Using an animal model of inflammatory itch and pain produced by allergic contact dermatitis, Qu et al. reveal hyperexcitability in two distinct populations of pruriceptive cutaneous nociceptors, due in part to enhanced sodium currents. Similar mechanisms may contribute to spontaneous itch and pain in human allergic and inflammatory diseases.
Keywords: itch, pain, MRGPRA3, MRGPRD, allergic contact dermatitis
Abstract
Itch is a common symptom of diseases of the skin but can also accompany diseases of other tissues including the nervous system. Acute itch from chemicals experimentally applied to the skin is initiated and maintained by action potential activity in a subset of nociceptive neurons. But whether these pruriceptive neurons are active or might become intrinsically more excitable under the pathological conditions that produce persistent itch and nociceptive sensations in humans is largely unexplored. Recently, two distinct types of cutaneous nociceptive dorsal root ganglion neurons were identified as responding to pruritic chemicals and playing a role in itch sensation. One expressed the mas-related G-coupled protein receptor MRGPRA3 and the other MRGPRD (MRGPRA3+ and MRGPRD+ neurons, respectively). Here we tested whether these two distinct pruriceptive nociceptors exhibited an enhanced excitability after the development of contact hypersensitivity, an animal model of allergic contact dermatitis, a common pruritic disorder in humans. The characteristics of increased excitability of pruriceptive neurons during this disorder may also pertain to the same types of neurons active in other pruritic diseases or pathologies that affect the nervous system and other tissues or organs. We found that challenging the skin of the calf of the hind paw or the cheek of previously sensitized mice with the hapten, squaric acid dibutyl ester, produced symptoms of contact hypersensitivity including an increase in skin thickness and site-directed spontaneous pain-like (licking or wiping) and itch-like (biting or scratching) behaviours. Ablation of MRGPRA3+ neurons led to a significant reduction in spontaneous scratching of the hapten-challenged nape of the neck of previously sensitized mice. In vivo, electrophysiological recordings revealed that MRGPRA3+ and MRGPRD+ neurons innervating the hapten-challenged skin exhibited a greater incidence of spontaneous activity and/or abnormal after-discharges in response to mechanical and heat stimuli applied to their receptive fields compared with neurons from the vehicle-treated control animals. Whole-cell recordings in vitro showed that both MRGPRA3+ and MRGPRD+ neurons from hapten-challenged mice displayed a significantly more depolarized resting membrane potential, decreased rheobase, and greater number of action potentials at twice rheobase compared with neurons from vehicle controls. These signs of neuronal hyperexcitability were associated with a significant increase in the peak amplitude of tetrodotoxin-sensitive and resistant sodium currents. Thus, the hyperexcitability of MRGPRA3+ and MRGPRD+ neurons, brought about in part by enhanced sodium currents, may contribute to the spontaneous itch- and pain-related behaviours accompanying contact hypersensitivity and/or other inflammatory diseases in humans.
Introduction
Itch is a fundamental sensory quality that is different from pain, but similar in its capacity to cause suffering and a decrease in the quality of life (Kini et al., 2011; Chen, 2012). Recent evidence suggests that chemical stimuli that elicit acute itch in humans and animals evoke action potentials in a specific subset of cutaneous nociceptors. These nociceptors, termed ‘pruriceptive’ because they respond to a pruritic agent, also respond to one or more noxious stimuli (Schmelz et al., 2003; Johanek et al., 2008; Ringkamp et al., 2011; Liu et al., 2012; Han et al., 2013). However, there is little information about whether activity in pruriceptive neurons might be abnormally generated during disorders that cause chronic itch. Neither is it known whether these neurons might become intrinsically hyperexcitable, thereby making them more responsive to endogenous stimuli normally present or pathologically generated. Our purpose here was to examine the changes in the excitability of pruriceptive neurons using a model of an inflammatory cutaneous disease, allergic contact dermatitis, which can be similarly produced in mice and humans (Scott et al., 2002; Camouse et al., 2008; Sikand et al., 2012). It is anticipated that the characteristics of the enhanced excitability of pruriceptive neurons accompanying this disease may be of relevance to the chronic itch and/or pruritic and nociceptive dysaesthesias that can accompany injuries or disorders of the peripheral nerve or other tissues or organs.
In mice, two distinct types of dorsal root ganglion neurons each expressing a different mas-related G-protein-coupled receptor have been implicated in chemically-evoked itch (Liu et al., 2012; Han et al., 2013). One type of neuron expresses MRGPRA3, the receptor for the agonist, chloroquine, and the other type, MRGPRD, the receptor for the agonist beta-alanine (Shinohara et al., 2004; Liu et al., 2012). Each neuronal type has unmyelinated axons that exclusively innervate the stratum granulosum of the epidermis (Liu et al., 2012; Han et al., 2013). MRGPRA3+ neurons are essential for normal histamine-dependent itch behaviour (Han et al., 2013). There is evidence that MRGPRD+ neurons contribute to mechanically evoked pain (Cavanaugh et al., 2009) but also to the histamine-independent itch evoked by the MRGPRD receptor agonist beta alanine (Liu et al., 2012). Though both types of nociceptors play a role in mediating itch evoked by acutely delivered pruritic stimuli, it is not known whether these neurons become more excitable in mice under a pathological condition that produces itch and nociceptive sensations in humans.
In the present study we examined the changes in the excitability of MRGPRA3+ and MRGPRD+ neurons innervating a cutaneous region of inflammation induced by a Type IV delayed contact hypersensitivity (CHS) reaction to an allergen to which the animal was previously sensitized—a model of allergic contact dermatitis. Preliminary results of this study were presented in abstract form (Fan et al., 2012).
Materials and methods
Animals
The mice were C57BL/6 males, 2 to 3 months of age and weighing 20–30 g. Wild-type mice were used for behavioural testing. The donors for our colony of MRGPRA3 and MRGPRD transgenic mice were provided by Dr Xinzhong Dong’s laboratory (John Hopkins University) and used for the electrophysiological recordings of cell bodies that expressed green fluorescent protein (GFP). For one type of mouse, the GFP was in neurons that expressed the MRGPRA3 receptor (MRGPRA3+ neurons; Han et al., 2013) whereas the other type of mouse exhibited the GFP in neurons that expressed the MRGPRD receptor (MRGPRD+ neurons; Zylka et al., 2005). MRGPRA3GFP-Cre;ROSA26DTR mice were generated as described (Han et al., 2013). Briefly, the MRGPRA3GFP-Cre transgenic line was crossed with Cre-dependent ROSA26DTR line, followed by diphtheria toxin (DTX; EMD Biosciences) injected intraperitoneally (40 µg/kg) twice, separated by 72 h (Han et al., 2013). All the experimental procedures were approved by the Institutional Animal Care and Use Committee of Yale University School of Medicine (or for the use of MRGPRA3GFP-Cre;ROSA26DTR mice, Johns Hopkins University School of Medicine) and were in accordance with the guidelines provided by the National Institute of Health and the International Association for the Study of Pain.
Model of allergic contact dermatitis
The contact sensitizer squaric acid dibutylester (SADBE) (Tokyo Chemical Co.) was used to elicit CHS in the mouse as a model of allergic contact dermatitis in humans (Scott et al., 2002; Camouse et al., 2008; Sikand et al., 2012). Mice were sensitized by the topical application of 25 µl of 1% SADBE in acetone (520 µg/cm2) to the shaved abdominal skin once a day for three consecutive days (Scott et al., 2002). Five days later, the SADBE-treated group was challenged with a topical application of 25 μl of 1% SADBE to the hairy skin of the foot and hind leg (for electrophysiology) or just onto the calf of one hind leg or, alternatively, to the right cheek (for behavioural testing) once a day for two consecutive days whereas acetone alone was used as the vehicle control. The thickness of calf skin was measured with a caliper before and 24 h after the second challenge as an assessment of inflammation. A similar protocol was used in a behavioural test using MRGPRA3GFP-Cre;ROSA26DTR mice to test the effects of ablating MRGPRA3+ neurons on CHS-induced scratching. However, in this test, the mice were challenged on the nape of the neck with 0.5% of SADBE delivered once daily for four consecutive days before behavioural testing.
