Tuberculosis (TB) is one of the top 10 causes of child mortality worldwide and an estimated 1 million children develop active TB annually.1 TB infection during infancy results in more rapid disease progression compared to adults.2 Between 20–40% of infants with TB infection develop active TB disease within one year, in contrast to a 10% lifetime risk of disease among TB-infected adults.2 Infants born to HIV-1 infected mothers may be particularly vulnerable to early TB infection due to increased TB exposure and diminished cellular response to BCG vaccination.3–5
In settings with high TB prevalence an estimated 20% of children have evidence of latent TB infection detected by tuberculin skin test (TST) by 5 years of age.6,7 However, less is known about the prevalence of TB infection during infancy, since detection of infant TB infection with TSTs is limited by cross-reactivity after recent BCG vaccination. Up to 30% of infants have TST reactions >10 mm due to BCG vaccination at birth, limiting utility of TST In this period.8 In contrast to TST, Mycobacterium tuberculosis-specific interferon gamma release assays (IGRAs) do not cross-react with BCG and provide a method to detect early infant TB infection.
We used the T-SPOT.TB IGRA assay to determine prevalence of early TB infection among 6-month old infants born to HIV-1 infected mothers in Kenya. In addition, we evaluated maternal and infant correlates of positive infant T-SPOT.TB to identify risk factors and clinical symptoms associated with early TB infection.
METHODS
Study Setting and Design
This study utilized cryopreserved infant peripheral blood mononuclear cells (PBMCs) from a perinatal HIV transmission cohort that has been previously described.9 In brief, HIV-1 infected mothers were enrolled at 32 weeks gestation at Kenyatta National Hospital in Nairobi, Kenya from 1999 through 2002. Mothers received short-course zidovudine to prevent maternal to child transmission of HIV-1. Clinical information was collected from pregnant mothers at enrollment and mother-infant pairs were followed monthly for 12 months after delivery. This study was approved by the University of Washington Institutional Review Board and the Kenyatta National Hospital Ethics and Research Committee.
Detection of Tuberculosis Infection
Cryopreserved PBMCs were used to retrospectively detect infant TB infection using the T-SPOT.TB interferon gamma release assay (Oxford Immunotec, Oxfordshire, UK). Tuberculin skin tests (TSTs) were not performed in real-time because evaluation of TB infection was not a study aim of the parent cohort. Anticoagulated whole blood was collected at study visits and PBMCs were isolated within 6 hours and cryopreserved in liquid nitrogen. For this nested study, PBMCs were thawed and incubated for 4 hours in 5 mL AIM-V (Invitrogen) serum-free media. Cells were manually counted and suspended at a concentration of 2.5 × 105 viable cells/100 µl per manufacturer specifications when sufficient cell recovery was obtained. Cells were plated below this concentration for 59 infants (median 1.1 × 105 cells/100 µl; IQR 7.0 × 104 – 1.8 × 105 cells/100 µl) and the results normalized to the number of spot forming cells (SFCs) per 2.5 × 105 PBMCs plated. The T-SPOT.TB test was otherwise performed according to manufacturer instructions. Individual wells containing 100 µl PBMCs were stimulated with 50 µl of phytohaemagglutinin (PHA) (positive control), M. tuberculosis-specific antigens ESAT-6, CFP-10, and AIM-V media (nil control) and incubated overnight at 37°C in 5% CO2. Plates were washed with PBS and SFCs were visualized after addition of a conjugate antibody and colorimetric reagent. Plates were dried overnight and SFCs were enumerated using ELISPOT Reader (CTL-Immunospot S4 Core Analyzer). An assay was considered failed if there were zero SFCs in the positive control well. For valid assays, results were interpreted using the T-SPOT.TB manufacturer instructions.10 A test was positive if there were ≥6 spots above the nil control for either antigen ESAT-6 or CFP-10. Indeterminate test results were reported if PHA <20 SFCs or the nil control had >10 SFCs. Results were reported as negative if they did not meet the above criteria.
