestion. 1998;59:547–555. doi: 10.1159/000007530. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 6.Pagliaro L, D’Amico G, Sörensen TI, et al. Prevention of first bleeding in cirrhosis. A meta-analysis of randomized trials of nonsurgical treatment. Ann Intern Med. 1992;117:59–70. doi: 10.7326/0003-4819-117-1-59. [DOI] [PubMed] [Google Scholar]
- 7.Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology. 1997;25:63–70. doi: 10.1053/jhep.1997.v25.pm0008985266. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 9.García-Pagán JC, Escorsell A, Moitinho E, Bosch J. Influence of pharmacological agents on portal hemodynamics: basis for its use in the treatment of portal hypertension. Semin Liver Dis. 1999;19:427–438. doi: 10.1055/s-2007-1007130. [DOI] [PubMed] [Google Scholar]
- 10.Bosch J, Pizcueta P, Feu F, Fernández M, García-Pagán JC. Pathophysiology of portal hypertension. Gastroenterol Clin North Am. 1992;21:1–14. [PubMed] [Google Scholar]
- 11.Kroeger RJ, Groszmann RJ. The effect of the combination of nitroglycerin and propranolol on splanchnic and systemic hemodynamics in a portal hypertensive rat model. Hepatology. 1985;5:425–430. doi: 10.1002/hep.1840050314. [DOI] [PubMed] [Google Scholar]
- 12.Bołdys H, Hartleb M, Rudzki K, Nowak A, Nowak S. Effect of propranolol on portosystemic collateral circulation estimated by per-rectal portal scintigraphy with technetium-99m pertechnetate. J Hepatol. 1995;22:173–178. doi: 10.1016/0168-8278(95)80425-0. [DOI] [PubMed] [Google Scholar]
- 13.Feu F, Bordas JM, Luca A, et al. Reduction of variceal pressure by propranolol: comparison of the effects on portal pressure and azygos blood flow in patients with cirrhosis. Hepatology. 1993;18:1082–1089. [PubMed] [Google Scholar]
- 14.Andreu V, Perello A, Moitinho E, et al. Total effective vascular compliance in patients with cirrhosis. Effects of propranolol. J Hepatol. 2002;36:356–361. doi: 10.1016/s0168-8278(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 15.Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Patch D, Burroughs AK. Assessment of the agreement between wedge hepatic vein pressure and portal vein pressure in cirrhotic patients. Dig Liver Dis. 2005;37:601–608. doi: 10.1016/j.dld.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Perelló A, Escorsell A, Bru C, et al. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology. 1999;30:1393–1397. doi: 10.1002/hep.510300628. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419–424. doi: 10.1002/hep.1840050313. [DOI] [PubMed] [Google Scholar]
- 18.Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol. 2003;38:S54–S68. doi: 10.1016/s0168-8278(02)00430-0. [DOI] [PubMed] [Google Scholar]
- 19.Feu F, García-Pagán JC, Bosch J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet. 1995;346:1056–1059. doi: 10.1016/s0140-6736(95)91740-3. [DOI] [PubMed] [Google Scholar]
- 20.De Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Lin HC, Soubrane O, Cailmail S, Lebrec D. Early chronic administration of propranolol reduces the severity of portal hypertension and portal-systemic shunts in conscious portal vein stenosed rats. J Hepatol. 1991;13:213–219. doi: 10.1016/0168-8278(91)90817-u. [DOI] [PubMed] [Google Scholar]
- 22.Sarin SK, Groszmann RJ, Mosca PG, et al. Propranolol ameliorates the development of portal-systemic shunting in a chronic murine schistosomiasis model of portal hypertension. J Clin Invest. 1991;87:1032–1036. doi: 10.1172/JCI115062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calés P, Oberti F, Payen JL, et al. Lack of effect of propranolol in the prevention of large oesophageal varices in patients with cirrhosis: a randomized trial. French-Speaking Club for the Study of Portal Hypertension. Eur J Gastroenterol Hepatol. 1999;11:741–745. doi: 10.1097/00042737-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Merkel C, Marin R, Angeli P, et al. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology. 2004;127:476–484. doi: 10.1053/j.gastro.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 26.Nevens F, Bustami R, Scheys I, Lesaffre E, Fevery J. Variceal pressure is a factor predicting the risk of a first variceal bleeding: a prospective cohort study in cirrhotic patients. Hepatology. 1998;27:15–19. doi: 10.1002/hep.510270104. [DOI] [PubMed] [Google Scholar]
