Abstract
BACKGROUND
The effects of primary tumor size on nodal involvement and of number of involved nodes on survival have not been examined in a national database of Merkel cell carcinoma.
OBJECTIVE
Analyze a retrospective cohort of MCC patients from the largest US national database to assess the relationships between these clinical parameters and survival.
METHODS
8,044 MCC cases in the National Cancer Data Base (NCDB) were analyzed.
RESULTS
There was a 14% risk of regional nodal involvement for 0.5 cm tumors which increased to 25% for 1.7cm (median sized) tumors and to >36% for ≥6 cm tumors. The number of involved nodes was strongly predictive of survival (0 nodes, 76% five-year relative survival; 1 node, 50%; 2 nodes, 47%; 3–5 nodes, 42%; and ≥ 6 nodes, 24%; p<0.0001 for trend). Younger and or male patients were more likely to undergo pathological nodal evaluation.
LIMITATIONS
NCDB does not capture disease-specific survival. Hence, relative survival was calculated by comparing overall survival to age- and sex-matched US population data.
CONCLUSION
Pathologic nodal evaluation should be considered even for patients with small primary MCC tumors. The number of involved nodes is strongly predictive of survival and may help improve prognostic accuracy and management.
Keywords: Merkel cell carcinoma, neuroendocrine carcinoma of the skin, National Cancer Data Base, prognosis, sentinel lymph node biopsy, average tumor size, nodal spread, regional node metastasis
Introduction
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine skin cancer with a five-year disease-associated mortality of 46%1. MCC is challenging to control due to its propensity for loco-regional recurrence and early microscopic spread to regional nodes and distant sites. Primary tumor size is the most commonly available prognostic factor for MCC; however its relationship with nodal disease has been controversial. One study concluded that sentinel lymph node biopsy (SLNB) is not justified for primary MCC tumors ≤1cm in size, as they were unlikely to harbor microscopic nodal disease2. Two other studies concluded that primary tumor ≤1cm had a 24–26% risk of nodal disease3,4.
The prognostic significance of number of involved regional nodes has not yet been determined for MCC. Analogous to melanoma5 and other cancers6,7, it is plausible that an increasing number of involved nodes may be associated with an increased risk of death from MCC. To address both of these issues, we analyzed data from the National Cancer Data Base (NCDB, established in 1989 as a joint project of the American College of Surgeons and the American Cancer Society)8. We sought to determine whether small primary MCC tumors have a significant risk of nodal involvement and whether a larger number of involved nodes is associated with poorer survival.
Methods
All studies were performed in accordance with Helsinki principles and institutionally approved (IRB approval #6585). 10,020 cases diagnosed between January 1, 1985 and December 31, 2004 were identified from the NCDB using MCC-specific histology code 8247. 1,684 cases missing all TNM information, and 292 distant metastatic cases that lacked tumor size information were not included resulting in a cohort of 8,044 cases.
All cases with primary tumor diameter data (n=5,722) were included for tumor size analysis (Figure 1) regardless of extent of disease or presence of follow up information. NCDB recorded the primary tumor size (maximum tumor diameter by pathological or clinical report in millimeters) up to 70 mm. Larger tumors were recorded as 70mm (1% of primary MCCs).
Figure 1. Size Distribution of Primary MCC Tumors among 5,722 cases.
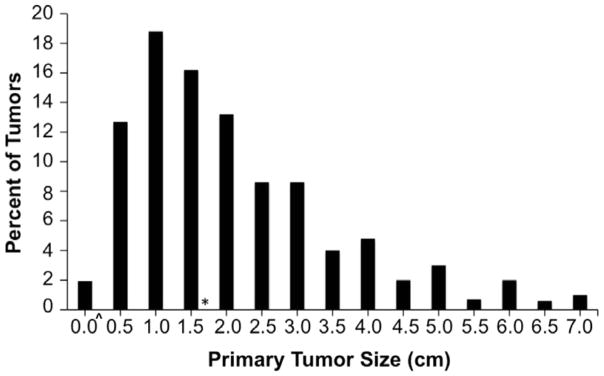
Primary tumor sizes from 5,722 cases were rounded to the nearest 0.5 cm (e.g. 0.8 – 1.2 cm tumors are shown as 1.0 cm). The median tumor size was 1.7 cm (shown by asterisk). ^Among 109 cases depicted as “0.0 cm”, 56 cases were 0.2 cm and 53 cases were 0.1 cm.
