Abstract
Excessive production of triglyceride-rich very low-density lipoproteins (VLDL-TG) contributes to hypertriglyceridemia in obesity and type 2 diabetes. To understand the underlying mechanism, we studied hepatic regulation of VLDL-TG production by (forkhead box O6) FoxO6, a forkhead transcription factor that integrates insulin signaling to hepatic metabolism. We showed that transgenic mice expressing a constitutively active FoxO6 allele developed hypertriglyceridemia, culminating in elevated VLDL-TG levels and impaired postprandial TG clearance. This effect resulted in part from increased hepatic VLDL-TG production. We recapitulated these findings in cultured HepG2 cells and human primary hepatocytes, demonstrating that FoxO6 promoted hepatic VLDL-TG secretion. This action correlated with the ability of FoxO6 to stimulate hepatic production of microsomal triglyceride transfer protein (MTP), a molecular chaperone that catalyzes the rate-limiting step in VLDL-TG assembly and secretion. FoxO6 was shown to bind to the MTP promoter and stimulate MTP promoter activity in HepG2 cells. This effect was inhibited by insulin, consistent with the ability of insulin to promote FoxO6 phosphorylation and disable FoxO6 DNA-binding activity. Mutations of the FoxO6 target site within the MTP promoter abrogated FoxO6-mediated induction of MTP promoter activity. Hepatic FoxO6 expression became deregulated in insulin-resistant mice with obesity and type 2 diabetes. FoxO6 inhibition in insulin-resistant liver suppressed hepatic MTP expression and curbed VLDL-TG overproduction, contributing to the amelioration of hypertriglyceridemia in obese and diabetic db/db mice. These results characterize FoxO6 as an important signaling molecule upstream of MTP for regulating hepatic VLDL-TG production.
Hypertriglyceridemia is the most common lipid disorder that is characterized by increased production in the liver and/or decreased clearance of triglyceride (TG)-rich lipoproteins in plasma (1–3). Hypertriglyceridemia along with its metabolic sequelae of plasma accumulation of TG-rich lipoprotein remnants is atherogenic, accounting for increased risk of coronary artery disease (4–10). Although the pathophysiology of hypertriglyceridemia is poorly understood, its close association with adiposity and type 2 diabetes implicates insulin resistance as a causative factor in the pathogenesis of hypertriglyceridemia (9, 11–13). Nonetheless, the molecular basis that links insulin resistance to hypertriglyceridemia remains incompletely defined. Critical for TG metabolism is the liver, in which TG-rich very low-density lipoproteins (VLDL-TG) are assembled for subsequent secretion into the blood. In response to fatty acid influx, the liver increases VLDL-TG assembly and secretion. This effect, which is regulated by insulin and fatty acid substrate availability, has been viewed as a mechanism for staving off hepatic fat accumulation in normal individuals (1, 14, 15). In insulin-resistant subjects, this mechanism becomes deregulated, contributing to the development of hypertriglyceridemia and steatosis in obesity and type 2 diabetes.
To better understand the molecular mechanism of hypertriglyceridemia, we investigated hepatic regulation of TG metabolism by forkhead box O6 (FoxO6), a nuclear transcription factor that belongs to the forkhead box O family. This subfamily of proteins (FoxO1, FoxO3, FoxO4, and FoxO6), characterized by a highly conserved winged-helix DNA binding motif, acts as substrates of Akt/PKB to mediate the inhibitory effect of insulin (or IGF-1) on key genes in diverse pathways including cell survival, proliferation, differentiation, oxidative stress, and metabolism in mammals (16). FoxO1 orthologs, namely dFoxO in Drosophila melanogaster and DAF16 in Caenorbhabditis elegans, contribute to the regulation of longevity (17, 18). In contrast, little is known about the role of FoxO6 in hepatic lipid metabolism and its potential contribution to hypertriglyceridemia in obesity and type 2 diabetes.