- 27.De Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez R, Zamora J, Gomez-Camarero J, Molinero LM, Bañares R, Albillos A. Meta-analysis: Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med. 2008;149:109–122. doi: 10.7326/0003-4819-149-2-200807150-00007. [DOI] [PubMed] [Google Scholar]
- 29.Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology. 2010;51:2214–2218. doi: 10.1002/hep.23689. [DOI] [PubMed] [Google Scholar]
- 30.Bañares R, Moitinho E, Piqueras B, et al. Carvedilol, a new nonselective beta-blocker with intrinsic anti- Alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology. 1999;30:79–83. doi: 10.1002/hep.510300124. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe K, Ohta Y, Nakazawa M, et al. Low dose carvedilol inhibits progression of heart failure in rats with dilated cardiomyopathy. Br J Pharmacol. 2000;130:1489–1495. doi: 10.1038/sj.bjp.0703450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalla Libera L, Ravara B, Gobbo V, et al. Skeletal muscle myofibrillar protein oxidation in heart failure and the protective effect of Carvedilol. J Mol Cell Cardiol. 2005;38:803–807. doi: 10.1016/j.yjmcc.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Nanjo S, Yamazaki J, Yoshikawa K, Ishii T, Togane Y. Carvedilol prevents myocardial fibrosis in hamsters. Int Heart J. 2006;47:607–616. doi: 10.1536/ihj.47.607. [DOI] [PubMed] [Google Scholar]
- 34.Ronsein GE, Guidi DB, Benassi JC, Filho DW, Pedrosa RC, Pedrosa RC. Cytoprotective effects of carvedilol against oxygen free radical generation in rat liver. Redox Rep. 2005;10:131–137. doi: 10.1179/135100005X38879. [DOI] [PubMed] [Google Scholar]
- 35.Hamdy N, El-Demerdash E. New therapeutic aspect for carvedilol: antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol Appl Pharmacol. 2012;261:292–299. doi: 10.1016/j.taap.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Forrest EH, Bouchier IA, Hayes PC. Acute haemodynamic changes after oral carvedilol, a vasodilating beta-blocker, in patients with cirrhosis. J Hepatol. 1996;25:909–915. doi: 10.1016/s0168-8278(96)80296-0. [DOI] [PubMed] [Google Scholar]
- 37.Bruha R, Vitek L, Petrtyl J, et al. Effect of carvedilol on portal hypertension depends on the degree of endothelial activation and inflammatory changes. Scand J Gastroenterol. 2006;41:1454–1463. doi: 10.1080/00365520600780403. [DOI] [PubMed] [Google Scholar]
- 38.Lin HC, Yang YY, Hou MC, Huang YT, Lee FY, Lee SD. Acute administration of carvedilol is more effective than propranolol plus isosorbide-5-mononitrate in the reduction of portal pressure in patients with viral cirrhosis. Am J Gastroenterol. 2004;99:1953–1958. doi: 10.1111/j.1572-0241.2004.40179.x. [DOI] [PubMed] [Google Scholar]
- 39.Bañares R, Moitinho E, Matilla A, et al. Randomized comparison of long-term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhosis. Hepatology. 2002;36:1367–1373. doi: 10.1053/jhep.2002.36947. [DOI] [PubMed] [Google Scholar]
- 40.De BK, Das D, Sen S, et al. Acute and 7-day portal pressure response to carvedilol and propranolol in cirrhotics. J Gastroenterol Hepatol. 2002;17:183–189. doi: 10.1046/j.1440-1746.2002.02674.x. [DOI] [PubMed] [Google Scholar]
- 41.Lo GH, Chen WC, Wang HM, Yu HC. Randomized, controlled trial of carvedilol versus nadolol plus isosorbide mononitrate for the prevention of variceal rebleeding. J Gastroenterol Hepatol. 2012;27:1681–1687. doi: 10.1111/j.1440-1746.2012.07244.x. [DOI] [PubMed] [Google Scholar]
- 42.Bosch J. Carvedilol for preventing recurrent variceal bleeding: Waiting for convincing evidence. Hepatology. 2013;57:1665–1667. doi: 10.1002/hep.26279. [DOI] [PubMed] [Google Scholar]
- 43.Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, Forrest EH, Hislop WS, Mills PR, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825–833. doi: 10.1002/hep.23045. [DOI] [PubMed] [Google Scholar]
- 44.Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634–1641. doi: 10.1136/gutjnl-2012-304038. [DOI] [PubMed] [Google Scholar]
- 45.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–908. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 46.Hernández-Gea V, Aracil C, Colomo A, et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with β-blockers. Am J Gastroenterol. 2012;107:418–427. doi: 10.1038/ajg.2011.456. [DOI] [PubMed] [Google Scholar]