Clinical and Laboratory Evaluation of Correlates
Detailed maternal and infant symptoms and clinical diagnoses of incident illnesses were collected with a structured questionnaire and physical examination every month after delivery. Intradermal BCG vaccination (Statens Serum Institute) was administered to infants at birth. Infants and mothers with suspected active TB were referred to the Ministry of Health TB clinic for evaluation and management. Children were diagnosed with active TB based on a scoring system per Kenyan National TB Guidelines.11 Routine TB contact screening was not performed during clinical follow-up. Maternal CD4 count and HIV-1 viral load were assessed at enrollment. Infant HIV-1 status was determined by DNA PCR dried blood spot testing.12
We evaluated maternal and infant cofactors hypothesized to be associated with either risk or protection from development of infant TB infection, including sociodemographic factors (education level, household crowding), male partner characteristics (HIV-1 status, history of TB and TB symptoms), and maternal clinical characteristics (CD4 count, active TB, and TB symptoms). For infants, we reviewed symptoms in the published literature associated with primary TB infection and active TB and evaluated their association with T-SPOT.TB results.13–17 These symptoms included current or persistent (>2 weeks) cough, current or persistent fever (>7 days, >1 month), weight loss (>5%), failure to thrive (crossing 2 percentiles in prior 3 months, weight for age z score ≤-2, or weight for height z score ≤-2), and unexplained neonatal pneumonia, hepatosplenomegaly or sepsis. Maternal symptoms of active TB were evaluated using symptom screening tools for HIV-1 infected individuals for variables available in the clinical database (report of fever or cough).13,18 Mothers were encouraged to notify their male partners about their study participation and a subset of mothers brought their male partners to the study clinic for additional data collection. In this subset of male partners, selected TB symptoms were assessed, including prolonged cough or fever (>1 month) or weight loss >5 kg.
Statistical Analysis
Statistical analysis was performed using STATA software, version 11 (StataCorp, College Station, TX). Maternal and infant correlates of infant TB infection were evaluated using t-tests for continuous variables and Fisher's exact tests for categorical variables. For maternal-infant comparisons of IFN-γ responses to PHA, ESAT-6, and CFP-10, IFN-γ was expressed as the absolute number of SFCs above the nil control, and median IFN-γ SFCs were compared for mothers and infants with a positive T-SPOT.TB using Wilcoxon rank sum tests.
RESULTS
Characteristics of the Cohort
Of the 470 live-born infants from the parent cohort, 182 infants had at least 1 cryopreserved PBMC sample at 6 months of age and no prior active TB (see Figure, supplemental Digital Content 1). Compared to the 185 infants who were not tested with T-SPOT.TB, the 182 infants tested had a lower prevalence of HIV-1 infection (7.7% vs 20.5%, p<.0001) and higher prevalence of prolonged fever (>1 month) (2.75% vs 0%, p=.03), but were otherwise similar with respect to the clinical and sociodemographic variables reported (data not shown). Of samples tested with the T-SPOT.TB, we excluded 22 failed assays (PHA=0 SFCs) and 32 indeterminate assays (10 with PHA<20 SFCs and 22 with nil control>10 SFCs) (see Figure, supplemental Digital Content 1). We included 128 infants with a determinate T-SPOT.TB result in our analysis of maternal and infant correlates. Among mothers of these infants, median age was 25 years (IQR 22–27.5) and mothers had completed a median of 8 years of education (IQR 8–12) (see Table, Supplemental Digital Content 2). Thirty-nine of 76 (51%) mothers with available antenatal T-SPOT.TB results had latent tuberculosis (defined by positive T-SPOT.TB in the absence of active TB, as previously reported).9 Three (2.8%) mothers developed active TB during postpartum follow-up. The median maternal CD4 count during pregnancy was 457 cells/mm3 (IQR 335–607). Nine (7.0%) infants were HIV-1 infected by 6 months of age. Infants had a median birth weight of 3.1 kg (IQR 2.9–3.5) and all were BCG vaccinated. At 6 months, 56% of infants were breastfed, median weight-for-age Z score was −0.5, median height-for-age Z score was −0.9 and median weight-for-height Z score was 0.2.
