Abstract
Background
Endoscopic retrograde cholangiopancreatography (ERCP) is now the exclusive endoscopic therapeutic modality for biliary as well as pancreatic diseases. The aim of the present study was to investigate patient- and procedure-related risk factors for post-ERCP complications in a large-scale study of procedures performed by a single experienced endoscopist.
Methods
This is a retrospective cohort study which included a total of 2,715 therapeutic ERCPs enrolled in the final analysis. Potential important patient- and procedure-related risk factors for overall post-ERCP complications, pancreatitis and post-endoscopic sphincterotomy (ES) bleeding were investigated by univariate and multivariate analyses.
Results
Following the first therapeutic ERCP, 327 patients suffered complications; pancreatitis was observed in 132 (4.9%) patients, hemorrhage in 122 (4.5%) patients, cholangitis in 63 (2.3%) patients, perforation in 3 (0.11%) patients, and basket impaction in 7 (0.26%) patients. History of acute pancreatitis was more common in patients with post-ERCP complications (P<0.001). Female gender, young age (<40 years), periampullary diverticulum, suspected sphincter of Oddi dysfunction, metal stent placement, opacification of main pancreatic duct and suprapapillary fistulotomy were not found to be risk factors for overall post-ERCP complications and post-ERCP pancreatitis (PEP). Multivariate analysis showed a history of acute pancreatitis, difficult cannulation, needle-knife papillotomy, transpancreatic sphincterotomy, opacification of first and second class pancreatic ductules and acinarization as independent risk factors for overall complications and PEP, whereas antiplatelet and anticoagulation drug use were not found to be independent risk factors for post-ES bleeding.
Conclusions
The results of this study demonstrate that the endoscopist’s experience reduces patient- and procedure-related risk factors for post-ERCP complications.
Keywords: Post-ERCP complications, risk factors, post-ERCP pancreatitis, post-endoscopic sphincterotomy bleeding
Introduction
Therapeutic endoscopic retrograde cholangiopancreatography (ERCP) remains one of the most complex endoscopic procedures in the non-surgical management of several pancreatobiliary diseases [1]. The main complications arising from therapeutic ERCP are well-recognized and include acute pancreatitis, bleeding, perforation, and cholangitis. The reported incidence of post-ERCP complications varies widely from study to study and ranges for pancreatitis between 1-5%, hemorrhage 1-4%, perforation 1-2% and cholangitis 1-5% [2-6]. Precise identification of risk factors for complications of ERCP is important because it can improve the safety of the procedure and recognize the high-risk cases in which ERCP should be avoided if possible or in which protective endoscopic (pancreatic stents) or pharmacologic measures should be considered to minimize the rate of post-ERCP pancreatitis (PEP). Moreover, the identification of the procedure’s risk factors may also aid in distinguishing patients at low risk for complications who are eligible for therapeutic ERCP on an outpatient basis, thereby reducing the cost of ERCP.
The aim of the present study was to reliably investigate the patient- and procedure-related risk factors of therapeutic ERCP complications by analyzing a large collected dataset in a single center of a single expert endoscopist.
Patients and methods
Data of patients who underwent therapeutic ERCP during the last 8 years (January 2005-December 2012), included in several studies, were retrospectively collected to determine the patient- and procedure-related risk factors for post-procedure complications. Permission to review a database kept for the clinical care of those patients was achieved by the Institutional Review Board of the Hospital. Data contained demographics, clinical history, blood test results, procedural details, technical procedures, procedural findings, diagnosis and grade of severity of post-ERCP complications.
Patients were excluded from the study for any of the following reasons: previous endoscopic sphincterotomy (ES); plastic stent removal; and placement of a new metal or plastic stent in an obstructed metal stent.
All patients were instructed to stop taking aspirin or antiplatelets 7 days prior to the procedure and to contact their physician as to whether they should be replaced with low molecular weight heparin.
ERCP procedure
Patients presented for the procedure at 09.00 after a 12 h fast and remained fasting for at least 24 h after the procedure. Before ERCP all patients or their relatives provided informed consent. All procedures were performed by an experienced endoscopist (P.K.), who has performed more than 400 ERCPs yearly over the last 13 years, using a standard therapeutic duodenoscope. ERCP was performed with patients under conscious sedation with midazolam and pethidine. Hyoscine-nbutyl (Buscopan; Boehringer, Ingelheim Ltd, UK) or glucagon was used as a smooth muscle relaxant at the endoscopist’s discretion. Arterial oxygen saturation, heart rate and blood pressure were monitored using automated devices.
For ductal opacification, contrast medium (50% sodium meglumine amide triaroate diluted in distilled water) was injected manually, under fluoroscopic guidance. Pancreatograms were graded according to the extent of pancreatic opacification: main pancreatic duct, first and secondary class pancreatic branches or acinarization. Acinarization was defined as any focal or diffuse pancreatic parenchymal blush of contrast.