Although categorized in the FoxO subfamily, FoxO6 differs from other FoxO members in fundamental ways (19): 1) FoxO6 has the lowest degree of homology (< 30%) in amino acid sequence with the FoxO subfamily; 2) FoxO6 contains only two consensus AKT/PKB phosphorylation sites at T26 and S184 within the DNA-binding domain. In contrast, other FoxO members contain 3 highly conserved phosphorylation sites (T24, S256, S319 in FoxO1); 3) FoxO6 lacks the consensus motif that corresponds to the nuclear export signal in other members of FoxO subfamily; and 4) FoxO6 does not undergo insulin-dependent nuclear export (20–22), a characteristic mechanism that is shared by other members of the FoxO subfamily (16). FoxO6 is expressed in the liver of both human and rodent origins (20). Our recent studies show that FoxO6 becomes deregulated in insulin-resistant liver, culminating in its marked up-regulation at both mRNA and protein levels in hepatocytes of dietary obese mice and diabetic db/db mice with altered lipid metabolism (20). These data have spawned the hypothesis that FoxO6 plays a significant role in hepatic lipid metabolism. FoxO6 deregulation in insulin-resistant liver may contribute to hypertriglyceridemia in diabetes. In this study, we addressed this hypothesis in transgenic mice with liver-specific FoxO6 production and diabetic db/db mice with selective FoxO6 depletion in the liver.
Materials and Methods
Animal studies
Male db/db mice and heterozygous db/+ littermates (6 weeks old) were purchased from The Jackson Laboratory. Mice were fed standard rodent chow and water ad libitum in sterile cages with a 12-hour light, 12-hour dark cycle. FoxO6 transgenic mice expressing a constitutively active allele FoxO6-S184A (FoxO6-CA) in the liver were described (20). Both male and female FoxO6 transgenic mice were used in this study. For blood chemistry, mice were fasted for 16 hours, and tail vein blood samples were collected into capillary tubes precoated with potassium-EDTA (Sarstedt). All procedures were approved by the Institutional Animal Care and Use Committee of University of Pittsburgh.
Cell culture
HepG2 cells were purchased from American Type Culture Collection (Manassas, Virginia). Cells were cultured in DMEM (Lonza) and were transduced with Adv-FoxO6-CA vector expressing constitutively active FoxO6 allele, using Adv-Empty vector as control as described elsewhere (20). Adv-FoxO6-small interfering RNA (siRNA) expressing the FoxO6-specific siRNA and Adv-Sc-siRNA encoding scrambled siRNA vectors have been described (20). Adv-Akt-CA encodes a constitutively active form of Akt, as described previously (23). All adenoviral vectors were produced in human embryonic kidney 293 cells, as described elsewhere (24).
Human primary hepatocytes
Human primary hepatocytes were isolated from donors and were obtained from the Tissue Cell Distribution System of University of Pittsburgh, which was funded by National Institutes of Health Contract HHSN276201200017C. Human primary hepatocytes from an adult donor (female, 51 year old) without obesity and type 2 diabetes were seeded at 2 × 105 cells per well and cultured in 12-well collagen-coated microplates, as described elsewhere (25).
Immunoprecipitation
Liver tissue (20 mg) was homogenized in 400 μL of M-PER buffer supplemented with protease inhibitor cocktail (Pierce). Aliquots of hepatic protein extracts (500 μg) were incubated with anti-FoxO6 (20) or anti-PPAR-γ (Santa Cruz Biotechnology), followed by protein-A agarose affinity immunoprecipitation. Immunoprecipitates were thoroughly washed with PBS plus Tween-20 (final concentration 0.25%), followed by immunoblot analysis using anti-FoxO6 and anti-PPAR-γ antibodies, respectively.
RNA isolation and real-time RT-PCR
RNA isolation from liver (20 mg) or HepG2 cells (∼2 × 106 cells) was performed using the RNeasy Mini Kit (QIAGEN). Real-time quantitative RT-PCR (qRT-PCR) was used for quantifying mRNA concentrations using the Roche LightCycler-RNA amplification kit (Roche Diagnostics), as described elsewhere (23). The primers were described in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. All primers were obtained commercially from Integrated DNA Technologies.