Infant and Maternal T-SPOT.TB Responses
Positive T-SPOT.TB responses were detected in 14 (10.9%) infants (95% CI: 6.1–17.7%). Among infants with positive T-SPOT.TB, median ESAT-6 and CFP-10 were 24 (IQR 12–43) and 20 (IQR 11–42) SFCs above the nil control, respectively. In comparison with positive maternal responses, infants with a positive T-SPOT.TB had significantly lower median PHA (324 vs 497 SFCs, p=.02), but comparable responses for ESAT-6 (24 vs 29 SFCs, p=.65) and CFP-10 (20 vs 29 SFCs, p=.52) (Figure 1).
Figure 1.
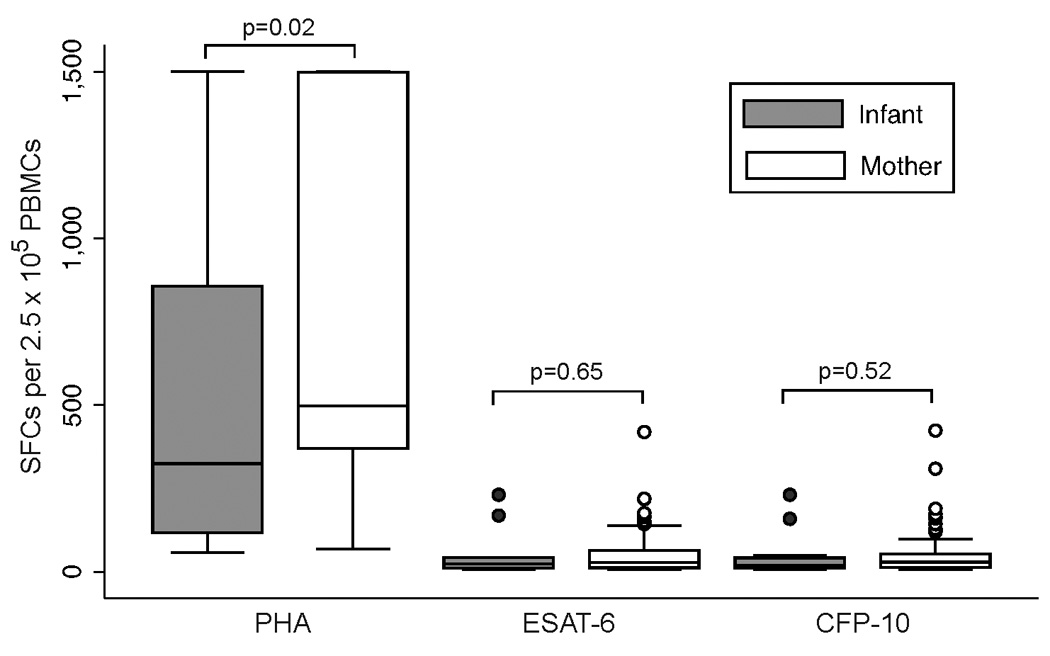
Magnitude of PHA, ESAT-6, and CFP-10 responses among mothers and infants with positive T-SPOT.TB
Seven infants with indeterminate responses due to high background (>10 SFCs nil control) had antigen-specific ESAT-6 and CFP-10 responses over twice the nil control, a threshold for “positive” that has been used in prior in-house ELISPOT assays.19 Using this alternative cutoff for a positive response would have resulted in a higher TB infection prevalence of 15.6% (95% CI: 9.9–22.8%).
Correlates of Positive Infant T-SPOT.TB
Infants with a positive T-SPOT.TB had a 15.5-fold increased odds of having a mother with active TB (95% CI: 1.30–184.09; p=.04). Maternal male partner HIV-1 status, TB history, or TB symptoms (cough or fever >1 month, or weight loss >5 kg) were not associated with positive infant T-SPOT.TB, although statistical power for these analyses was limited. Report of infant fever or cough at the time of IGRA testing or a history of persistent cough (>2 weeks) in the first 6 months of life were not associated with a positive infant T-SPOT.TB. However, infants with a positive T-SPOT.TB were more likely to have a history of prolonged fever (>1 month) in the first 6 months of life (14.3% vs 0.9%, p=.03).