Behavioural testing
All behavioural tests of SADBE sensitized mice were performed just before administering the first challenge of SADBE (‘pre-challenge’ test) and then again 24 h after each SADBE challenge or application of acetone vehicle. Behavioural responses were video recorded by a camcorder. The video recording was subsequently played back offline in slow-motion to permit detailed measurements of behavioural responses directed toward the challenge site.
For the calf model, the duration of each biting or licking action directed toward the calf was timed by a stopwatch and summed to provide the cumulative biting or licking time over a period of 1 h as described (LaMotte et al., 2011). Licking behaviour consisted of a series of long-stroke, bobbings of the head (∼4 Hz) during which the tongue could sometimes be seen moving across the skin. Biting behaviour was characterized not by head movements, but by gnawing-like movements of the mandible (∼15 Hz) (LaMotte et al., 2011). For the nape of the neck model, scratching behaviour with the hind paw was quantified by recording the number of incidences of scratching bouts for 1 h (Liu et al., 2009). For the cheek model, the number of bouts of spontaneous scratching with the hindpaw and wiping with the forepaw (Shimada and LaMotte, 2008) were counted for 2 h (a longer period of observation than for the calf or neck because of the fewer frequencies of spontaneous site-directed behaviours).
In vivo electrophysiological recordings
The properties of dorsal root ganglion neurons innervating the skin of the hind limb of mice were recorded in vivo using extracellular recording as described (Ma et al., 2010, 2012). Briefly, under isoflurane anaesthesia, after laminectomy at the levels of L2–L6, the L3 dorsal root ganglion was fixed to a plate, and superfused with warm (∼37°C) oxygenated artificial CSF at flow rate of 3 ml/min within a pool formed by a ring to which the skin was sewn. After removal of the epineurium, the neurons on the surface of the dorsal root ganglion were viewed by reflection microscopy and epifluorescence (to identify the presence of GFP indicative of MRGPRD+ or MRGPRA3+ expression).
Extracellular recordings were made on individual dorsal root ganglion neurons using a micropipette electrode with a tip of 20–25 µm. The sensory submodality of a neuron was classified according to its responses to chemical, heat and/or mechanical stimuli applied to its peripheral receptive field. These stimuli included innocuous stroking with a cotton-tipped probe, a gentle pinch using the experimenter’s fingers and von Frey filaments with a fixed tip diameter (200 µm) but delivering different bending forces (for noxious mechanical stimuli), and a temperature-controlled chip-resistor heating probe (for noxious heat stimuli) (Ma et al., 2010). The noxious heating stimulus had a trapezoidal temperature waveform with a ramp (1 s) from a base of 38°C to a plateau of 51°C (5 s) followed by a ramp back to the base. Conduction velocity was obtained by electrically stimulating the peripheral receptive field with two wire electrodes or sometimes a saline soaked cotton probe and calculated by dividing the latency of a spike peak by the distance between the stimulation electrode and the soma of the recorded neurons. The neuron was classified as spontaneously active only if spontaneous ongoing discharges occurred during a 3-min period without any external stimulation (Ma and LaMotte, 2007).
Acute culture of dorsal root ganglion neurons
The cell bodies of dorsal root ganglion neurons from adult MRGPRD+ and MRGPRA3+ mice were acutely dissociated as described (Qu et al., 2012). Briefly, L3 and L4 lumbar dorsal root ganglions, ipsilateral to either acetone control or SADBE-treated skin, were harvested from mice 24 h after the second SADBE challenge or application of acetone vehicle and transferred into oxygenated complete saline solution for cleaning and mincing. The complete saline solution contained (mM): 137 NaCl, 5.3 KCl, 1 MgCl2, 3 CaCl2, 25 sorbitol, and 10 HEPES, adjusted to pH 7.2 with NaOH. The dorsal root ganglions were then digested with Liberase TM (0.35 U/ml; Roche Diagnostics) for 20 min, before another 15 min with Liberase TL (0.25 U/ml; Roche Diagnostics) and papain (30 U/ml, Worthington Biochemical) in complete saline solution containing 0.5 mM EDTA at 37°C. The dorsal root ganglion tissue was triturated with a fire-polished Pasteur pipette. Dispersed neurons were suspended in Dulbecco’s modified Eagle medium containing 1 mg/ml bovine serum albumin and 1 mg/ml trypsin inhibitor (Sigma-Aldrich) and plated onto poly-D-lysine/laminin coated glass coverslips (BioCoat, BD Biosciences). The Dulbecco’s modified Eagle medium contained equal amounts of Dulbecco’s modified Eagle medium and F12 (Gibco) with 10% foetal calf serum (Sigma) and 1% penicillin and streptomycin (Invitrogen). The cells were incubated at 37°C and used within 24 h after plating.
Whole-cell patch clamp recordings from acutely cultured dorsal root ganglion neurons
Whole-cell recordings were made from MRGPRA3+ and MRGPRD+ dorsal root ganglion neurons identified by the fluorescence of GFP using epifluorescence microscopy (Olympus, Japan). Whole-cell current and voltage-clamp experiments were performed at room temperature (20–22°C) using a Multiclamp 700A amplifier with pClamp 9 software (Molecular Device), as described (Qu et al., 2011, 2012). Signals were sampled at 10 kHz or 20 kHz and filtered at 2 kHz. The patch pipettes were pulled from borosilicate glass capillaries (Sutter Instrument; Novato) using a P97 micropipette puller (Sutter Instrument) and had a resistance of 3–4 MΩ and 1–2 MΩ for current- and voltage-clamp recordings, respectively. The series resistance was routinely compensated at 60–80%.
Resting membrane potential was recorded for each neuron under the current clamp mode after stabilization (within 3 min). A neuron was included only if the resting potential was more negative than −40 mV and the spike overshoot was >15 mV. Action potentials were evoked by a series of depolarizing current steps, each 500 ms duration, in increments of 50 pA up to 1 nA delivered through the recording electrode. The number of action potentials evoked by a suprathreshold stimulus was estimated by injecting a 500-ms depolarizing current of a magnitude at twice rheobase. Input resistance was obtained from the slope of a steady-state current-voltage plot in response to a series of hyperpolarizing currents steps from −200 to −50 pA. For current clamp recordings, the internal solution contained (in mM): 120 K+-gluconate, 20 KCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES-K+, 2 MgATP, with pH adjusted to 7.2 using Tris-base and osmolarity adjusted to 290–300 mOsm with sucrose. The external solution contained the following (in mM): 145 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted at 7.4 with NaOH. The liquid junction potential of 11 mV was corrected.
Na+ current recordings were started ∼3 min after establishing whole-cell voltage-clamp configuration, as described (Fan et al., 2011). The neurons with the ratio of peak tetrodotoxin (TTX)-resistant to TTX-sensitive currents <10% were excluded for analysis (Fan et al., 2011). Linear leakage currents were digitally subtracted on-line using a P/4 procedure. The internal solution contained the following (in mM): 140 CsF, 10 NaCl, 2 MgCl2, 0.1 CaCl2, 1 EGTA, and 10 HEPES, pH adjusted to 7.2 with CsOH. The bath solution contained (in mM): 35 NaCl, 105 choline Cl, 1 MgCl2, 1 CaCl2, 0.1 CdCl2, 20 tetraethylammonium Cl, 10 glucose, and 10 HEPES, pH adjusted at 7.4 with NaOH. The liquid junction potential for Na+ current recordings was calculated to be 11.5 mV but was not corrected, as it was constant across experimental groups (Fan et al., 2011).
