Abstract
Missing meals and fasting have long been reported as headache triggers. Stress also has received attention for its role in precipitating headaches. This study explored the effects of eating behaviors on new-onset headache. Analyzing only the 1070 of 1648 (64.9%) diary days that followed a non-headache day, the study included 34 migraineurs who contributed a median (25th, 75th percentile) of 28 (22, 40) days of diary entries. Multivariable survival modeling with random effects was conducted, and hazards ratios and 95% confidence intervals were calculated. Nighttime snacking was associated with a 40% reduction in the odds of experiencing a headache compared to having no food (p = 0.013). Eating a late dinner was associated with a 21% reduction in the odds of headache when compared to no additional food, but this association was not statistically significant (p = 0. 22). These results demonstrate the potential for eating behaviors to be targeted in headache management, as regulated eating habits may have the potential to reduce the occurrence of headache. Although no causal relationship can be established, these results indicate that further research into the mechanisms of the association between eating behaviors and headache activity is warranted.
Keywords: Fasting, Headache precipitant, Headache trigger, Migraine, Stress
1. Introduction
Among the putative “triggers” of migraine and tension-type headache (TTH) attacks, missing meals/fasting is frequently endorsed in self-report studies among adults.1,2 Though estimates vary from 39–82% across most studies,2,3,4,5 the largest recent study on 1207 migraineurs with and without aura, found that not eating was a headache precipitant for 57% of patients, ranking it alongside stress, hormonal fluctuations (among women), and sleep disturbance as the most common and consistent migraine triggers.6 The role of fasting in headache onset has also been supported by findings of increased headache incidence and exacerbation of pre-existing migraine and TTH among individuals who fast for long periods as part of religious practices such as Yom Kippur7,8 and Ramadan.9,10 Data from these studies also suggested that longer durations of fasting were associated with a proportionally increased risk of headache onset.11
Prospective designs, though few, have provided some direct empirical support for fasting and missing meals as triggers of migraine.12,13 In the best exemplar to date, Martin and Seneviratne assigned 56 individuals with migraine or TTH to one of four conditions involving the presence or absence of 19 hour food deprivation (allowing only water from dinner to the next morning’s laboratory session) and a laboratory stressor.13 A main effect on headache intensity was observed for both hunger and stress, indicating that food deprivation and stress contributed to headache. Headache occurred or worsened in 93% of those in the hunger-stress condition and 58% of those in the hunger-no stress condition. There was no interaction between these conditions over experimental phases or with headache diagnosis, indicating that hunger and stress operate independently as triggers for both migraine and TTH sufferers.
Although common clinical advice is to maintain a regular eating schedule, dietary regimentation of meal timing is not a consensus recommendation for headache prophylaxis. Some studies have failed to identify missing meals as a significant trigger for migraine,14,15,16 and prospective designs underpinning the recommendation to eat small, regular meals remain scarce. In view of a need for more prospective data concerning potential associations between eating behavior and migraine, the present study used survival analyses on twice-daily headache diary data to examine the relationship between timing of eating and risk of next-day migraine after taking into account daily stress. Stress was accounted for because of its well-documented role as a headache precipitant1 and to avoid potential variable confounding among individuals who might have missed (or eaten) meals as a function of current high stress or an unknown third variable. We hypothesized that, after controlling for the effects of stress on subsequent migraine, nighttime meals or snacking would be associated with reduced likelihood of next-day migraine, and that timing of food intake would moderate this association such that more recent eating would be associated with least likelihood of migraine.
2. Materials and methods
Daily diary data from 34 migraine sufferers was included in this interim analysis of an ongoing study sponsored by the USA National Institutes of Health (1R01NS06525701). Institutional Review Board approval was obtained from Wake Forest School of Medicine, and each participant provided informed consent. To participate, individuals were required to be at least 18 years old, meet the International Headache Society Diagnostic criteria for migraine either with (ICHD-II 1.2) or without aura (ICHD-II 1.1),17 and suffer from at least two headache attacks per month with headaches on 4–14 days per month. Participants were recruited through collaboration with local neurology clinic offices and through direct television advertising in the community. Participants were compensated for their time and effort.
