Abstract
Background
Combined transcranial magnetic stimulation (TMS) and electroencephalography (EEG) can provide insights into how differing cognitive contexts produce different brain states, through TMS-based measures of effective connectivity. For instance, in a recent study, the amplitude of the TMS-evoked response (TMS-ER) derived during the delay-period of a spatial short-term memory (STM) task had a larger amplitude, and greater spread to distal cortical areas, than the TMS-ER from a fixation condition [1]. This indicated that the brain's electrical response to TMS is influenced by the cognitive context (STM or fixation) at the time of stimulation. This study also showed significant individual differences in the shape of the TMS-ER. Further, delay-period spectrograms revealed patterns of activity, the sustained pattern of delay-period activity (SPDPA), which were different across individuals.
Objective/Hypothesis
The present study addressed whether individual differences in the SPDPA predict spectral properties of the TMS-ER. We predicted that significant relationships would exist in task-relevant areas, such as the prefrontal cortex in the case of STM.
Methods
The TMS-ER was derived using TMS-EEG and source-localization methods.
Results
The SPDPA varied significantly across subjects, and these differences predicted individual differences in several frequency-dependent parameters of the TMS-ER that were specific to task-relevant areas, including prefrontal cortex for STM. Furthermore, a follow-up test-retest study revealed that the SPDPA was stable over sessions.
Conclusions
These observations offer a window into how individual differences in the effects of TMS are related to trait-like individual differences in physiological profile.
INTRODUCTION
Studies using combined transcranial magnetic stimulation (TMS) and electroencephalography (EEG) have begun to provide important insights into how differing behavioral and cognitive contexts map onto patterns of effective connectivity quantified using the scalp-recorded TMS-evoked response (TMS-ER). Effective connectivity refers to the ability of one neuronal group or brain area to causally influence another [2]. The TMS-ER is a direct measure of effective connectivity between the area stimulated and temporally downstream effects in distal brain areas, because the time and location of stimulation are known. Studies using this method have revealed reduced connectivity during non-rapid eye movement sleep [3], coma [4], and anesthesia-induced coma [5] compared to waking states. Our own research has shown that TMS-EEG-based measures of effective connectivity can also distinguish between waking states [1]. In this prior study, single pulses of TMS were delivered to the superior parietal lobule (SPL) during the delay-period of a spatial STM task and during a perceptually-identical period of passive fixation. SPL was chosen as the stimulation site because it has been implicated in both visual STM [6] and working memory [7]. Results revealed increased connectivity between the SPL and distal brain areas during STM versus fixation. Connectivity was quantified using synthetic measures derived from the TMS-ER and included the significant current density (SCD) and significant current scatter (SCS) [8]. These differences in connectivity were particularly strong in task-relevant brain areas including, but not limited to, the prefrontal, parietal, and extrastriate cortices.
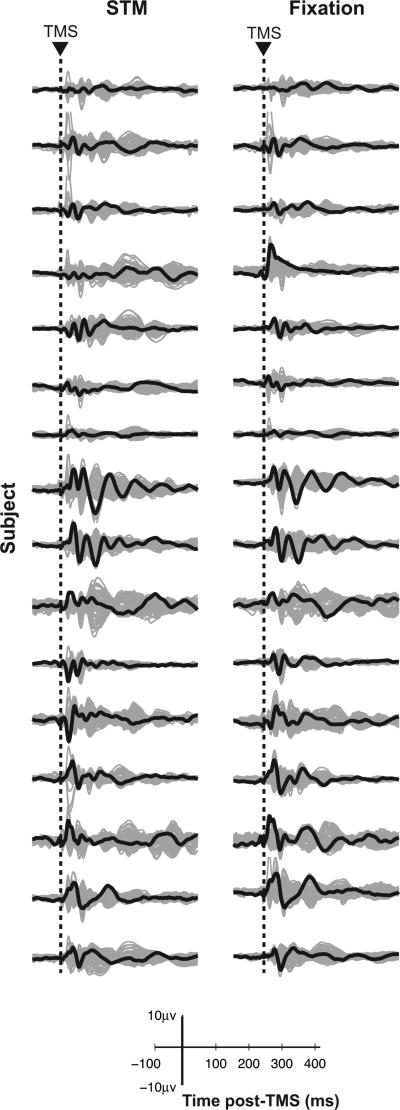
The present report was prompted by a striking observation that we made during analyses of the data for the study just described [1]. As illustrated in Figure 1, the TMS-ER differed significantly between subjects, but appeared to be quite stable within subject but across cognitive state (STM and fixation). Given that all subjects performed well above chance on the behavioral task, and complied during fixation, it seems unlikely that these individual differences in the TMS-ER can be attributable to performance differences. That is, inspection of Figure 1 suggests that the idiosyncratic shape of a subject's TMS-ER was highly similar when comparing the ER from task performance to the ER derived from when the subject was maintaining steady fixation, a condition for which performance measures such as accuracy and reaction time do not apply. This raises the intriguing possibility that the patterns illustrated in Figure 1 reflect individual differences in stable, trait-like features of the underlying physiology of these subjects.
Figure 1. TMS-ER differs between individuals across contexts.
TMS-ERs for all subjects in the STM (left column) and fixation (right column) conditions. Butterfly plots with channel under coil in black, all other channels in gray.