Immunoblot assay
HepG2 cells (∼1 × 106 cells) were lysed in 200-μl M-PER (Pierce Chemical Co) containing 2-μL Halt Protease Inhibitor Cocktail (Pierce). Protein extracts were obtained by centrifugation at 13 000 rpm for 10 minutes. To obtain protein extracts from liver tissue, 20 mg liver tissue was homogenized in 400-μL M-PER supplemented with 4-μL Halt Protease Inhibitor Cocktail (Pierce), followed by centrifugation at 13 000 rpm for 10 minutes. Proteins were resolved on 4%–20% sodium dodecyl sulfate-polyacrylamide gels and subjected to immunoblot analysis, using rabbit anti-FoxO6 (20), anti-PPAR-γ coactivator (PGC)-1β (Santa Cruz), anti-PPAR-γ (Santa Cruz), anti-apolipoprotein B (ApoB) (Abcam), anti-lipoprotein lipase (LPL) (Santa Cruz), anti-hepatic lipase (HL) (Santa Cruz), anti-microsomal triglyceride transfer protein (MTP) (23), antialbumin (Cell Signaling Technology), or antiactin antibody (Sigma-Aldrich). Protein bands were detected by autoradiography and their relative intensities were quantified by densitometry using NIH (National Institutes of Health, Bethesda, MD) image software, as described elsewhere (26).
LPL assay
Mice were injected iv with 300 IU heparin/kg body weight, and tail-vein blood (20 μL) was sampled 10 minutes after heparin infusion. Heparinized sera were prepared for the determination of LPL activity using the LPL activity kit (Roar Biochemical, Inc.), as described previously (24, 27).
VLDL-TG production assay
Mice were fasted for 16 hours, followed by ip injection of tyloxapol (1 g/kg, Sigma Aldrich) for inhibiting systemic TG clearance. Aliquots of blood (20 μL) were taken from tail vein at different times for determining plasma TG levels. VLDL-TG production rates were defined as the amount of hepatic TG produced per unit time, as described elsewhere (23).
Fat tolerance test
Mice were fasted for 16 hours, followed by an oral bolus of olive oil (10 μL/g). Aliquots of blood (20 μL) were taken from tail vein at different times for determining plasma TG, as described (24).
Hepatic lipid content
Aliquots of liver tissue (20 mg) were homogenized in 400 μL HPLC-grade acetone. After incubation with agitation at room temperature overnight, aliquots (50 μL) of acetone-extract lipid suspension were used for the determination of TG concentrations using the Infinity TG reagent (Thermo Electron). Hepatic lipid content was defined as mg of TG per gram of total liver proteins, as described elsewhere (23, 28).
Fecal lipid content
Feces were collected from individual mice during a 24-hour period under fed and fasting conditions. Aliquots of feces (150 mg) were homogenized in 400 μL HPLC-grade acetone, followed by incubation with agitation at room temperature overnight. Fecal lipid content, defined as mg of triglyceride per gram of feces, was determined in acetone-extract lipid suspension, using the Infinity TG reagent (Thermo Electron).
FPLC fractionation of lipoproteins
Aliquots (400 μL) of plasma pooled from FoxO6-tg mice and control littermates were applied to 2 head-to-tail linked Tricorn high performance Superose S-6 10/300GL columns using an FPLC system (GE Health Care), followed by elution with PBS at a constant flow rate of 0.25 mL/min. Fractions (500 μL) were eluted for determining TG levels, as described previously (23, 24).
PPAR-γ activity assay
PPAR-γ activity was based on its ability to bind and stimulate PPAR response element (PPRE)-directed luciferase expression. HepG2 cells were cotransduced with PPREx3-TK-Luc (expressing Firefly luciferase, Addgene) and pGL4.75 (expressing Renilla luciferase, Promega), followed by transduction with Adv-Empty or Adv-FoxO6 vector (200 pfu/cell). After 24-hour incubation, cells were subjected to dual luciferase activity assay. PPAR-γ activity was defined as the ratio of Firefly and Renilla luciferase activities.