To characterize the relationship between tumor size and nodal information (Figure 2), cases presenting with distant metastases (n=199) and patients without tumor size information (n=2,322) were eliminated from this analysis. 4,027 cases that also had nodal data were included for this analysis.
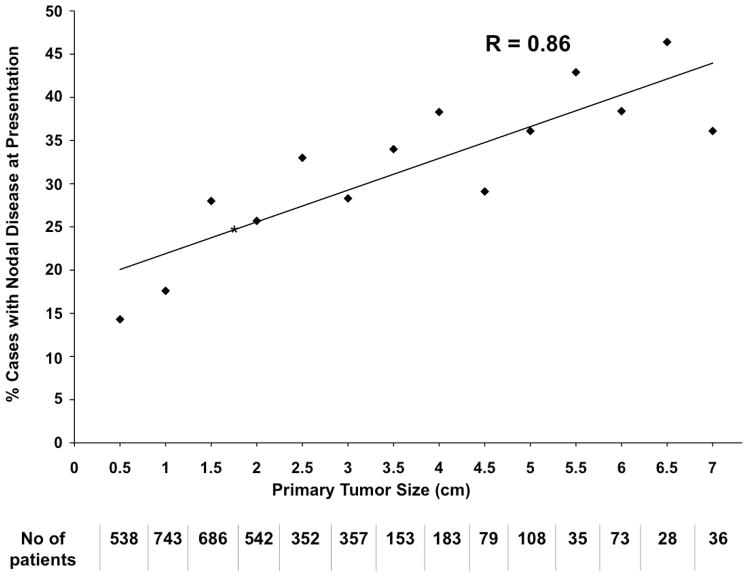
Figure 2. Relationship of Primary Tumor Size to Regional Nodal Disease.
Among 4,027 evaluable patients, the percentage with clinically or pathologically involved lymph nodes at presentation is shown relative to primary tumor size (Pearson correlation coefficient (R) = 0.86 with 95% confidence interval of 0.61–0.95), p<0.001). The number of patients presenting with each primary tumor size is shown below the X axis. An asterisk (*) at 1.7 cm indicates the median tumor size at presentation. 114 cases are in the “0 cm” bin (56 cases were 0.2 cm, 53 cases were 0.1 cm, and 5 cases were recorded as 0.0 cm; these may have represented ‘unknown primary’ or in situ tumors). 21% of these 114 cases with “0” cm size in fact had nodal disease at presentation.
To assess the relationship between number of involved regional nodes and survival (Figure 3), we studied all patients with no metastatic disease, available follow up and regional nodal data (n=1,305).
Figure 3. Relationship between number of positive regional nodes at diagnosis and survival.

Overall survival (3a) and relative survival (3b) is plotted for 1,305 patients who had no distant metastasis at the time of diagnosis and for whom nodal status at presentation was available. Percent surviving is indicated below ‘Years from Diagnosis” for each number of positive nodes.
To examine factors affecting the likelihood of pathological nodal evaluation (Figure 4), 7,845 cases who had no distant metastatic disease at diagnosis were analyzed. Differences in pathological evaluation between “younger” (<70 years) versus “older” (over 70 years) cases, and between men versus women were determined using chi square test and error bars were calculated using binomial standard error.
Figure 4. Proportion of patients who underwent pathological regional nodal evaluation.

Among patients with no distant metastatic disease at presentation, more men underwent pathological nodal staging of their lymph nodes than women in each age category. Pathological staging of nodes decreased with advancing age for both sex. Error bars represent binomial standard error. Data from 4,809 males and 3,036 females are shown. The frequency of pathologic node evaluation was significantly different as a function of age and sex (young versus old male, p<0.0001; young female versus old female, p<0.0001; young male versus young female, p = 0.008; old male versus old female p<0.0001).
