Sir, We thank Gillespie et al. (2014) for their letter. They summarize data that suggest that in autism, vicarious activations can be relatively normal under instructions explicitly encouraging vicarious processes, while being abnormal in conditions in which instructions do not. This points to an important opportunity to improve our understanding of empathy—both with regards to deficits in patients and individual differences in the normal population.
To date, the ‘cake’ called empathy has been cut in various ways. Some cut it in two pieces, cognitive versus emotional empathy (Baron-Cohen and Wheelwright, 2004), acknowledging that somewhat separate systems support the capacity to think what another is thinking (cognitive empathy) and to feel what another is feeling (emotional empathy). This has influenced our thinking about individual variability in empathy and psychiatric disorders, with some disorders, like autism, thought to be impaired more in cognitive empathy whereas others, like psychopathy, are impaired more in emotional empathy (Blair, 2005). Based on neuroscience, we have ‘cut’ empathy into four pieces: motor, emotional, tactile and cognitive empathy (Keysers and Gazzola, 2009). Mirror neurons in motor cortices and functional MRI activations in motor cortices during action observation suggest motor empathy: vicariously activating one’s own actions while witnessing the actions of others (Gallese et al., 1996; Gazzola and Keysers, 2009; Keysers, 2009; Caspers et al., 2010). Later, neuroimaging data showed that one also vicariously activate neural substrates involved in one’s own disgust (Wicker et al., 2003) and pain (Lamm et al., 2011) while witnessing the disgust or pain of others, suggesting emotional empathy. Somatosensory brain regions were also found to be vicariously active when viewing the tactile and haptic sensations of others, suggesting somatosensory empathy (Keysers et al., 2004, 2010; Gazzola and Keysers, 2009; Caspers et al., 2010). Finally, reasoning about the beliefs of others vicariously activates ventral medial prefrontal regions involved in reasoning about one’s own beliefs, suggesting cognitive empathy (Mitchell et al., 2006). Because these forms of empathy recruit partially distinct neural substrates, and specific lesions can impair specific domains of empathy (Calder et al., 2000; Pazzaglia et al., 2008), neuroscientific data suggest that empathy is indeed composed of partly separated modalities.
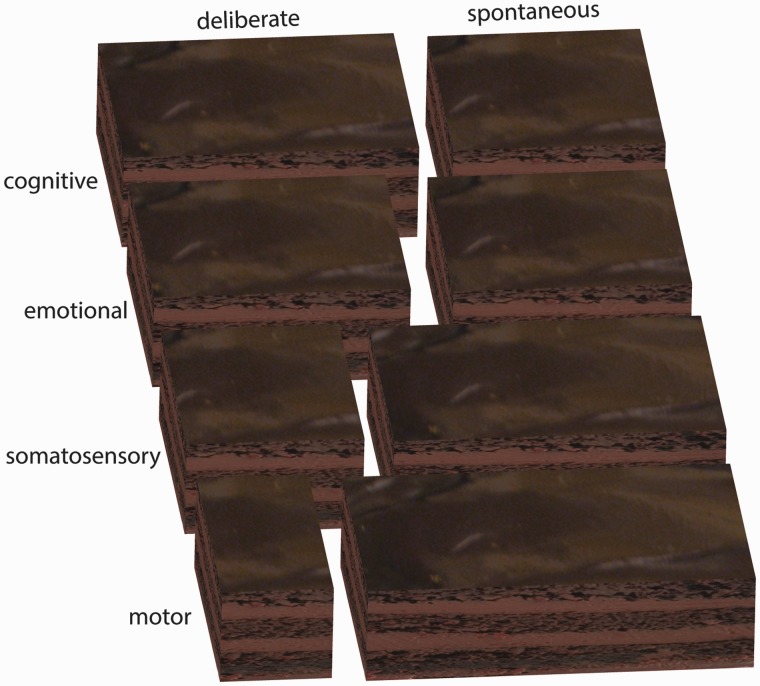
What our data (Meffert et al., 2013), and those reviewed by Gillespie et al. (2014), suggest is that the cake of empathy can be cut in another, psychiatrically and neurologically relevant, direction as well: not by domain (motor, emotional, somatosensory or cognitive) but by how deliberately one empathizes. In our psychopathic individuals, we found that spontaneous motor, somatosensory and emotional empathy is reduced while their deliberate counterparts are not (Meffert et al., 2013). The data reviewed by Gillespie et al. (2014) suggest that in autism, deliberate motor empathy might be intact, whereas spontaneous motor empathy might not be. In addition, neurological data suggest, that after lesions to the amygdala, spontaneous emotion processing is compromised, whereas deliberate processing under instructions to look at eye regions might be preserved (Adolphs et al., 2005). From a neurological or psychiatric point of view, the question is then no longer: ‘is that patient’s empathy intact or not?’, instead, one would need to check the four modalities of empathy separately, and entertain the possibility, that for each of these, deliberate and spontaneous empathy might not necessarily be equally functional (Fig. 