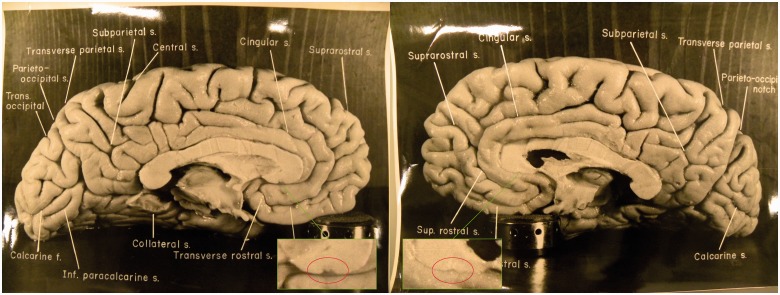
Sir, Albert Einstein was arguably the greatest physicist in the 20th century and his extraordinary intelligence has long intrigued both scientists and the general public. Despite several studies that focused mainly on the histological and morphological features of Einstein’s brain after his death, the substrates of Einstein’s genius are still a mystery (Diamond et al., 1985; Anderson and Harvey, 1996; Kigar et al., 1997; Hines, 1998; Witelson et al., 1999a, b; Colombo et al., 2006; Falk, 2009). Recently, Falk et al. (2013) analysed 14 newly discovered photographs and found that Einstein’s brain had an extraordinary prefrontal cortex, and that inferior portions of the primary somatosensory and motor cortices were greatly expanded in the left hemisphere. Among these 14 images were photographs of the left and right medial surface of Einstein’s brain, on which the corpus callosum was shown with great resolution and accuracy. The corpus callosum is the largest nerve fibre bundle that connects the cortical regions of the cerebral hemispheres in human brains and it plays an essential role in the integration of information transferred between the hemispheres over thousands of axons (Aboitiz et al., 1992). The two photographs of the medial surfaces of Einstein’s cerebral hemispheres provide the basis for the present study.
To examine whether there are regional callosal differences between the brain of Einstein and those of ordinary people, and to minimize potential differences in corpus callosum morphology due to cause of death, brain atrophy, age, and sex, in vivo MRI data sets from two different age groups were used. The high-resolution photographs of Einstein’s left and right hemispheres were supplied by Dean Falk with permission from the National Museum of Health and Medicine (Fig. 1). Because Einstein was right-handed and died at the age of 76, our first control group consisted of 15 elderly, healthy right-handed males, aged 70 to 80 years (mean: 74.20 ± 2.60 years). All participants were college graduates or beyond college, and non-demented (Clinical Dementia Rating = 0, Mini-Mental State Examination was from 28 to 30, mean ± SD: 29.53 ± 0.64) (Marcus et al., 2007, 2010). The information regarding the subjects’ racial/ethnic backgrounds is unavailable. The T1-weighted MRI data of these 15 older males were obtained from the Open Access Series of Imaging Studies (OASIS, http://www.oasis-brains.org/). All images were acquired on a 1.5 T Vision scanner (Siemens) and a T1-weighted MPRAGE sequence, with the following parameters: repetition time/echo time/inversion time = 18 ms/10 ms/20ms, 128 contiguous 1.25 mm sagittal slices, and voxel size =1 × 1 × 1.25 mm3. Our second control group consisted of 52 younger, healthy right-handed Caucasian males, aged 24 to 30 years (mean: 26.60 ± 2.19 years). The reasons for selection are described in the Supplementary material. The high resolution T1-weighted MRI data of these 52 Caucasian males were obtained from the International Consortium for Brain Mapping (ICBM) database (www.loni.ucla.edu/ICBM). Thirty-five of the MRI data sets were acquired on a Philips 1.5 T ACSIII scanner (Philips Intera, Philips Medical System) and a 3D T1-weighted sequence (T1-FFE) with the following parameters: repetition time/echo time = 18 ms/10 ms, ∼160–190 contiguous 1 mm sagittal slices, and voxel size = 1 × 1 × 1 mm3. The remaining 17 MRI data sets were acquired on a GE 1.5 T Signa scanner (General Electric) and a 3D T1-weighted sequence with the following parameters: repetition time/echo time = 24 ms/4 ms, 124 contiguous 1.2 mm sagittal slices, and voxel size = 0.9766 × 0.9766 × 1.2 mm3.
Figure 1.
Photographs of the left and right midsagittal sections of Einstein’s brain with original labels (Falk et al., 2013), reproduced here with permission from the National Museum of Health and Medicine, Silver Spring, MD. The red circles indicate two breaches on each hemisphere of Einstein’s corpus callosum that have different shapes, which may have been introduced when the two hemispheres were separated in 1955.