Cannulation of the common bile duct (CBD) was firstly attempted with a sphincterotome (Clever-cut; Olympus, Athens). If the endoscopist failed to cannulate the CBD with the sphincterotome after 10 min, a hydrophilic guide wire (Jagwire or Dreamwire; Boston Scientific, Athens) was used for another 10 min. If the techniques failed, a precut access papillotomy was attempted; after catheterization of CBD the procedure was completed with a standard sphincterotome. All ESs were performed via a hydrophilic guide wire to achieve controlled cutting and avoid the “zipper cut” phenomenon. All ESs were done using blended current (cut 45W, coagulation 30W) from an Olympus electrosurgical unit (PSD-30). The length of the ES depended on the indication: small for stent placement, or as large as possible for choledocholithiasis.
Data collected by the endoscopist conducting the procedures comprised: specific details concerning the procedure including the presence of periampullary diverticulum; method of CBD cannulation (sphincterotome, sphincterotome plus hydrophilic guide wire); CBD diameter; type of precut access papillotomy [conventional needle-knife papillotomy (NKP), suprapapillary fistulotomy (SPF) or transpancreatic sphincterotomy (TPS)]; extent of pancreatic opacification; and type of therapy performed.
Venous blood was drawn from each patient for serologic testing for amylase activity using an automated analyzer before the procedure, at 6 h post-procedure in all patients and at 24 h in hospitalized patients.
Definitions
Sphincter of Oddi dysfunction (SOD) was defined according to the revised Milwaukee SOD classification system [7]. Biliary manometry was not performed. Cannulation was considered difficult if more than 10 min were needed to achieve deep CBD cannulation using the sphincterotome with or without the hydrophilic guide wire.
The definition of complications and the grading of their severity were based on consensus criteria [8]. PEP was diagnosed when new-onset or increased abdominal pain lasting more than 24 h caused an unplanned admission of an outpatient for more than one night or prolonged hospitalization of an inpatient and was associated with an at least 3-fold increase in serum amylase at approximately 6 or 24 h post-procedure. Specifically, PEP was graded as follows: 1) mild symptoms lasting 3 days and mildly edematous appearance of the pancreas at computed tomography (CT) scan; 2) moderate, requiring specific therapeutic measures for 4-10 days post-procedure (Balthazar’s grade B/C on CT) and 3) severe local or systemic complications lasting longer than 10 days post-treatment (Balthazar’s grade D/E or death. CT findings that included presence of either tissue necrosis involving >30% of the pancreatic gland or peripancreatic fluid collection were also used to classify pancreatitis as severe.
Procedure-related hemorrhage was defined as intra-procedural when bleeding occurred during the procedure; immediate and delayed when the bleeding was observed in the first 24 h and within 15 days, respectively.
Cholangitis was defined as an elevation in the temperature to more than 38oC because of a biliary cause without evidence of other concomitant infections.
Follow up
After the procedure, all patients were monitored in the ward with frequent, close and direct contact with the endoscopist who performed the ERCP to decide whether the patient could be discharged. All patients and their escorts were given detailed standardized written information about possible post-procedure complications and family doctors and local hospitals were also informed about the intervention. All were instructed to contact the on-call ERCP staff immediately, should any of the symptoms described arise. Finally, we suggested that the discharged patients should fast (except for water) until the following morning, at which time a clear liquid breakfast could be ingested and followed, if tolerated, by a regular diet. In addition, patients were followed-up in an outpatient clinic or by telephone call on days 1, 10 and 30, post-ERCP to detect any delayed complications.
Study outcomes
The primary study outcome was to investigate patient and procedure risk factors predisposing to the development of complications. The secondary endpoint was the type of PEP (mild, moderate, severe) and type of post-ES bleeding (intraprocedural, immediate, delayed).
Statistical analysis
Categorical variables were analyzed with chi-squared and Fisher’s exact tests, as appropriate, while continuous variables were expressed as means with standard deviation (SD) and analyzed using the Student t test. Factors associated with increased risk for complication development were examined by univariate and multivariate analyses and calculated with odds ratio (OR) with 95% confidence interval (CI), using a logistic regression method. Statistical significance was set at P<0.05. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 16.0, Chicago, IL, USA).
Results
During the study period, 2,837 patients with naïve papilla underwent therapeutic ERCP. Of these, 2,715 were enrolled in the final analysis. One hundred and twenty two patients were excluded because of insufficient data regarding the type of complications and details of the procedure.