Histology
Mice were deeply anaesthetized with ketamine and xylazine and perfused transcardically with 0.1 M PBS followed by 4% paraformaldehyde in PBS. L3, L4 and L5 dorsal root ganglions from control and CHS MRGPRA3+ or MRGPRD+ mice (n = 3 mice for each group) were collected and fixed in the same fixative for 4 h. Then the dorsal root ganglions were cryoprotected overnight in 0.1 M PBS containing 30% sucrose. Dorsal root ganglions were cryosectioned at 20 µm thickness and mounted on slides. The nucleus was stained with 4’,6-diamidino-2-phenylindole (2 µg/ml) (Sigma). Images were captured using a Nikon Eclipse Ti-S fluorescent microscope equipped with an Andorra Technology camera (Nikon Instruments Inc.) and analysed using Adobe Photoshop CS5 software. For quantitative analysis, at least one dorsal root ganglion was chosen from each mouse. A total of at least eight sections from each dorsal root ganglion were randomly selected. The proportions of MRGPRA3+ or MRGPRD+ neurons were calculated by the number of GFP-expressing neurons divided by the total number of neuronal profiles. Only the neurons with a clearly visible nucleus in the tissue section were counted.
Data analysis
Electrophysiological data were analysed using pClamp 9 software (Molecular Devices) and Origin 6.0 (OriginLab). For the analysis of voltage versus current relationships, the current density (pA/pF) was calculated by dividing the peak current by the cell capacitance. For the analysis of steady-state activation, Na+ conductance (G) at each test pulse voltage (V) was calculated from the corresponding current (I) using the equation G = I / (V − Vrev), where Vrev is the reversal potential of Na+ current which was determined for each cell individually. Activation curves were obtained by plotting normalized conductance (G / Gmax) against test pulse voltage (V) and then fitting with Boltzmann functions in the forms of G / Gmax = 1 / {1 + exp [(V1/2, act − V) / k]}, where Gmax is the maximal Na+ conductance, V1/2, act is the voltage for half-maximum activation and k is the slope factor. Steady-state inactivation was examined using a series of 500 ms prepulses from −130 to −10 mV with 10 mV increments, before a 30 ms test pulse of −10 mV (Fan et al., 2011). The inactivation of TTX-resistant Na+ currents was assumed to occur positive to −50 mV. Peak inward currents (I) obtained from steady-state inactivation protocol were normalized to the maximal peak current (Imax) and fitted with a negative Boltzmann function of the form: I / Imax = 1 / {1 + exp[(V − V1/2, inact) / k]}, where V represents the inactivation prepulse potential, V1/2, inact represents the voltage at which activation is half-maximal and k is the slope factor.
Means are presented with the standard error of the mean. Statistical analyses were performed using a student’s t-test or one-way repeated measures analysis of variance with Bonferroni adjustments for pairwise comparisons or Tukey's post hoc test as appropriate. Comparisons of proportions were made using Fisher’s exact test. Significance was set at P < 0.05.
Results
SADBE challenge induced contact hypersensitivity accompanied by itch and pain behaviour
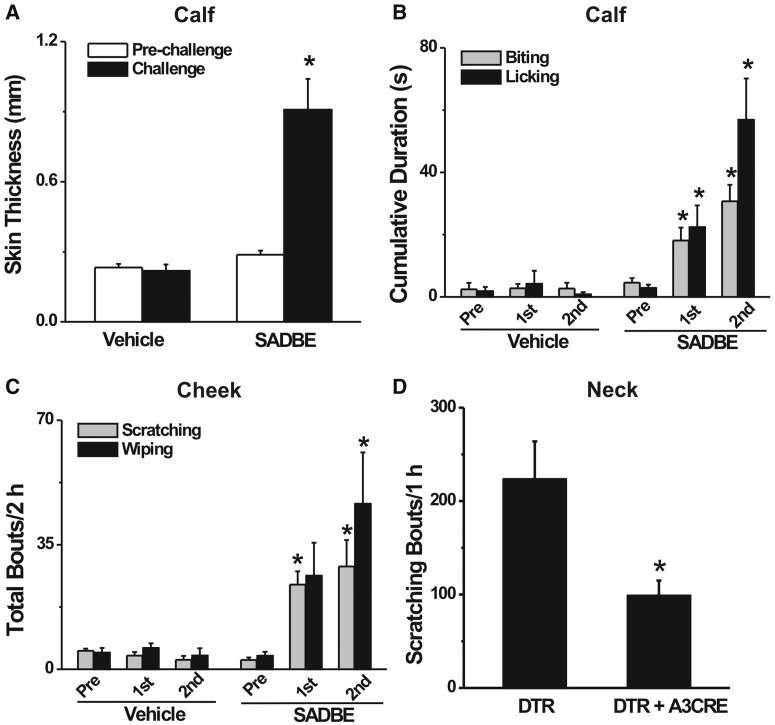
SADBE challenge to the calf of sensitized mice induced progressive CHS characterized by skin swelling, consistent with previous findings with SADBE and other contact sensitizers (Christensen and Haase, 2012; Honda et al., 2013). In comparison with pre-challenge values, the thickness of the calf skin was significantly increased after the second SADBE challenge (n = 13 mice) but not after the second application of the acetone vehicle (n = 6) (Fig. 1A, paired t-tests). In addition, SADBE challenge produced spontaneous biting and licking directed to the SADBE-treated calf skin of the mouse. In comparison with pre-challenge values, the cumulative durations of spontaneous biting and licking were each significantly greater after the first and the second SADBE challenge (Fig. 1B; repeated measures analysis of variance, Bonferroni adjustments for pairwise comparisons; n = 20). There were no significant differences in the cumulative duration of spontaneous biting and licking before versus after each application of acetone alone (Fig. 1B; n = 6).
Figure 1.
SADBE challenge induced CHS and spontaneous site-directed behaviours in previously sensitized mice. (A) In comparison with pre-challenge values, the thickness of calf skin was significantly increased 24 h after the second SADBE challenge (n = 13 mice) whereas applications of acetone alone (vehicle) had no significant effect (n = 6) (paired t-test). (B) After SADBE challenge of the calf, cumulative durations of spontaneous biting and licking of the calf were each significantly increased over pre-challenge (Pre) values (n = 20) but not after applications of acetone (n = 6) (repeated measures analysis of variance with Bonferroni adjustments). (C) After SADBE challenge of the cheek, the number of bouts of spontaneous scratching and wiping were each significantly increased over pre-challenge values (n = 10) whereas the acetone vehicle itself had no significant effect (n = 6) (repeated measures analysis of variance with Bonferroni adjustments). (D) SADBE challenge to the nape of the neck in previously sensitized mice evoked significantly fewer bouts of site-directed scratching in MRGPRA3GFP-Cre;ROSA26DTR mice with selective ablation of MRGPRA3+ neurons (n = 7) than control ROSA26DTR mice (n = 8) (unpaired t-test).*P < 0.05.
To test further the validity of the use of biting versus licking on the calf as behavioural indicators of itch and pain, we used the ‘cheek model’ in which the behavioural indicators of itch and pain on the cheek were scratching the cheek with the hind limb versus wiping the cheek with the forelimb, respectively (Shimada and LaMotte, 2008). In comparison with pre-challenge values, there was a significantly greater number of bouts of site-directed scratching after each SADBE challenge to the cheek and a significantly greater number of site-directed wipes after the second SADBE challenge (Fig. 1C; repeated measures analysis of variance with Bonferroni adjustments, n = 10). In contrast, no such significant increases were observed after each application of acetone (Fig. 1C, n = 6). Together, these data support the validity of measuring itch and nociceptive behaviours in the mouse during the development of SADBE-induced CHS.
If MRGPRA3+ neurons can mediate scratching behaviour evoked by pruritic chemicals (Han et al., 2013), one would expect that ablation of these neurons might result in a decrease in spontaneous scratching accompanying CHS. We therefore induced CHS in MRGPRA3GFP-Cre;ROSA26DTR mice in which the MRGPRA3+ neurons were selectively ablated and DTX-treated ROSA26DTR control mice (Han et al., 2013). When challenged with SADBE on the nape of the neck, the total number of bouts of site-directed scratching was significantly lower in the mice with the ablated MRGPRA3 neurons (n = 7) than in the control mice (n = 8, unpaired t-test; Fig. 1D).