STATISTICAL METHODS
NCDB does not record the cause of death for the cases it tracks. Given that the median age of diagnosis for MCC is 76, deaths among this cohort will often be due to age-associated non-MCC causes. To adjust for decreased survival associated with advanced age and sex in this cohort, we used a “proportional excess hazard model9” to analyze survival relative to age- and sex-matched population data from the Centers for Disease Control and Prevention (http://www.cdc.gov/nchs/data/statab/lewk3_2003.pdf). This approach assesses the expected survival for the cohort (based on age and sex) and thereby determines the “excess” risk of death that is associated with MCC beyond that attributable to the patient’s age and sex (Lemos et al and Dickman et al1,9). The overall survival used an unadjusted proportional hazards model. SAS software (SAS Institute Inc, Cary, North California) was used.
Results
The demographic characteristics of 8,044 MCC patients diagnosed between 1985–2004 are presented in Table 1. The NCDB had primary tumor size information on 5,722 cases (71.1%) and no tumor size information on 1,976 cases. The NCDB collects follow-up data from the time of initial diagnosis at five-year intervals. Follow-up data was available for 4,542 of the 8,044 cases. Among 1,830 live patients, median follow-up was 64.2 months (range 0.5 – 198 months).
Table 1.
Demographics of MCC cases used for each Figure.
| Any figure (n=8044) | Figure 1 (n=5722) | Figure 2 (n=4027) | Figure 3 (n=1305) | Figure 4 (n=7845) | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 4943 (61) | 3440 (60) | 2463 (61) | 866 (66) | 4809 (61) |
| Female | 3101 (39) | 2282 (40) | 1564 (39) | 439 (34) | 3036 (39) |
| Age | |||||
| <50 | 306 (4) | 209 (4) | 154 (4) | 72 (6) | 302 (4) |
| 50–59 | 677 (8) | 451 (8) | 339 (8) | 151 (12) | 654 (8) |
| 60–69 | 1424 (18) | 1011 (18) | 722 (18) | 289 (22) | 1391 (18) |
| 70–79 | 2861 (36) | 2001 (35) | 1401 (35) | 508 (39) | 2790 (36) |
| 80–89 | 2272 (28) | 1658 (29) | 1158 (29) | 258 (20) | 2211 (28) |
| 90+ | 504 (6) | 392 (7) | 253 (6) | 27 (2) | 497 (6) |
| Race | |||||
| White | 7747 (96) | 5512 (96) | 3894 (97) | 1265 (97) | 7556 (96) |
| Black | 102 (1) | 77 (1) | 45 (1) | 15 (1) | 95 (1) |
| Native American | 12 (<1) | 10 (<1) | 7 (<1) | 1 (<1) | 12 (<1) |
| Asian, Pacific Is. | 64 (1) | 50 (1) | 36 (1) | 6 (<1) | 64 (1) |
| Other/Unknown | 119 (1) | 73 (1) | 45 (1) | 18 (1) | 118 (2) |
| Stage | |||||
| Local | 4437 (55) | 2994 (52) | 2994 (74) | 630 (48) | 4437 (57) |
| Regional | 1836 (23) | 1033 (18) | 1033 (26) | 675 (52) | 1836 (23) |
| Local/regional | 1572 (20) | 1496 (26) | -- | -- | 1572 (20) |
| Metastatic | 199 (2) | 199 (3) | -- | -- | -- |
| N. of involved nodes | |||||
| 0 | 1592 (20) | 1006 (18) | 999 (25) | 630 (48) | 1585 (20) |
| 1 | 742 (9) | 436 (8) | 411 (10) | 331 (25) | 717 (9) |
| 2 | 218 (3) | 139 (2) | 131 (3) | 101 (8) | 210 (3) |
| 3–5 | 250 (3) | 151 (3) | 144 (4) | 120 (9) | 243 (3) |
| 6+ | 252 (3) | 156 (3) | 143 (4) | 123 (9) | 239 (3) |
| Unknown | 4990 (62) | 3834 67) | 2199 (55) | -- | 4851 (62) |
| Tumor size | |||||
| 0–2.0 cm | 3579 (44) | 3579 (63) | 2538 (63) | 464 (36) | 3488 (44) |
| 2.1–5.0 cm | 1862 (23) | 1862 (33) | 1305 (32) | 271 (21) | 1774 (23) |
| >5.0 cm | 281 (3) | 281 (5) | 184 (5) | 40 (3) | 261 (3) |
| Unknown | 2322 (29) | -- | -- | 530 (41) | 2322 (30) |
See Methods for details of case selection. Basic criteria were as follows: Figure 1 – all patients with tumor size known, regardless of stage; Figure 2 -- tumor size known, local and regional stage; Figure 3 – number of nodes and vital status known, local and regional stage; Figure 4 – all patients with any non-metastatic stage at presentation. Numbers in parenthesis for each individual parameter represent percent values.
