Abstract
Purpose
Glioblastoma multiforme (GBM) is the most common primary brain cancer in adults. Chemotherapy with temozolomide (TMZ) significantly prolongs the survival of GBM patients. However, the 3-year survival is still ~5%. Herein we combined intratumoral administration of an adenoviral vector expressing Flt3L (Ad-Flt3L) with systemic TMZ in order to assess its impact on therapeutic efficacy.
Experimental Design
Wild type or immunodeficient mice bearing intracranial GBM or metastatic melanoma were treated with an intratumoral injection of Ad-Flt3L alone or in combination with the conditionally cytotoxic enzyme thymidine kinase (Ad-TK), followed by systemic administration of ganciclovir and TMZ. We monitored survival and measured the tumor-infiltrating immune cells.
Results
While treatment with TMZ alone led to a small improvement in median survival, when used in combination with gene therapy-mediated immunotherapy it significantly increased the survival of tumor-bearing mice. The anti-tumor effect was further enhanced by concomitant intratumoral administration of Ad-TK, leading to 50–70% long-term survival in all tumor models. Although TMZ reduced the content of T cells in the tumor, this did not affect the therapeutic efficacy. The anti-tumor effect of Ad-Flt3L+Ad-TK+TMZ required an intact immune system, since the treatment failed when administered to KO mice that lacked lymphocytes or dendritic cells.
Conclusions
Our results challenge the notion that chemotherapy leads to a state of immune-suppression which impairs the ability of the immune system to mount an effective anti-tumor response. Our work indicates that TMZ does not inhibit antitumor immunity and supports its clinical implementation in combination with immune-mediated therapies.
Keywords: Immunotherapy, glioma, brain tumors, gene therapy, adenoviral vectors
INTRODUCTION
Glioblastoma multiforme WHO grade IV (GBM) is the most aggressive and frequent primary brain tumor (1), with an incidence of 4–5 per 100,000 inhabitants per year in industrialized countries. The current standard treatment for GBM is surgical resection followed by radiotherapy and chemotherapy with temozolomide (TMZ) (2). TMZ is an alkylatying agent, which upon spontaneous conversion to its active metabolite 3-methyl-(triazen-1-yl) imidazole-4-carboxamide (MITC), methylates DNA leading to the death of proliferating cells. TMZ has a higher efficacy and better toxicology profile in GBM patients than other chemotherapeutic agents. TMZ exhibits adequate penetration of the blood brain barrier (BBB), which could not be achieved by previous generation chemotherapeutics without dose-limiting systemic side effects (3). Since GBM cells may express MGMT, a DNA repairing enzyme that repairs TMZ-induced DNA damage, GBM can be resistant to TMZ-induced apoptosis. However, in 25–50% of GBM patients MGMT promoter is spontaneously methylated and thus, TMZ-induced DNA damage cannot be repaired. It has been shown that, in GBM patients with methylated MGMT promoter, TMZ has significantly higher therapeutic benefit than in those patients with active MGMT (4). Nevertheless, three years post-diagnosis less than 5% of patients remain alive; this compares unfavorably with other cancers such as breast, lung and prostate, and therefore new treatments are urgently needed.
Our laboratory has previously shown the antitumor effect of combined adenovirus (Ad) mediated gene therapy in several rat and mouse brain tumor models. Combination of Ad vectors that encode the conditionally cytotoxic herpes simplex virus type 1–thymidine kinase (Ad-TK) and the immune stimulatory fms-like tyrosine kinase-3 ligand (Ad-Flt3L) induces an antitumor immune response that leads to tumor regression and long term survival in mice and rats bearing intracranial GBM (5–7). This therapeutic approach also shows a very safe neurotoxic profile when compared to other proapoptotic agents or immune stimulant molecules (5,8).
Our results in preclinical models have shown that the antitumor effect of Ad-TK+Ad-Flt3L is dependent on the immune system (6). Considering that chemotherapeutic agents may have a strong immunosuppressive effect, it is important to investigate the impact of concomitant chemotherapy with TMZ on the efficacy of Ad-TK+Ad-Flt3L treatment. We evaluated the survival of mice bearing intracranial GL26 (9) and GL261 (10) GBM, as well as intracranial B16 melanoma tumors after treatment with intratumoral delivery of Ad-TK+Ad-Flt3L (+GCV) followed by systemic administration of TMZ. We also assessed the effect of these treatments on the expansion and infiltration of immune cell populations into the tumor microenvironment and the spleen. The efficacy of the combined therapies was also evaluated in KO mice that lack specific immune cell populations. Our results indicate that concomitant treatment with TMZ does not impair the antitumor effect of Ad-TK+Ad-Flt3L and supports the clinical implementation of immunotherapeutic approaches in patients with GBM receiving chemotherapy with TMZ.
MATERIALS AND METHODS
Adenoviral vectors
Ad vectors used are based on adenovirus type 5 (Ad5), in which the left end of E1 and a portion of the E3 regions are deleted (E1−/E3−), and a cassette containing a recombinant exogenous gene and promoter is inserted in place of the E1 deletion. Two vectors were used: Ad-TK [expresses HSV1-thymidine kinase under the control of the hCMV promoter (11)] and Ad-Flt3L [expresses human soluble fms-like tyrosine kinase ligand under the control of the hCMV promoter (5,11). Ads were grown and purified as previously described (12).
Animals and cell lines
Wild-type C57BL/6, CD4−/−, CD8−/−, Rag1−/−, Igh-6−/− and IFNγ−/− mice, all on a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). CD11c knockout mice were bred in-house as previously described (13,14). GL26 and GL261 cells were obtained from the National Cancer Institute repository (http://www.dtp.nci.nih.gov/branches/btb/tumor-catalog.pdf) in 2005 and 2007, respectively, while B16-F10 cells were obtained from ATCC (Manssas, VA) in 2007. Cells were grown in DMEM (CellGro), supplemented with 10% FCS, 1% L-glutamine, 1% Pen-Strep, 1% nonessential amino acids, and passaged routinely. The tumorigenicity of the three cell lines was authenticated by histologic characterization of the intracranial tumors grown in syngeneic C57BL/6 mice. The day of surgery, cells were trypsinized, resuspended in DMEM without supplements, and kept on ice for up to 4 h.