Chromatin immunoprecipitation (ChIP) assay
ChIP was used to study the molecular interaction between FoxO6 and MTP promoter DNA, as described elsewhere (24). HepG2 cells were transduced with Adv-FoxO6-CA or control Adv-Empty vector, followed by transfection with pGH11-WT (or pGH11-MT), encoding wild-type (or mutant) MTP promoter-directed luciferase reporter system, as described previously (23). After 24-hour incubation, cells were subjected to ChIP assay using rabbit anti-FoxO6 antibody or preimmune IgG as control. Immunoprecipitated DNA was analyzed by PCR assay using primers (5′-CTTCAGGGATAAAAGGCCTA-3′ and 5′-ATGTTTCATCCCAGAAGGAA-3′) flanking the consensus insulin-responsive element (IRE, −767/−369 nucleotides [nt]) in the MTP promoter. Primers (5′-CTCTGACAGACCTTACTTCT-3′ and 5′-AGTTGGGATTACAGAATTTT-3′) flanking a distal MTP promoter region (−3528/−3045 nt) were used as off-target control.
MTP promoter activity assay
HepG2 cells were transduced in 6-well plates with 200 pfu/cell of Adv-FoxO6-CA or Adv-Empty vector, followed by transfection with 2-μg of pGH11-WT or pGH11-MT plasmid encoding wild-type or mutant version of the mouse MTP promoter-directed luciferase reporter system. Plasmid pGL3-basic encoding promoter-less luciferase was used as control for determining the baseline luciferase activity in HepG2 cells. Plasmid pGL4.75 expressing Renilla luciferase (Promega) was included as control for normalizing transfection efficiency. After a 24-hour incubation, cells were subjected to dual luciferase activity assay for determining the MTP promoter activity.
Statistics
Statistical analyses of data were performed by ANOVA using StatView software (Abacus Concepts). ANOVA post hoc tests were performed to study the significance between different conditions. Data were expressed as the mean ± SEM. P values < .05 were considered significant.
Results
FoxO6 transgenic mice exhibit hypertriglyceridemia
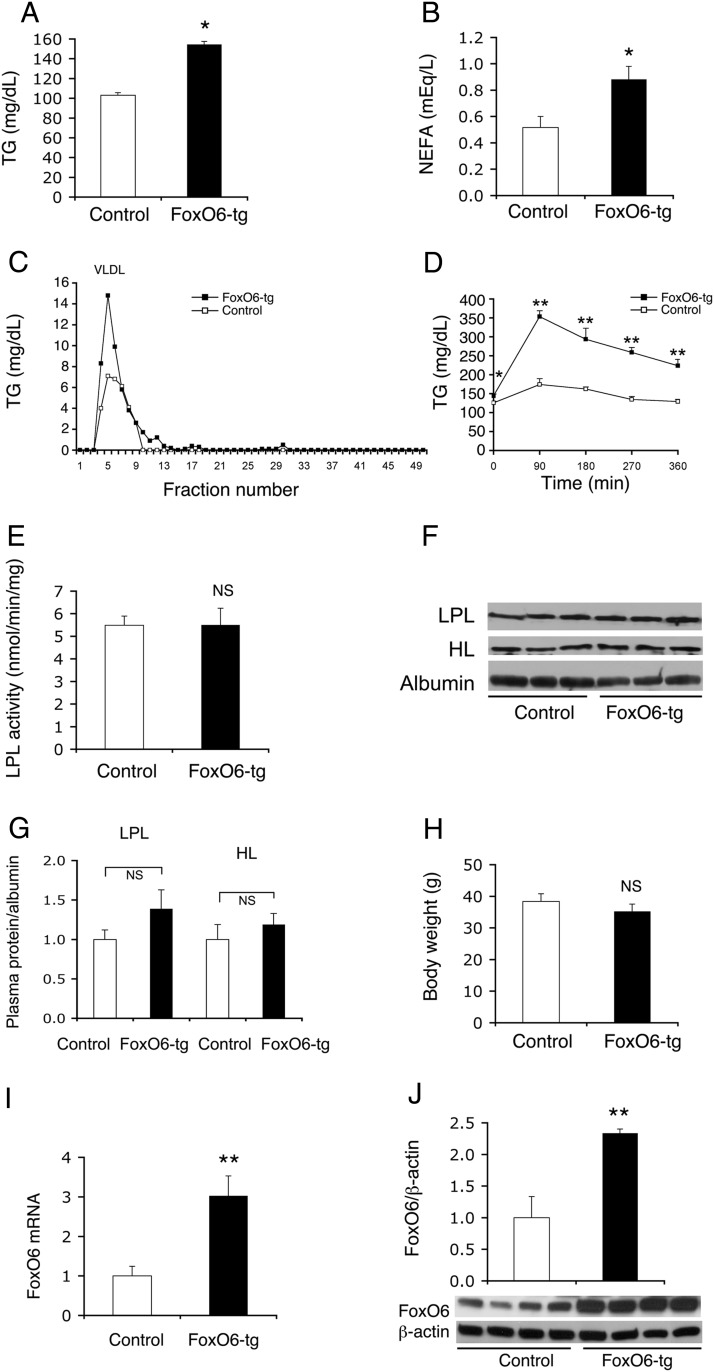
To characterize the role of FoxO6 in hepatic lipid metabolism, we generated FoxO6 transgenic mice expressing the constitutively active FoxO6-CA allele from the liver-specific transthyretin promoter. This transgenic line expressed FoxO6-CA selectively in the liver with nondetectable expression in other peripheral tissues including brain, muscle, adipose tissue, and pancreatic β-cells (20). Male FoxO6-CA transgenic mice (FoxO6-tg), as opposed to age/sex-matched control littermates, developed hypertriglyceridemia, culminating in significantly elevated plasma TG levels (Figure 1A), plasma nonesterified fatty