Clinical Symptoms among Infants with Positive T-SPOT.TB
Ten of 14 (71%) of infants with a positive T-SPOT.TB test result had at least 1 symptom in the prior 6 months suggestive of active TB.13 In subsequent 6-month follow-up, some infants experienced symptom resolution, while others developed persistent TB symptoms (Table 2). Two infants with persistent TB symptoms died and one was diagnosed clinically with active TB. One infant with a positive T-SPOT.TB test at 6 months remained asymptomatic throughout the first 12 months of life. TB symptoms were non-specific and occurred frequently in all infants; the prevalence of at least one TB symptom did not differ between infants with positive and negative T-SPOT.TB results (71% vs 53%, p=.26).
Table 2.
Longitudinal TB symptoms and outcomes in infants with positive T-SPOT.TB at 6 months of age
| Infant ID |
T-SPOT.TB Response* |
Age in Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESAT-6 | CFP-10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Asymptomatic | 189 | 169 | 2 | ||||||||||||
| Symptom resolution | 41 | 7 | 17 | ++ | |||||||||||
| 394 | 10 | 1 | + | + | |||||||||||
| 46 | 24 | 9 | + | ||||||||||||
| 76 | 1 | 8 | + | ||||||||||||
| 91 | 12 | 32 | + | ||||||||||||
| 110 | 0 | 12 | + | ||||||||||||
| 129 | 25 | 34 | + | ||||||||||||
| Persistent symptoms | 238 | 13 | 159 | ++ | D | ||||||||||
| 359 | 12 | 15 | + | + | + | + | + | D | |||||||
| 19 | 34 | 231 | + | ++ | + | +TB | + | ||||||||
| 118 | 231 | 23 | + | + | + | + | § | ||||||||
| 160 | 0 | 6 | ++ | + | + | + | |||||||||
| 146 | 43 | 50 | + | + | + | ||||||||||
+ = Symptom or sign suggestive of TB; D = Death; § = Lost to follow up; TB = Active tuberculosis;
SFCs above nil control Time of T-SPOT.TB testing
Clinical Diagnosis of Active TB and Infant T-SPOT.TB Response
Two infants whose 6-month specimens were tested by T-SPOT.TB were diagnosed with active TB during follow-up by study clinicians using Kenyan National TB Guidelines.11 Of these, one infant diagnosed with active TB at 8 months of life had a positive 6-month T-SPOT.TB (ESAT-6 34 SFCs and CFP-10 231 SFCs above the nil control). The other infant, diagnosed at 10 months, had an indeterminate T-SPOT.TB test at 6 months (ESAT-6 and CFP-10 <6 SFCs above the nil control and nil control >10 SFCs). An additional 6 infants were diagnosed clinically with active TB in this cohort after 6 months of life; however 6-month PBMC specimens from these infants were not available for T-SPOT.TB testing.
DISCUSSION
We found an appreciable prevalence of early tuberculosis infection (10.9%) among 6 month-old Kenyan infants born to HIV-1 infected mothers. Assuming a constant risk of TB acquisition over the first year of life, this corresponds to an annual rate of infection of 20.6%.20 Including indeterminate assays with TB-specific responses that were excluded due to a high background (nil control >10 SFCs) would yield an even higher annual rate of infection of 29%. This high rate of TB infection among Kenyan HIV-1 exposed infants may contribute to substantial morbidity and mortality and requires improved detection and prevention.