Brain tumor models
Intracranial tumor models were generated as previously described (6,9). For a brief description of the tumor implantation procedure see Supp Materials and Methods. Fourteen days later saline or gene therapy vectors were administered into the tumor using the following doses: Ad-TK: 0.35×108 pfu/site and Ad-Flt3L: 0.65×108pfu/site. Twenty four h later groups of animals were treated intraperitoneally (i.p.) with TMZ [100 mg/kg of body weight/day, (Selleck chemicals, Houston, TX)] for 2 weeks and/or Ganciclovir (GCV) (25 mg/kg/day) i.p. for 7 days.
Animals were allowed to recover and their health status was closely monitored. Mice were euthanized at specific time points or when their health status reached criteria established by the guidelines of the Institutional Animal Care and Use Committee at The University of Michigan School of Medicine. Groups of mice were euthanized at specific time points and blood, spleen and tumors were collected immediately and processed for flow cytometric analysis or ELISA.
Isolation and characterization of immune cells in blood, spleen, bone marrow and tumor
Immune cells were purified from tumors and lymphoid organs and analyzed by flow cytometry as decribed before (6,15). For a brief description of this procedure see Supp Materials and Methods.
HMGB1 ELISA
HMGB1 release was determined in mouse serum using a specific anti-HMGB1 ELISA (IBL-Transatlantic LLC) following the manufacturer's protocol. For a brief description of this technique see Supp Materials and Methods.
Statistical analysis
Kaplan-Meier survival curves were analyzed using the Mantel log-rank test (GraphPad Prism version 3.00, GraphPad Software). Levels of HMGB1 and immune cell numbers were analyzed by one-way ANOVA followed by Tukey's test (NCSS) or by Student's t test. Pearson's test was used to determine correlation coefficient (r) between HMGB1 release and survival rates (GraphPad Prism). p values of <0.05 were used to determine the null hypothesis to be invalid.
RESULTS
TMZ improves the survival of brain tumor-bearing mice treated with Ad-Flt3L
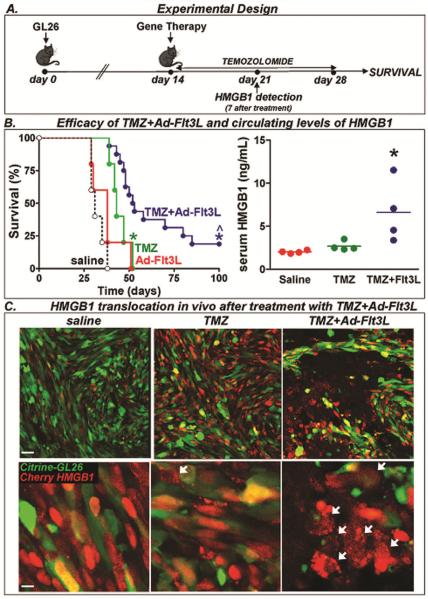
We first assessed the efficacy of TMZ alone or in combination with Ad-Flt3L in immune-competent mouse models of brain cancer. We implanted GL26 GBM cells in the brain of C57BL/6 mice and 14 days later they received an intratumoral injection of saline or Ad-Flt3L. A group of mice received i.p injections of TMZ (100 mg/kg/day) once a day for 14 days (Fig. 1A). We found that while Ad-Flt3L alone did not modify the median survival of tumor-bearing mice (saline: 31d; Ad-Flt3L: 43d), TMZ led to only a small improvement in the median survival (TMZ: 52.5d, p<0.05 vs saline, Log-rank test) (Fig. 1B). However, combination of intratumoral Ad-Flt3L and systemic TMZ induced tumor regression and long term survival in ~20% of the mice (3/16 mice, p<0.05 vs saline, vs Ad-Flt3L alone and vs TMZ alone, Log-rank test).
Fig. 1. Efficacy of TMZ in combination with Ad-Flt3L in GL26 tumor model.
A, C57BL/6 mice were implanted in the striatum with GL26 glioma cells. 14 days later, mice (n= 5–16/group) were treated by intratumoral injections of Ad-Flt3L, or saline followed by daily administration of TMZ. B, Kaplan-Meier survival curves. * p < 0.05 versus saline, ^p<0.05 vs TMZ alone (Mantel log-rank test). This experiment has been performed three times. In a set of mice the serum levels of the proinflammatory nuclear protein HMGB1 were assessed by ELISA 7 days after the treatment. * p < 0.05 versus saline (one-way ANOVA followed by Tukey's test). This experiment has been performed twice. C, C57BL/6 mice were implanted in the striatum with citrine-GL26-Cherry-HMGB1, which were stably transfected to express the green fluorescent protein citrine and HMGB1 fused to red fluorescent protein cherry. 14 days later, they were treated with saline, Ad-Flt3L, or Ad-Flt3L+TMZ. Five days after treatment, the cellular location of cherry-HMGB1 in these cells was assessed by Confocal microscopy. Arrows indicate tumor cells (green) with cytoplasmic HMGB1 (red).