Because MRI data are not available for Einstein’s brain, we used the measurements from two photographs obtained from his preserved brain to compare with the MRI data of the control brains. Justification for this approach comes from a previous study in which 44 preserved cadaver brains and 30 in vivo brain MRI data sets in two age- and sex-matched groups were compared, and a remarkable similarity was found between the two groups’ callosal measurements (Gupta et al., 2008). We developed a novel method for determining callosal thickness, which was used to test whether Einstein’s corpus callosum differed significantly from those of the control groups. The connectivity of bilateral symmetrical brain regions of various subdivisions of Einstein’s corpus callosum was assessed and compared with corresponding measurements in controls, with greater area of a subregion in Einstein or the controls indicating relatively greater interhemispheric connectivity (Aboitiz et al., 1992).
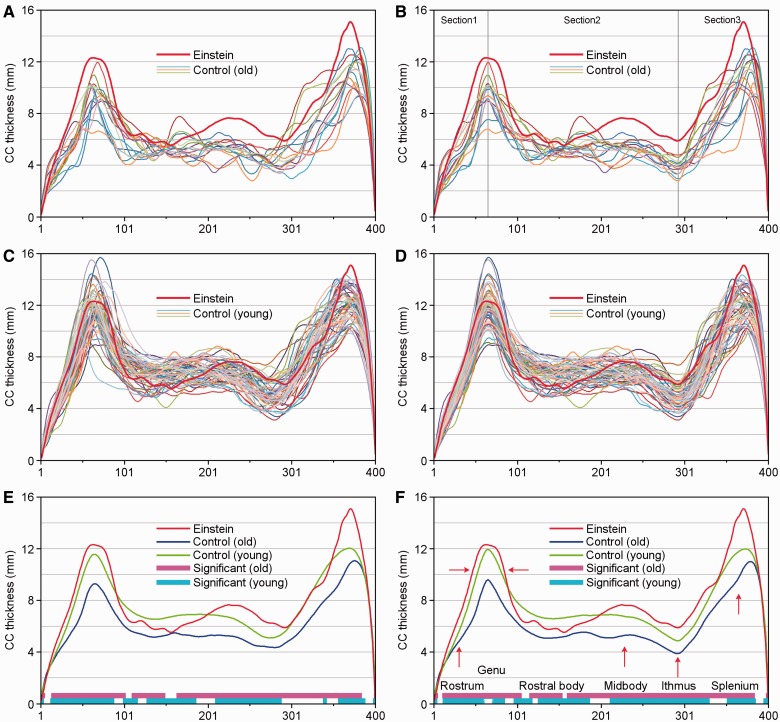
Briefly, the scale/callibration of two photographs of Einstein’s brain was determined by using the lengths of Einstein’s hemispheres (17.2 cm left/16.4 cm right) reported in the literature (Anderson and Harvey, 1996). The contours of both corpus callosums were outlined by one rater (M.W.), and the top and bottom edges were defined relative to anterior and posterior end points. The middle line of Einstein’s corpus callosum (i.e. that courses rostrocaudally through the centre of the corpus callosum approximately parallel to its superior and inferior edges) was defined by the Symmetry-Curvature Duality Theorem (Leyton, 1987) and then sectioned into 400 equidistant points, with 400 corresponding points on the top edge and bottom edge. The distance between corresponding points at the top and bottom edges was defined as the thickness of the corpus callosum at that level. The value of the 400 thicknesses were coded in colour and mapped onto Einstein’s left callosal space. The 400 values were averaged and defined as the mean thickness of the corpus callosum, whereas the summed distances between the 400 adjacent points was defined as the length of the middle line of the corpus callosum. The callosal area, perimeter and maximal length of corpus callosum were measured from the callosal mask; the circularity of corpus callosum accorded with the definition of Ardekani et al. (2013). We identified subdivisions of the corpus callosum by partitioning it at specified intervals along the anterior–posterior length as described and illustrated in the Supplementary material. The maximum thicknesses and positions along the callosum of the genu, midbody and splenium, and the minimum thickness and position of the isthmus were then determined. Computational analysis was done with an in-house Matlab program (MATLAB 7, Mathworks). For contour reliability of corpus callosum, the same rater (W.M.) contoured Einstein’s left and right callosum five times, and the repeatability errors of total callosal areas were 0.40% for left hemisphere and 0.90% for right hemisphere. Einstein’s brain was separated into two hemispheres after it was harvested, which caused slightly different distortions in their corpus callosums. In order to reduce error, both of Einstein’s corpus callosums were measured multiple times and the results averaged. Because the corpus callosums of the in vivo hemispheres had no such distortion, we only measured the corpus callosum of controls on one hemisphere (right). Other details about the processing of Einstein’s photographs and MRI data of the control groups are described in the Supplementary material, and the measurements of Einstein’s brain and that of the two control groups are shown in Fig. 2. Corpus callosum plots for the individuals in our study are shown in Fig. 3A and C. To compare the difference between Einstein’s callosal thickness and that of the control brains, the callosal thickness distribution was partitioned into three sections along the corpus callosum, with divisions at the maximum thickness in the genu and the minimum thickness in the isthmus (Fig. 3B), and the sections of the control groups were registered to corresponding sections of Einstein’s brain. The registered plots of the control groups are shown in Fig. 3B and D, the registered thickness maps are shown in the right columns of Figs 4 and 5. The details of the corpus callosum thickness measurement and registration are provided in the Supplementary material.