Accurate estimates of prevalent TB infection in infancy have been limited by unknown TST specificity after recent BCG vaccination at birth. Since the effect of BCG on TST assay specificity diminishes with time, estimates of childhood latent TB infection using TST assays have been performed on school-age children.18,21 In South Africa, 10–25% of children age 5 years have TST >10 mm, corresponding to an annual rate of infection of 3.9% for children under 5 years.6 HIV-1 exposed children may have higher risk for TB due to increased exposure to active TB, impaired BCG vaccine responses, and alterations in innate and adaptive immunity that may affect TB susceptibility.4,5,22 The IMPAACT 1041 trial detected latent TB among 2.6% of HIV-1 exposed children below 2 years using TST; however, this study excluded infants with known active TB contacts, had high incidence of active TB prior to 2 years, and did not assess earlier time-points for latent TB. Extrapolating from their observed 4.25% annual rate of active TB and assuming that 20–40% of infants with TB infection develop active TB, we estimate an annual rate of infant TB infection between 11 and 21%, consistent with our findings.2,23
Positive infant T-SPOT.TB was strongly associated with maternal active TB (OR=15.5). This finding underscores the importance of household TB contact screening to identify infants with TB infection. However, maternal active TB identified only 14% of infants with a positive T-SPOT.TB. Our study did not include systematic ascertainment of household TB exposure; thus, it is unknown whether intensive TB contact screening could have identified additional infants with TB infection. Mandalakas et al. demonstrated that TB exposure (elicited using a detailed screening tool) was associated with positive infant IGRA.24 However, intensive TB contact screening may not be sufficient to identify early TB infection in HIV-1 exposed infants. Identifying a TB contact through screening may depend on the questions asked and whether a home visit is conducted. In settings of high TB transmission, TB exposure may also occur from non-household members in the community. For example, in the IMPAACT 1041 trial, only 36% of the HIV-1 exposed children (<2 years of age) who developed active TB had an identifiable TB contact.23,25 Early IGRA screening among HIV-1 exposed infants may complement TB contact screening and could potentially be used as an indicator to target provision of IPT.
We found that infants with positive T-SPOT.TB were more likely to have prolonged fever but not current fever, current cough or cough >2 weeks. While a positive T-SPOT.TB does not discriminate between TB infection and disease, the presence of TB symptoms in the context of TB infection suggests progression to active disease. In historical studies of children, primary TB infection was associated with fever of variable duration (days to weeks).16 Prolonged fever >2–4 weeks was associated with active TB disease in a study of pediatric TB suspects in South Africa.15 Of the 2 children in our cohort with fever >1 month, both had cough >2 weeks and one developed failure to thrive. Thus, these may have represented cases of clinically unrecognized active TB.
Overall, most (>50%) infants in our cohort had signs or symptoms that could be suggestive of active TB disease.13 Given the high prevalence of these fairly non-specific symptoms, IGRA testing may be useful to prioritize infants who need additional diagnostic evaluation for active TB disease. Due to the retrospective design of our study, clinicians were not aware of T-SPOT.TB results at the time of clinical evaluation. In subsequent 6-month clinical data on infants with positive IGRAs, 6 of 14 infants (42%) had persistent symptoms or mortality suggestive of undiagnosed active TB. Further longitudinal studies to determine predictors of active TB in IGRA positive infants would be useful to improve understanding of TB pathogenesis. Given poor outcomes of active TB in infancy, it is critical to improve TB prevention in HIV-1 exposed infants. There are conflicting data on isoniazid preventive therapy (IPT) among HIV-1 exposed and infected infants; one RCT suggested mortality benefit in HIV-1 infected children while another noted no benefit of IPT in HIV-1 exposed children <2 years without known TB exposure.23,26 IGRA-targeted IPT may be an alternative strategy to prevent active TB in this high-risk population. Current WHO guidelines do not recommend the use of IGRAs in low- and middle-income countries due to cost and insufficient performance data.27 However, HIV-1 exposed infants may be a specific population that benefits from IGRA screening, because of the limited utility of TST in the context of recent BCG vaccination. Prospective studies to evaluate the predictive value and cost-effectiveness of IGRAs among high-risk infants in TB-endemic settings are warranted.