Considering that soluble HMGB1 seems to be required for the activation of tumor infiltrating APCs recruited by Ad-Flt3L, we evaluated whether this treatment affects the metabolism of nuclear HMBG1. We found that treatment with TMZ alone did not affect circulating levels of HMGB1 (Fig. 1B). However, combined treatment with intratumoral Ad-Flt3L and systemic TMZ increased circulating levels of HMGB1 ~3-fold 7 days after the treatment (Fig. 1B, p<0.05 vs saline and vs TMZ, One-way ANOVA). Using GL26 cells that express a cytoplasmic green fluorescent protein (citrine) and in which HMGB1 is fused to the red fluorescent protein cherry (citrine-GL26-Cherry-HMGB1 cells), we evaluated the cytoplasmic translocation of HMGB1 in response to TMZ alone or in combination with Ad-Flt3L (Fig. 1C). Supp. Fig S1 shows citrine-GL26-Cherry-HMGB1 cells treated in vitro with TMZ, Ad-TK+GCV or both. In healthy cells, citrine fluorescence is very intense and yields a green signal while HMGB1 is visualized in red, as it is fused to Cherry, and is confined to the nucleus, colocalizing with DAPI. When these cells undergo apoptotic cell death, citrine seems to undergo rapid degradation while HMGB1 is translocated to the cytoplasm and then released from the cell. In Supp Fig S1 the upper panels show citrine (green) and HMGB1 (red). When citrine is degrading the fluorescence intensity is lower, and when it colocalizes with HMGB1, it yields a yellow signal. Lower panels in Supp Fig S1 show HMGB1 (red) and DAPI. While TMZ treatment induced HMGB1 translocation in a few cells, most of the cells treated with Ad-TK+GCV in the presence or absence of TMZ exhibited cytoplasmic HMGB1. Similar results were obtained in vivo (Fig. 1C). Mice bearing intracranial citrine-GL26-Cherry-HMGB1 tumors were treated with systemic TMZ and local Ad-Flt3L. Confocal images show loss of citrine staining and cytoplasmic HMGB1 localization in the tumors treated with Ad-Flt3L and systemic TMZ (Fig. 1C). Again tumor-bearing mice treated with TMZ, exhibited only a few cells with cytoplasmic HMGB1. These findings correlated with the circulating levels of HMGB1 measured in these mice. While TMZ treatment increased slightly serum HMGB1 levels, combination with Ad-Flt3L treatment substantially raised the circulating levels of HMGB1 (Fig. 1B).
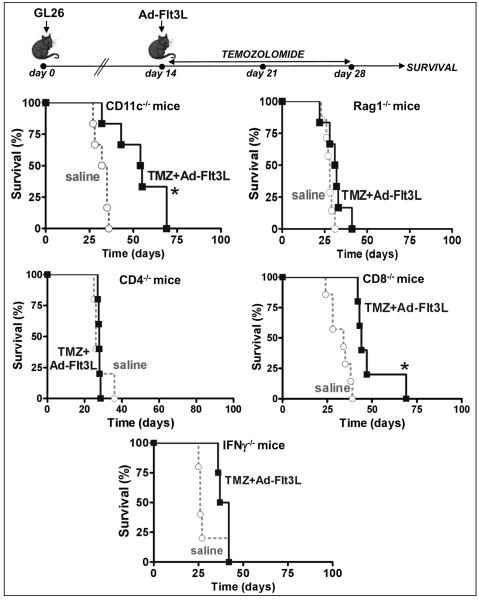
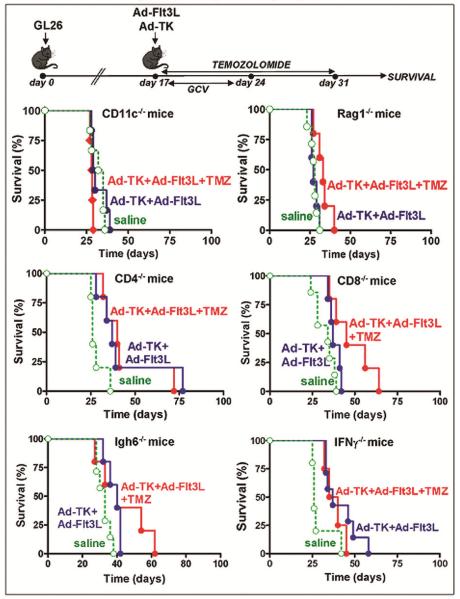
To evaluate whether the efficacy of this treatment was dependent on the immune system, we implanted GL26 tumors in the brain of KO mice that lack specific immune cell populations and treated them with an intratumoral injection of Ad-Flt3L and systemic TMZ for 14 days (Fig. 2). The treatment with Ad-Flt3L and TMZ did not lead to long term survival in mice that lacked T lymphocytes (Rag1 KO, CD4 KO and CD8 KO) or that were unable to produce IFN-γ (IFN-γ KO) (Fig. 2). In mice that lack dendritic cells [DCs, CD11c KO] the combined treatment improved the median survival of GL26 tumor-bearing mice (saline: 33.5d, TMZ+Ad-Flt3L: 54.5d, p<0.05, Log-rank test). However, while 3/17 GL26 tumor-bearing wild type mice treated with Ad-Flt3L+TMZ survived for over 100 days, all the mice lacking CD11c cells succumbed to tumor burden (Fig. 2). These observations are in agreement with our previous reports that indicate that TLR2 signaling in DCs is required for the efficacy of our immunotherapy (6).
Fig. 2. Efficacy of TMZ and Ad-Flt3L in immunosuppressed mice bearing GL26 tumors.
GL26 glioma cells were implanted in the brain of immune-deficient mice lacking dendritic cells (CD11c−/−), T and B cells (Rag1−/−), CD4 or CD8 T cells (CD4−/−, CD8−/−), IFN-gamma producing cells (IFNγ−/−) or B cells (Igh6−/−). 14 days after tumor implantation, mice were treated by intratumoral injection of Ad-Flt3L followed by daily administration of TMZ. *p<0.05 vs saline (Mantel log-rank test). This experiment has been performed twice.