One possibility is that individual differences in the TMS-ER (a snapshot of the state of cortical networks at the time of stimulation) are related to, or perhaps governed by, differences in the underlying, ‘oscillatory state’ of the brain. The state of oscillatory coupling in the brain is gaining support as a candidate factor influencing cognition and perhaps even consciousness [9,10]. Confirmation of this possibility could have important implications for understanding individual differences in many domains of behavior, including cognition and, possibly, neurological and psychiatric deviations from typical behavior. Thus, the focus of the present study was to determine whether underlying trait-like physiological factors may explain the individual differences illustrated in Figure 1. To anticipate the outcome, and because our results were consistent with this idea, we consider possible functional implications in the Discussion.
Alternative explanations for the pattern of individual differences in the TMS-ER illustrated in Figure 1 include the possibility that they may reflect task-unrelated contextual differences specific to the experimental session, such as, for example, what the subject was thinking about while completing the task. Alternately, they could reflect variation in individual brain anatomy. Although source localization methods can map an individual's brain activity into a common coordinate space, intrinsic differences in gray and white matter structure might underlie differences in the TMS-ER.
The oscillatory state of the brain can be estimated through a spectral decomposition of delay- or fixation-related activity (in the absence of TMS). An underlying assumption of group-level analyses of task-related neural activity is that individual variation in spectra simply reflects noise that needs to be averaged out to find the “true” task-related signal. However, a recent series of studies show that individual differences in network properties associated with the delay-period of a visuospatial STM task predict individual differences in behavior [11,12]. Additionally, TMS may be sensitive to individual differences in ongoing oscillations. For instance, an individual's phosphene threshold (the stimulation intensity required to produce phosphenes and an indirect measure of cortical excitability) varies with the individual's dominant, resting alpha-band frequency [13,14]. Finally, as described more fully below, inspection of the delay-period spectrograms from [1] revealed patterns of delay-period activity that varied markedly between individuals, a phenomenon we will refer to as the ‘sustained pattern of delay-period activity’ (SPDPA).
This report presents results of two studies. In Study 1 we interrogated the data from [1] to address whether individual differences in the SPDPA related to differences in spectral properties of the TMS-ER. Upon confirming that they did, Study 2 addressed the test-retest reliability of each subject's SPDPA, thereby ruling out the possibility that individual differences in the SPDPA may have merely reflected a temporary state of the brain, unique to the time of observation and, instead, lending credence to the idea that these individual differences may reflect a more stable, trait-like property.
Study 1
Here, we tested the following two hypotheses: 1) that individual differences in the SPDPA correlated with differences in spectral properties of the TMS-ER, and 2) that these effects would localize to areas unique to the current cognitive context (STM or fixation). Specifically, we predicted that individual differences in SPDPA would relate to TMS-evoked power in prefrontal cortex (PFC) during STM. A body of evidence implicates the PFC as a source of ‘top-down control’, particularly during working memory and STM [15]. Of relevance to the present study, disruption of left PFC using theta burst repetitive TMS caused disruption of working memory performance. Furthermore, individual differences in this change in performance were correlated with change in left PFC functional magnetic resonance imaging (MRI) activity and with compensatory, increased activity in right PFC [16]. Finally, training subjects on a working memory task strengthened effective connectivity between the SPL (the site of single-pulse TMS) and areas of the PFC during performance of a spatial STM task similar to the one used in the present study [17].
Results from Study 1 showed that the SPDPA varied significantly between subjects, and that these differences, particularly in the theta (4-7Hz) frequency band in two prefrontal areas, predicted individual differences in TMS-evoked theta band power in those areas. Importantly, these effects varied with cognitive context, occurring in PFC during STM but not during fixation. These findings suggest there to be a dynamic nature to the cortical networks underlying different cognitive contexts, and how these networks might vary systematically across subjects. To better understand these findings, it was important to establish whether individual differences in the SPDPA remain stable over time.
Study 2
Here, we measured the test-retest reliability of the SPDPA. Results revealed that individual differences in the SPDPA were highly stable over sessions.
METHODS
Study 1
Subjects
16 subjects (8 males, mean age = 21.88 years [SD=2.94]), described in [1], were recruited from the University of Wisconsin-Madison community. The UW-Madison Health Sciences Institutional Review Board approved the study protocols. All subjects gave informed consent and were screened for the presence of neurological and psychiatric conditions and other risk factors related to the application of TMS.
Behavioral procedures
Simultaneous TMS-EEG was administered during two consecutive cognitive conditions. Full methodological details can be found in [1]. Briefly, single pulses of TMS were delivered to the SPL during the delay-period of a test of spatial STM (STM) and during fixation in the absence of a cognitive task (fixation). Conditions were blocked and block order was counterbalanced across subjects.
STM
Each trial began with a 1000-ms fixation period, followed by sequential presentation of four memory targets at different, randomly selected screen locations. This was followed by a 3750-ms delay period, during which, the central fixation cross remained visible. Lastly, there was a probe stimulus. When the probe appeared, subjects made a yes/no button press indicating whether its location matched that of one of the four memory targets (P=.5). The shape of the stimulus was irrelevant. On 50% of trials (randomly interleaved), two TMS pulses were delivered during the delay period: The first pulse was delivered 750±250 ms after delay-period onset, followed by the second pulse 2000±250 ms later. Trials were separated by a 1000-ms intertrial interval. A total of 160 TMS pulses were delivered across 80 experimental trials (STM TMSon), intermixed with an equal number of trials in which the STM task was performed in the absence of TMS (STM TMSoff).