We found an appreciable number of indeterminate infant T-SPOT.TB results due to a high background defined by T-SPOT.TB manufacturer guidelines (nil control >10 SFCs). HIV-1 exposed infants have been noted to have higher background responses compared to HIV-1 unexposed infants, perhaps due to generalized immune activation.3 The ESAT-6 and CFP-10 levels we observed suggest that T-SPOT.TB cut-offs may be similar for infants and adults. However, modification of T-SPOT.TB criteria to include TB antigen-specific responses over a high background may decrease indeterminate assays and enhance applicability for HIV-1 exposed infants.
There are several important strengths and limitations of this study. The cohort included standardized longitudinal examinations of mothers and infants, enabling retrospective evaluation of clinical correlates of TB infection without clinician bias. In contrast to cohorts of household TB contacts, we evaluated infants born to mothers with HIV-1 infection. We had limited power to detect associations with a positive T-SPOT.TB due to small sample size. We did not have microbiologic confirmation of active TB. Mothers received a short-course antiretroviral regimen to prevent mother-to-child transmission of HIV-1 (PMTCT); in newer cohorts we would anticipate lower risk for infant exposure to active TB with use of maternal combination antiretroviral therapy (cART) for PMTCT. Cryopreserved PBMCs and suboptimal cell concentration may have decreased assay sensitivity.28 Cross-reactivity with non-tuberculous mycobacteria may have compromised IGRA specificity, though we expect this to be minimal given the young age of our study participants. Finally, T-SPOT.TB testing was performed at a single time point with insufficient longitudinal data to determine value to predict active TB. Prior studies have shown that TST and IGRAs have similar accuracy to predict incident active TB among adults.29
In conclusion, we found a high prevalence of TB infection in this Kenyan cohort of HIV-1 exposed infants. A substantial proportion of infants with TB infection developed persistent symptoms suggestive of TB disease that was not clinically diagnosed. Our study points to the need for increased clinical suspicion of TB disease in HIV-1 exposed infants and systematic contact screening to identify infants with TB infection who could benefit from IPT. In the future, IGRAs may be useful to define infant TB incidence, target provision of IPT, and guide diagnostic decision-making. Optimizing cut-offs and determining predictive ability of infant IGRAs for active TB will be important to enhance utility of these assays among HIV-1 exposed infants.
Supplementary Material
Infants tested with T-SPOT.TB (figure)
Demographic and clinical characteristics of mothers and infants (table)
Table 1.
Maternal and infant correlates of positive infant 6-month T-SPOT.TB
| T-SPOT.TB | ||||
|---|---|---|---|---|
| Positive | Negative | |||
| Correlate | n/N (%) or Median |
n/N (%) or Median |
Coefficient or OR (95% CI) |
p^ |
| Mothers | ||||
| Sociodemographic characteristics | ||||
| Age | 25 | 25 | 0.9 (−1.5 – 3.3) | .6 |
| Years of Education | 8 | 8 | −0.2 (−1.7 – 1.2) | .8 |
| Number of people in home | 3 | 3 | 0.1 (−0.6 – 0.9) | .8 |