Figure 2.
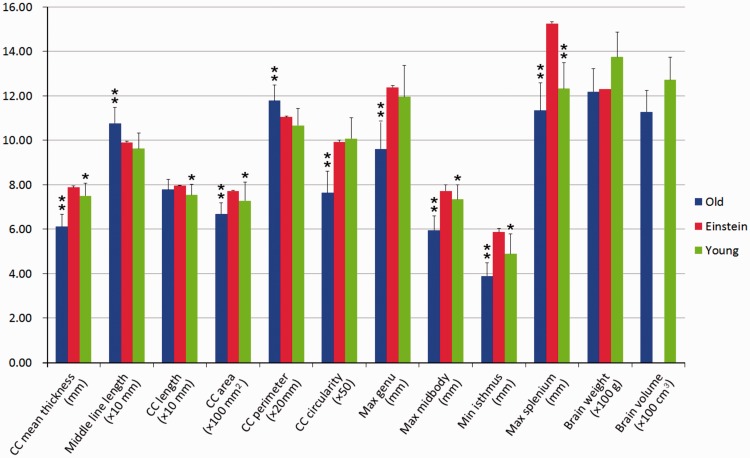
Measurements of corpus callosum (CC) morphology and brain between Einstein and the two different age control groups. The red, blue and green bars represent the measurements of Einstein, the old age control group and the young control group, respectively. Measurements should be multiplied as indicated in their labels. The asterisks on the top of bars indicate that there are significant differences between the control group and Einstein, *P < 0.05, **P < 0.001.
Figure 3.
The corpus callosum (CC) thickness plots, with left to right sequentially representing genu to splenium (as labelled in F). (A) Measured thickness plots of Einstein (red thick line) and elderly controls (coloured thin lines). (B) Each control thickness plot sectioned into three segments (at the maximum thickness in genu and minimum thickness in isthmus) and registered to Einstein’s callosal thickness plot. (C) Measured thickness plots of Einstein (red thick line) and young controls (coloured thin lines). (D) The callosal thickness plots of the young group were sectioned and registered to Einstein’s corpus callosum thickness plot. (E) Measured average corpus callosum thickness plots of Einstein (red), the elderly control group (blue) and the young control group (green), the purple (old controls) and cyan (young controls) spans indicate that these regions differ significantly (P < 0.05, FDR corrected) between Einstein and the two age control groups. (F) The sectioned and registered average corpus callosum thickness plots, Einstein (red), the elderly control group (blue) and the young control group (green); labels after Witelson (1989). The meaning of purple and cyan spans are the same as (E). Red arrows indicate that Einstein’s callosal thickness is 10% thicker than the mean for the young group, especially in the splenium, whereas the width of Einstein’s corpus callosum is noticeably larger in the genu.
Figure 4.