The distribution of MCC primary tumor sizes is shown in Figure 1. The median primary MCC tumor size among 5,722 patients with available tumor size information was 1.7 cm (0.1cm – 7.0 cm) (Figure 1). Note: Fewer than 1% of lesions were over 7 cm in size. These were truncated at 7 cm at the time of accrual. Patients with no distant metastatic disease for whom both tumor size and regional nodal status were available (n=4,027), are depicted in Figure 2. Key findings included: 1) increased risk of nodal involvement with larger primary tumors, 2) For 0.5 cm tumors, the observed risk of nodal involvement is 14%, while the estimated risk per the fitted regression line is 20.7% (95% CI, 16.2–25.1). This difference is likely due to the lack of a linear relationship at the extreme ends of the data. 3) For 1.7 cm (median sized tumors), the observed risk of nodal involvement is 25%, while the estimated risk per the fitted regression line is 24.9% (95% CI, 21.5–28.3). (Figure 2).
The prognostic significance of the number of positive lymph nodes was analyzed among 1,305 patients with no distant metastatic disease at presentation who had nodal information available. The overall survival (Figure 3A) for patients with 0, 1, 2, 3–5 and 6+ nodes were 62%, 38%, 38%, 32% and 19% respectively (0 node: HR=1.0 [reference], 1 node: HR = 1.88, CI: 1.6–2.3, p<0.0001; 2 nodes: HR = 1.89, CI: 1.4–2.5, p<0.0001; 3–5 nodes: HR = 2.51, CI: 2.0 –3.2, p<0.0001; 6+ nodes: HR = 3.9, CI=3.1 – 5.0, p<0.0001). Although the difference between the overall survival curve for 3–5 nodes (38%) and 1 node (32%) is statistically significant (p=0.002), from a clinical standpoint these percentages are similar. The 5-year relative survival (Figure 3B) for patients with 0, 1, 2, 3–5 and 6+ nodes were 76%, 50%, 47%, 42% and 24% (0 node: HR=1.0 [reference], 1 node: HR = 2.16, CI: 1.6–3.0, p<0.0001; 2 nodes: HR = 2.27, CI: 1.5–3.5, p<0.0002; 3–5 nodes: HR = 3.43, CI: 2.4 – 5.0, p<0.0001; 6+ nodes: HR = 5.66, CI = 4.0 – 8.0, p<0.0001).
The frequency of pathologic node evaluation decreased with age for both sex (Figure 4), and more men had pathological staging of their lymph nodes than women among any age group. These differences were statistically significant (young versus old male, p<0.0001; young female versus old female, p<0.0001; young male versus young female, p = 0.008; old male versus old female p<0.0001).
Discussion
This study examined 8,044 cases from the National Cancer Database to determine the relationship of MCC regional nodal involvement with primary tumor size and the relationship of number of involved nodes with survival. Our findings indicate that patients with small primary MCC tumors have significant risk of nodal disease at the time of presentation and that the number of involved nodes is strongly predictive of survival.