Fixation
In this trial block, TMS was applied at an average rate of 0.5 Hz in groups of four pulses during a period of fixation that was perceptually identical to the delay period of the STM condition (fixation TMSon). Following each group of four pulses, participants were instructed to “rest and blink” for 2000 ms. A total of 160 TMS pulses were delivered during fixation. Data for fixation without TMS was derived from the 2000-ms fixation interval at the start of each trial (fixation TMSoff).
TMS targeting and stimulation
TMS was delivered with a Magstim Standard Rapid magnetic stimulator equipped with a 70-mm figure-of-eight stimulating coil (Magstim, Whitland, UK). TMS was applied to a portion of the left SPL [Brodmann's Area (BA) 7] dorsal and medial to the intraparietal sulcus and posterior to the postcentral sulcus (see, Figure 1 of [1]). The SPL was identified on the basis of individual anatomy from whole-brain T1-weighted anatomical MRIs acquired with a GE MR750 3T MRI scanner for each subject prior to the study (176 axial slices with a resolution of 1 mm isotropic). TMS targeting was achieved using a Navigated Brain Stimulation (NBS) system (Helsinki, Finland) that uses infrared-based frameless stereotaxy and the individual's high-resolution MRI. TMS intensity was 110-140 V/m (for a given subject, intensity and coil position were held constant across the STM and fixation blocks). EEG auditory artifacts were masked by noise played through headphones. During the set-up, the noise volume was adjusted such that the subject could not hear heard the TMS clicks. This noise masking was done throughout the session.
EEG recording
EEG was recorded with a 60-channel TMS-compatible amplifier (Nexstim; Helenski, Finland). Electrode impedance was < 5k . A single electrode placed on the forehead was used as the reference and eye movements were recorded with two additional electrodes placed near the eyes. Data were sampled at 1450 Hz with 16-bit resolution.
Data pre-processing
Data were processed offline using the EEGLab toolbox (version 6.01b) running in Matlab R2007b (Mathworks, Natick, MA, USA). The data were down-sampled to 500 Hz, band-pass filtered between 2-80 Hz, and notch filtered at 60 Hz. Individual electrodes exhibiting excessive noise were interpolated using spherical spline interpolation [18]. Independent components analysis (ICA) was then used to identify and remove components reflecting residual muscle activity, eye movements, blink-related activity, and residual TMS-related artifacts [19]. In general, very few large TMS artifacts were evident in the raw data (mean electrodes exhibiting an artifact = 2.03 [min = 0; max = 5]), and what artifacts were present were effectively removed using ICA with little distortion of the EEG waveform [1]. All data were then average-referenced.
Source modeling
Source modeling procedures are described fully in [8] and [1]. Briefly, individual cortical meshes were created using the Statistical Parametric Mapping version 5 (SPM5) software. This involved warping the binary masks of the skull and scalp obtained from individual MRIs to the corresponding meshes of the Montreal Neurological Institute (MNI) atlas. A 3-spheres BERG method [20] was used to model conductive head volume and calculate the lead field matrix using the Brainstorm software package. The inverse solution was then calculated on a trial-by-trial basis using an empirical Bayesian approach as implemented in SPM5 [21]. Finally, to compute the overall current evoked by TMS in different cortical areas, individual cortical surfaces were attributed to different Brodmann areas (BAs) using an automatic anatomical classification method that maps the individual cortical surface to the region-of-interest (ROI) masks provided by the WFUPickAtlas tool [22]. Statistically significant sources were determined using a bootstrapping procedure as outlined in [8].
Spectral Analysis
TMS-Evoked power
For each ROI and condition, power from 0 to 500 ms after TMS onset was calculated using a Morlet wavelet with 3 cycles for the lowest frequency (4 Hz) increasing linearly to 18 cycles for the highest frequency analyzed (50 Hz). Responses were baseline corrected trial-by-trial by subtracting the mean power from 500 ms preceding TMS onset.
STM and fixation spectrograms
An estimate of an individual subject's SPDPA (i.e. the spectrogram) during STM, for each ROI, was derived from STM TMSoff trials, epoched from 500 to 2500 ms after delay onset. To acquire an estimate of an individual subject's oscillatory profile during fixation, data from the fixation period of each trial (2000 ms) were epoched to make up the data of the fixation TMSoff condition. Spectral profiles for the STM TMSoff and fixation TMSoff conditions were then calculated as above. No baseline correction was done.
All data were averaged across conventional frequency bands: theta (4-7 Hz), alpha (8-14 Hz), beta (15-29 Hz), and gamma (30-50 Hz).
Statistical Analysis
Cluster analyses
Cluster-based permutation tests implemented using the Fieldtrip toolbox [23], were used to assess individual differences in both the TMS-ER and the SPDPA, as measured at the scalp. Clusters were defined as two or more contiguous electrodes in which the F-statistic of voltage (or power, μV2/Hz, in the case of the frequency domain) values within individual 2-ms time bins (and 1 Hz frequency bins, in the case of power) exceeded a threshold of p<0.05. Above-threshold samples were bootstrapped against 500 random sets of permutations (significance value of .05 determined significant clusters). Individual differences in the TMS-ER from 0-500 ms post-TMS were assessed for each cognitive condition (STM, fixation), entering Subject (1-16) as a factor. Individual differences in the SPDPA (baseline corrected to the fixation period) were assessed from 0-3600 ms, entering Subject (1-16) as a factor.