- 47.Merli M, Lucidi C. Bacterial resistance in cirrhotic patients: an emerging reality. J Hepatol. 2012;56:756–757. doi: 10.1016/j.jhep.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022–2033. doi: 10.1002/hep.23264. [DOI] [PubMed] [Google Scholar]
- 49.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56:S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 50.Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223–229. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 51.Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Navasa M, Rimola A, Rodés J. Bacterial infections in liver disease. Semin Liver Dis. 1997;17:323–333. doi: 10.1055/s-2007-1007209. [DOI] [PubMed] [Google Scholar]
- 53.Soares-Weiser K, Brezis M, Tur-Kaspa R, Paul M, Yahav J, Leibovici L. Antibiotic prophylaxis of bacterial infections in cirrhotic inpatients: a meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2003;38:193–200. doi: 10.1080/00365520310000690. [DOI] [PubMed] [Google Scholar]
- 54.Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207–1212. doi: 10.1002/hep.510270504. [DOI] [PubMed] [Google Scholar]
- 55.Hou MC, Lin HC, Liu TT, et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39:746–753. doi: 10.1002/hep.20126. [DOI] [PubMed] [Google Scholar]
- 56.Arvaniti V, D’Amico G, Fede G. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 57.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 59.Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556–563. doi: 10.1136/gut.2004.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- 61.Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock. 2002;18:465–470. doi: 10.1097/00024382-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Green BT, Lyte M, Chen C, et al. Adrenergic modulation of Escherichia coli O157:H7 adherence to the colonic mucosa. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1238–G1246. doi: 10.1152/ajpgi.00471.2003. [DOI] [PubMed] [Google Scholar]
- 63.Iwao T, Toyonaga A, Ikegami M, et al. Effects of vasopressin and nicardipine on hemodynamics and liver function in patients with cirrhosis: comparison with vasopressin alone. J Hepatol. 1993;19:345–352. doi: 10.1016/s0168-8278(05)80542-2. [DOI] [PubMed] [Google Scholar]
- 64.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–S131. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 65.Pérez-Paramo M, Muñoz J, Albillos A, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43–48. doi: 10.1002/hep.510310109. [DOI] [PubMed] [Google Scholar]
- 66.Cholongitas E, Papatheodoridis GV, Manesis EK, Burroughs AK, Archimandritis AJ. Spontaneous bacterial peritonitis in cirrhotic patients: Is prophylactic propranolol therapy beneficial? J Gastroenterol Hepatol. 2006;21:581–587. doi: 10.1111/j.1440-1746.2005.03982.x. [DOI] [PubMed] [Google Scholar]
- 67.Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell’Era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol. 2006;101:506–512. doi: 10.1111/j.1572-0241.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 68.Soylu AR, Dökmeci G, Tezel A, Amuca H, Umit H. Propranolol does not affect incidence of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2003;98:1446–1448. doi: 10.1111/j.1572-0241.2003.07507.x. [DOI] [PubMed] [Google Scholar]
- 69.Lo GH, Chen WC, Lin CK, et al. Improved survival in patients receiving medical therapy as compared with banding ligation for the prevention of esophageal variceal rebleeding. Hepatology. 2008;48:580–587. doi: 10.1002/hep.22358. [DOI] [PubMed] [Google Scholar]
- 70.Senzolo M, Cholongitas E, Burra P, et al. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int. 2009;29:1189–1193. doi: 10.1111/j.1478-3231.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 71.Reiberger T, Ferlitsch A, Payer BA, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Thalheimer U, Bosch J, Burroughs AK. How to prevent varices from bleeding: shades of grey--the case for nonselective beta blockers. Gastroenterology. 2007;133:2029–2036. doi: 10.1053/j.gastro.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 73.Wong F, Salerno F. Beta-blockers in cirrhosis: friend and foe? Hepatology. 2010;52:811–813. doi: 10.1002/hep.23852. [DOI] [PubMed] [Google Scholar]
- 74.Valeriano V, Funaro S, Lionetti R, et al. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200–3205. doi: 10.1111/j.1572-0241.2000.03252.x. [DOI] [PubMed] [Google Scholar]