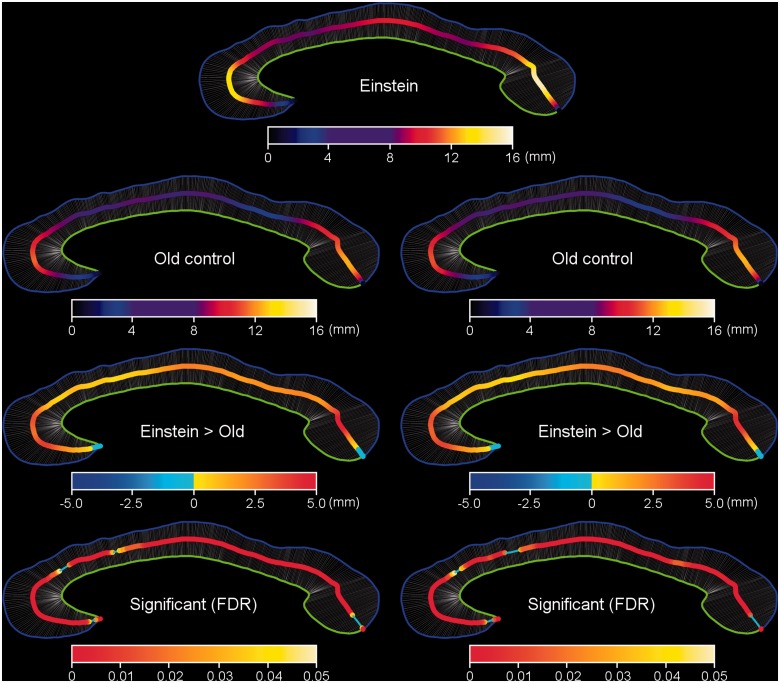
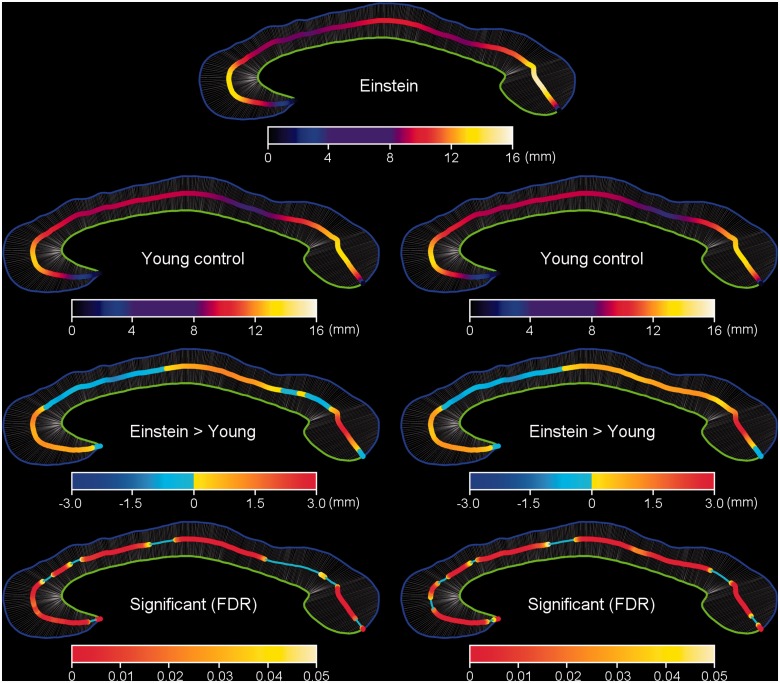
Distribution maps of corpus callosum thickness between Einstein and the elderly controls. The corpus callosum thickness map of Einstein (top row); maps for old age control group (second row), with the actual measured callosal thickness on the left and the registered callosal thickness on the right. The corpus callosum thicknesses of Einstein are greater than respective thicknesses in the elderly controls (third row), as indicated by the actual (left) and registered (right) significance maps between Einstein and the old age control group (fourth row, P < 0.05 corrected with FDR).
Figure 5.
Distribution maps of corpus callosum thickness between Einstein and the young age control group. The corpus callosum thickness map of Einstein (top row) is compared to those for young controls (second row). Row 3 illustrates the extent to which Einstein’s corpus callosum is regionally thicker than those of young controls; Row 4 graphs the statistical significance of these differences. For Rows 2–4, the actual measured callosal thickness is on the left while the registered callosal thickness is on the right.
A non-parametric test, the Mann–Whitney U test (Mann and Whitney, 1947), was used in this study to test for significant differences, and was used in a previous study of Einstein’s brain (Anderson and Harvey, 1996). The same test was used to compare the difference of the callosal thickness between Einstein and the control groups, for multiple comparisons using False Discovery Rate (FDR) with a cut-off threshold at 0.05 (Benjamini and Hochberg, 1995), and the corrected P-values were colour-coded and mapped onto Einstein’s callosal space. These statistics were implemented by a Matlab script.