1). This distinction of deliberate versus spontaneous should not be confused with the cognitive versus affective empathy distinction, although it bears some resemblance. Cognitive empathy, prototyped by false belief tasks, is mainly thought to rely on internal discourses that feel deliberate. In contrast, the pain we feel in our finger when seeing another cut theirs feels spontaneous. However, some forms of cognitive empathy can be spontaneous (German and Cohen, 2012), and emotional empathy can be deliberately up- or downregulated through a variety of processes. For instance, people can actively avoid putting themselves in a situation that would induce empathy (Shaw et al., 1994), and even once they are in an empathy-inducing situation, they can focus attention towards or away from emotional cues and reappraise social stimuli (Ochsner and Gross, 2005), and there is thus also a deliberate side to emotional empathy. Hence, there is a deliberate and a spontaneous side to all four domains of empathy, but the respective size of these slices might differ.
Figure 1.
If we consider empathy as a ‘cake’, it could be cut, to first approximation, into four domains (rows) and in a deliberate and spontaneous form (columns). Because some domains may have a more spontaneous side to them, and others a more deliberate side, the two axes of cutting are oblique rather than orthogonal.
In reality, the distinction between deliberate versus spontaneous empathy is not a simple binary distinction, but a complex landscape. For instance, males spontaneously show strong vicarious activations to the pain of a fair, but not an unfair individual, whereas females spontaneously show vicarious activations to both (Singer et al., 2006). Other research shows that spontaneous empathy can additionally be influenced by factors such as race, in-group, out-group, etc. (Engen and Singer, 2013). Even rodents show signs of spontaneous vicarious emotions for the distress of others, but only when they have previous experience with the particular stressor and have shared a cage with the object of their empathy (Atsak et al., 2011). This suggests that spontaneous empathy does not have a single ‘magnitude’, but can be influenced by many different contextual factors and by previous experience in a way that might be subject-specific. A similar story is probably true for more deliberate forms of empathy, as these very likely depend on, for example, the type of instruction given to participants. However, it will typically be impractical for clinicians or scientists to systematically explore all these various factors in order to characterize the empathy deficit. Hence, dividing the cake into eight might be a pragmatic first step.
Distinguishing deliberate and spontaneous processes in the clinic has a strong tradition in the study of physical disability. The World Health Organization’s International Classification of Functioning, Disability and Health (http://www.who.int/classifications/icf/en/) explicitly distinguishes two constructs, performance and capacity, that are related to what we call spontaneous and deliberate: ‘The first describes what a person does in their actual environment. The second describes what a person does in … a standardized evaluation setting’ (http://www.who.int/entity/classifications/drafticfpracticalmanual.pdf). This distinction derives from the frequent clinical observation that after stroke, a patient’s capacity to use an affected limb, as measured in optimal conditions, often recovers faster than the everyday use of the same limb (performance), begging for a dissociation between the deliberate and spontaneous use of a motor function. Here, we apply a similar dissociation to a mental function—empathy.