Clinical characteristics of the patients are demonstrated in Table 1, and the two groups were not significantly different between the two groups, except for the history of acute pancreatitis which was statistically more frequent in patients with complications (Table 1). Choledocholithiasis was the main indication for therapeutic ERCP (Table 1). Complications were observed in 327 patients (Table 2). PEP occurred in 132 patients (4.9%); mild in 102 patients (77.3%), moderate in 22 patients (16.7%), severe in 7 patients (5.3%) and an old woman died from multi-organ failure due to severe PEP (Table 2). Post-ES bleeding occurred in 122 patients (4.5%); intraprocedural in 85 patients (69.7%), immediate in 9 patients (7.4%), delayed in 27 patients (22.1%) and a 77-year-old man was operated but died from respiratory failure. All cases except one with post-ES hemorrhage were successfully treated conservatively or endoscopically with injection of adrenaline solution (1:10,000) with dextrose 50% or hydroxyl-methyl starch (Voluven®) or thermocoagulation. Blood transfusions were administered in 11 (9%) patients. Cholangitis was observed in 63 (2.3%) patients (Table 2) and and resolved with re-endoscopy and removal of residual stones or placement of a plastic stent in 58 (92.1%) patients and substitution of a migrated plastic stent in 4 (6.3%) patients; one patient with SOD type I experienced cholangitis after ES and died of uncontrolled sepsis.
Table 1.
Characteristics of the patients
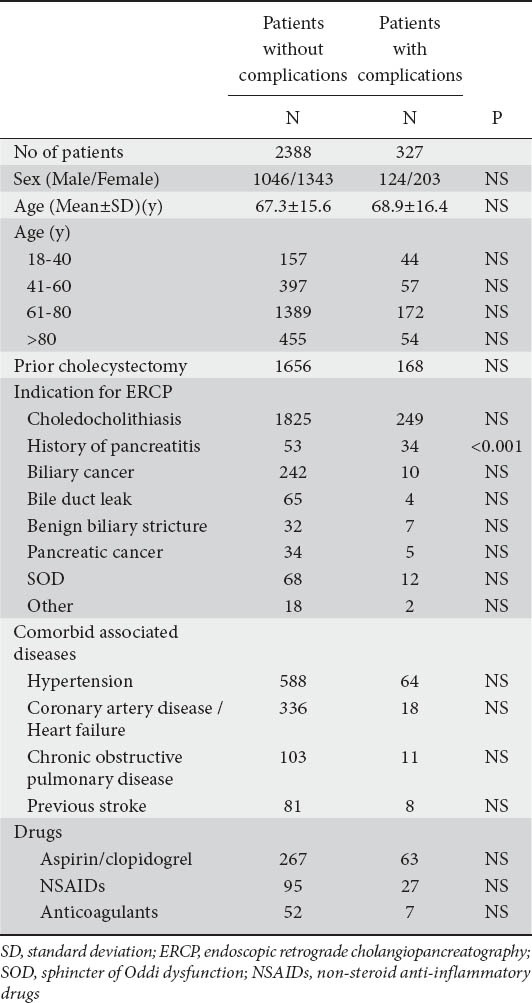
Table 2.
Overall complication rate of the first-only 2,715 therapeutic ERCPs
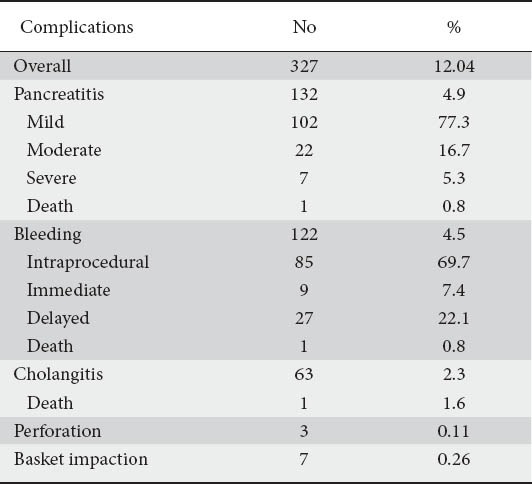
Three patients (0.11%) presented ES-related perforation (Table 2); one was operated, in one patient the perforation was closed with endoscopic endoclipping [9] and one patient was treated conservatively; all patients had an uneventful course.
Basket impaction occurred in 7 patients (0.26%) (Table 2); one patient with intradiverticular papilla was operated with excellent outcome and in 6 patients the basket impaction was managed successfully by large-balloon (CRE, Boston Scientific, Athens) dilation of the biliary orifice and extraction en bloc of the basket and stone [10].
Analysis of risk factors was performed only for overall post-ES complications, PEP and post-ES bleeding; the numbers of other complications were too small for further analysis in our series.