Correlation analyses
Pearson's correlation coefficient was used to compare individual differences in the TMS-evoked power with individual differences in spectral profiles without TMS, using source-space data. Specifically, for each ROI (16 ROIs total; left and right BAs 6, 7, 8, 9, 17, 18, 19, 46), individual differences in mean power in either the theta, alpha, or beta bands for STM TMSoff (i.e. the SPDPA) were correlated with mean power during STM TMSon in the same or a different frequency band. Similarly, individual differences in mean power during fixation TMSoff were correlated with mean power during fixation TMSon across frequency band combinations. The gamma band was not included due to a relative lack of significant individual differences and overall power compared to lower frequency bands as seen in Figure 2. Additionally, spectral profiles observed during STM TMSoff and fixation TMSoff were correlated across frequency band combinations. Bonferroni correction for multiple comparisons was applied.
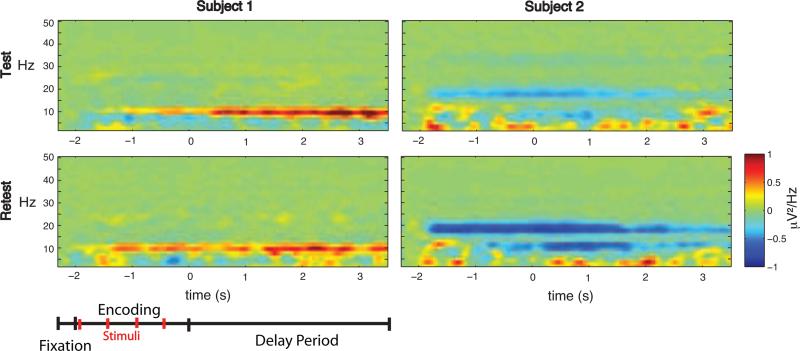
Figure 2. Time-frequency representations of the SPDPA for two representative subjects across testing sessions, channel P1.
The SPDPAs were highly unique to each subject (left and right columns of each panel), but were remarkably similar across test (top) and retest (bottom) sessions for a given subject. Schematic of task timing shown at the bottom. Baseline corrected to fixation period.
Study 2
Subjects
Five subjects from Study 1 plus an additional group of five newly recruited subjects (1 male, mean age = 22.60 [SD=2.79]) participated in this follow-up experiment. Recruitment procedures were identical to Study 1.
Behavioral procedures
Five subjects from Study 1 performed the STM TMSoff trials (from the STM task described above; 80 total trials) at retest. Newly recruited subjects only performed the STM TMSoff trials of the STM task at both test and retest sessions. The lag between sessions was either 1 week (n=5) or 3 months (n=5).
EEG recording, data pre-processing, and source modeling
Procedures were identical to Study1.
Statistical Analysis
Cluster-based permutation tests were used to assess test-retest differences in the SPDPA using scalp-derived data. Clusters were defined as two or more contiguous sensors in which the t- statistic of power values within individual 2-ms time bins and 1 Hz frequency bins exceeded a threshold of p<0.025 (two-sided). Bootstrapping was done as above. Test-retest differences were assessed from 0 (start of the delay) to 3600 ms, with Testing Session (1, 2) as the within-subjects factor.
RESULTS
Study 1
Behavioral data
As reported in [1], mean accuracy (% correct) on the STM task was 84.06 (SD=8.75) and 84.38 (SD=8.37) for the TMSoff versus TMSon conditions, respectively. Mean reaction time (RT) was 735.90 (SD=121) ms and 722.66 (SD=128.09) ms for the TMSoff versus TMSon conditions, respectively. Repeated measures ANOVA showed no significant difference between conditions for either performance measure (ps < 0.05). Of note, in the course of the initial analyses of these data as reported for the publication by Johnson et al. (2012), we found there to be no correlation between synthetic measures of TMS-based effective connectivity (the SCD and SCS) and behavioral performance (unpublished result). The possible behavioral implications of the present findings are considered in the Discussion section.
The TMS-ER differs between individuals
The present study sought to determine the factors contributing to variation in the TMS-ER observed across subjects in [1] (Fig. 1). Results confirmed that the TMS-ER differed significantly across subjects in both cognitive conditions (tested separately, cluster present across all electrodes and time points, all p's < .0001), suggesting that the amplitude and spatial spread of the TMS-ER shows significant individual differences within either cognitive context. A possible clue to understanding these differences in the TMS-ER is suggested by Figure 2 (left and right columns), which depicts the SPDPA for two representative subjects during TMSoff trials. As can be seen, each subject shows a distinctive SPDPA.