In order to evaluate the efficacy of this treatment in alternative tumor models we used mice bearing intracranial GL261 GBM. Although GL26 and GL261 have a similar origin, they are two different cell lines (16). Both tumors were induced in C57BL/6 mice by methylcholanthrene injection, GL261 tumor was developed by Seligman and Shear (17), and GL26 tumor, by Sugiura (18). Both tumors were propagated by serial s.c. transplantation in C57BL/6 mice (16,19) until in vitro growing cell cultures were established (16). The average of the median survival times of animals with GL26 and GL261 tumors implanted in the brain is very similar (~28–35 days). GL26 and GL261 tumors are histopathologically similar, but GL26 tumors are more hemorrhagic and necrotic than GL261 (16,20). These two tumor cell lines have been used side by side in preclinical neuro-oncology research (6,21,22). Alternatively, we used mice implanted with B16 melanoma cells in the brain as a model of brain metastasis. We treated GL261 and B16 tumor-bearing mice with TMZ alone or in combination with Ad-Flt3L (Supp Fig. S2A). We found that TMZ alone increased 40–50% the median survival of tumor-bearing mice (GL261: saline: 31.5d, TMZ: 43.5; B16: saline: 27, TMZ: 41; p<0.05, Log-rank test), but did not lead to long-term survival (Supp Fig. S2B). However, administration of TMZ in combination with Ad-Flt3L injection led to 30% long term survival in GL261 tumor-bearing mice (3/10 mice, median survival: 58d; p<0.05, Log-rank test).
Although this treatment did not lead to long term survival in B16 tumor-bearing mice, it significantly improved the median survival (46d, p<0.05, Log-rank test) when compared to TMZ treatment alone (Supp Fig. S2B). In a set of mice euthanized 7 days after the treatment, combined treatment but not TMZ alone increased the circulating levels of HMGB1 in both tumor models (Supp Fig. S2C, p<0.05 vs saline and TMZ alone, One-way ANOVA).
TMZ does not reduce the efficacy of combined conditional cytotoxic/immune stimulatory gene therapy in brain tumor-bearing mice
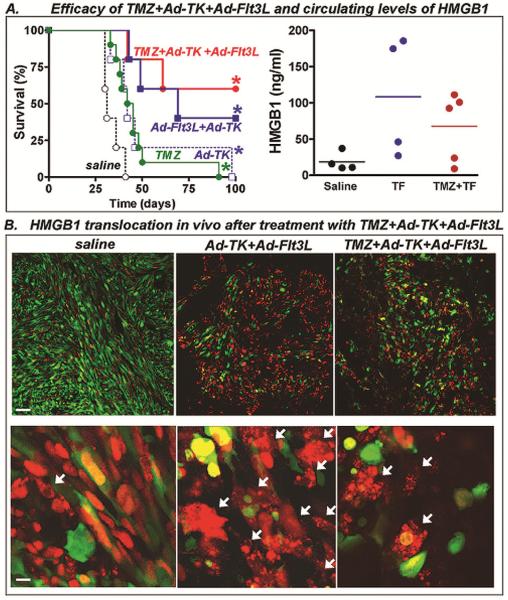
We next evaluated whether addition of systemic TMZ affects the efficacy of immunotherapy with Ad-TK+Ad-Flt3L. Tallying with our previous results (6,15), treatment with Ad-TK in combination with Ad-Flt3L induced long term survival in ~40% of GL26 (Fig. 3A, p<0.05 vs saline, Log-rank test). The efficacy of this treatment was not inhibited by the concomitant administration of TMZ.
Fig. 3. Efficacy of TMZ in combination with Ad-TK and Ad-Flt3L in GL26 tumor model.
A, Wild type C57BL/6 mice were implanted in the striatum with GL26 glioma cells. 14 days after tumor implantation, mice were treated by intratumoral injection of Ad-TK alone or in combination with Ad-Flt3L followed by daily administration of GCV and TMZ. *p<0.05 vs saline (Mantel log-rank test). This experiment has been performed three times. In a set of mice (4–5/group) serum levels of the proinflammatory nuclear protein HMGB1 were assessed by ELISA 7 days post-treatment. This experiment has been performed twice. B, C57BL/6 mice were implanted in the striatum with citrine-GL26-Cherry-HMGB1, which were stably transfected to express the green fluorescent protein citrine and HMGB1 fused to red fluorescent protein cherry. 14 days later, they were treated with saline, Ad-TK+Ad-Flt3L, or Ad-TK+Ad-Flt3L+TMZ. Five days after treatment, the cellular location of cherry-HMGB1 in these cells was assessed by Confocal microscopy. Arrows indicate tumor cells (green) with cytoplasmic HMGB1 (red).
When we evaluated soluble HMGB1 in serum, we found that TMZ chemotherapy did not affect the circulting levels of HMGB1 induced by Ad-TK+Ad-Flt3L treatment (Fig. 3A). We found similar results when we evaluated the cytoplasmic translocation of HMGB1 in citrine-GL26-cherry-HMGB1 tumors (Fig. 3B). Ad-TK+Ad-Flt3L-treated tumors exhibted loss of tumor structure with massive cell death and cytoplasmic HMGB1 translocation (Fig. 3B). A similar pattern was observed when mice were administered systemic TMZ in addition to Ad-TK+Ad-Flt3L treatment (Fig. 3B)
In order to assess the translational implications of our findings, we assessed the effect of TMZ treatment on the efficacy of Ad-TK+Ad-Flt3L in two additional brain tumor models. Systemic administration of TMZ did not inhibit the antitumor immunity elicited by Ad-TK+Ad-Flt3L in mice bearing intracranial GL261 or B16 tumors (Supp Fig. S3A). We found that Ad-TK+Ad-Flt3L led to long-term survival of ~40% and ~25% of Gl261 and B16 tumor-bearing mice, respectively (Supp Fig. S3A), in accordance to our previous reports (6,15). Treatment with Ad-TK+Ad-Flt3L significantly increased the circulating levels of soluble HMGB1 in both tumor models, and this effect was not affected by concomitant administration of TMZ (Supp Fig. S3B). Considering that our findings indicate that only the treatment combinations that increased the circulating levels of HMGB1 led to therapeutic efficacy in the three models of brain cancer, we evaluated whether there was a correlation between the serum levels of HMGB1 and the median survival of tumor-bearing mice. The correlation analysis between the circulating levels of HMGB1 (mean of each treatment group) and the median survival of each treatment group in the three different tumor models studied indicated that the efficacy of the treatment positively correlated (Pearson r=0.56, p<0.05) with the circulating levels of HMGB1 (Supp. Fig. S4).