Overall complications
A total of 17 variables including nine patient-related factors and eight procedure-related factors were investigated (Table 3). Univariate analysis showed that history of acute pancreatitis (P<0.001, OR 2.78, 95%CI 1.74-4.55), non-steroid anti-inflammtory drug (NSAID) or aspirin/clopidogrel use (P=0.02, OR 1.55, 95%CI 1.02-2.75), and anticoagulant use (P=0.03, OR 1.84, 95%CI 1.27-3.82) were significant patient-related factors for complications (Table 3); significant procedure-related factors were found to be: difficult cannulation (P=0.008, OR 2.25, 95%CI 1.24-4.36); first and second class pancreatic ductules (P<0.001, OR 2.21, 95%CI 1.47-4.27); acinarization (P<0.001, OR 1.96, 95%CI 1.32-3.56); NKP (P=0.005, OR 1.64, 95%CI 1.26-2.84); and TPS (P<0.001, OR 1.98, 95%CI 1.23-3.38) (Table 3).
Table 3.
Risk factors for overall complications after therapeutic ERCP in univariate and multivariate analyses
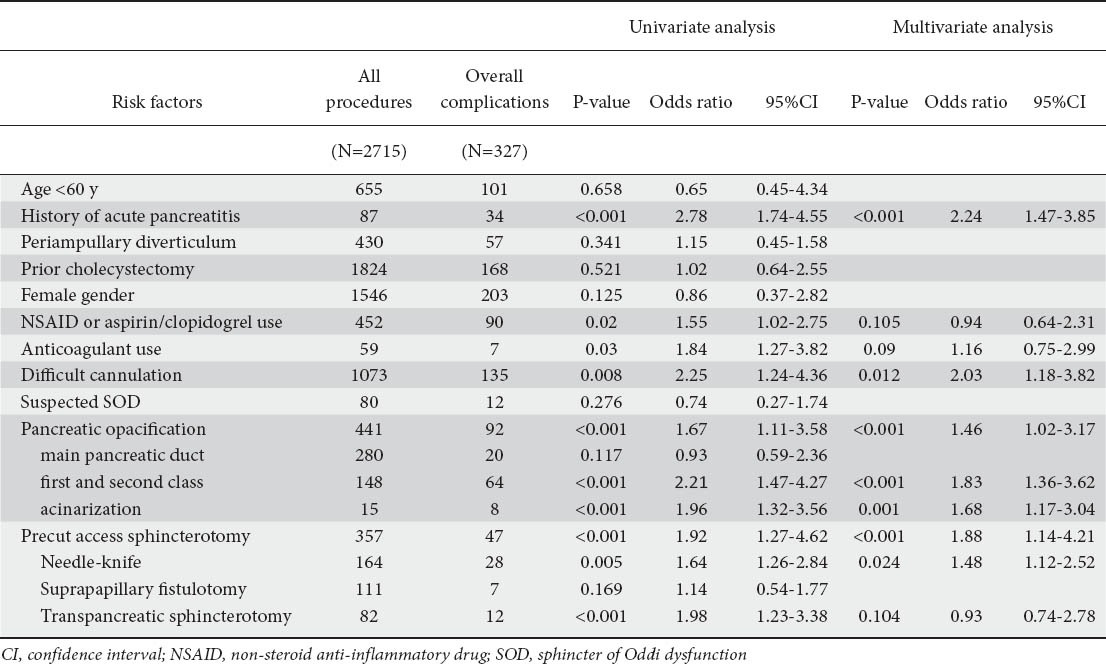
Multivariate analysis after forward stepwise binary logistic regression from the pool of 17 potential risk factors for overall complications identified: history of acute pancreatitis as a patient-related independent risk factor (P<0.001, OR 2.24, 95%CI 1.47-3.85); and: difficult cannulation (P=0.012, OR 2.03, 95%CI 1.18-3.82); NKP (P=0.024, OR 1.48, 95%CI 1.12-2.52); TPS (P=0.012, OR 1.87, 95%CI 1.23-2.35); opacification of first-and second-class pancreatic ductules (P<0.001, OR 1.83, 95%CI 1.36-3.62); and acinarization (P=0.001, OR 1.68, 95%CI 1.17-3.04) as procedure-related independent risk factors for post-ERCP complications (Table 3).
PEP
Patients with chronic pancreatitis and ampullary cancer were excluded from the analysis of risk factors for PEP because of the very low risk of PEP. Therefore in the final analysis 2,688 patients were included.