Callosal dimensions and brain weight for Einstein and the two control groups are shown in Table 1 and Fig. 2. The corpus callosum measurements of Einstein’s brain are greater than those of the two control groups except for the middle line length and corpus callosum perimeter, which are both longer in the old age group, and the corpus callosum circularity, which is negligibly longer than Einstein's in the young controls. There are significant differences in all of the corpus callosum measurements except corpus callosum length between Einstein and the old age group (P < 0.001). Einstein’s corpus callosum also differs statistically from those in the younger group in the corpus callosum mean thickness, corpus callosum length, corpus callosum area, maximum thickness in the midbody, minimum thickness in the isthmus (all P-values < 0.05), and maximum thickness in the splenium (P < 0.001). Einstein’s brain weight is 1230 g (Anderson and Harvey, 1996) and very similar to the mean brain weight of the elderly control group (1219 ± 102.93 g), but less than that of the young control group (1374.13 ± 111.56 g). Falk et al. (2013) suggested that the weight of Einstein’ brain is consistent with his age. However Einstein’s body height was 171.5 cm when he was 22 years old (http://www.relativity.li/en/epstein2/read/d0_en/d7_en/), which was below the average height of similarly aged people (176 cm, 22–30 years old) (Dekaban, 1978). Schreider (1966) found that there was a positive correlation between brain weight and the body height, indicating that Einstein should have a relatively small brain/head. However his brain weight is slightly heavier than the mean brain weight of the elderly controls in this study, which could infer that his brain was healthy with little atrophy when he died; this inference is in line with previous findings described by Dr. Harry Zimmerman, ‘Einstein’s brain was normal for his age’ (Lepore, 2001). The shape of the corpus callosum, characterized by its circularity, is sensitive to brain atrophy (Ardekani et al., 2013). Einstein’s corpus callosum circularity is significantly larger than that of the elderly control group (P < 0.001) and slightly smaller than that of the younger group (P = 0.4160), which further indicates that Einstein’s brain was healthy and had little atrophy when he died.
Table 1.
Measurements of corpus callosum morphology for Einstein and two different age control groups
| measurements | Einstein |
Control (old) |
Control (young) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | SD# | mean | SD | z | P | mean | SD | z | P | |
| CC mean thickness (mm) | 7.88 | 0.05 | 6.11 | 0.56 | 4.13 | 0.0000** | 7.50 | 0.56 | 2.29 | 0.0222* |
| Middle line length (mm) | 99.07 | 0.65 | 107.61 | 7.09 | −3.58 | 0.0003** | 96.23 | 7.01 | 1.35 | 0.1772 |
| CC length(mm) | 79.49 | 0.24 | 77.87 | 4.48 | 0.81 | 0.4208 | 75.34 | 4.89 | 2.84 | 0.0045* |
| CC area (mm2) | 771.71 | 2.34 | 668.91 | 50.69 | 3.58 | 0.0003** | 728.47 | 82.34 | 2.02 | 0.0435* |
| CC perimeter (mm) | 221.00 | 0.61 | 235.69 | 14.15 | −3.02 | 0.0000** | 213.40 | 15.25 | 1.71 | 0.0867 |
| CC circularity (×50) | 9.93 | 0.07 | 7.63 | 0.97 | 4.13 | 0.0000** | 10.08 | 0.93 | −0.81 | 0.4160 |
| Max in genu (mm) | 12.39 | 0.07 | 9.61 | 1.28 | 4.13 | 0.0000** | 11.96 | 1.42 | 1.75 | 0.0799 |
| Max in midbody (mm) | 7.72 | 0.28 | 5.96 | 0.64 | 3.86 | 0.0001** | 7.34 | 0.67 | 2.21 | 0.0271* |
| Min in isthmus (mm) | 5.87 | 0.17 | 3.89 | 0.61 | 4.13 | 0.0000** | 4.88 | 0.90 | 3.19 | 0.0014* |
| Max in splenium (mm) | 15.25Δ | 0.08 | 11.35 | 1.24 | 4.13 | 0.0000** | 12.32 | 1.18 | 4.97 | 0.0000** |
| Brain weight (g) | 1230 | - | 1219.01 | 102.93 | - | - | 1374.13 | 111.56 | - | - |
| Brain volume (cm3) | - | 1127.67 | 95.22 | - | - | 1271.17 | 103.20 | - | - | |
The Mann–Whitney U test was used to compare measurements of Einstein’s corpus callosum with the two different age control groups, respectively.
# Standard deviation of measurements.
ΔEinstein’s maximum callosal thickness is significantly greater than that of both the old and young control groups.
The asterisks indicate statistically significant differences between the control groups and Einstein, *P < 0.05, **P < 0.001.
CC = corpus callosum.