Whereas motor performance can be assessed in a relatively straightforward manner, the same is not true for the assessment of empathy, as empathy is a ‘hidden’ process that happens within a person, rather than an overt behaviour that can directly be measured. One solution might be the development of self-report questionnaires that would sample how much empathy individuals experience in the four domains when empathy is encouraged, versus when empathy is accidental. For instance questionnaires could ask questions such as ‘if I want to, I’m pretty good at feeling how other people feel’ (deliberate emotional empathy) versus ‘I sometimes have to look away from a movie because I get overwhelmed by the emotions of the actors’ (spontaneous emotional empathy). However, the problem with self-report instruments is that impression management and lack of insight can bias results. Alternatively, one could think of physiological measures that are potentially less likely to be manipulated by the participant such as functional MRI, EEG or transcranial magnetic stimulation to probe domains of empathy under more deliberate and/or spontaneous situations (Box 1). Standardizing these physiological assessments, which are currently being developed in research laboratories, to the point where they can be used at the bedside in clinical practice might be a worthwhile effort in the long-run.
Box 1 Some physiological methods to assess subforms of empathy.
Functional MRI: functional MRI can be used to measure a neural proxy for subforms of empathy. This is done with a two step approach: (i) participants are presented with social stimuli that trigger empathy for the actions, emotions, sensations and/or believes of others; and (ii) participants are allowed to perform similar actions, experience similar emotions, sensations and/or reflect about their own beliefs. Step (ii) localizes brain regions involved in the first-hand experience of these domains. By quantifying during (i) how strongly the social stimuli trigger activations in regions localized in (ii), one can quantify vicarious activations in the blood oxygenation level-dependent (BOLD) signal related to these four domains. These vicarious activations measured from the BOLD signal serve as a neural proxy of how strongly participants recruit their own actions, emotions, sensations and beliefs, while witnessing those of others. This approach has two important caveats. First, each voxel identified in (ii) encompasses millions of neurons, only some of which will have caused the activations measured in (ii). That the same voxel is also active in (i) might be a result of the reactivation of the same neurons (e.g. mirror neurons in the premotor cortex), which is what we would call true neural vicarious activations, and/or to the activation of some of the neurons in the same voxel not involved in (ii). However, that vicarious activations measured from the BOLD signal correlate with individual differences in trait empathy (Gazzola et al., 2006), predict costly helping (Hein et al., 2010), and that mirror neurons are found in some of these voxels in the monkey (Keysers, 2009) suggest that true neural vicarious activations are part of the signal found in vicarious activations measured from the BOLD signal, providing us with a valuable, albeit imperfect proxy for empathy. Second, functional MRI data are noisy. Accordingly, functional MRI leads to reliable findings when comparing groups of individuals, but is not suited to diagnose or characterize a single individual. Technical advances may help circumvent this problem in the future.
EEG: EEG has the advantage of being more easily brought to forensic institutions. Mu-suppression can be used to probe deliberate and spontaneous motor empathy as illustrated in the letter by Gillespie et al. (2014). Also the modulation of sensori-evoked potentials by observed touch or pain in others (Bufalari et al., 2007) could be used to probe deliberate and spontaneous somatosensory empathy.
Transcranial magnetic stimulation: the social modulation of motor-evoked potentials triggered using transcranial magnetic stimulation could also probe deliberate and spontaneous motor empathy (Fadiga et al., 1995), and their inhibition while viewing the pain of others can indirectly inform emotional empathy (Avenanti et al., 2009).
For a long time, many scholars have suspected that empathy motivates prosocial behaviour and inhibits instrumental aggression (Hoffman, 2000; Smith, 1976). The distinction we propose refines the question of whether empathy motivates prosociality and inhibits instrumental aggression: the question becomes which (if any) of the deliberate or spontaneous forms of the four modalities of empathy have these effects. Does it suffice to have a stronger deliberate empathy to engage in more prosocial behaviour and less instrumental aggression? Or is instrumental aggression only reduced for people with stronger spontaneous empathy, as this is a form of empathy that would be triggered even in situations in which the current goals of a person would motivate him to avoid deliberate empathy (e.g. while hurting people in a violent theft).