Effect of TMZ on the infiltration of immune cells into the tumor mass induced by Ad-TK+Ad-Flt3L gene therapy
Treatment with Ad-TK+Ad-Flt3L stimulates the immune system to mount an antitumor immune response that detects and erradicates tumor cells spread throughout the brain. Considering that high doses of TMZ could exert a lymphodepletive effect, we first evaluated the effect of TMZ (100mg/Kg) administered for 7 and 14 days to GL26 tumor-bearing mice. Complete blood counts (CBC) and flow cytometry were performed to assess the levels of circulating lymphocytes and the degree of lymphodepletion. We found that TMZ induced a profound lymphodepletion when administered for 7 or 14 days (Supp. Fig. S5). CBC indicated that there was a ~95% reduction in circulating lymphocytes and we determined by flow cytometry that the depletion affected both CD4+ and CD8+ T cells (Supp. Fig. S5). We also observed a substantial reduction in the content of CD4+ and CD8+ T cells in the spleen and bone marrow of these mice (Supp. Fig S6). Our findings indicate that TMZ exerts a lymphodepletive effect when administered for 7 or 14 days and they are in agreement with reports that show that high doses of TMZ are lymphodepletive in humans and rodents (23,24).
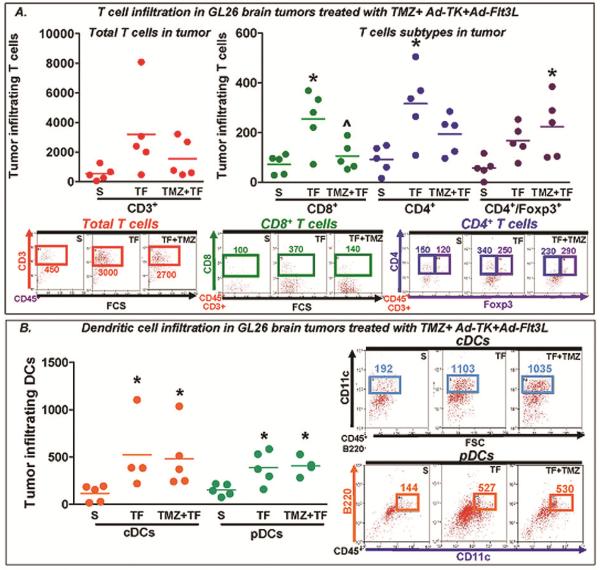
In order to evaluate the effect of TMZ on the expansion and infiltration of immune cells induced by Ad-TK+Ad-Flt3L, we implanted GL26 cells in the brain of C57BL/6 mice and 14 days later we treated them with an intratumoral injection of Ad-TK and Ad-Flt3L followed by systemic adminitration with TMZ. Seven days later immune cells were purified from the tumor and the spleen and analyzed by flow cytometry. We assessed the infiltration of T cells and DCs within the tumor mass. We found that Ad-TK+Ad-Flt3L treatment induced a significant increase in the number of tumor-infiltrating CD8+ and CD4+ T cells. Concomitant treatment with TMZ reduced the infiltration of CD8+ T cells and increased the infiltration of regulatory T cells (Fig. 4A). TMZ treatment did not affect the recruitment of plasmacytoid (pDCs) and conventional DCs (cDCs) induced by Ad-TK+Ad-Flt3L in the brain (Fig. 4B).
Fig. 4. Immune cell infiltration in GL26 brain tumors treated with TMZ in combination with Ad-TK+Ad-Flt3L.
Wild type C57BL/6 mice were implanted in the striatum with GL26 glioma cells. 14 days after tumor implantation, mice were treated by intratumoral injection of Ad-Flt3L+Ad-TK followed by daily administration of GCV and TMZ. Seven days later tumor-infiltrating immune cells were purified and analyzed using flow cytometry. This experiment has been performed twice. A, Scatter plots show the content of T cells in the tumor. Total immune cells were assessed by gating live cells with CD45, and then T cells were determined plotting against CD3, CD8 and CD4. B, Scatter plots show the content of DCs in the tumor, which were assessed by live cells with CD45, then plotting against B220 and CD11c. pDC were identified as CD11c+ B220+ CD45+ and cDC as CD11c+B220−CD45+. * p< 0.05 vs saline; ^p < 0.05 vs TF (one-way ANOVA followed by Tukey's test).
Mice treated with TMZ in combination with Ad-TK+Ad-Flt3L exhibited an increased percentage of T cells in the spleen, specifically CD8+ T cells, when compared to mice that received only gene therapy (Supp. Fig S7 A–B). Addition of TMZ to the treatment with Ad-TK+Ad-Flt3L did not affect the number of total B cells in the spleen (Supp Fig. S7D), but increased the content of Marginal Zone B cells (Supp. Fig. S7E).
In view of our observations in tumor-infiltrating immune cells, we aimed to assess whether the antitumor effect of Ad-TK+Ad-Flt3L in combination with chemotherapy with TMZ was dependent on the immune system. Thus, we implanted GL26 tumors in the brain of KO mice that lack specific immune cell populations and treated them with an intratumoral injection of Ad-TK+Ad-Flt3L and systemic TMZ for 14 days (Fig. 5). The treatment with Ad-TK+Ad-Flt3L alone or in combination with TMZ did not promote long term survival in tumor-bearing CD11c KO mice. The therapy with Ad-TK+Ad-Flt3L alone or in combination with TMZ chemotherapy also failed to provide long-term survival in mice that lack T lymphocytes (Rag1 KO, CD4 KO and CD8 KO), B lymphocytes (Igh6 KO) or that were unable to produce IFN-γ (IFN-γ KO).