After study enrollment procedures, each participant was evaluated by a neurologist and completed a packet of questionnaires that included the Center for Epidemiologic Studies Depression Scale (CES-D),18 the State-Trait Anxiety Inventory Form Y-2 (STAI-T),19 and the Migraine Disability Scale (MIDAS).20 The CES-D is a 20 question valid and reliable scale commonly used to measure symptoms associated with depression.18 This scale is suitable for assessing depressive symptoms in the general population. The STAI-T is a reliable and valid 20 question scale used to assess trait anxiety, the natural tendency to feel threatened by environmental factors.19 This scale is also appropriate for assessing symptoms of anxiety in the general population. The MIDAS is a reliable and valid 5 item scale used in clinical practice and research to assess migraine-related disability.20,21
Participants were provided an electronic diary to use at home for the duration of the 6 week observational study. The migraineurs completed time-stamped diary entries each morning and evening. The diaries collected information on headache activity (clock time for the initiation and cessation of an attack) and pain characteristics (pain intensity: 0 to 10). In addition, the diaries contained several items that assessed eating practices including the presence or absence of snacking. One item in the morning diary assessed the previous day’s late evening and early morning eating patterns by inquiring, “Have you eaten since your last diary entry?” Participants selected from the choices of “no food,” “late dinner,” “snack,” or “breakfast.” The evening rating contained a measure of daily hassles, the Daily Stress Inventory (DSI).22 The DSI is a well-validated 58 item measure of daily stress. Participants rated stressful events on a 1 (“occurred but was not stressful”) to 7 (“caused me to panic”) scale. For the present analysis the sum of daily stress ratings was used.
2.1. Statistical analysis
Prior to initiating the analysis, we examined the statistical power available to find clinically significant effect sizes for detecting a new-onset headache attack while considering the hierarchical structure of the data. Assuming 289 daily diaries (reduced from the available 1070 due to an assumed within-person intra-class correlation of r = 0.10) there was 94% power to detect a class predictor that exhibited an effect size that is associated with 30% new-onset headaches in one group and 10% in the other. Thus, although this analysis was conducted based on the available data and was not designed to specifically address our hypothesis, sufficient statistical power existed to detect a clinically meaningful effect size.
Descriptive statistics are presented as median (25th percentile, 75th percentile) or frequency counts with percentages for categorical data. To examine the associations between daily characteristics and the presence of new-onset headache, a multivariable survival model was created. To account for the fact that participants each contributed multiple days to the analysis, random effects survival models were utilized that treated subjects as a random effect. Two models were specified based on forced entry of stress, modeled as a continuous predictor, and the categories of dinner-breakfast snacking (no food, late dinner, snack, breakfast). In the first model, these predictors were considered to be simple predictors; a second model examined interactions between stress and snacking. In essence, the models examined the odds of experiencing a headache for each hour of the day (hazard ratio [HR]) as a function of predictors in the model. The proportional-hazards assumption was tested and satisfied prior to interpretation of the model. To express the relative risks of the stress and eating predictors, HR with respective 95% confidence intervals (CI) are reported. The HR presents the odds ratio of experiencing a new headache attack for each hour during the day. All analyses were conducted using SAS 9.2 (SAS, Inc., Cary, NC, USA). Where appropriate, all hypotheses are two-tailed with p < 0.05.
3. Results
3.1. Sample characteristics
The database contained 1648 entries from 34 participants who completed diaries between September 2009 and November 2011. Adherence to the diaries was excellent with 95.1% of the 1732 possible AM and PM diary entries completed by the participants. Using the inclusion criteria, 1070 (64.9%) diary days could be found where a participant was without a headache for a full 24 hours prior to the beginning of a new day. All 34 participants provided diary days to the analysis with each participant contributing a median of 28 (22, 40) days. Although there was substantial variability in the timing of diary entries, the median time of morning diary entries was 6:38 AM, and the median time of evening diary entries was 9:52 PM. As shown in Table 1, patients were primarily middle-aged females with episodic migraine. The participants reported only mild symptoms of depression and anxiety with modest levels of disability from their headaches. During the observation period, the participants reported new-onset headaches on 25% of the days. On most days (65%) participants did not engage in a late dinner, a nighttime snack, or an early breakfast, but on the remaining days participants engaged in at least one of these eating practices (Table 1).
Table 1.