SADBE challenge produced spontaneous activity in a portion of cutaneous MRGPRA3+ and MRGPRD+ neurons in vivo
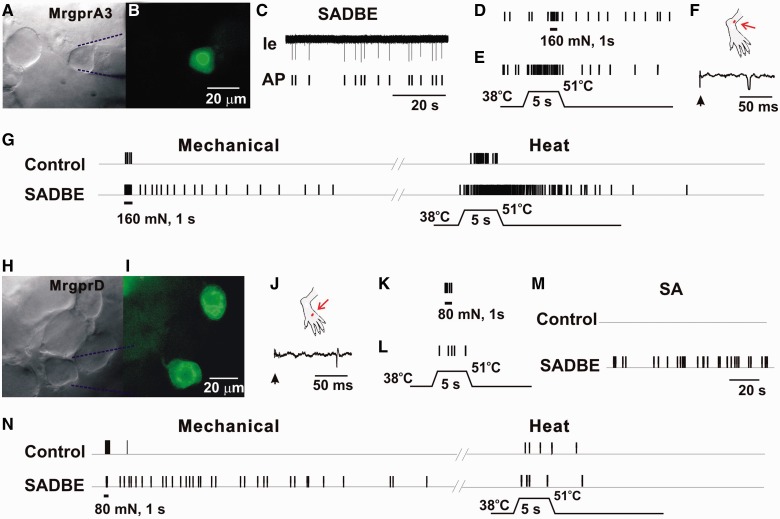
As MRGPRA3+ and a subset of MRGPRD+-expressing neurons were recently identified as two specific types of pruriceptive cutaneous dorsal root ganglion neurons, we next examined whether these putative itch-mediating dorsal root ganglion neurons became spontaneously active after SADBE challenge in a physiologically relevant setting with an in vivo recording method. Fourteen MRGPRA3+ and 22 MRGPRD+ neurons were recorded from CHS mice and 12 MRGPRA3+ and 19 MRGPRD+ -expressing neurons were recorded from control (acetone treated) mice. All the neurons tested were cutaneous C-nociceptive units with a peripheral receptive field within vehicle- or SADBE-treated skin area (Fig. 2). In control mice, both types of neurons were silent, i.e. exhibited no spontaneous activity, in agreement with previous studies (Liu et al., 2012; Han et al., 2013). All MRGPRA3+ neurons tested responded to both noxious heat and mechanical stimuli, and thus were classified as C-mechanoheat (CMH) polymodal nociceptors. Less than half of the MRGPRD+ neurons tested were CMH nociceptors, whereas the remaining neurons responded only to noxious mechanical stimulus and were classified as C-mechano (CM) nociceptors. In the animals with CHS, 6 of 14 (43%) MRGPRA3+ neurons tested exhibited spontaneous activity in the absence of previous cutaneous stimulation (Fig. 2A–F). Moreover, 4 of 14 (29%) MRGPRA3+ neurons from CHS mice displayed abnormal after-discharges in response to heat and to mechanical stimuli. In contrast, no after-discharges occurred in response to such stimuli in MRGPRA3+ neurons from control mice (Fig. 2G). The proportion of MRGPRA3+ neurons with spontaneous activity and after-discharges was significantly increased in CHS mice (57%) as compared with control animals (0%) (Fisher’s exact test). Only 1 of 22 MRGPRD+ neurons recorded from CHS mice exhibited spontaneous activity (Fig. 2H–M). In addition, mechanically evoked after-discharges were observed in 3 (including the spontaneous activity neuron) of 22 MRGPRD+ neurons from CHS mice, but not in any of the MRGPRD+ neurons from control animals (Fig. 2N). No abnormal heat-evoked after-discharges occurred in MRGPRD+ neurons from both control and CHS mice (Fig. 2N). There was no significant difference in the proportion of MRGPRD+ neurons with abnormal activity between control (0%) and CHS mice (14%) (Fisher’s exact test). Nevertheless, the presence of spontaneous activity in one neuron and abnormal mechanically evoked discharges in this and two others are consistent with the idea that the excitability of the peripheral terminals of a portion of the MRGPRD+ neurons was increased after CHS.
Figure 2.
CHS produced spontaneous activity and enhanced stimulus-evoked responses in MRGPRA3+ and MRGPRD+ neurons. (A) Bright-field image of a neuron under recording with the extracellular electrode (outlined with dashed blue lines). (B) Fluorescent microscopy revealed the expression of GFP (i.e. MRGPRA3) in this neuron. (C) Original extracellular recording trace (Ie) and action potential markers (AP) indicated the presence of abnormal spontaneous activity (SA) of this neuron, without any external stimulation. (D) Responses of this MRGPRA3-GFP+ neuron to 160 mN force through a 200 μm diameter probe (1 s). (E) Response to heat stimulation (38 to 51°C, 5 s), in the presence of ongoing spontaneous activity. (F) Location of cutaneous receptive field (red dot) of this neuron on the hairy skin of hind paw (the region challenged with SADBE), and conduction velocity (0.67 m/s, lower trace) obtained with electrical stimulation (arrow) to the peripheral receptive field. (G) Responses of the MRGPRA3+ neuron innervating the vehicle control or SADBE-challenged skin to mechanical stimulus of 160 mN force through a 200 μm probe for 1 s, and to heat stimuli (38 to 51°C, 5 s). The neuron innervating SADBE-challenged skin was initially silent but showed prolonged after-discharge following the 160 mN mechanical stimulation and heating. No stimulus-evoked after-discharges were observed in MRGPRA3 neurons from control animals. (H) Bright-field image of a MRGPRD+ neuron under recording with the extracellular electrode (outlined with dashed blue lines). (I) Fluorescent microscopy revealed the expression of GFP (or MRGPRD) in this neuron. (J) Location of cutaneous peripheral receptive field (red dot) of this neuron on the hairy skin of hind paw (the region challenged with SADBE), and conduction velocity (0.57 m/s, lower trace) obtained with electrical stimulation (arrow) to the peripheral receptive field. (K and L) Responses of MRGPRD-GFP+ neuron to 80 mN force through a 200 μm diameter probe (1 s), and to heat stimulation (38 to 51°C, 5 s). This neuron did not exhibit spontaneous activity. (M) Spontaneous activity was observed in a subset of MRGPRD+ neurons from CHS mice, but not from controls. (N) MRGPRD+ neurons from CHS mice exhibited prolonged after-discharges in response to noxious mechanical stimulus (80 mN, 1 s) but not heat (38 to 51°C, 5 s). No prolonged after-discharges were evoked by noxious mechanical and heat stimuli in MRGPRD+ neurons from control animals.
As a test of whether CHS may have caused an upregulation in the expression of MRGPRA3+ or MRGPRD+ in other types or greater numbers of the same types of neurons, we measured, in individual dorsal root ganglions, the proportion of the total number of counted cells that expressed GFP (indicating expression of MRGPRA3 or MRGPRD). The proportions were averaged for the number of dorsal root ganglions sampled from mice serving as controls (acetone treated) versus mice that developed SADBE-induced CHS. Similar to previous studies (Liu et al., 2008), the percentages of MRGPRA3+ and MRGPRD+ neurons from control mice were 4.4 ± 0.3% (n = 3 dorsal root ganglions) and 23.8 ± 2.9% (n = 4), respectively. In CHS mice, the percentages of MRGPRA3+ and MRGPRD+ neurons were 4.8 ± 0.2%; (n = 3) and 24.6 ± 1.8% (n = 3), each of which was not significantly different from corresponding control values (Fisher’s exact tests). Thus, there was no evidence for a change in the proportion of neurons expressing each type of MRGPRs after CHS.