Univariate analysis revealed history of acute pancreatitis, either non-ERCP or ERCP-related, to be the only significant patient-related risk factor for PEP (P<0.001, OR 2.14, 95% CI 1.41-4.17) (Table 4). Multivariate analysis demonstrated the same result (P<0.001, OR 1.95, 95% CI 1.26-3.12) (Table 5). Of the procedure-related risk factors, precut access sphincterotomy and, especially its subtypes NKP and TPS and opacification of first and second class pancreatic ductules and acinarization were significantly associated with PEP according to univariate analysis (P<0.001, OR 1.94, 95%CI 1.18-3.41, P<0.01, OR 1.78, 95% CI 1.33-2.63, P<0.001, OR 1.91, 95% CI 1.27-2.59, P<0.001, OR 1.88, 95% CI 1.14-3.01, and P<0.001, OR 2.34, 95%CI 1.45-4.57, respectively) (Table 4).
Table 4.
Risk factors for pancreatitis using univariate analysis
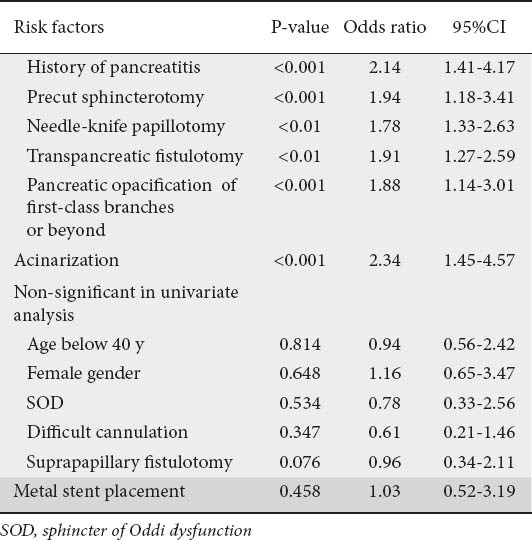
Table 5.
Risk factors for pancreatitis after ERCP in multivariate analysisRisk factors P-value Odds ratio 95%CI
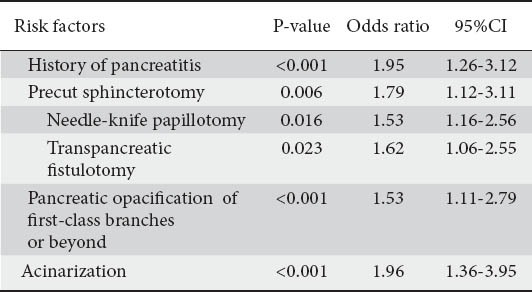
The results of multivariate analysis, apart from the history of acute pancreatitis, identified five additional risk factors; these were all independently associated with PEP: precut access sphincterotomy and especially its subtypes NKP, TPS, opacification of first and second class pancreatic ductules and acinarization (P=0.006, OR 1.79, 95% CI 1.12-3.11, P=0.016, OR 1.53, 95% CI 1.16-2.56 P=0.023, OR 1.62, 95% CI 1.06-2.55, P<0.001, OR 1.53, 95% CI 1.11-2.79, and P<0.001, OR 1.96, 95%CI 1.36-3.95 respectively) (Table 5).
Age below 40 years, female gender, SOD, difficult cannulation and metal stent placement were not significantly associated with PEP by both analyses (Tables 4 and 5).
Post-ES hemorrhage
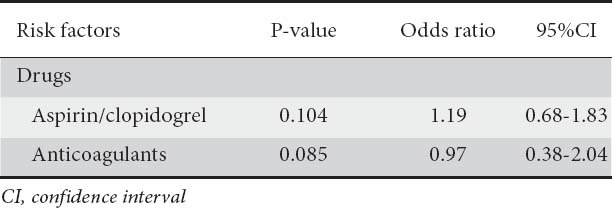
Univariate analysis showed aspirin/clopidogrel use (P=0.032, OR 1.84, 95%CI 1.45-2.88) and anticoagulant use (P=0.012, OR 1.65, 95%CI 1.15-2.89) as significant risk factors for post-ES bleeding (Table 6). However in multivariate analysis, no independent risk factors for post-ES bleeding were found (Table 7).
Table 6.
Risk factors for post-endoscopic sphincterotomy bleeding after therapeutic ERCP in univariate analysis
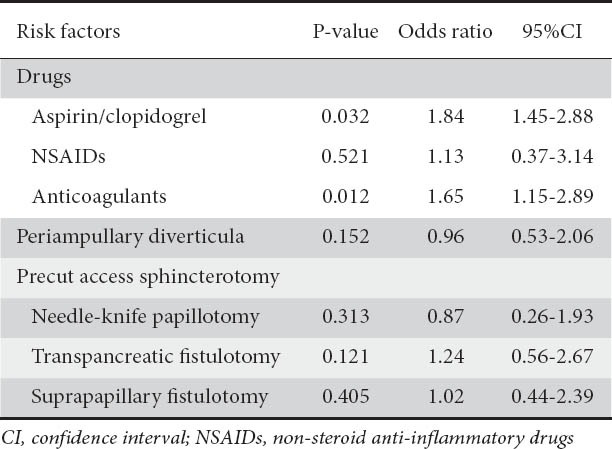
Table 7.