Demographics and sample characteristics of migraineurs
| Sample characteristics (n = 34) | Diary characteristics (n = 1070 entries) | ||
|---|---|---|---|
| No headache n = 802 (75%) |
Headache n = 268 (25%) |
||
| Sex, female | 33 (97%) | ||
| Diagnosis | |||
| Migraine without aura | 31 (91%) | ||
| Migraine with aura | 3 (9%) | ||
| Age, years | 42 [30, 54] | ||
| CESD | 8 [3, 16.75] | ||
| STAI | 35 [26, 42] | ||
| MIDAS | 15 [4.25, 30] | ||
| Stress SUM | 10.5 [3, 21] | 17 [8, 33] | |
| No food | 516 (74%) | 181 (26%) | |
| Early breakfast | 56 (73.7%) | 20 (26.3%) | |
| Late dinner | 73 (71.6%) | 29 (28.4%) | |
| Snack | 133 (80.6%) | 32 (19.4%) | |
| Worst pain rating (0 to 10) | 0 | 6 [4, 7.5] | |
Data are presented as either median [25th percentile, 75th percentile] or n (%).
CESD = Center for Epidemiologic Studies Depression Scale, MIDAS = Migraine Disability Scale, SUM = sum of daily stress ratings, STAI-T = State-Trait Anxiety Inventory Form Y-2.
3.2. Multivariable model
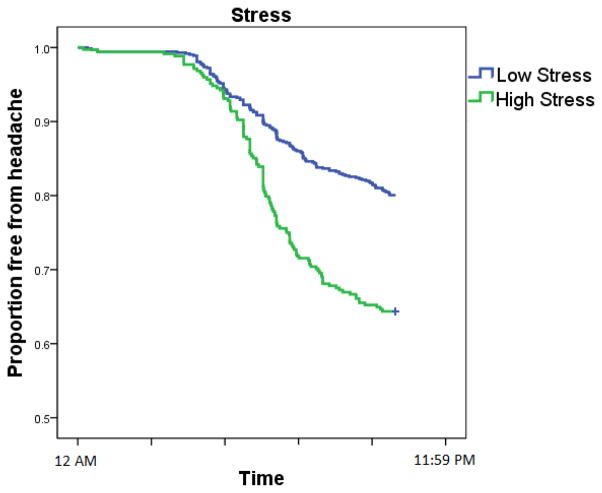
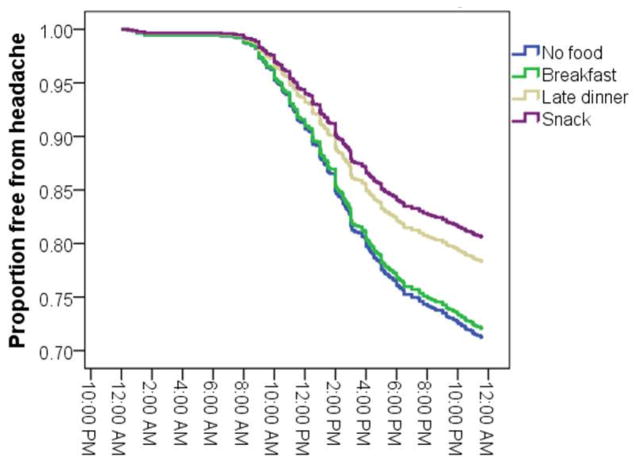
Both stress and snacking behaviors were statistically significant predictors of a new-onset headache during the 24 hours of the diary observation period. Table 2 displays the model coefficients that reflect the unique contribution of each predictor in the odds of experiencing a headache after accounting for the other predictors. Each standard deviation increase in same-day stress was associated with a 35% increase in the odds of a headache for each hour of the day (Fig. 1). Several aspects of between-meal eating behaviors were also related to the risk of experiencing a new headache. Eating an early breakfast was not associated with any benefit compared to eating no additional food. Eating a late dinner was associated with a 21% decrease in the odds of experiencing a headache when compared to no additional food, but this effect was not statistically significant (p = 0. 22). However, nighttime snacking was associated with reduction in the odds of experiencing a headache, with a 40% reduction in the odds of headache compared to no food (p = 0.013). The survival curve in Figure 2 graphically depicts this benefit by plotting the probability of experiencing a headache by type of eating behavior.
Table 2.
Multivariable survival model predicting the probability of experiencing a migraine attack
| Predictor | HR | 95% CI | p value |
|---|---|---|---|
| Stress | 1.35 | 1.19 to 1.54 | < 0.0001 |
| No food | Reference | Reference | Reference |
| Early breakfast | 0.92 | 0.65 to 1.47 | 0.98 |
| Late dinner | 0.79 | 0.55 to 1.15 | 0.22 |
| Snack | 0.60 | 0.40 to 0.90 | 0.013 |
Stress is coded such that the hazard reflects each one standard deviation increase in stress.