SADBE challenge increased the excitability of dissociated MRGPRA3+ and MRGPRD+ neurons
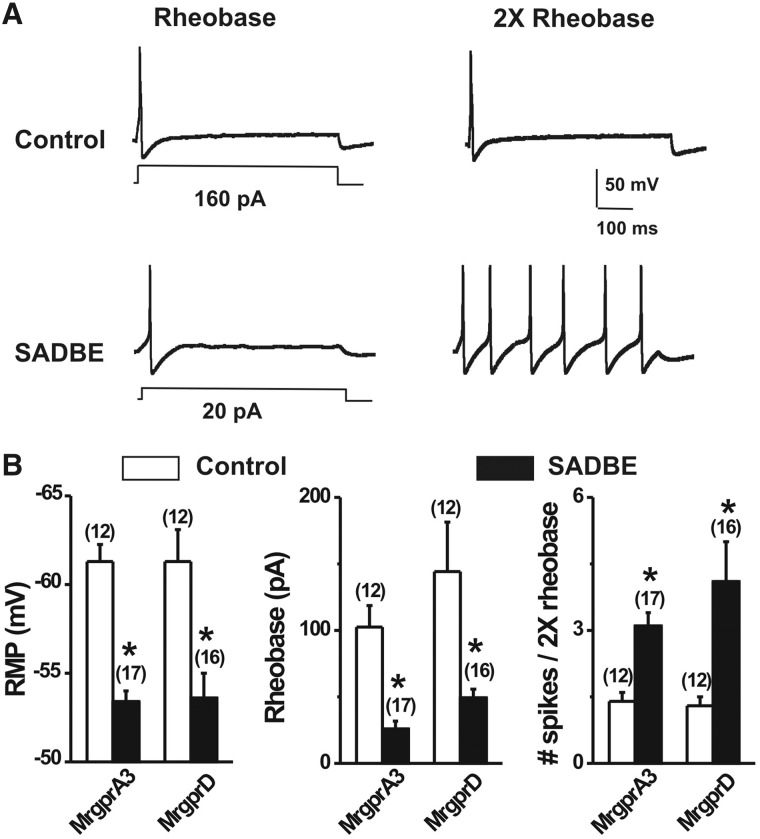
To further investigate the excitability of cutaneous itch-mediating neurons, whole-cell recordings were made from acutely dissociated, fluorescently labelled MRGPRA3+ and MRGPRD+ neurons after the second challenge either with SADBE (CHS group) or acetone (control group) (Table 1 and Fig. 3). Because both types of neurons have been shown to innervate the skin, it was assumed that these neurons had innervated the chemically treated skin of the hind leg. For each type of neuron (MRGPRA3+ and MRGPRD+), the excitability was measured under current-clamp conditions and the mean values of each parameter were compared for CHS and control conditions using unpaired t-tests (Table 1). In comparison with controls, both MRGPRA3+ and MRGPRD+ neurons from CHS mice exhibited significantly more depolarized resting membrane potentials, lower rheobases, and increased numbers of action potentials evoked at twice rheobase (Table 1 and Fig. 3). In contrast, for either MRGPRA3+ or MRGPRD+ neurons, no significant differences were found in cell capacitance, input resistance, or action potential voltage threshold (Table 1).
Table 1.
Membrane properties of MRGPRA3+ and MRGPRD+ neurons from control (vehicle-treated) and CHS (SADBE-challenged) mice
| MRGPRA3+ |
MRGPRD+ |
|||
|---|---|---|---|---|
| Control (n = 12) | SADBE (n = 17) | Control (n = 12) | SADBE (n = 16) | |
| Cell capacitance (pF) | 17.8 ± 1.7 | 14.8 ± 1.4 | 15.5 ± 1.3 | 14.1 ± 1.3 |
| Resting membrane potential (mV) | −62.2 ± 1.0 | −53.4 ± 3.2*** | −61.3 ± 1.0 | −53.7 ± 1.3** |
| Rheobase (pA) | 102.5 ± 16.2 | 25.9 ± 5.9*** | 144.2 ± 37.7 | 49.3 ± 6.5* |
| Input resistance (MΩ) | 560.3 ± 67.7 | 624.8 ± 64.1 | 651.0 ± 77.8 | 631.0 ± 75.8 |
| Action potential voltage threshold (mV) | −23.7 ± 1.9 | − 23.6 ± 2.2 | −26.8 ± 4.0 | −18.7 ± 1.1 |
| Number of action potentials at twice rheobase | 1.4 ± 0.2 | 3.1 ± 0.6** | 1.3 ± 0.2 | 4.6 ± 0.9** |
Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; as compared with control group, unpaired t-test.
Figure 3.
SADBE challenge increased the excitability of MRGPRA3+ and MRGPRD+ neurons. (A) Representative traces of action potentials elicited at rheobase and twice rheobase in MRGPRA3+ neurons from vehicle control and SADBE-treated mice. SADBE challenge results in a reduction of rheobase while increasing the number of action potentials evoked by a 2× rheobase current injection. (B) MRGPRA3+ and MRGPRD+ neurons from SADBE-challenged mice each exhibited a more depolarized resting membrane potential (RMP), lower mean rheobase, and greater number of action potentials at twice rheobase, as compared to control group. Cell numbers tested are indicated in the parentheses. *P < 0.05 versus vehicle control, unpaired t-test.
SADBE challenge increased voltage-gated Na+ currents
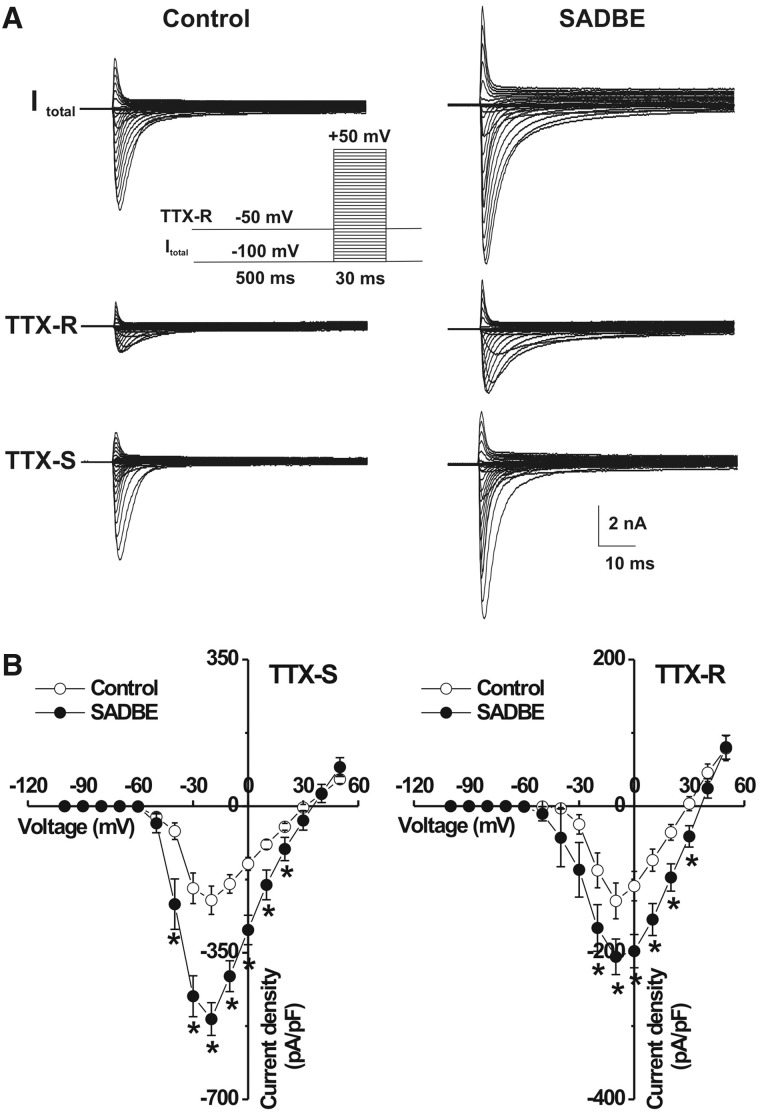
As no change was observed in the membrane input resistance of MRGPRA3+ and MRGPRD+ neurons from CHS mice, the increased excitability seemed likely to be a result of alterations in voltage-gated ion channels. The total Na+ currents were generated by a series of test pulses, each 30 ms in duration, from −100 mV to +50 mV in 5-mV steps, preceded by a 500-ms prepulse to −100 mV (Fan et al., 2011). In addition, we applied a prepulse inactivation protocol with a 500-ms step of −50 mV to isolate TTX-resistant Na+ currents (Fig. 4A). The TTX-sensitive Na+ current was obtained by digitally subtracting the TTX-resistant component from the total Na+ current. As the properties of the TTX-sensitive (or TTX-resistant) Na+ currents were similar for MRGPRA3+ and MRGPRD+ under control conditions and the effects of SADBE challenge in changing these currents were the same for each type of neuron, we combined the results for these two types of neurons for ease of presentation. The mean peak current density of the TTX-sensitive Na+ currents was significantly greater for the CHS group (n = 27) compared with controls (n = 24) (Fig. 4; repeated measures analysis of variance and Tukey post hoc comparisons). Similarly, the TTX-resistant Na+ peak current density was also increased significantly for CHS neurons (n = 27) compared to neurons from control animals (Fig. 4, n = 24).