Fig. 5. Role of immune cell populations in the antitumor effect of TMZ+Ad-TK+Ad-Flt3L.
Kaplan-Meier curves show the survival of immune-deficient mice lacking dendritic cells (CD11c−/−), T and B cells (Rag1−/−), CD4 or CD8 T cells (CD4−/−, CD8−/−), IFN-gamma producing cells (IFNγ−/−) or B cells (Igh6−/−) that were implanted in the brain with GL26 tumors and were treated 14 days later Ad-Flt3L+Ad-TK and TMZ. This experiment has been performed twice.
Thus, we conclude that lymphocytes and antigen presenting cells are required for the antitumor effect of Ad-Flt3L+Ad-TK in combination with TMZ. In spite of its apparent immunosuppressive effect, TMZ does not impair the efficacy of immunotherapy with Ad-Flt3L alone or in combination with Ad-TK. These findings suggest that immunotherapeutic approaches can be admininistered simultaneously with chemotherapy with TMZ in brain cancer patients.
DISCUSSION
Although TMZ has traditionally been ascribed with immunosuppressive activities, this notion has shifted, thus the use of immunotherapy in combination with chemotherapy may provide a boost to current therapeutic outcomes in patients with GBM. Our aim was to assess whether chemotherapy, using TMZ would impair the antitumor efficacy of immunotherapy with Ad-TK+Ad-Flt3L. Our results show that treatment with TMZ does not reduce the efficacy of Ad-TK+Ad-Flt3L gene therapy in mouse models of brain cancer. Moreover, combination of TMZ with intracranial administration of Ad-Flt3L significantly improved the median survival when compared to each treatment alone, leading to long-term survival in 20–25% of mice bearing intracranial GBM, while the mice that received TMZ or Ad-Flt3L succumbed to tumor burden. Tallying with our results, it has been observed that chemotherapy with TMZ enhances T cell-dependent antitumor immunity induced by several immunotherapeutic strategies in murine models of brain cancer. Concomitant administration of TMZ enhances the efficacy of tumor antigen-pulsed DC vaccines in mice bearing intracranial GBM, while mice treated with either therapy alone fails (25,26). Chemotherapy with TMZ greatly enchanced the efficacy of CCL2 blockade using anti-CCL2 antibodies in GL261 glioma-bearing mice (27). While single treatment with anti-CCL2 induced only a very modest increase in the median survival, addition of TMZ treatment led to long-term survival in 80% of treated mice (27).
Although TMZ seems to improve the efficacy of many imunotherapeutic approaches, it is well recognized that TMZ induces lymphopenia in the majority of treated patients. Bone marrow cells seem to be particularly sensitive to TMZ due to a low MGMT activity, which results in myelosuppression (25). Our findings indicate that administration of TMZ may reduce the total number of CD8+ T cells present within the tumor mass. A reduction in the circulating levels of CD8+ T cells that was accompanied by a proportional increase in circulating Treg levels was also observed in GBM patients receiving systemic TMZ treatment (28). Low-dose (0.2–5mg/Kg) chemotherapy treatment lowers the levels of tumor-infiltrating and splenic Tregs in rats bearing intracranial RG2 tumors, which was not observed with the standard treatment with TMZ (10 mg/kg for 21 days or 30 mg/kg for 5 days) (29). Our results show that treatment with higher doses of TMZ did not inhibit the infiltration of Tregs, and in fact, concomitant treatment with TMZ induced a slight increase in the intratumoral infiltration of Tregs. This has been also observed in GBM patients receiving dose intensified treatment with TMZ (100mg/m2 for 21 days) (23). On the other hand, we found that TMZ treatment did not affect the recruitment of cDCs and pDCs in the brain tumor induced by the immunotherapy and induced an expansion in the population of MZB cells in the spleen, which, as our previous results indicate, are required for the antitumor immunity induced by this treatment (15). Although TMZ reduces the intratumor infiltration of CD4+ and CD8+ T cells induced by Ad-TK+Ad-Flt3L and increases the infiltration of Tregs, this chemotherapeutic agent did not abolish the therapeutic efficacy of the immunotherapy. Tallying with our findings, doses of 125 mg/Kg in mice bearing intracranial melanoma metastasis were shown to lead to a profound reduction in circulating CD4+ and CD8+ T-cell (24). An increase in circulating levels of Tregs was detected when brain metastasis-bearing mice were injected with high doses of TMZ (30). However, an increase in serum levels of IL-2 that facilitated optimal T-cell expansion was also observed in these mice (24). It has been suggested that depletion of Tregs using neutralizing antibodies administered systemically may enhance the efficacy of TMZ chemotherapy in combination with immunotherapeutic approaches (30).
A proliferative response of lymphocytes following TMZ-induced lymphopenia has been proposed to favor the development of adaptative antitumor immunity (24). It has been recently reported that administration of myeloablative doses of TMZ (125 mg/Kg) to brain tumor-bearing mice leads to a profound and sustained lymphopenia, but stimulates the expansion of antigen-specific CD8+ T cells in response to an antitumor vaccine, leading to an improve in median survival (24). T cells from mice receiving myeloablative doses of TMZ exhibited enhanced secretion of pro-inflammatory cytokines when compared to mice treated with a lower non-myeloablative dose of TMZ (60 mg/ml) (24). It has been proposed that lymphopenia may amplify the immune response by decreasing competition at the surface of antigen-presenting cells, which improves cytokine availability, amplifying T-cell function (25). Lymphodepletion may also reset the host's immune system, reducing the tolerance towards endogenous antigens (25).