Although Einstein’s brain weight is 10% less than the mean brain weight of the young controls, six of Einstein’s corpus callosum measurements are significantly greater than those of the young controls (Fig. 2). To further examine the regional callosal differences between Einstein and the controls (Aboitiz et al., 1992), a novel method was developed to explore the relative degrees of connectivity in certain subdivisions of the corpus callosum. The callosal thickness distribution between Einstein’s corpus callosum and the two control groups are shown in Figs 3, 4 and 5. Figure 3 shows the corpus callosum thickness plots between Einstein’s brain and those of the two control groups, after being sectioned and registered to the callosal thickness plot of Einstein’s brain. Einstein’s total callosal thickness (red) is greater than the mean corpus callosum thickness of the older control group (blue), except at the tip of the rostrum and posterior splenium (Fig. 3F). The purple spans at the bottom of the graphs indicate the areas with significant differences between Einstein’s corpus callosum and those of the elderly controls (P < 0.05, FDR corrected). In most of the genu, midbody, isthmus and part of the splenium, Einstein’s corpus callosum is thicker than the mean callosal thickness of the young controls (green), but thinner in the most rostral body (Fig. 3F). The cyan belt indicates the areas with significant differences between Einstein’s corpus callosum and those of the young controls (P < 0.05, FDR corrected). Similar results appear in the right column of Figs 4 and 5, respectively. Einstein’s corpus callosum in the genu is wider than that of both the control groups (Fig. 3F).
The corpus callosum is the largest bundle of white matter neural fibres in the brain that connects the interhemispheric cortices, and it may be involved in any neuroanatomical substrate of hemisphere specialization (Witelson, 1989). Underlying assumptions of this research are that an increased callosal area indicates an increased total number of fibres crossing through the corpus callosum and that post-mortem shrinkage of the corpus callosum is uniform across its subregions (Aboitiz et al., 1992, 2003). We therefore focused on the corpus callosum thickness which indicates the fibres crossing through the regional callosal cross-section area, rather than on the 3D volume of the corpus callosum, which would be impossible to measure in Einstein’s brain.
Several in vivo diffusion tensor imaging studies revealed the connectivity of cortical regions between hemispheres through the corpus callosum (Hofer and Frahm, 2006; Park et al., 2008; Chao et al., 2009). The fibres that pass through the callosal rostrum and genu appear to connect the interhemispheric regions of orbital gyri and prefrontal cortices corresponding with the left and right Brodmann areas 11/10, which are involved in planning, reasoning, decision-making, memory retrieval and executive function. According to Aboitiz et al. (1992, 2003), thin fibres are denser in these rostral and genu regions of the corpus callosum compared to its midbody and some of the caudal regions, and are involved in transfer of cognitive information. Einstein’s callosum is thicker and greater than those of young controls in the rostrum and genu, which suggests that the orbital gyri and prefrontal cortices may have been unusually well connected in his brain. This hypothesis is consistent with the finding that Einstein had relatively expanded prefrontal cortices (Falk et al., 2013). The morphology of both his corpus callosum and prefrontal cortex may have provided underpinnings for his exceptional cognitive abilities and remarkable thought experiments (Einstein, 1979).
The neural fibre bundle that passes though the callosal midbody and isthmus mainly connects corresponding interhemispheric premotor cortices (Brodmann area 6), primary motor cortices (Brodmann area 4), primary somatosensory cortices (Brodmann areas 1/2/3), secondary somatosensory cortices (Brodmann area 5) and parts of the parietal region (Park et al., 2008; Chao et al., 2009). These fibres have the largest and most heavily myelinated axons, which transfer information faster (Aboitiz et al., 1992). Einstein had an enlarged omega-shaped fold (known as the ‘knob’) in his right primary motor cortex, which probably represented motor cortex for his left hand, an unusual feature that may have been associated with the fact that he was a right-handed violin-player from childhood (Falk, 2009; Falk et al., 2013). Einstein’s callosum was thicker than the comparable region of the young controls in the region that was likely to have corresponded with his ‘knob’.