Figure 4.
Voltage-gated Na+ currents of MRGPR neurons were increased after the second SADBE challenge. (A) Representative traces of voltage-gated Na+ currents in two MRGPRA3+ neurons, one from an acetone-treated control mouse (left) and the other from a SADBE-treated mouse. Total Na+ currents (Itotal; top) were generated by stepwise 30-ms test pulses in 5-mV steps from −100 to +50 mV, preceded by a 500-ms prepulse of −100 mV (inset). TTX-resistant (TTX-R) currents (middle) were generated using the same series of test pulses but preceded by a 500-ms prepulse of −50 mV. TTX-sensitive (TTX-S) currents (bottom) were obtained by digitally subtracting TTX-resistant currents from total Na+ currents. (B) The peak current densities of both TTX-sensitive (left) and TTX-resistant (right) Na+ currents was significantly larger in SADBE-treated mice (closed circles, n = 27 neurons), as compared with controls (open circles, n = 24 neurons). Data for MRGPRA3+ and MRGPRD+ neurons were similar and thus combined (see main text). *P < 0.05, SADBE versus vehicle control, one-way repeated measures analysis of variance with Tukey post hoc comparisons.
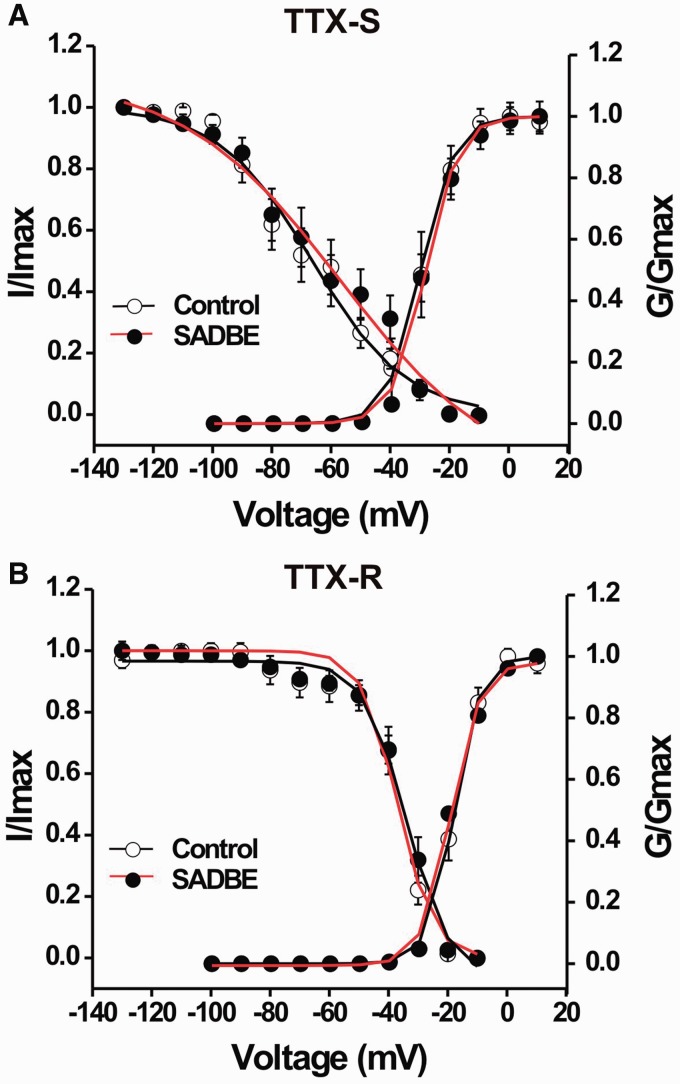
We further tested whether an increase in Na+ current density observed in the CHS group was because of significant changes in the voltage dependence of activation and inactivation of Na+ currents. We found that the voltage-dependence of TTX-sensitive or TTX-resistant Na+ currents was not significantly different between control and CHS groups (Fig. 5; unpaired t-tests). For TTX-sensitive Na+ currents, the mean activation midpoint (V1/2) from fitting the data with a Boltzmann function did not significantly differ for control and CHS (−28.0 ± 3.2 mV, n = 8, versus. −25.7 ± 0.3 mV, n = 15, respectively). Neither was there a significant difference in the mean slope factor for activation (control: 4.0 ± 0.8, n = 8; CHS: 4.7 ± 0.1, n = 15; Fig. 5A). Similarly, for TTX-resistant Na+ currents, we also found no significant differences for control versus CHS groups in the mean V1/2 (−16.3 ± 1.2 mV, n = 13, versus −15.6 ± 1.4 mV, n = 14, respectively) or in the mean slope factor for activation (4.7 ± 0.9, n = 13, versus 6.6 ± 0.8, n = 14, respectively; Fig. 5B). In addition, SADBE challenge did not significantly affect the voltage-dependence of steady-state inactivation of TTX-sensitive or TTX-resistant Na+ currents as demonstrated by non-significant differences between control and CHS groups in mean values of either the V1/2 or the slope factor for current inactivation for either TTX-sensitive or TTX-resistant Na+ currents (Fig. 5 and data not shown).
Figure 5.
Comparison of voltage-dependent activation and steady-state inactivation curves of TTX-sensitive and TTX-resistant Na+ currents in MRGPR neurons between vehicle-treated and SADBE-challenged mice. Data were pooled for MRGPRA3+ and MRGPRD+ neurons. (A) For activation curves, normalized conductance (G / Gmax) was plotted against test pulse voltage and fitted to a Boltzmann function. (B) For inactivation curves, normalized current (I / Imax) was plotted against prepulse voltage and fitted to a negative Boltzmann function. Line represents the average of the individual curve fits. There were no significant differences in the levels of activation or inactivation for TTX-sensitive (A) and TTX-resistant (B) currents between the neurons from control (open circles) and SADBE-treated mice (closed circles).
Discussion
Peripheral afferent and neuronal hyperexcitability accompanying contact hypersensitivity
Although the pathogenesis of allergic contact dermatitis is widely studied, little is known of the effects of allergic contact dermatitis on the functional properties of pruriceptive cutaneous dorsal root ganglion neurons. The present study specifically focused on the spontaneous itch and pain behaviours and the changes in the excitability of cutaneous MRGPRA3+ and MRGPRD+ neurons after CHS, the murine model of allergic contact dermatitis in humans. These neurons, or a subset, have been implicated in the encoding of itch or pain (Cavanaugh et al., 2009; Liu et al., 2012; Han et al., 2013). We provide several observations that support the presence of hyperexcitability of these neurons after CHS. First, in vivo electrophysiological recordings revealed spontaneous activity in a subpopulation of MRGPRA3+ and MRGPRD+ neurons innervating SADBE-challenged skin, but not in neurons from control animals. Second, mechanical stimuli evoked abnormal after-discharges in a subset of MRGPRA3+ and MRGPRD+ neurons with receptive fields in SADBE-challenged skin. Also, some MRGPRA3+ neurons from CHS mice exhibited prolonged after-discharges when noxious heat was applied to their receptive fields. Whether these abnormal discharges or possible changes in response thresholds (not presently measured) are associated with an increase in stimulus evoked itch or pain behaviour in the mouse analogous to enhanced stimulus evoked pain and itch in humans (Sikand et al., 2012) awaits further experimentation.