The SPDPA differs between individuals
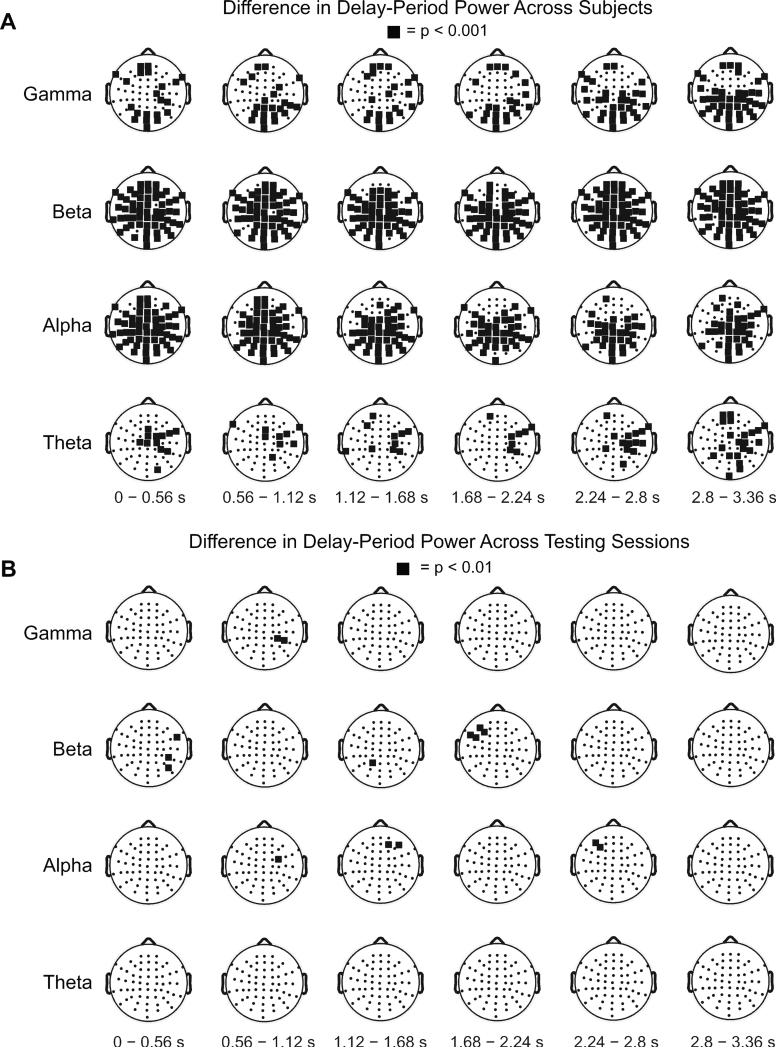
We conducted a cluster-based analysis to quantify the individual differences across the scalp-derived SPDPA. Figure 3A shows clusters exhibiting significant differences in the SPDPA between subjects, segregated into four conventional frequency bands. Significant clusters of electrodes existed throughout the delay-period in each frequency band. The most pronounced differences were in the alpha and beta bands. Given these differences, we went on to assess whether variance in the SPDPA across subjects explained variance in spectral properties of the TMS-ER.
Figure 3. SPDPA varies between individuals but is stable over time, group-level analysis.
Topographical plots showing results of cluster analyses comparing delay-period spectral power between- and within- subjects. In each plot, black squares highlight electrodes exhibiting significant differences in delay-period spectral power. (A) Illustrates differences between the 16 subjects participating in (Study 1); (B) illustrates within-subject differences from five of these original subjects, plus five additional, who performed the STM task on two separate occasions, thereby giving an empirical estimate of test-retest reliability (Study 2).
Variation in TMS-evoked power is correlated with individual differences in SPDPA
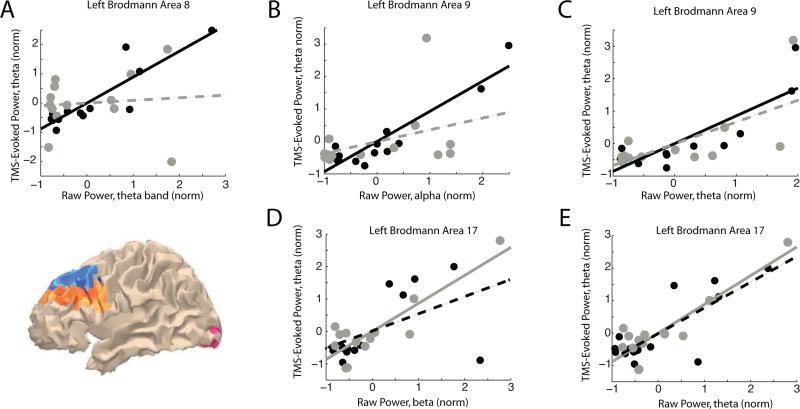
Separate correlation analyses were conducted at the source level to determine whether intersubject variance in power correlated with variation in TMS-evoked power at each ROI, across frequency bands. Results showed that significant correlations were all in the positive direction and were restricted to certain areas (Table 1 and Fig. 4). Beginning with the STM TMSoff condition, individual differences in two frequency bands correlated with the magnitude of the theta-band component of the TMS-ER. In particular, variation in theta-band power during STM TMSoff related to individual variation in TMS-evoked theta-band power in BAs 8 and 9 (Table 1, Fig. 4 A and C). Variation in STM TMSoff alpha-band power also correlated with variation in TMS-evoked theta-band power in BA 9 (Table 1, Fig. 4 B). In BAs 8 and 9, the correlations between theta-band and alpha-band power for STM TMSoff and theta-band power for STM TMSon were significantly different from analogous comparisons made during fixation (Table 1, Fig. 4 A and B), suggesting that the effects in PFC were specific to the cognitive context.
Table 1.