Risk factors for post-ES bleeding after therapeutic ERCP in multivariate analysis
Discussion
Diagnostic ERCP has been abandoned with the increased availability of magnetic resonance cholangiopancreatography (MRCP), multidetector helical CT, and endoscopic ultrasound [1]. In contrast, since its introduction forty years ago, therapeutic ERCP continues to remain an essential therapeutic modality for a variety of biliary and pancreatic diseases. Over the last 2 decades significant advances have been made in ERCP, i.e. intensive training, novelties in accessories (hydrophilic guide wires [11], steerable catheters, diathermy with microprocessors [12]), which facilitate the cannulation of a desired duct, and contribute to controlled cutting of ampullary sphincter, minimizing the trauma of major papilla. However, the incidence of post-ERCP complications has not changed during last ten years [1,13].
Therefore, identifying patient-and procedure-related risk factors for post-ERCP complications has a significant impact on clinical practice helping in the implementation of appropriate pharmacological [14,15] and technical measures [16-18] (pancreatic stents, ES via hydrophilic guide wire to avoid the “zipper cut” phenomenon) to reduce the likelihood of post-ERCP complications. Moreover, the assessment of risk factors allows better identification of patients who might be candidates for immediate discharge after therapeutic ERCP and might reduce the financial cost of the procedure.
A number of prospective and retrospective multicenter studies [3,13,19-22] have investigated patient- and procedure-related risk factors of therapeutic ERCP. In this regard, the present study differs from previous studies by investigating therapeutic ERCP-related risk factors because it is the largest study in which all procedures were performed by an experienced pancreatobiliary endoscopist. Moreover, the present study differs from other studies in the respect that the three subtypes of precut access papillotomies were estimated as risk factors for post-ERCP complications.
Univariate and multivariate analysis showed history of acute pancreatitis (ERCP or non-ERCP-related) as the only patient-related risk factor for overall complications and PEP and this finding is consistent with previous studies [2,5,23]. Younger age (<40 years), female sex and periampullary diverticulum were not associated risk factors for overall complications, PEP and post-ES bleeding. Although these three patient-related parameters were reported in earlier studies [2,5,23] as risk factors for PEP and post-ES bleeding and perforation, more recent studies and multivariate analysis [21,24] did not consider these parameters per se as risk factors for post-ERCP complications. We believe that it is preferable to perform ES in a periampullary diverticulum via a hydrophilic guide wire; thus, a better control in cutting the sphincter is achieved avoiding post-ES hemorrhage and perforation.
Technical variables are of obvious importance as procedure-related factors in overall post-ERCP complications and especially PEP. By both analyses, first-and second-class pancreatic branches and acinarization were independent risk factors for PEP and this is consistent with most previous reports [1,23]. It is interesting that when visualization is limited to the main pancreatic duct, the risk is not significant. These findings confirm the hypothesis that hydrostatic injury from pancreas overfilling is the main trigger of activation of PEP mechanism [25].
Precut access papillotomy in the present study was a significant risk factor for overall complications and especially for PEP, a finding not different with previous studies and meta-analyses [6,22,23,26,27]. It is interesting that when analyzing the influence of the three subtypes of precut access papillotomy, we found that NKP and TPS on both analyses were significant risk factors for PEP. Contrary to this finding, SPF was demonstrated to be related with very low risk of PEP. In this precut access papillotomy subtype thermal injury and subsequent edema at the level of the papilla is avoided, thus preserving pancreatic flow after the cannulation. The choice of type of precut access papillotomy used to achieve CBD cannulation is highly individual. We prefer the SPF technique especially in a protuberant papilla with visible intraduodenal course of CBD.
Surprising, in our study, we found that SOD and difficult cannulations were not risk factors for PEP by both analyses, in spite of the fact that most studies and meta-analyses [2,3,5,6,13,21,22,24] recorded them as independent predictors of PEP. This difference is explained by the fact that SOD diagnosis in our study was based on clinical and laboratory findings (Milwaukee criteria) and not on sphincter of Oddi manometry. We acknowledge this as a limitation of our study since all our patients were SOD I or SOD II. In these patients the pathogenesis of SOD is probably related to stenosis resulting from passive obstruction at the sphincter of Oddi caused by fibrosis, inflammation, or both. Dyskinesia of the sphincter, which results from intermittent obstruction caused by sphincter muscle spasm, is more common in patients with SOD III. Patients with suspected SOD III but no manometric confirmation were not included in this study and these are the SOD patients that are at greater risk for PEP. Moreover, it is a common belief that papillary trauma from repeated attempts to achieve selective bile duct cannulation leads to edema with a subsequent major impact on sphincter hypertension-related impairment of pancreatic drainage and PEP development [26,28]. Papillary trauma is different when the cannulation is attempted by a trainee, an endoscopist with low experience or a very experienced endoscopist. Therefore, we believe the 10 min of unsuccessful attempts by our experienced endoscopist was not adequate to produce papillary edema and trigger the PEP development and explains our findings that SOD and difficult cannulation are not risk factors for PEP. Although in a recent study [29] covered and uncovered self-expanding metal stent placement (SEMS) was reported as a risk factor for PEP, in the present study we found no association of SEMS placement with PEP.