CI = confidence interval, HR = hazard ratio.
Fig. 1.
The proportion of patient-days free from headache (y-axis) as a function of clock time (x-axis) and stress. Each day began without a headache with each passing hour leading to a reduction in days free from headache. Stress was artificially dichotomized into high (above the median) or low (at or below the median) for each patient for graphical display purposes; the multivariable model considered stress as a continuous predictor.
Fig. 2.
The proportion of patient-days free from headache (y-axis) as a function of clock time (x-axis) and eating behavior. Each day began without a headache with each passing hour leading to a reduction in days free from headache.
A sensitivity analysis was conducted to examine an alternative explanation of the snacking association. The proportion of days that someone snacked was entered into the model as a person-level predictor instead of the day-level snacking. This association essentially tests whether individuals who tend to snack have lower levels of headache as opposed to the previous association where the act of snacking is associated with reduced headache. A statistically significant association was found (p < 0.001), indicating that in this sample, people who tended to snack reported fewer new-onset headache attacks. For example, the 50% of individuals who recorded a nighttime snack on more than 1 day per month experienced a headache on 93/381 (24.4%) days without a snack but experienced a headache on only 31/160 (19.4%) days with a nighttime snack. The 50% of individuals who rarely snacked experienced a headache on 143/524 (27.3%) days without a snack but experienced a headache on only 1/5 (20%) the rare days with a nighttime snack. When snacking proclivity (the proportion of days with snacking) was entered into the model along with actual daily snacking behavior, both predictors lost their statistical significance. Thus, these patterns of associations complicate the interpretation of snacking’s role in headache onset in this modest sample size, given that it is difficult to untangle the daily effect of snacking versus person-level differences that lead to snacking.
In a final model, the interaction between stress and eating behavior was examined for evidence of a differential benefit to eating a snack as a function of stress. The interaction between same-day stress and eating behavior was not statistically significant (p = 0.159), refuting the notion that stress moderates the relationship. The point estimate of effect suggested that snacking might produce a survival curve that is more similar to the low stress days than the other eating patterns (Snack × Stress HR: 0.78 [95%CI 0.62–0.99]) in a future better-powered study. Figure 3 displays this statistically non-significant interaction.
Fig. 3.
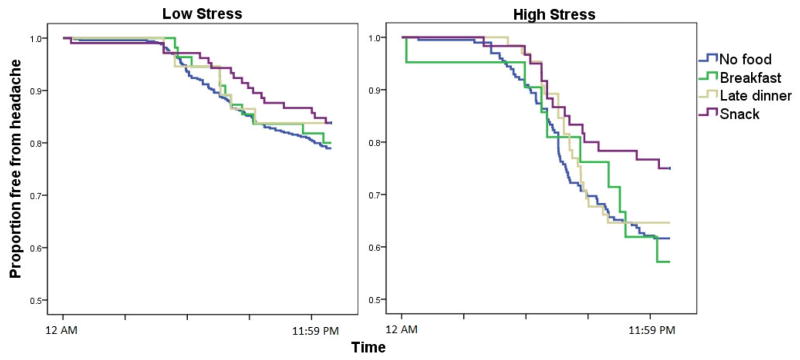
The proportion of patient-days free from headache (y-axis) as a function of clock time (x-axis), eating behavior, and stress. Each day began without a headache with each passing hour leading to a reduction in days free from headache. For this plot, stress was artificially dichotomized into high (above the median, right panel) or low (at or below the median, left panel) for each patient.
4. Discussion
This study demonstrated that the previous evening’s snacking behaviors and the current day’s stress predict a new-onset headache attack. The data presented here support via association the long-held notion that missing meals/food is related to subsequent headache onset in those with a headache history and those without a headache history.23 Precisely why hunger triggers headache remains unclear. Both observational7,8 and experimental studies13 suggest that dehydration is not the putative mechanism, though caffeine withdrawal may be involved to some degree among those who consume caffeine regularly.11 Although unlikely to be the sole mechanism,11 a promising contributor is the increase in sympathetic nervous system activity occasioned by resulting hypoglycemia, which is particularly contributory in prolonged fasts begetting liver glycogen depletion.6,24 Maintenance of steady relative glucose levels by eating more frequent small meals and snacks has indeed been proposed as one strategy for preventing headaches triggered by fasting.25 This advice is supported by findings that eating certain foods may be protective against headache14 and uncontrolled studies indicating that correcting hypoglycemia in diabetic migraineurs26 and eating regular meals among those with reactive hypoglycemia27,28 improve migraine. Although we did not examine glucose levels directly, our data suggest that eating can be protective against headache. However, the mechanism of action for this finding remains mysterious due to the fact that an 8–12 hour fast is not expected to induce hypoglycemia. An experimental study would be required to support this mechanism.