| Single room home | 11/14 (79) | 89/113 (79) | 0.9 (0.2 – 3.8) | >.9 |
| Male partner characteristics | ||||
| HIV-1 positive | 2/3 (67) | 15/25 (89) | 1.3 (.11–16) | >.9 |
| History of TB | 0/4 (0) | 1/27 (3.7) | NA | >.9 |
| TB symptoms§ | 1/4 (25) | 5/29 (17) | 1.6 (.14–19) | >.9 |
| Clinical characteristics | ||||
| Cough | ||||
| Current | 5/13 (39) | 22/93 (24) | 2.0 (0.6 – 6.8) | .3 |
| History >2 weeks | 4/14 (29) | 23/94 (25) | 1.2 (0.4 – 4.3) | .8 |
| Fever | ||||
| Current | 0/13 (0) | 7/93 (7.5) | NA | .6 |
| History >1 month | 0/14 (0) | 1/94 (1.1) | NA | >.9 |
| CD4 count (cells/mm3) | 498 | 446 | 97 (−25 – 219) | .3 |
| Log10 plasma RNA (copies/mL) | 4.6 | 4.8 | −0.2 (−0.6 – 0.2) | .6 |
| Latent tuberculosis¥ | 3/9 (33) | 36/67 (54) | 0.4 (0.1 – 1.9) | .3 |
| Active tuberculosis | 2/14 (14) | 1/94 (1.1) | 15.5 (1.3 – 184) | .04 |
| Infants | ||||
| Breastfedº | 9/14 (64) | 62/113 (55) | 0.6 (0.3 – 1.5) | .5 |
| Cough | ||||
| Current | 8/14 (57) | 53/114 (47) | 1.5 (0.5 – 4.7) | .6 |
| History >2 weeks* | 4/14 (29) | 32/114 (28) | 1.0 (0.3 – 3.5) | >.9 |
| Fever | ||||
| Current | 1/14 (7.1) | 24/114 (21) | 0.3 (.04 – 2.3) | .3 |
| History >1 weeks* | 1/13 (7.1) | 13/114 (11) | 0.6 (.07 – 5.0) | >.9 |
| History >1 month | 2/14 (14) | 1/114 (0.9) | 18.8 (1.6 – 223) | .03 |
| Weight loss > 5%* | 0/14 (0) | 6/114 (5.3) | NA | >.9 |
| Failure to thrive* | 4/14 (29) | 29/114 (25) | 1.2 (0.3 – 4.0) | .8 |
| Neonatal pneumonia 0–60d* | 3/14 (21) | 9/114 (7.9) | 3.2 (0.8 – 13) | .1 |
| Hepatosplenomegaly 0–60d* | 1/14 (7.1) | 1/114 (0.9) | 8.7 (0.5 – 147) | .2 |
| Sepsis 0–60d* | 0/14 (0) | 0/114 (0) | NA | NA |
| Any TB sign or symptom | 10/14 (71) | 61/114 (54) | 2.2 (0.6 – 7.3) | .3 |
Cough > 1 month, fever > 1 month or weight loss > 5 kg
Assessed at 6 months of age
Signs or symptoms suggestive of active TB included in Clinical Case Definition for Intrathoracic TB in Children12
Defined by one or more signs or symptoms suggestive of TB12
Positive T-SPOT.TB without clinical diagnosis of active TB
Fisher’s exact test
Acknowledgements
We thank members of the Kizazi Collaborative Group (UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh)) for helpful discussions during the development of this manuscript. We thank Ken Tapia and Anjuli Wagner for graphical assistance. We also thank the participants of the perinatal cohort and the clinical and laboratory staff at the Paediatric Research Laboratory, Kenyatta National Hospital.
Source of Funding:
Supported by the US National Institutes Health NIH HD058477-01. L.C. is a Fellow of the Pediatric Scientist Development Program (PSDP) supported by an American Pediatric Society/American Academy of Pediatrics grant and NICHD K12-HD000850. B.L.-P. and D.W. were scholars in the International AIDS Training and Research Program, NIH Research Grant D43 TW000007, funded by the Fogarty International Center and the Office of Research on Women's Health. G.J.-S. is supported by NIH Research Grant K24 HD054314-04. This publication was made possible with help from the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA).
Footnotes
Conflicts of Interest:
The authors have no other funding or conflicts of interest to disclose.