The dissociated cell bodies of MRGPRA3+ and MRGPRD+ neurons became hyperexcitable after CHS, as indicated by a depolarized resting membrane potential, a significant decrease in rheobase and an increase in the number of action potential discharges evoked at twice rheobase. An increase in the excitability of the cell bodies of MRGPRA3+ neurons from SADBE-treated mice may be linked to the hyperexcitability of their peripheral terminals, which might account for the after-discharges observed when their nerve terminals were stimulated by noxious heat and mechanical stimuli.
Although the cell bodies of MRGPRD+ neurons exhibited signs of enhanced excitability, their peripheral terminals were less likely to exhibit signs of hyperexcitability than those of the MRGPRA3+ neurons as a result of CHS. No after-discharges were observed in MRGPRD+ neurons when noxious heat was delivered to their peripheral receptive fields. And, only a small proportion of MRGPRD+ neurons from CHS mice exhibited spontaneous activity or abnormal after-discharges in response to mechanical force compared to MRGPRA3+ neurons. One possible reason for these differences at the peripheral terminals is that, in contrast to MRGPRD+ neurons, MRGPRA3+ neurons express TRPV1 and respond to more types of pruritic and algesic chemical stimuli (Liu et al., 2012; Han et al., 2013) thereby possibly making them more responsive to endogenous inflammatory and other chemical mediators after the development of CHS. In the future it will be interesting to test the responses of both types of neurons to chemical pruritogens that, for human subjects with SADBE-induced allergic contact dermatitis elicited enhanced itch sensations (Sikand et al., 2012).
Mechanisms of the hyperexcitability of MRGPRA3+ and MRGPRD+ neurons
Our findings demonstrated that CHS produced a significant increase in the magnitude of TTX-sensitive and TTX-resistant Na+ currents but without any changes in the kinetics of activation and inactivation, which may underlie neuronal hyperexcitability observed in cutaneous MRGPRA3+ and MRGPRD+ neurons from CHS mice. Changes in these currents have been proposed to underlie the increased neuronal excitability found in other studies of peripheral inflammation. Peripheral inflammation of skin produced by an injection of carrageenan (Tanaka et al., 1998) or complete Freund’s adjunct (Gould et al., 2004) caused an increase in both TTX-sensitive and TTX-resistant Na+ currents without affecting the kinetics of activation or inactivation in small-diameter dorsal root ganglion neurons. Unlike our model of allergic contact dermatitis in humans, these models of inflammatory pain are toxic and cannot be used in humans. The mechanisms whereby peripheral inflammation modulates voltage-gated Na+ channels require further investigation. The inflammatory milieu surrounding the nerve terminals in the skin contains numerous inflammatory mediators during CHS (Kondo et al., 1994; Westphal et al., 2003; Christensen and Haase, 2012), such as tumor necrosis factor alpha (TNFα) and interleukin-1β (IL-1β), which may be retrogradely transported to cell bodies of dorsal root ganglion neurons and contribute to the increased Na+ currents observed in this study. Indeed, TNFα and (IL-1β) have been demonstrated to augment TTX-sensitive and TTX-resistant Na+ currents in nociceptive dorsal root ganglion neurons (Binshtok et al., 2008; Czeschik et al., 2008). Although an increase in Na+ currents likely contributes to the lower rheobase observed in the present study, we cannot exclude a role for other ion channels. As no significant changes in input resistance of dorsal root ganglion neurons were observed after CHS, it is unlikely that leak channels account for neuronal hyperexcitability in the murine model of allergic contact dermatitis.
Implications for itch and pain associated with allergic contact dermatitis
Itch and pain often accompany skin diseases, such as allergic contact dermatitis and atopic dermatitis. Although the immune mechanisms of CHS have been extensively explored in murine models of allergic contact dermatitis (Christensen and Haase, 2012; Honda et al., 2013), less is known of the itch and pain behaviours and their underlying neural mechanisms. Behavioural assays of itch have typically used a single response indicator such as scratching the site of chemical application to the ear or nape of the neck (Liu et al., 2013). By applying SADBE to the cheek or to the calf of the hindlimb, i.e. locations that allow the opportunity for distinguishing itch- and pain-like site-directed behaviours (Shimada and LaMotte, 2008; Akiyama et al., 2010a; LaMotte et al., 2011), we found that both types of behaviour were evoked during the elicitation phase of CHS. Analogously, sensations of itch and nociceptive sensations were reported when SADBE was applied to the skin of previously sensitized human subjects (Sikand et al., 2012). Moreover, SADBE-treated human skin exhibited alloknesis, hyperknesis, and hyperalgesia to mechanical stimuli, itch sensation to noxious heat and an enhanced itch to intradermal injection of histamine, BAM8-22 (ligand for MRGPRC11 in mice and MRGPRX1 in humans), or beta-alanine (MRGPRD ligand in both humans and mice) (Sikand et al., 2012). In humans, MRGPRX1 shares homology with murine MRGPRA3 and MRGPRC11. Thus, it is possible that peripheral pruriceptive neurons expressing MRGPRD or MRGPRX1 may become more excitable after experimental allergic contact dermatitis in humans. MRGPRA3+ and MRGPRD+ neurons were recently identified as candidates for mediating acute itch in mice evoked by pruritic chemicals that also evoke itch and nociceptive sensations in humans (Liu et al., 2012; Han et al., 2013). MRGPRA3+ neurons play a critical role in histamine-dependent acute itch (Han et al., 2013) whereas subpopulations of MRGPRD+ neurons mediate the histamine-independent itch elicited by beta-alanine (Liu et al., 2012) and mechanically evoked pain behaviour (Cavanaugh et al., 2009) in mice under normal conditions. Also, MRGPRA3+ neurons were recently shown to be involved in dry skin chronic itch and allergic itch (Han et al., 2013).
Our findings indicate the possibility that the enhanced excitability of MRGPRA3+ and MRGPRD+ neurons may contribute to the itch and pain associated with CHS and the accompanied dysaesthesias. First, ablation of MRGPRA3+ neurons significantly reduced the spontaneous scratching on the nape of the neck of CHS mice. Although the behavioural responses of scratching the nape of neck may also indicate pain in addition to itch sensation, we consider that MRGPRA3+ neurons contribute to CHS-induced itch rather than pain as MRGPRA3+ neurons were specifically linked to itch but not pain regardless of the type of stimulus that activates the neuron (Han et al., 2013). MRGPRD knockout mice would be useful in future studies of the role of MRGPRD in inflammatory itch and pain. Second, cutaneous MRGPRA3+ neurons innervating SADBE-treated skin exhibited spontaneous firing, which might indicate a chronic sensitization of pruriceptive primary afferent fibres and itch-signalling superficial dorsal horn neurons and, as a consequence, lead to chronic hyperknesis and alloknesis. Spontaneously ongoing activity in C-pruriceptors was also identified in patients with chronic itch (Ikoma et al., 2004). Indeed, peripheral sensitization of pruriceptive primary sensory afferents has been suggested as a mechanism underlying the chronic itch of atopic dermatitis (Schmelz et al., 2003). Third, noxious heat and mechanical stimuli evoked abnormal after-discharges at peripheral terminals of MRGPRA3+ neurons after CHS, suggesting that this subset of peripheral pruriceptors is potentially sensitized by CHS and exhibits increased sensitivity to certain cutaneous stimuli. The increased mechanosensitivity (i.e. the after-discharges) observed at the peripheral terminals of MRGPRA3+ neurons may contribute to increased mechanically evoked itch (hyperknesis and alloknesis) that humans experience after SADBE (Sikand et al., 2012). Enhanced responses of peripheral pruriceptors to pruritic chemicals have also been found in a rodent model of itch from dry skin (Akiyama et al., 2010b). Further studies are required to determine whether MRGPRA3+ and MRGPRD+ neurons exhibit enhanced responses to pruritic chemical stimuli after CHS.