Correlation between oscillatory power and TMS-evoked power
| Area | TMSoffband:TMSonband | STM | Fixation | Comparison | |||
|---|---|---|---|---|---|---|---|
| df, r | p | df, r | p | z | p | ||
| 9L | theta:theta | 15, 0.86* | <0.001 | 13, 0.67 | 0.01 | 2.11 | 0.03 |
| 9L | alpha:theta | 15, 0.93* | <0.001 | 13, 0.36 | 0.22 | 2.99 | 0.003+ |
| 8L | theta:theta | 15, 0.89* | <.001 | 13, 0.09 | 0.77 | 3.11 | 0.002+ |
| 17L | theta:theta | 15, 0.79 | <0.001 | 13, 0.89* | <0.001 | −0.82 | 0.41 |
| 17L | beta:theta | 15, 0.53 | 0.04 | 13, 0.86* | <0.001 | −1.64 | 0.10 |
ps < 1.54−4, corrected
ps<0.01, corrected
Pearson's correlation coefficient and associated p-value for correlation between STM TMSoff versus STM TMSon in columns 3 and 4 and between fixation TMSoff versus fixation TMSon in columns 5 and 6. Columns 7 and 8 show the z-value and associated p-value testing if there is a significant difference between the two correlations.
Figure 4. Correlation between spectral profiles of the TMS-ER and SPDPA during either STM TMSoff or fixation TMSoff.
Solid lines illustrate regression lines for significant correlations (corrected for multiple comparisons), and dashed lines show non-significant linear trends. Note that, with the exception of BA 8, these effects are highly similar across conditions. Black: individual subject data during STM. Gray: individual subject data during fixation. All correlations coefficients and associated p-values found in Table 1. Inset MNI brain shows spatial extent of BAs with significant correlations; BA 9 in orange, BA 8 in blue, and BA 17 in pink.
For fixation, oscillatory power in both the beta and theta bands during fixation TMSoff correlated with the magnitude of TMS-evoked theta-band power during fixation, emanating from visual cortex (left BA 17; Table 1, Fig. 4 D and E). However, neither of these effects statistically differed from the analogous effects observed during STM.
Because the TMS-ER for STM and fixation shared similar features (Fig. 1), we wondered to what degree the oscillatory profiles of the two cognitive contexts were similar across brain areas. Multiple factors might contribute to such a correlation including not only subject-specific brain anatomy, but also commonality in the underlying functional neural circuitry that might be used for both STM and maintaining fixation. To address this, we computed correlations between the spectral profiles observed during STM TMSoff and fixation TMSoff. Note that these data were source-localized to alleviate some of the exogenous factors that might contribute to such a correlation such as scalp-to-cortex distance, etc. This analysis showed high levels of correlation across brain areas that were highest between the same bands (e.g. theta-band power during STM TMSoff correlated with theta-band power during fixation TMSoff; Fig. 5) or adjacent spectral bands (e.g. theta-band power during STM TMSoff correlated with alpha-band power during fixation TMSoff). This raises the possibility that there is a high degree of similarity between the actual networks engaged during these two cognitive contexts.
Figure 5. Correlation between raw power during STM and fixation.
Correlations were greater for adjacent bands, and highly similar between cognitive contexts. Only significant correlations colored (p's corrected for multiple comparisons), black squares show no significant correlation.
Study 2
Individual SPDPAs are reliable across testing sessions
The results of Study 1 showed that individual differences in the power of the TMS-ER correlated with individual differences in the SPDPA, which could reflect unique features of an individual's underlying physiology. Alternatively, these differences might reflect incidental factors, such as the arrangement of the electrode cap on the subject's head, or the subject's emotional state on the day of testing. To rule these out, we assessed the stability of an individual's unique SPDPA over time.
As a first step, we conducted separate one-way ANOVAs comparing RT and accuracy measures of performance across testing sessions. All tests were non-significant (all ps > 0.30). The lack of behavioral differences allowed for direct comparison of neural measures. Individual differences in the SPDPA between the two sessions were assessed using cluster-based permutation tests (Fig. 3B). Although significant clusters were found during some time epochs, no significant differences were sustained across the duration of the delay-period, suggesting that individual differences in delay-period activity are stable over time, and may thus reflect a trait-like property (see examples of test-retest reliability in two example subjects in Figure 2).
DISCUSSION
The prevalence of group-level analyses in many areas of cognitive neuroscience demonstrates an implicit assumption that individual-level variation in physiological signals amount to noise that needs to be “averaged out” to find the “true”, task-related signal. In the present study we analyzed a dataset in which we had observed marked individual variability in the SPDPA to determine what influence, if any, such differences had on the TMS-ER. Contrary to the assumption outlined above, we hypothesized that individual differences in the TMS-ER would be systematically related to properties of the underlying context-specific oscillatory brain activity. Furthermore, we reasoned that if such a relationship exists, it would most likely be observed in brain networks that are specific to the current context.
Results showed that the SPDPA varied significantly across subjects and that differences in delay period theta- and alpha-band power correlated with individual differences in delay-period TMS-evoked power in the theta band. These effects were seen in frontal regions (BAs 8 and 9) known to have strong anatomical connections with SPL, and to display elevated effective connectivity with SPL during STM-task performance [1,17]. The latter may explain why the individual differences effects reported here were stronger in frontal cortex during the STM than the fixation condition. During fixation, power in the theta and beta bands predicted TMS-evoked power in the theta band in primary visual cortex (BA 17), though this relationship was not significantly different from the analogous relationship seen during STM. Since the SPDPA is effectively “in place” when TMS is delivered, it necessarily precedes TMS in time, and allows us to speculated that individual differences in the SPDPA might have a deterministic effect on the TMS-ER.