References
- 1.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Suppl 3):S184–S194. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 2.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 3.Van Rie A, Madhi SA, Heera JR, et al. Gamma interferon production in response to Mycobacterium bovis BCG and Mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Clin Vaccine Immunol. 2006;13:246–252. doi: 10.1128/CVI.13.2.246-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miles DJ, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guerin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129:446–454. doi: 10.1111/j.1365-2567.2009.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis. 2008;12:225–227. [PubMed] [Google Scholar]
- 6.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–412. [PMC free article] [PubMed] [Google Scholar]
- 7.Shanaube K, Sismanidis C, Ayles H, et al. Annual risk of tuberculous infection using different methods in communities with a high prevalence of TB and HIV in Zambia and South Africa. PLoS One. 2009;4:e7749. doi: 10.1371/journal.pone.0007749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burl S, Adetifa UJ, Cox M, et al. The tuberculin skin test (TST) is affected by recent BCG vaccination but not by exposure to non-tuberculosis mycobacteria (NTM) during early life. PLoS One. 2010;5:e12287. doi: 10.1371/journal.pone.0012287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonnalagadda S, Lohman Payne B, Brown E, et al. Latent tuberculosis detection by interferon gamma release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. J Infect Dis. 2010;202:1826–1835. doi: 10.1086/657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.T-SPOT.TB [package insert vOIL. Oxfordshire, UK: 2009. [Google Scholar]
- 11.Division of Leprosy Tuberculosis and Lung Disease MoPHaS, Kenya. National NLTP guideline: what the health care worker needs to know. [Accessed 14 December 2012];Ministry of Public Health and Sanitation Division of Leprosy, Tuberculosis and Lung Disease website. http://wwwnltpcoke/docs/National_NLTP_Guidelinepdf Published August 2005. [Google Scholar]
- 12.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S199–S208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marais BJ, Obihara CC, Gie RP, et al. The prevalence of symptoms associated with pulmonary tuberculosis in randomly selected children from a high burden community. Arch Dis Child. 2005;90:1166–1170. doi: 10.1136/adc.2004.060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marais BJ, Gie RP, Obihara CC, Hesseling AC, Schaaf HS, Beyers N. Well defined symptoms are of value in the diagnosis of childhood pulmonary tuberculosis. Arch Dis Child. 2005;90:1162–1165. doi: 10.1136/adc.2004.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallgren A. The time-table of tuberculosis. Tubercle. 1948;29:245–251. doi: 10.1016/s0041-3879(48)80033-4. [DOI] [PubMed] [Google Scholar]
- 17.Organization. WH. Provisional guidelines for the diagnosis and classification of the EPI target diseases for primary health care, suveillance, and special studies. Geneva, Switzerland: 1983. EPI/GEN/83/4. [Google Scholar]
- 18.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 19.Beattie T, Kaul R, Rostron T, et al. Screening for HIV-specific T-cell responses using overlapping 15-mer peptide pools or optimized epitopes. Aids. 2004;18:1595–1598. doi: 10.1097/01.aids.0000131362.82951.b2. [DOI] [PubMed] [Google Scholar]
- 20.Rieder H. Annual risk of infection with Mycobacterium tuberculosis. Eur Respir J. 2005;25:181–185. doi: 10.1183/09031936.04.00103804. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Chandrasekhar A, Gupte N, et al. Symptom screening among HIV-infected pregnant women is acceptable and has high negative predictive value for active tuberculosis. Clin Infect Dis. 2011;53:1015–1018. doi: 10.1093/cid/cir605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slyker JA, Lohman-Payne B, John-Stewart GC, et al. The impact of HIV-1 infection and exposure on natural killer (NK) cell phenotype in Kenyan infants during the first year of life. Front Immunol. 2012;3:399. doi: 10.3389/fimmu.2012.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365:21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandalakas AM, Kirchner HL, Lombard C, et al. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int J Tuberc Lung Dis. 2012;16:1033–1039. doi: 10.5588/ijtld.12.0027. [DOI] [PubMed] [Google Scholar]
- 25.Nachman S, Zeldow B, Dittmer S, Krzesinski E, Archary M, Jean-Philippe P, Mofenson L, Violari A. Lack of Identification of Adult TB Contacts in Infants with Microbiologically Confirmed or Clinically presumed TB (MCCP TB) in Clinical Trial P1041. International AIDS Society. 2011 [Google Scholar]
- 26.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. Bmj. 2007;334:136. doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Organization WH. Use of tuberculosis interferon-gamma release assays (IGRAs) in low-and middle-income countries: policy statement. 2011 [PubMed]
- 28.Smith SG, Joosten SA, Verscheure V, et al. Identification of major factors influencing ELISpot-based monitoring of cellular responses to antigens from Mycobacterium tuberculosis. PLoS One. 2009;4:e7972. doi: 10.1371/journal.pone.0007972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Infants tested with T-SPOT.TB (figure)
Demographic and clinical characteristics of mothers and infants (table)