- 75.Merli M, Calicchia A, Ruffa A, et al. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med. 2013;24:172–176. doi: 10.1016/j.ejim.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001;49:268–275. doi: 10.1136/gut.49.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Møller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179–190. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 78.Ruiz-del-Arbol L, Monescillo A, Jimenéz W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–586. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 79.Panos MZ, Moore K, Vlavianos P, et al. Single, total paracentesis for tense ascites: sequential hemodynamic changes and right atrial size. Hepatology. 1990;11:662–667. doi: 10.1002/hep.1840110420. [DOI] [PubMed] [Google Scholar]
- 80.Moreau R, Asselah T, Condat B, et al. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut. 2002;50:90–94. doi: 10.1136/gut.50.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sersté T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017–1022. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 82.Senzolo M, Nadal E, Cholongitas E, Burroughs AK. Is hydrophobia necessary for the hepatologist prescribing nonselective beta-blockers in cirrhosis? Hepatology. 2011;53:2149–2150. doi: 10.1002/hep.24176. [DOI] [PubMed] [Google Scholar]
- 83.Sersté T, Francoz C, Durand F, et al. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol. 2011;55:794–799. doi: 10.1016/j.jhep.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 84.Pozzi M, Osculati G, Boari G, et al. Time course of circulatory and humoral effects of rapid total paracentesis in cirrhotic patients with tense, refractory ascites. Gastroenterology. 1994;106:709–719. doi: 10.1016/0016-5085(94)90706-4. [DOI] [PubMed] [Google Scholar]
- 85.Ginès A, Fernández-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 86.Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 87.Longacre AV, Imaeda A, Garcia-Tsao G, Fraenkel L. A pilot project examining the predicted preferences of patients and physicians in the primary prophylaxis of variceal hemorrhage. Hepatology. 2008;47:169–176. doi: 10.1002/hep.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bolognesi M, Balducci G, Garcia-Tsao G, et al. Complications in the medical treatment of portal hypertension. In: de Franchis R, editor. Portal Hypertension III: Proceedings of the Third Baveno International Consensus Workshop on Definitions, Methodology, and Therapeutic Strategies. Oxford: Blackwell Science; 2001. pp. 180–201. [Google Scholar]
- 89.Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;32:930–934. doi: 10.1053/jhep.2000.19322. [DOI] [PubMed] [Google Scholar]
- 90.Bureau C, Péron JM. Alric L“A La Carte” treatment of portal hypertension: Adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology. 2002;36:1361–1366. doi: 10.1053/jhep.2002.36945. [DOI] [PubMed] [Google Scholar]
- 91.Thalheimer U, Mela M, Patch D, Burroughs AK. Targeting portal pressure measurements: a critical reappraisal. Hepatology. 2004;39:286–290. doi: 10.1002/hep.20061. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764–1772. doi: 10.1002/hep.22273. [DOI] [PubMed] [Google Scholar]
- 93.La Mura V, Abraldes JG, Raffa S, et al. Prognostic value of acute hemodynamic response to i.v. propranolol in patients with cirrhosis and portal hypertension. J Hepatol. 2009;51:279–287. doi: 10.1016/j.jhep.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 94.Villanueva C, Aracil C, Colomo A, et al. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119–128. doi: 10.1053/j.gastro.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 95.Senzolo M, Cholongitas E, Marelli L, Thalheimer U, Patch D, Burroughs AK. The low incidence of bacterial infections could be a protective factor against variceal bleeding per se in hemodynamic responders to propranolol. Am J Gastroenterol. 2006;101:2436–2437. doi: 10.1111/j.1572-0241.2006.00742_5.x. [DOI] [PubMed] [Google Scholar]
- 96.Triantos C, Samonakis D, Thalheimer U, Patch D, Burroughs A. The relationship between liver function and portal pressure: what comes first, the chicken or the egg? J Hepatol. 2005;42:146–147. doi: 10.1016/j.jhep.2004.07.023. [DOI] [PubMed] [Google Scholar]