Fibres of the posterior isthmus and splenium are thought to connect corresponding parts of the superior parietal lobules (Brodmann area 7), inferior parietal lobules (Brodmann areas 39/40), and temporal cortices (Brodmann areas 20/21/37), whereas other fibres of the splenium have been shown to connect extensive cortical regions including occipital cortex (Brodmann areas 17/18/19) (Luders et al., 2007; Park et al., 2008; Chao et al., 2009). Most of Einstein’s callosal thickness distributions in the splenium (especially in the mid-splenium) are significantly greater than comparable regions of the young controls. The fibres crossing through this sub-area are usually small diameter axons, which transfer cognitive information between hemispheres and facilitate higher-order processing in the parietal, temporal and occipital lobes (Aboitiz et al., 1992). The superior parietal lobules are involved in visuomotor coordination, spatial attention, and spatial imagery (Formisano et al., 2002). Recent functional MRI studies indicate that the superior parietal lobule and the intraparietal sulcus are both activated during mental arithmetic and digit memory tasks (Arsalidou and Taylor, 2011; Tanaka et al., 2012). The inferior parietal lobules are concerned with language, mathematical operations (especially on the left), spatial perception, and visuomotor integration (Hugdahl et al., 2004). The occipital cortices are in charge of visual processing and can be activated during imagery with eyes closed (O'Craven and Kanwisher, 2000). The inferior temporal gyri (Brodmann area 20) are involved in high-level visual processing, recognition memory, face and body recognition, and processing of colour information (Buckner et al., 2000). Witelson et al. (1999a) demonstrated that the parietal lobes of Einstein’s brain were 15% wider than those of controls. Falk et al. (2013) showed that Einstein’s right superior parietal lobule (Brodmann area 7) was considerably wider than the left, his right intraparietal sulcus was highly unusual, his left inferior parietal lobule appeared to be relatively expanded compared to the right, and the cortical surfaces of Einstein’s occipital lobes were very convoluted. The ratio of glial to neuronal cells was significantly greater in Einstein’s left compared to right Brodmann area 39 and relatively increased in the bilateral temporal neocortices compared with the average for controls (Diamond et al., 1985). The glia affect neuronal excitability, synaptic transmission and coordinate activity across networks of neurons (Fields and Stevens-Graham, 2002). Luders et al. (2007) observed significant positive correlations between posterior callosal thickness and intelligence measures. However, the corpus callosum of Einstein is not always thicker than those of the young controls, especially in the rostral body, where the fibres mostly connect right and left middle superior frontal gyri (Brodmann area 8), which is involved in the management of uncertainty (Volz et al., 2005). Nonetheless, our overall findings strongly suggest that Einstein had more extensive connections between certain parts of his cerebral hemispheres compared to both younger and age-matched controls, which is consistent with the studies discussed above and adds another level to the growing evidence that Einstein’s extraordinary spatial imagery and mathematical gifts were grounded on definable neurological substrates. Although the intelligence of human beings cannot be fully explained by regional cortical volumes (Gazzaniga, 2000), our findings suggest that Einstein’s extraordinary cognition was related not only to his unique cortical structure and cytoarchitectonics, but also involved enhanced communication routes between at least some parts of his two cerebral hemispheres.
In summary, to the best of our knowledge, this study is the first to investigate the connectivity of Einstein’s cerebral hemispheres by comparing the morphology of his corpus callosum with that of 15 elderly healthy males and 52 young healthy males. We found that Einstein’s corpus callosum was thicker in the vast majority of subregions than their corresponding parts in the corpus callosum of elderly controls, and that Einstein’s corpus callosum was thicker in the rostrum, genu, midbody, isthmus, and (especially) the splenium compared with younger controls. These findings show that the connectivity between the two hemispheres was generally enhanced in Einstein compared with controls. The results of our study suggest that Einstein’s intellectual gifts were not only related to specializations of cortical folding and cytoarchitecture in certain brain regions, but also involved coordinated communication between the cerebral hemispheres. Last but not the least, the improved approach for corpus callosum measurement used in this study may have more general applications in corpus callosum studies.
Supplementary Material
Acknowledgements
The authors would like to thank the U. S. National Museum of Health and Medicine for permitting us access to the high resolution photographs of Einstein’s brain. We thank the Open Access Series of Imaging Studies (OASIS; Daniel S. Marcus, PhD) for permitting us to download the 15 old age MRI data. We also thank the International Consortium for Brain Mapping (ICBM; Principal Investigator: John Mazziotta, MD, PhD) for allowing us to download and publish the brain MRI data of 52 healthy males.
Funding
The acquisition of these data and support for data analysis were provided by NIH grants P50 AG05681, P01 AG03991, R01 AG021910, P50 MH071616, U24 RR021382 and R01 MH56584. This study was partly supported by ‘12th Five-Year Plan supporting project of Ministry of Science and Technology of the People’s Republic of China’ (grant no. 2013BAI10B03).
Supplementary material
Supplementary material is available at Brain online.