Post-ES bleeding was observed at a rate of 4.5%, in accordance with previous studies [2,3,5,6,13,21-24], with most bleedings being intraprocedural (69.7%) (Table 2). Because it is our policy to intervene immediately in any ES-related hemorrhage, it is difficult to appreciate the significance of bleeding episodes according to consensus criteria.
We examined as risk factors for post-ES hemorrhage the use of oral anticoagulants and aspirin/clopidogrel, precut access papillotomy with its subtypes and the presence of periampullary diverticulum. Although univariate analysis showed aspirin/clopidogrel and anticoagulant use as risk factors for post-ES bleeding, on multivariate analysis which has more credibility, no relationship was found between the use of drugs influencing the platelet’s function or coagulation cascade and post-ES hemorrhage. The guidelines of the European Association of Gastrointestinal Endoscopy (ESGE) [30] recommend that aspirin and other NSAIDs should be continued in patients undergoing ES, especially in patients at high risk of thromboembolic events, because the risk of bleeding is not different between patients with/without their use. However, the recommendations are less clear regarding the use of clopidogrel in endoscopic interventions.
The principal limitations of our study are that the data were collected retrospectively. In addition, the globalization of our findings is questionable because all procedures were performed by an experienced endoscopist, but this does not represent the common practice of an average endoscopist; the synergistic effect of multiple risk factors which might have led to the post-ERCP complications was not analyzed.
In conclusion, our study demonstrates that patient- and procedure-related risk factors for therapeutic ERCP complications could be reduced in the hands of an experienced pancreatobiliary endoscopist.
Summary Box.
What is already known:
Therapeutic ERCP remains one of the most complex endoscopic procedures in the non-surgical management of several pancreatobiliary diseases
Main complications of therapeutic ERCP include acute pancreatitis, bleeding, perforation, and cholangitis
Identifying patient-and procedure-related risk factors for post-ERCP complications has a significant impact on clinical practice
What the new findings are:
Endoscopist’s experience reduces patient- and procedure-related risk factors for post-ERCP complications
History of acute pancreatitis was identified as the only patient-related risk factor for overall complications and post-ERCP pancreatitis
Opacification of first and second class pancreatic ductules, acinarization, precut access papillotomy, and more specifically needle-knife papillotomy and transpancreatic sphincterotomy were significant risk factors for overall complications and especially for post-ERCP pancreatitis
No relationship was found between the use of drugs influencing the platelet’s function or coagulation cascade and post-ES hemorrhage
Biography
G. Gennimatas General Hospital; Aristotle University of Thessaloniki, Ippokration Hospital, Thessaloniki, Greece
Footnotes
Conflict of Interest: None
References
- 1.Freeman ML. Complications of endoscopic retrograde cholangiopancreatography: avoidance and management. Gastrointest Endosc Clin N Am. 2012;22:567–586. doi: 10.1016/j.giec.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 3.Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417–423. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]
- 4.Feurer ME, Adler DG. Post-ERCP pancreatitis: review of current preventive strategies. Curr Opin Gastroenterol. 2012;28:280–286. doi: 10.1097/MOG.0b013e3283528e68. [DOI] [PubMed] [Google Scholar]
- 5.Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652–656. doi: 10.1067/mge.2002.129086. [DOI] [PubMed] [Google Scholar]
- 6.Wang P, Li ZS, Liu F, et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104:31–40. doi: 10.1038/ajg.2008.5. [DOI] [PubMed] [Google Scholar]
- 7.Geenen JE, Hogan WJ, Dodds WJ, et al. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter of Oddi dysfunction. N Engl J Med. 1989;320:82–87. doi: 10.1056/NEJM198901123200203. [DOI] [PubMed] [Google Scholar]