Of note, no such correlations were observed at the site of stimulation, the SPL, for either behavioral condition. This surprised us considering that Johnson et al. (2012) show that the TMS-ER in SPL is larger in amplitude during STM versus fixation [1]. Based on this, we expected there to be a correlation between underlying brain activity and TMS-evoked power in SPL for STM. However, this was not the case. In fact, the SPDPA as measured at parietal electrodes was highly similar across behavioral conditions (Fig 5), what was different between conditions is that the effective connectivity of SPL was stronger with frontal areas during STM, whereas its was stronger with primary visual cortex during fixation.
As mentioned, oscillatory profiles during STM and fixation observed in the present study were strongly correlated, suggesting a high degree of similarity between the networks engaged during the two cognitive contexts. The degree to which these correlations reflect neutrally relevant activity and the degree to which they reflect nonspecific effects of factors such as volume conduction cannot be parsed with the current analysis.
An additional possible confound could be the tapping sensation produced by discharge of the TMS coil, which was present during both STM and fixation. We think this is unlikely to explain our results, however, because the majority of individual differences findings from the present study were in the left hemisphere, whereas peripheral stimulation of the scalp on the left side of the midline (where TMS was delivered) would be expected to be manifest as neural activity in the right hemisphere.
Why might these effects concentrate in lower frequency bands of the EEG? One possibility is that they reflect a specific role for low-frequency oscillations in promoting coordination between cortical areas during task performance (as suggested in, e.g. [24], see [25] for a review). In keeping with this possibility, prominent theta-band oscillations in frontal, control-related cortical areas have been proposed to synchronize activity in posterior brain areas [26], the putative site of the mnemonic representations underlying STM [27]. Additionally, theta-band power increases as memory load increases [28], and a measure of theta:gamma coupling plateaus in posterior areas at supra-capacity memory loads [29]. Also, fronto-parietal alpha-band phase synchronization predicts individual differences in STM capacity [12].
A further question addressed here is whether one's distinct SPDPA represents a temporary state of the brain, unique to the date and time of observation, or whether it represents a stable trait-like physiological property of the observer. Study 2 revealed that individual SPDPAs were stable over repeated testing sessions separated by anywhere from one week to several months. Thus, an individual's SPDPA may indeed be trait-like. In keeping with the present findings, previous research has shown high test-retest reliability of EEG spectra, both during the delay-period of a modified Sternberg STM task, and with eyes closed in the absence of an explicit task [30,31]. These analyses, however, focused on the average spectral power collapsed across the delay-period. The analysis reported here extends these observations to the reliability of the full time-frequency spectrogram throughout the delay- and fixation- intervals. Additionally, the TMS-ER itself has shown test-retest reliability [32]. Thus an individual's unique SPDPA may be akin to a fingerprint of an individual's brain activity. Such differences could be determined, in part, by molecular and genetic factors [33,34], which can be mapped by neurogenesis [35] and changes in effective connectivity arising as a result of experience [17].
An important future direction for this work is to explore the behavioral implications of these results. Defining the direct behavioral relationship between individual differences in the TMS-ER and the SPDPA was not the purpose of the current study. The central problem in addressing this larger question is that it is unclear which components of the TMS-ER should be expected to predict or relate to performance. Formal testing of this question using these data would require a multivariate regression of all measures of the TMS-ER across frequency bands onto a single measure of behavioral performance. In view of the relatively small sample size in the present study, and the narrow range of behavioral variability (which was by design for this study), it is unlikely that meaningful correlations would come from such an analysis. Results from the present study, however, do offer a concrete range of frequencies and ROIs of interest that can now be used to test the relationship between STM performance and TMS-based effective connectivity. Of relevance, in a recently published study we explored the question of whether training on a working memory task changes measures of task- related effective connectivity [17]. We found that working memory training increased effective connectivity between frontal and parietal brain areas, but found no consistent relationship between individual differences in memory capacity and singular metrics of TMS-based effective connectivity (unpublished observation). Using those same measures in the current dataset, we also found no significant correlation between measures of TMS-based effective connectivity and neither accuracy nor RT. Most likely the relationship between behavior and such TMS-based measures is multivariate in nature and requires a tailored experimental approach.