Ultimately, the quest will then become to develop interventions that can selectively alter spontaneous and deliberate forms of empathy in order to focus interventions to the specific subform that they might lack or have in excess. For instance, can forms of meditation help, and if so, what form of empathy do different forms of meditation boost?
Part of the question should of course also be why certain forms of empathy are affected in certain psychiatric populations and not in others. Gillespie et al. (2014) concluded from our paper, that in psychopathy high-level visual regions of the temporal lobe (including the fusiform gyrus and the superior temporal sulcus) were recruited spontaneously to normal levels, whereas regions involved in vicarious activations were not. This would have differed from data in autism, where both sets of regions are either recruited or not. However, the fusiform gyrus and regions involved in biological motion processing around the superior temporal sulcus were significantly less activated in our psychopathic individuals during our spontaneous condition (Meffert et al., 2013). These differences outside of vicarious brain regions disappeared when we masked the group differences with regions involved in experiencing similar emotions, and we therefore did not discuss these reduced visual activations as much in the paper. Further research is thus needed to differentiate the mechanisms behind the varying empathy deficits.
In summary, the distinction between spontaneous and deliberate empathy, which emerges from an increasing number of findings, raises some urgent questions that future research could address to shed new light on empathy and its deficits. It is exciting to see that by looking more closely at the subtleties of empathy, we may start to understand this fascinating capacity better. It will hopefully lead us to understand why a number of disorders can all be associated with an empathy deficit, and yet feel deeply different.
Finally, it is important to keep in mind that empathy operates in the context of many other cognitive and emotional functions, and that differences across clinical groups in their behavioural presentation may result from differences in this context rather than, or in addition to, a deficit in one of the empathy components we distinguish.
Acknowledgements
We thank Michael Spezio and Dan Batson for discussions that shaped these thoughts.
Funding
V.G. was supported by a VENI grant 451-09-006 from the Netherlands Organization for Scientific Research (N.W.O.), C.K. by a European Research Council grant 312511 of the European Commission. H.M. was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health under grant number 1-ZIA-MH002860-08.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, et al. Experience modulates vicarious freezing in rats: a model for empathy. PLoS One. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A, Minio-Paluello I, Bufalari I, Aglioti SM. The pain of a model in the personality of an onlooker: influence of state-reactivity and personality traits on embodied empathy for pain. Neuroimage. 2009;44:275–83. doi: 10.1016/j.neuroimage.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17:2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen HG, Singer T. Empathy circuits. Curr Opin Neurobiol. 2013;23:275–82. doi: 10.1016/j.conb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–11. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824–9. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–55. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German TC, Cohen AS. A cue-based approach to ‘theory of mind': re- examining the notion of automaticity. Br J Dev Psychol. 2012;30:45–58. doi: 10.1111/j.2044-835X.2011.02055.x. [DOI] [PubMed] [Google Scholar]
- Gillespie S, McCleery J, Oberman L. Spontaneous versus deliberate vicarious representations: different routes to empathy in psychopathy and autism. Brain. 2014:137–e272. doi: 10.1093/brain/awt364. [DOI] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron. 2010;68:149–60. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hoffman M. Empathy and moral development: the implications for caring and justice. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Keysers C. Mirror neurons. Curr Biol. 2009;19:R971–3. doi: 10.1016/j.cub.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol. 2009;19:666–71. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–46. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Meffert H, Gazzola V, den Boer JA, Bartels AA, Keysers C. Reduced spontaneous but relatively normal deliberate vicarious representations in psychopathy. Brain. 2013;136(Pt 8):2550–62. doi: 10.1093/brain/awt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Pizzamiglio L, Pes E, Aglioti SM. The sound of actions in apraxia. Curr Biol. 2008;18:1766–72. doi: 10.1016/j.cub.2008.09.061. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. The theory of moral sentiments. Oxford: Clarendon Press; 1976. [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G. Both of us disgusted in My Insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Shaw LL, Batson CD, Todd RM. Empathy avoidance-forestalling feeling for another in order to escape the motivational consequences. J Pers Soc Psychol. 1994;67:879–87. [Google Scholar]