The evidence of an association between eating behaviors and a reduction in headache occurrence has considerable implications for treatment. In behavioral management of headache, it is often recommended that patients avoid skipping meals, and findings from this observational study add data to that common clinical wisdom. Much attention has been paid to what people eat. While this study did not examine the type of foods, it does support the timing. Snacking in the late evening was the best choice for preventing headache as compared to dinner and breakfast. Although this study was not able to support the interaction between high stress and snacking, there appears to be subtle evidence that on days when stress was higher, snacking was more beneficial.
4.1. Limitations and future direction
The diary measurement of eating behavior used in this study is not a validated assessment. This data collection tool did not include information on the amount or type of food eaten, which likely is important in assessing the impact of eating behaviors on headache. The exact time food was eaten also was not collected. Although the type of meal eaten was collected, participants may have had varying interpretations of what constitutes a snack, a late dinner, or even breakfast (that is, at what time or how much is eaten). Providing precise definitions of all meal and snack options would produce more accurate results. Another limitation is the retrospective nature of the diary entries. Although the entries were completed two times per day, participants might have had trouble remembering when they ate between diary entries. A sensitivity analysis demonstrated that the association between snacking behaviors and headache was related to person-level characteristics and that, as in any other observational study, unmeasured predictors also associated with individual traits could be confounding the relationship. Additionally, the study’s headache sufferers were not confirmed as diabetic or non-diabetic. Whereas an 8–12 hour fasting regimen may not induce hypoglycemia in someone without diabetes, those individuals with poor glucose regulation might be susceptible to this fasting state. Future studies would benefit from confirmation of this information.
While nighttime snacking and the occurrence of headache are associated, it is necessary to explicitly acknowledge that correlation does not mean causation. The complete nature of this association is yet to be fully explored. Future studies are needed to examine this phenomenon in light of other potential contributing factors. Data on the exact timing of eating and foods eaten should be collected and analyzed in such studies. Another consideration is the sequence of events in the migraine attack process. Feeling less hungry the evening before a migraine could be a prodromal symptom of the attack. In this case, eating no food after dinner might be the result of any early migraine attack. Experimental research designs would provide valuable information on the sequence of events and mechanisms of eating behaviors and migraine attack.
Acknowledgments
Research reported in this paper was supported by the USA National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1R01NS06525701.
Footnotes
Conflicts of Interest/Disclosures
Dana P. Turner: unrestricted grant funding from Merck, Inc.
Todd A. Smitherman: unrestricted grant funding from Merck, Inc.
Donald B. Penzien: unrestricted grant funding from Merck, Inc.
John A. H. Porter: speaker’s bureau for Novartis, UCB Pharma, Teva, and Impax; research funding from Roche.
Vincent T. Martin: consultant and speaker with Allergan; consultant with Nautilus and Zogenix; grant funding from GlaxoSmithKline.