MRGPRD+ neurons from CHS mice exhibited prolonged after-discharges in response to mechanical, but not heat stimulation of their receptive fields. Mechanical- and mechanoheat-sensitive MRGPRD+ neurons may encode pain and itch, respectively (Liu et al., 2012). Our in vitro study did not distinguish between MRGPRD+ neurons that are responsive to noxious heat and beta-alanine and are thought to be pruriceptive (Liu et al., 2012) and other MRGPRD+ neurons that only respond to noxious mechanical stimuli. Perhaps the latter contributes to mechanical nociception, which was found to be impaired after ablation of MRGPRD neurons (Cavanaugh et al., 2009). Thus, the sensitization of MRGPRD+ neurons might in part contribute to pain behaviour observed in CHS. Although itch can be transiently relieved by scratching, the itch–scratch cycle can further exacerbate the inflammation and damage the skin, probably activating mechanosensitive nociceptors thereby increasing pain as well as itch (Takaoka et al., 2007; Hashimoto et al., 2011).
We used GFP markers for MRGPRA3 and MRGPRD to identify the cell bodies of same subset of neurons in vivo (functional studies in the intact neuron) and in vitro (mechanistic studies after acute dissociation). We did not investigate a possible role of the MRGPRA3 or MRGPRD receptors in SADBE-induced itch versus pain behaviour. Our findings do not allow us to speculate on the role of other receptors or other types of sensory neurons in these behaviours. For example, there is evidence that TRPA1-expessing neurons are critically involved in a murine model of CHS produced by repeated delivery of the contact allergen, oxazolone (Liu et al., 2013). Nonetheless, our results have identified neuronal targets and ionic mechanisms of neuronal hyperexcitability that might contribute to spontaneous itch and pain sensations and the related dysaesthesias associated with CHS. These neurons and the associated mechanisms of enhanced excitability could be targeted by new therapeutic strategies designed to treat the pain and itch from inflammatory diseases of the skin and other pruritic disorders as well.
Acknowledgements
We thank Pu Zhang, Yumei Li for genotyping of transgenic animals
Glossary
Abbreviations
- CHS
contact hypersensitivity
- GFP
green fluorescent protein
- SADBE
squaric acid dibutyl ester
- TTX
tetrodotoxin
Funding
This work was supported by NIH grants NS047399 and NS014624 (RHL); GM087369 and NS054791 (XD); National Science Foundation of China #81271239 (CM) and PUMC/CAMS/IBMS Funding #2011RC01 (CM). L. Qu is the recipient of a fellowship from the Canadian Institutes of Health Research (CIHR). K Fu is a recipient of a scholarship from Jinan University, China.
References
- Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and micro-opoid modulation in mice. Acta Derm Venereol. 2010a;90:575–81. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010b;151:378–83. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camouse MM, Swick AR, Ryan CA, Hulette B, Gerberick F, Tinkle SS, et al. Determination of in vivo dose response and allergen-specific T cells in subjects contact-sensitized to squaric acid dibutyl ester. Dermatitis. 2008;19:95–9. [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. 2009;106:9075–80. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC. Pruritus. Dermatol Clin. 2012;30:309–21, ix. doi: 10.1016/j.det.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120:1–27. doi: 10.1111/j.1600-0463.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–8. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Fan N, Donnelly DF, LaMotte RH. Chronic compression of mouse dorsal root ganglion alters voltage-gated sodium and potassium currents in medium-sized dorsal root ganglion neurons. J Neurophysiol. 2011;106:3067–72. doi: 10.1152/jn.00752.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan N, Qu L, Ma C, Shimada SG, Zhang P, Han L, et al. New Orleans LA: Society for Neuroscience; 2012. Allergic contact dermatitis induces both itch behavior and hyperexcitability in a subpopulation of cutaneous dorsal root ganglion neurons in the mouse. Abstract Program No. 675.08. [Google Scholar]
- Gould HJ, III, England JD, Soignier RD, Nolan P, Minor LD, Liu ZP, et al. Ibuprofen blocks changes in Na v 1.7 and 1.8 sodium channels associated with complete Freund's adjuvant-induced inflammation in rat. J Pain. 2004;5:270–80. doi: 10.1016/j.jpain.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–82. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Takaoka A, Sugimoto M, Honma Y, Sakurai T, Futaki N, et al. Itch-associated scratching contributes to the development of dermatitis and hyperimmunoglobulinaemia E in NC/Nga mice. Exp Dermatol. 2011;20:820–5. doi: 10.1111/j.1600-0625.2011.01337.x. [DOI] [PubMed] [Google Scholar]
- Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133:303–15. doi: 10.1038/jid.2012.284. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–7. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–69. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153–6. doi: 10.1001/archdermatol.2011.178. [DOI] [PubMed] [Google Scholar]
- Kondo S, Pastore S, Shivji GM, McKenzie RC, Sauder DN. Characterization of epidermal cytokine profiles in sensitization and elicitation phases of allergic contact dermatitis as well as irritant contact dermatitis in mouse skin. Lymphokine Cytokine Res. 1994;13:367–75. [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol. 2011;20:778–82. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–63. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, et al. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32:14532–7. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–65. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, et al. Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci. 2008;28:125–32. doi: 10.1523/JNEUROSCI.4472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Donnelly DF, LaMotte RH. In vivo visualization and functional characterization of primary somatic neurons. J Neurosci Methods. 2010;191:60–5. doi: 10.1016/j.jneumeth.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27:14059–68. doi: 10.1523/JNEUROSCI.3699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Nie H, Gu Q, Sikand P, Lamotte RH. In vivo responses of cutaneous C-mechanosensitive neurons in mouse to punctate chemical stimuli that elicit itch and nociceptive sensations in humans. J Neurophysiol. 2012;107:357–63. doi: 10.1152/jn.00801.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Li Y, Pan X, Zhang P, LaMotte RH, Ma C. Transient receptor potential canonical 3 (TRPC3) is required for IgG immune complex-induced excitation of the rat dorsal root ganglion neurons. J Neurosci. 2012;32:9554–62. doi: 10.1523/JNEUROSCI.6355-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Zhang P, LaMotte RH, Ma C. Neuronal Fc-gamma receptor I mediated excitatory effects of IgG immune complex on rat dorsal root ganglion neurons. Brain Behav Immun. 2011;25:1399–407. doi: 10.1016/j.bbi.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–9. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Hilliges M, Schmidt R, Orstavik K, Vahlquist C, Weidner C, et al. Active “itch fibers” in chronic pruritus. Neurology. 2003;61:564–6. doi: 10.1212/01.wnl.0000078193.64949.08. [DOI] [PubMed] [Google Scholar]
- Scott AE, Kashon ML, Yucesoy B, Luster MI, Tinkle SS. Insights into the quantitative relationship between sensitization and challenge for allergic contact dermatitis reactions. Toxicol Appl Pharmacol. 2002;183:66–70. doi: 10.1006/taap.2002.9469. [DOI] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–7. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, et al. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–64. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- Sikand P, King BA, LaMotte RH. New Orleans LA: Society for Neuroscience; 2012. Psychophysical measurements of itch and nociceptive sensations in an experimental model of allergic dermatitis in humans. Abstract Program No. 675.07. [Google Scholar]
- Takaoka A, Arai I, Sugimoto M, Futaki N, Sakurai T, Honma Y, et al. Role of scratch-induced cutaneous prostaglandin D production on atopic-like scratching behaviour in mice. Exp Dermatol. 2007;16:331–9. doi: 10.1111/j.1600-0625.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport. 1998;9:967–72. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- Westphal GA, Schnuch A, Moessner R, Konig IR, Kranke B, Hallier E, et al. Cytokine gene polymorphisms in allergic contact dermatitis. Contact Dermatitis. 2003;48:93–8. doi: 10.1034/j.1600-0536.2003.480208.x. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]