Prior studies of primary tumor size (27 to 153 cases), reported a median size of 1.2 cm or 1.9 cm3,10,11. Among 5,722 cases in the present study, median tumor size was 1.7cm, close to the cut-off (2.0 cm) between stage 1 and stage 2 MCC in the current MCC staging system12.
Prior studies examining the relationship between primary tumor size and nodal disease have had conflicting conclusions. Stokes et al reported that only two of 54 patients with tumors ≤1 cm had grossly apparent clinical nodal disease, and that all others were both clinically and microscopically negative. They concluded that pathological lymph node evaluation was not indicated for patients with MCC tumors ≤1 cm. In contrast, Sarnaik et al reported that 42% of clinically occult regional nodal metastases occurred in patients with tumors that were ≤1 cm13. We previously reported that 32% of MCC patients with clinically uninvolved regional lymph nodes had occult involvement when examined pathologically14. Similarly, Fields et al3 reported a 26% risk of occult nodal involvement among 66 patients with ≤1cm tumors and Schwartz et al4 reported a 24% risk of occult nodal involvement among 42 patients with <1cm lesion. The present study, based on national registry data of a far larger number of patients than any prior study, corroborates the conclusions by several other groups3,4 that even small MCC tumors have a significant risk of nodal involvement at the time of diagnosis.
The effect of the number of involved nodes on survival has not been previously described for MCC. In this study, similar to results in breast cancer7, melanoma16,17, and esophageal cancer6, we found that the number of metastatic regional lymph nodes was an important predictor of survival in patients with MCC. While this dataset is the first to demonstrate an association between survival and the number of involved nodes in MCC, unfortunately, it is not feasible to determine whether or not inclusion of this variable would improve prognostication beyond the current AJCC staging system. This is because the NCDB did not (until recently) record the clinical nodal status for patients who underwent pathologic nodal evaluation. It is thus not possible to determine if pathologically node-positive patients had clinically apparent (stage IIIB) or clinically occult (stage IIIA) nodal disease. In the future, it will be possible to carry out multivariate analyses to determine whether the number of involved nodes improves prognostic accuracy in staging because the NCDB currently captures clinical nodal information for all patients.
Similar to other cancers including breast and prostate18,19–21, we found the likelihood of patients undergoing pathological nodal evaluation decreased with advancing age. Because advancing age and comorbid conditions may limit a patient’s ability to undergo pathological nodal staging22 this observation is not surprising. In this study, we also observed that women were less likely in all age groups than men to have pathologic nodal evaluation. Subset analyses indicated that the greater likelihood of men to undergo pathological nodal evaluation cannot be ascribed to factors including primary site (face versus extremity) or lesion size as these factors were similar between men and women. This trend of increased pathologic nodal evaluation among males was previously observed for liver, pancreatic, esophageal, and stomach cancers18,23 and ascribed to differences in socioeconomic and insurance coverage (women are more likely to have financial or insurance constraints with advancing age as compared to men). We could not explore such associations for MCC as socioeconomic data were unavailable for this cohort.
The current dataset has several limitations. In the past, NCDB did not frequently capture clinical nodal status if pathological node status was recorded. Hence we were unable to discern whether a patient’s nodal involvement was occult or clinically apparent. This limitation will be less significant in future analyses as both clinical and pathological node data are now being collected by the NCDB. Another limitation of the dataset is that it does not have MCC-specific mortality information. Hence, analyses were performed using relative survival of the subjects within this cohort as compared to age-and sex-matched population data1. However these analyses are not race-matched.
In conclusion, the findings from this national dataset demonstrate that there is no primary tumor size for which the risk of nodal involvement is clinically negligible. Although pathologic nodal evaluation for MCC is not indicated in certain circumstances (eg if the regional nodes will be treated by radiation even if the node biopsy is negative and/or if improved prognostic information is not desired by the patient), this study supports the notion that small primary tumor size should not be used to eliminate a patient from consideration of pathologic nodal evaluation. This study also demonstrates that the number of involved nodes is strongly predictive of survival and thus may be relevant for clinical management and potentially useful in future MCC staging systems.