References
- Aboitiz F, Lopez J, Montiel J. Long distance communication in the human brain: timing constraints for inter-hemispheric synchrony and the origin of brain lateralization. Biol Res. 2003;36:89–99. doi: 10.4067/s0716-97602003000100007. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–53. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Anderson B, Harvey T. Alterations in cortical thickness and neuronal density in the frontal cortex of Albert Einstein. Neurosci Lett. 1996;210:161–4. doi: 10.1016/0304-3940(96)12693-8. [DOI] [PubMed] [Google Scholar]
- Ardekani B, Bachman A, Figarsky K, Sidtis J. Corpus callosum shape changes in early Alzheimer’s disease: an MRI study using the OASIS brain database. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0503-0. Jan 16. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Taylor MJ. Is 2+2=4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage. 2011;54:2382–93. doi: 10.1016/j.neuroimage.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a pratical and powerful approach to multiple testing. J R Statist Soc. 1995;57:289–300. [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123(Pt 3):620–40. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172–87. doi: 10.1002/hbm.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo JA, Reisin HD, Miguel-Hidalgo JJ, Rajkowska G. Cerebral cortex astroglia and the brain of a genius: a propos of A. Einstein's. Brain Res Rev. 2006;52:257–63. doi: 10.1016/j.brainresrev.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–56. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Scheibel AB, Murphy GJ, Harvey T. On the brain of a scientist: Albert Einstein. Exp Neurol. 1985;88:198–204. doi: 10.1016/0014-4886(85)90123-2. [DOI] [PubMed] [Google Scholar]
- Einstein A. Autobiographical Notes. Paul Arthur Schilpp (Centennial ed.). Chicago: Open Court Publishing Company; 1979. p. 48–51. [Google Scholar]
- Falk D. New Information about Albert Einstein's Brain. Front Evol Neurosci. 2009;1:3. doi: 10.3389/neuro.18.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D, Lepore FE, Noe A. The cerebral cortex of Albert Einstein: a description and preliminary analysis of unpublished photographs. Brain. 2013;136(Pt 4):1304–27. doi: 10.1093/brain/aws295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–62. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E, Linden DE, Di Salle F, Trojano L, Esposito F, Sack AT, et al. Tracking the mind's image in the brain I: time-resolved fMRI during visuospatial mental imagery. Neuron. 2002;35:185–94. doi: 10.1016/s0896-6273(02)00747-x. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Gupta T, Singh B, Kapoor K, Gupta M, Kochar S. Corpus callosum morphometry: comparison of fresh brain, preserved brain and magnetic resonance imaging values. Anat Sci Int. 2008;83:162–8. doi: 10.1111/j.1447-073X.2008.00227.x. [DOI] [PubMed] [Google Scholar]
- Hines T. Further on Einstein's brain. Exp Neurol. 1998;150:343–4. doi: 10.1006/exnr.1997.6759. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Rund BR, Lund A, Asbjornsen A, Egeland J, Ersland L, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161:286–93. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- Kigar D, Witelson S, Glezer I, Harvey T. Estimates of cell number in temporal neocortex in the brain of Albert Einstein. Soc Neurosci Abst. 1997;23:89–9. [Google Scholar]
- Lepore FE. Dissecting genius: Einstein's brain and the search for the neural basis of intellect. New York: Dana Press; 2001. pp. 11–26. [Google Scholar]
- Leyton M. Symmetry-curvature duality. Comput Vision Graphics Image Process. 1987;38:327–41. [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, et al. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37:1457–64. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;1:50–60. [Google Scholar]
- Marcus DS, Fotenos AF, Csernansky JG, Morris JC, Buckner RL. Open access series of imaging studies: longitudinal MRI data in nondemented and demented older adults. J Cogn Neurosci. 2010;22:2677–84. doi: 10.1162/jocn.2009.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19:1498–507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J Cogn Neurosci. 2000;12:1013–23. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, et al. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp. 2008;29:503–16. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreider E. Brain weight correlations calculated from original results of Paul Broca. Am J Phys Anthropol. 1966;25:153–8. doi: 10.1002/ajpa.1330250207. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Seki K, Hanakawa T, Harada M, Sugawara SK, Sadato N, et al. Abacus in the brain: a longitudinal functional MRI study of a skilled abacus user with a right hemispheric lesion. Front Psychol. 2012;3:315. doi: 10.3389/fpsyg.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Variants of uncertainty in decision-making and their neural correlates. Brain Res Bull. 2005;67:403–12. doi: 10.1016/j.brainresbull.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL, Harvey T. The exceptional brain of Albert Einstein. Lancet. 1999a;353:2149–53. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL, Harvey T. Authors' reply. Lancet. 1999b;354:1822. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.