Timothy T. Houle: unrestricted grant funding from Merck, Inc.; consultant with Allergan.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483–95. doi: 10.1016/j.cpr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Rockett FC, de Oliveira VR, Castro K, et al. Dietary aspects of migraine trigger factors. Nutr Rev. 2012;70:337–56. doi: 10.1111/j.1753-4887.2012.00468.x. [DOI] [PubMed] [Google Scholar]
- 3.Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache. 2010;50:1366–70. doi: 10.1111/j.1526-4610.2010.01753.x. [DOI] [PubMed] [Google Scholar]
- 4.Robbins L. Precipitating factors in migraine: A retrospective review of 494 patients. Headache. 1994;34:214–6. doi: 10.1111/j.1526-4610.1994.hed3404214.x. [DOI] [PubMed] [Google Scholar]
- 5.Spierings LH, Ranke AH, Honkoop PC. Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41:554–8. doi: 10.1046/j.1526-4610.2001.041006554.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 7.Mosek A, Korczyn AD. Yom Kippur headache. Neurology. 1995;45:1953–5. doi: 10.1212/wnl.45.11.1953. [DOI] [PubMed] [Google Scholar]
- 8.Mosek A, Korczyn AD. Fasting headache, weight loss, and dehydration. Headache. 1999;39:225–7. doi: 10.1046/j.1526-4610.1999.3903225.x. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Salameh I, Plakht Y, Ifergane G. Migraine exacerbation during Ramadan fasting. J Headache Pain. 2010;11:513–17. doi: 10.1007/s10194-010-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awada A, al Jumah M. The First-of-Ramadan headache. Headache. 1999;39:490–3. doi: 10.1046/j.1526-4610.1999.3907490.x. [DOI] [PubMed] [Google Scholar]
- 11.Torelli P, Evangelista A, Bini A, et al. Fasting headache: A review of the literature and new hypotheses. Headache. 2009;49:744–52. doi: 10.1111/j.1526-4610.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarty A, Mukherjee A, Roy D. Trigger factors in childhood migraine: A clinic-based study from eastern India. J Headache Pain. 2009;10:375–80. doi: 10.1007/s10194-009-0147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin PR, Seneviratne HM. Effects of food deprivation and a stressor on head pain. Health Psychol. 1997;16:310–8. doi: 10.1037//0278-6133.16.4.310. [DOI] [PubMed] [Google Scholar]
- 14.Connelly M, Bickel J. An electronic daily diary process study of stress and health behavior triggers of primary headaches in children. J Pediatr Psychol. 2011;36:852–62. doi: 10.1093/jpepsy/jsr017. [DOI] [PubMed] [Google Scholar]
- 15.Milde-Busch A, Blaschek A, Borggrafe I, et al. Associations of diet and lifestyle with headache in high-school students: Results from a cross-sectional study. Headache. 2010;50:1104–14. doi: 10.1111/j.1526-4610.2010.01706.x. [DOI] [PubMed] [Google Scholar]
- 16.Takeshima T, Ishizaki K, Fukuhara Y, et al. Population-based door-to-door survey of migraine in Japan: The Daisen study. Headache. 2004;44:8–19. doi: 10.1111/j.1526-4610.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 17.International Headache Society. The international classification of headache disorders. Cephalalgia. (2nd) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 19.Spielberger CD, Gorssuch RL, Lushene PR, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 20.Stewart WF, Lipton RB, Simon D, et al. Reliability of an illness severity measure for headache in a population sample of migraine sufferers. Cephalalgia. 1998;18:44–51. doi: 10.1046/j.1468-2982.1998.1801044.x. [DOI] [PubMed] [Google Scholar]
- 21.Stewart WF, Lipton RB, Simon D, et al. Validity of an illness severity measure in a population sample of migraine headache sufferers. Pain. 1999;79:291–301. doi: 10.1016/s0304-3959(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 22.Brantley PJ, Waggoner CD, Jones GN, et al. A Daily Stress Inventory: Development, reliability, and validity. J Behav Med. 1987;10:61–74. doi: 10.1007/BF00845128. [DOI] [PubMed] [Google Scholar]
- 23.Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am. 2001;85:911–41. doi: 10.1016/s0025-7125(05)70351-5. [DOI] [PubMed] [Google Scholar]
- 24.Peroutka SJ. Serum glucose regulation and headache. Headache. 2002;42:303–8. doi: 10.1046/j.1526-4610.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- 25.Hufnagl KN, Peroutka SJ. Glucose regulation in headache: Implications for dietary management. Expert Rev Neurotherapeutics. 2002;2:311–7. doi: 10.1586/14737175.2.3.311. [DOI] [PubMed] [Google Scholar]
- 26.Blau JN, Pike DA. Effect of diabetes in migraine. Lancet. 1970;2:241–3. doi: 10.1016/s0140-6736(70)92588-2. [DOI] [PubMed] [Google Scholar]
- 27.Blau JN. Migraine: clinical, therapeutic, conceptual and research aspects. London: Chapman and Hall; 1987. [Google Scholar]
- 28.Dexter JD, Roberts J, Byer JA. The five hour glucose tolerance test and effect of low sucrose diet in migraine. Headache. 1978;18:91–4. doi: 10.1111/j.1526-4610.1978.hed1802091.x. [DOI] [PubMed] [Google Scholar]