Acknowledgments
Supported by NIH K02-AR50993, American Cancer Society RSG-08-115-01-CCE, NIH K24-CA139052, the David & Rosalind Bloom Endowment for MCC Research, the Michael Piepkorn Endowment and the UW MCC Patient Gift Fund.
Abbreviations
- SLNB
Sentinel lymph node biopsy
- NCDB
National Cancer Data Base
Footnotes
Reprint requests to: pnghiem@uw.edu
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST: The author(s) indicated no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stokes JB, Graw KS, Dengel LT, Swenson BR, Bauer TW, Slingluff CL, Jr, et al. Patients with Merkel cell carcinoma tumors < or = 1.0 cm in diameter are unlikely to harbor regional lymph node metastasis. J Clin Oncol. 2009;27:3772–3777. doi: 10.1200/JCO.2008.20.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fields RC, Busam KJ, Chou JF, Panageas KS, Pulitzer MP, Kraus DH, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol. 2011;18:2529–2537. doi: 10.1245/s10434-011-1662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz JL, Griffith KA, Lowe L, Wong SL, McLean SA, Fullen DR, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036–1041. doi: 10.1200/JCO.2010.33.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch CM, Soong SJ, Atkins MB, Buzaid AC, Cascinelli N, Coit DG, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–149. doi: 10.3322/canjclin.54.3.131. quiz 182–134. [DOI] [PubMed] [Google Scholar]
- 6.Rizk N, Venkatraman E, Park B, Flores R, Bains MS, Rusch V. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006;132:1374–1381. doi: 10.1016/j.jtcvs.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 7.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 10.Veness M, Howle J. Patients with clinically node negative extremity Merkel cell carcinoma: The importance of identifying and treating patients with microscopic nodal metastases. Australas J Dermatol. 2010;51:274–278. doi: 10.1111/j.1440-0960.2010.00702.x. [DOI] [PubMed] [Google Scholar]
- 11.Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938–945. doi: 10.1093/jnci/djp139. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 13.Sarnaik AA, Zager JS, Cox LE, Ochoa TM, Messina JL, Sondak VK. Routine omission of sentinel lymph node biopsy for merkel cell carcinoma <= 1 cm is not justified. J Clin Oncol. 2010;28:e7. doi: 10.1200/JCO.2009.25.9937. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SG, Wang LC, Penas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 15.Howle JR, Hughes TM, Gebski V, Veness MJ. Merkel cell carcinoma: An Australian perspective and the importance of addressing the regional lymph nodes in clinically node-negative patients. J Am Acad Dermatol. 2012;67:33–40. doi: 10.1016/j.jaad.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Ding S, Byrd DR, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrill RM, Sloan A, Anderson AE, Ryker K. Unstaged cancer in the United States: a population-based study. BMC Cancer. 2011;11:402. doi: 10.1186/1471-2407-11-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. Jama. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 20.Bradley CJ, Clement JP, Lin C. Absence of cancer diagnosis and treatment in elderly Medicaid-insured nursing home residents. J Natl Cancer Inst. 2008;100:21–31. doi: 10.1093/jnci/djm271. [DOI] [PubMed] [Google Scholar]
- 21.Mettlin CJ, Murphy GP, Cunningham MP, Menck HR. The National Cancer Data Base report on race, age, and region variations in prostate cancer treatment. Cancer. 1997;80:1261–1266. [PubMed] [Google Scholar]
- 22.Koroukian SM, Xu F, Beaird H, Diaz M, Murray P, Rose JH. Complexity of care needs and unstaged cancer in elders: a population-based study. Cancer Detect Prev. 2007;31:199–206. doi: 10.1016/j.cdp.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato N, Ito Y, Ioka A, Tanaka M, Tsukuma H. Gender differences in stomach cancer survival in Osaka, Japan: analyses using relative survival model. Jpn J Clin Oncol. 2009;39:690–694. doi: 10.1093/jjco/hyp084. [DOI] [PubMed] [Google Scholar]