TMZ may enhance the antitumor effect of the immunotherapy not only through its direct effects on the immune system, but also through indirect mechanisms that involve changes in tumor cells. In recent years, the induction of immunogenic cell death by proapoptotic agents has been involved in the stimulation of antitumor immunity (31). It has been observed that TMZ increases the expression of proinflamatory molecules, such as calreticulin, on the surface of GBM cells (26). It has been proposed that TMZ could enhance the immunogenicity of tumor cells by generating novel antigens due to its mutagenic properties (32).
Our results indicate that treatment with TMZ in combination with intracranial injection with Ad-Flt3L led to cytoplasmic translocation of HMGB1 and increased circulating levels of HMGB1, which may affect the onset of the antitumor immune response. We have previously shown that TLR2 activation of DCs via soluble HMGB1 released from apoptotic tumor cells is required for the antitumor immune response triggered by Ad-TK+Ad-Flt3L treatment (6). In fact, we found a positive correlation between circulating HMGB1 and survival in the tumor models studied here. These findings support the use of HMGB1 circulating levels as a noninvasive surrogate marker of therapeutic efficacy of cytotoxic/immunestimulatory strategies.
Treatment with TMZ has been shown to enhance antitumor immunity when combined with immunotherapeutic approaches not only in murine models of GBM, but also in GBM patients undergoing clinical trials. Cervical intranodal vaccination with dendritic cells pulsed with tumor lysates following radiotherapy and TMZ chemotherapy resulted in an increased frequency of interferon-gamma-positive CD4+ T cells that was associated with prolonged survival of GBM patients (28). In a Phase I clinical trial GBM patients that received TMZ chemotherapy following the administration of Trp2-pulsed DC vaccines exhibited slower tumor progression and prolonged survival than those receiving either therapy alone (33). In a phase II clinical trial undertaken to assess the immunogenicity of an EGFRvIII-targeted peptide vaccine, concomitant administration of TMZ did not impair antitumor immunity. In spite of developing a grade 2–3 lymphopenia, all GBM patients treated with TMZ developed EGFRvIII-specific humoral and cellular immunity upon administration of a peptide vaccine (23). Combination of proapoptotic gene therapy and chemotherapy with TMZ is also a valuable therapeutic strategy for the treatment of GBM patients. Intracranial administration of Ad-TK followed by GCV administration can kill GBM cells even if they habor functional MGMT, which confers resistance to TMZ and can be detected in ~50% of GBM patients (34). This vector has proved safe when administered in the brain tumor bed of GBM patients during surgery in combination with radiotherapy and chemotherapy with TMZ (35). A synergic effect of Ad-TK and TMZ has been observed, which appears to be related to the inhibition of DNA repair enzymes by phosphorylated GCV (36).
Although the models tested here evaluate the efficacy of the immune stimulant treatment during the TMZ consolidation phase and we are not assessing the effect of concomitant radiothearpy, clinical trials suggest that addition of radiotherapy favor rather than impair the therapeutic benefit of immunotherapies in GBM patients. In a pilot study 5/8 GBM patients exhibited antitumor immunity after receiving intradermal vaccines of tumor lysate-loaded DC following radiotherapy and TMZ (37). In 10 patients with GBM DC vaccination following radiation and TMZ led to tumor-specific immunity that was associated with prolonged survival (38). Results from a phase II clinical trial indicate that GBM patients receiving DC vaccines after radiation and chemotherapy have an improved survival compared with patients receiving the standard treatment alone (39). Our previous preclinical results suggest that both TMZ and radiation induce tumor cell death that leads to the release of HMGB1 that activates TLR2 in tumor-infiltrating DCs recruited by Ad-Flt3L, which in turn trigger the antitumor immune response that erradicates intracranial GBM (6). In GBM patients, gene therapy vectors are injected in the tumor bed after neurosurgery. Administration of Ad-Flt3L in the brain of these patients could lead to long-term expression of Flt3L, which would elicit recruitment of antigen presenting cells and lymphocytes. When TMZ is administered to these patients, it will induce apoptosis of tumor cells that remain in the brain, further promoting the release of tumor antigens and proinflammatory molecules, such as HMGB1. This will in turn boost the activation of immune cells recruited in response to expression of Flt3L within the tumor cavity.
In summary, our results indicate that concomitant administration of TMZ chemotherapy does not inhibit the antitumor effect of Ad-TK+Ad-Flt3L in murine models of brain cancer. These findings suggest that chemotherapy with TMZ would not impair the antitumor immune response triggered by immune-stimulatory strategies. Our results support the administration of immunotheraputic approaches in patients with brain cancer undegoing chemotherapy with TMZ.
Supplementary Material
Statement of translational relevance.
As a prelude to a Phase I clinical trial in patients with glioblastoma multiforme (GBM), we evaluated whether concomitant administration of the alkylating drug, temozolomide (TMZ), would affect the anti-GBM immune mechanisms and efficacy of immune-stimulatory gene therapy. Our findings indicate that, in spite of the well known immunesuppressive effects of TMZ, its administration does not impair gene therapy mediated anti-tumor immunity. These results support the concomitant administration of TMZ and immunotherapeutic approches in patients undergoing Phase I clinical trials for GBM.
ACKNOWLDGEMENTS
We are grateful to Dr. Karin Murasko for her academic leadership, to M. Dahlgren for superb administrative support, and to R. Lemons and M. Dzaman for superb technical assistance.
Financial support: This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants U01-NS052465, U01-NS052465-S1, R01-NS074387, R01-NS057711, and MICHR Pilot R12 to M.G.C.; NIH/NINDS Grants R01-NS054193, R01-NS061107, R01-NS082311, and R21-NS084275 to P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH UL1-TR000433 and MICHR U040007; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863. M.C. was supported by NIH/NINDS 1F32 NS058156, the Consejo Nacional de Ciencia y Tecnologia (CONICET PIP 114-201101-00353) and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT-2012-0830).