- 8.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 9.Katsinelos P, Beltsis A, Chatzimavroudis G, et al. Endoscopic management of occluded biliary uncovered metal stents: a multicenter experience. World J Gastroenterol. 2011;17:98–104. doi: 10.3748/wjg.v17.i1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsinelos P, Fasoulas K, Beltsis A, et al. Large-balloon dilation of the biliary orifice for the management of basket impaction: a case series of 6 patients. Gastrointest Endosc. 2011;73:1298–1301. doi: 10.1016/j.gie.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Artifon EL, Sakai P, Cunha JE, Halwan B, Ishioka S, Kumar A. Guidewire cannulation reduces risk of post-ERCP pancreatitis and facilitates bile duct cannulation. Am J Gastroenterol. 2007;102:2147–2153. doi: 10.1111/j.1572-0241.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 12.Perini RF, Sadurski R, Cotton PB, Patel RS, Hawes RH, Cunningham JT. Post-sphincterotomy bleeding after the introduction of a microprocessor-controlled electrosurgery: does the new technology make the difference? Gastrointest Endosc. 2005;61:53–57. doi: 10.1016/s0016-5107(04)02454-x. [DOI] [PubMed] [Google Scholar]
- 13.Cotton P, Garrow DA, Gallagher J, Romaqnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11497 procedures over 12 years. Gastrointest Endosc. 2009;70:80–88. doi: 10.1016/j.gie.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsinelos P, Fasoulas K, Paroutoglou G, et al. Combination of diclofenac plus somatostatin in the prevention of post-ERCP pancreatitis: a randomized, double-blind, placebo-controlled trial. Endoscopy. 2012;44:53–59. doi: 10.1055/s-0031-1291440. [DOI] [PubMed] [Google Scholar]
- 16.Sofuni A, Maguchi H, Mukai T, et al. Endoscopic pancreatic duct stent reduces the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastroenterol Hepatol. 2011;9:851–859. doi: 10.1016/j.cgh.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Cha SW, Leung WD, Lehman GA, et al. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest Endosc. 2013;77:209–216. doi: 10.1016/j.gie.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Katsinelos P, Gatopoulou A, Gkagkalis S, et al. A prospective analysis of factors influencing fluoroscopy time during therapeutic ERCP. Ann Gastoenterol. 2012;25:338–344. [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper ST, Slivka A. Incidence, risk factors and prevention of post-ERCP pancreatitis. Gastroenterol Clin N Am. 2007;36:259–276. doi: 10.1016/j.gtc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Jeurnink SM, Siersema PD, Steyerberg EW, et al. Predictors of complications after endoscopic retrograde cholangiopan-creatography: a prognostic model for early discharge. Surg Endosc. 2011;25:2892–2900. doi: 10.1007/s00464-011-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 22.Williams EJ, Taylor S, Fairclouqh P, et al. Risk factors for complications following ERCP: results of a large-scale prospective multicenter study. Endoscopy. 2007;39:793–801. doi: 10.1055/s-2007-966723. [DOI] [PubMed] [Google Scholar]
- 23.Freeman ML, DiSario JA, Nelosn DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 24.Testoni PA. Preventing post-ERCP pancreatitis: where are we? JOP. 2003;4:22–32. [PubMed] [Google Scholar]
- 25.George S, Kulkarni AA, Stevens G, et al. Role of osmolality of contrast media in the development of post-ERCP pancreatitis: a meta-analysis. Dig Dis Sci. 2004;49:503–508. doi: 10.1023/b:ddas.0000020511.98230.20. [DOI] [PubMed] [Google Scholar]
- 26.Katsinelos P, Gkagkalis S, Chatzimavroudis G, et al. Comparison of three types of precut technique to achieve common bile duct cannulation: a retrospective analysis of 274 cases. Dig Dis Sci. 2012;57:3286–3292. doi: 10.1007/s10620-012-2271-8. [DOI] [PubMed] [Google Scholar]
- 27.DaVee T, Garcia JA, Baron TH. Precut sphincterotomy for selective biliary duct cannulation during endoscopic retrograde cholangiopancreatography. Ann Gastroenterol. 2012;25:291–302. [PMC free article] [PubMed] [Google Scholar]
- 28.Cennamo V, Fuccio L, Zaqari RM, et al. Can early precut implementation reduce endoscopic retrograde cholangiopancreatography-related complication? Meta-analysis of randomized controlled trials. Endoscopy. 2010;42:381–388. doi: 10.1055/s-0029-1243992. [DOI] [PubMed] [Google Scholar]
- 29.Cote GA, Kumar N, Ansstas M, et al. Risk of post-ERCP pancreatitis with placement of self-expandable metallic stents. Gastrointest Endosc. 2010;72:748–754. doi: 10.1016/j.gie.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Boustiere C, Veitch A, Vanbiervliet G, et al. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) Guidelines. Endoscopy. 2011;43:445–461. doi: 10.1055/s-0030-1256317. [DOI] [PubMed] [Google Scholar]