Although at present it is little more than speculation, we are intrigued by the possibility that one factor underlying our results may be the phenomenon of long-range temporal correlations (LRTCs), or temporal dependencies between different parts of a neuronal signal that decays according to a power law. They may also relate to the phenomenon of neural avalanches, in which one ‘unit’ of a system reaches a threshold and “turns”, initiating a cascade of events that propagate throughout a system, the nature of which can also be described by power laws. Both of these phenomena have been shown to be related to performance on cognitive tasks [36,37]. Additionally, LRTCs are highly stable over time [38] and appear to be genetically determined to some extent [39]. Furthermore, LRTCs and avalanches seen during task and rest states are closely related [36]. This raises the possibility that the TMS-ER may reflect a similar ‘neuronal avalanche’, albeit one generated by an injection of current directly into a cortical area, rather than through stimulus input. The relationship between individual differences in TMS-evoked power and endogenously generated neural oscillations observed during different cognitive contexts in the present study suggests that this might be the case. Assessing this possibility, however, will require further research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors declare no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Johnson JS, Kundu B, Casali AG, Postle BR. Task-dependent changes in cortical excitability and effective connectivity: A combined TMS-EEG study. J Neurophysiol. 2012;107:2383–92. doi: 10.1152/jn.00707.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal- component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 3.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 4.Rosanova M, Gosseries O, Casarotto S, Boly M, Casali AG, Bruno M-AA, et al. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain. 2012;135:1308–20. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–6. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamidi M, Tononi G, Postle BR. Evaluating the role of prefrontal and parietal cortices in memory-guided response with repetitive transcranial magnetic stimulation. Neuropsychologia. 2009;47:295–302. doi: 10.1016/j.neuropsychologia.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29:14980–6. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casali AG, Casarotto S, Rosanova M, Mariotti M, Massimini M. General indices to characterize the electrical response of the cerebral cortex to TMS. Neuroimage. 2010;49:1459–68. doi: 10.1016/j.neuroimage.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Buzsaki G. Rhythms of the Brain. Oxford University Press; New York: 2006. [Google Scholar]
- 10.Palva S, Palva JM. Discovering oscillatory interaction networks with M/EEG: challenges and breakthroughs. Trends Cogn Sci. 2012;16:219–30. doi: 10.1016/j.tics.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Palva S, Kulashekhar S, Hamalainen M, Palva JM. Localization of cortical phase and amplitude dynamics during visual working memory encoding and retention. J Neurosci. 2011;31:5013–25. doi: 10.1523/JNEUROSCI.5592-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci U S A. 2010;107:7580–5. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–8. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romei V, Rihs T, Brodbeck V, Thut G. Resting electroencephalogram alpha-power over posterior sites indexes baseline visual cortex excitability. Neuroreport. 2008;19:203–8. doi: 10.1097/WNR.0b013e3282f454c4. [DOI] [PubMed] [Google Scholar]
- 15.Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16:129–35. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TG, D'Esposito M. The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI-TMS study. J Neurosci. 2012;32:15458–66. doi: 10.1523/JNEUROSCI.0627-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kundu B, Sutterer DW, Emrich SM, Postle BR. Strengthened effective connectivity underlies transfer of working memory training to tests of short-term memory and attention. J Neurosci. 2013;33:8705–15. doi: 10.1523/JNEUROSCI.5565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrin F, Pernier J, Bertrand O, Giard MH, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr Clin Neurophysiol. 1987;66:75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- 19.Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiol. 2000;37:163–78. [PubMed] [Google Scholar]
- 20.Berg P, Scherg M. A fast method for forward computation of multiple-shell spherical head models. Electroencephalogr Clin Neurophysiol. 1994;90:58–64. doi: 10.1016/0013-4694(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 21.Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–83. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- 22.Maldjian J a., Laurienti PJ, Kraft R a., Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 23.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Meth. 2007;164:177–90. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev. 2010;34:1015–22. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Von Stein a, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–13. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 26.Sauseng P, Klimesch W, Doppelmayr M, Hanslmayr S, Schabus M, Gruber WR. Theta coupling in the human electroencephalogram during a working memory task. Neurosci Lett. 2004;354:123–6. doi: 10.1016/j.neulet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Riggall AC, Postle BR. The Relationship between Working Memory Storage and Elevated Activity as Measured with Functional Magnetic Resonance Imaging. J Neurosci. 2012;32:12990–8. doi: 10.1523/JNEUROSCI.1892-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–9. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- 29.Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim A a, et al. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19:1846–52. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 30.Näpflin M, Wildi M, Sarnthein J. Test-retest reliability of resting EEG spectra validates a statistical signature of persons. Clin Neurophysiol. 2007;118:2519–24. doi: 10.1016/j.clinph.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Näpflin M, Wildi M, Sarnthein J. Test-retest reliability of EEG spectra during a working memory task. Neuroimage. 2008;43:687–93. doi: 10.1016/j.neuroimage.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Casarotto S, Romero Lauro LJ, Bellina V, Casali AG, Rosanova M, Pigorini A, et al. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One. 2010;5:e10281. doi: 10.1371/journal.pone.0010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Störmer VS, Passow S, Biesenack J, Li S-C. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: insights from molecular genetic research and implications for adult cognitive development. Dev Psychol. 2012;48:875–89. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]
- 34.Koten JW, Wood G, Hagoort P, Goebel R, Propping P, Willmes K, et al. Genetic contribution to variation in cognitive function: an FMRI study in twins. Science. 2009;323:1737–40. doi: 10.1126/science.1167371. [DOI] [PubMed] [Google Scholar]
- 35.Freund J, Brandmaier a. M, Lewejohann L, Kirste I, Kritzler M, Kruger a., et al. Emergence of Individuality in Genetically Identical Mice. Science. 2013;340:756–9. doi: 10.1126/science.1235294. [DOI] [PubMed] [Google Scholar]
- 36.Palva JM, Zhigalov A, Hirvonen J, Korhonen O, Linkenkaer-Hansen K, Palva S. Neuronal long-range temporal correlations and avalanche dynamics are correlated with behavioral scaling laws. Proc Natl Acad Sci U S A. 2013;110:3585–90. doi: 10.1073/pnas.1216855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monto S, Palva S, Voipio J, Palva JM. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci. 2008;28:8268–72. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikulin V V, Brismar T. Long-range temporal correlations in alpha and beta oscillations: effect of arousal level and test-retest reliability. Clin Neurophysiol. 2004;115:1896–908. doi: 10.1016/j.clinph.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Linkenkaer-Hansen K, Smit DJ a, Barkil A, van Beijsterveldt TEM, Brussaard AB, Boomsma DI, et al. Genetic contributions to long-range temporal correlations in ongoing oscillations. J Neurosci. 2007;27:13882–9. doi: 10.1523/JNEUROSCI.3083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]