Footnotes
Conflict of interests: There are no conflicts of interest to disclose.
REFERENCES
- 1.Kitange GJ, Templeton KL, Jenkins RB. Recent advances in the molecular genetics of primary gliomas. Curr Opin Oncol. 2003;15:197–203. doi: 10.1097/00001622-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Iacob G, Dinca EB. Current data and strategy in glioblastoma multiforme. J Med Life. 2009;2:386–93. [PMC free article] [PubMed] [Google Scholar]
- 4.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–99. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 5.Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–14. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–27. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candolfi M, King GD, Yagiz K, Curtin JF, Mineharu Y, Muhammad AK, et al. Plasmacytoid dendritic cells in the tumor microenvironment: immune targets for glioma therapeutics. Neoplasia. 2012;14:757–70. doi: 10.1593/neo.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–48. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erpolat OP, Akmansu M, Goksel F, Bora H, Yaman E, Buyukberber S. Outcome of newly diagnosed glioblastoma patients treated by radiotherapy plus concomitant and adjuvant temozolomide: a long-term analysis. Tumori. 2009;95:191–7. doi: 10.1177/030089160909500210. [DOI] [PubMed] [Google Scholar]
- 11.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Southgate T, Kroeger KM, Liu C, Lowenstein PR, Castro MG. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci. 2008;Chapter 4(Unit 4):23. doi: 10.1002/0471142301.ns0423s45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen SJ, Mott KR, Chentoufi AA, BenMohamed L, Wechsler SL, Ballantyne CM, et al. CD11c controls herpes simplex virus 1 responses to limit virus replication during primary infection. J Virol. 2011;85:9945–55. doi: 10.1128/JVI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Rodgers JR, Perrard XY, Perrard JL, Prince JE, Abe Y, et al. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol. 2004;173:297–306. doi: 10.4049/jimmunol.173.1.297. [DOI] [PubMed] [Google Scholar]
- 15.Candolfi M, Curtin JF, Yagiz K, Assi H, Wibowo MK, Alzadeh GE, et al. B Cells Are Critical to T-cell-Mediated Antitumor Immunity Induced by a Combined Immune-Stimulatory/Conditionally Cytotoxic Therapy for Glioblastoma. Neoplasia. 2011;13:947–60. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe R, Nakasu Y, Tashiro H, Mitsuya K, Ito I, Nakasu S, et al. O6-methylguanine DNA methyltransferase expression in tumor cells predicts outcome of radiotherapy plus concomitant and adjuvant temozolomide therapy in patients with primary glioblastoma. Brain Tumor Pathol. 2011;28:127–35. doi: 10.1007/s10014-011-0022-8. [DOI] [PubMed] [Google Scholar]
- 17.Seligman A, Shear M. Studies in Carcinogenesis. VIII. Experimental Production of Brain Tumors in Mice with Methylcholanthrene. American J Cancer. 1939;37:364–95. [Google Scholar]
- 18.Sugiura K. Tumor Transplantation. In: Gay W, editor. Methods of Animal Experimentation. Academic Press, Inc; New York: 1969. pp. 171–222. [Google Scholar]
- 19.Lee JH, Jung TY, Jung S, Kim IY, Jang WY, Moon KS, et al. Performance status during and after radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma multiforme. J Clin Neurosci. 2013;20:503–8. doi: 10.1016/j.jocn.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Szatmari T, Lumniczky K, Desaknai S, Trajcevski S, Hidvegi EJ, Hamada H, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer science. 2006;97:546–53. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minniti G, Amelio D, Amichetti M, Salvati M, Muni R, Bozzao A, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010;97:377–81. doi: 10.1016/j.radonc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Minniti G, Salvati M, Arcella A, Buttarelli F, D'Elia A, Lanzetta G, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in elderly patients with glioblastoma treated with radiotherapy plus concomitant and adjuvant temozolomide. J Neurooncol. 2011;102:311–6. doi: 10.1007/s11060-010-0324-4. [DOI] [PubMed] [Google Scholar]
- 23.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–33. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C, Johnson LA, et al. Myeloablative temozolomide enhances CD8(+) T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS One. 2013;8:e59082. doi: 10.1371/journal.pone.0059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta S, Marrinan J, Frishman C, Sampath P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol. 2012;2012:831090. doi: 10.1155/2012/831090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TG, Kim CH, Park JS, Park SD, Kim CK, Chung DS, et al. Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma model. Clin Vaccine Immunol. 2010;17:143–53. doi: 10.1128/CVI.00292-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadul CE, Fisher JL, Gui J, Hampton TH, Cote AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. 2011;13:393–400. doi: 10.1093/neuonc/noq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–34. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, et al. Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011;118:3003–12. doi: 10.1182/blood-2011-02-334565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 32.Tentori L, Graziani G. Recent approaches to improve the antitumor efficacy of temozolomide. Curr Med Chem. 2009;16:245–57. doi: 10.2174/092986709787002718. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–26. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 34.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 35.Chiocca EA, Aguilar LK, Bell SD, Kaur B, Hardcastle J, Cavaliere R, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–9. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rainov NG, Fels C, Droege JW, Schafer C, Kramm CM, Chou TC. Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther. 2001;8:662–8. doi: 10.1038/sj.cgt.7700355. [DOI] [PubMed] [Google Scholar]
- 37.Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol. 2010;99:261–72. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 38.Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114–21. doi: 10.1016/j.jocn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Cho DY, Yang WK, Lee HC, Hsu DM, Lin HL, Lin SZ, et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: a phase II clinical trial. World Neurosurg. 2012;77:736–44. doi: 10.1016/j.wneu.2011.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.