acid (NEFA) levels (Figure 1B), plasma VLDL-TG levels (Figure 1C), and impaired fat tolerance (Figure 1D). In response to oral fat administration, plasma TG levels in FoxO6-tg mice were markedly raised from 144 ± 5 mg/dL to 353 ± 16 mg/dL at 90 minutes and remained at higher levels (223 ± 14 mg/dL) at 6 hours after fat administration. In contrast, plasma TG levels were moderately induced from 125 ± 4 to 174 ± 15 mg/dL in control mice at 90 minutes after olive oil administration, followed by declination to basal levels (129 mg/dL) within 6 hours. Likewise, female FoxO6-tg mice exhibited significantly elevated plasma TG levels, accompanied by increased VLDL-TG production and impaired fat tolerance, when compared with age-matched female littermates (Supplemental Figure 1).
Figure 1.
FoxO6-tg mice develop hypertriglyceridemia. A, Plasma TG levels. B, Plasma NEFA levels. C, Plasma VLDL-TG levels. Aliquots (400 μL) of plasma pooled from FoxO6-tg (with FoxO6-CA transgenic production in the liver) and control wild-type littermates were fractionated by gel filtration in a FPLC system. Fractions (500 μL) were eluted for the determination of TG levels. D, Plasma TG profiles in response to fat tolerance. E, Plasma LPL activity. F, Western blots of plasma LPL, HL, and albumin. G, LPL and HL protein levels relative to albumin. H, Body weight. I, Hepatic FoxO6 mRNA levels relative to β-actin mRNA. J, Hepatic FoxO6 protein levels relative to β-actin. Data were obtained from male FoxO6-tg (n = 6) and control wild-type littermates (n = 4) at 30–32 weeks of age under fasting conditions. *, P < .05; and **, P < .001 vs control. NS, not significant.
To address the underlying pathophysiology, we determined plasma activity of LPL, a key enzyme that is responsible for postprandial plasma VLDL-TG clearance (29, 30). No significant difference in plasma LPL activity was detected between male FoxO6-tg and control littermates (Figure 1E). As control, we determined plasma LPL protein and HL protein levels. Both plasma LPL and HL protein levels remained unchanged in male FoxO6-tg vs control mice (Figure 1, F and G). Likewise, no significant differences in body weight were seen between male FoxO6-tg and control groups (Figure 1H). Female FoxO6-tg mice also had a mean body weight similar to that in age-matched female controls (Supplemental Figure 1). We then used male mice for understanding the mechanism by which FoxO6 regulates hepatic lipid metabolism. As control, we determined FoxO6 expression in the liver, demonstrating that hepatic FoxO6 abundance was increased by 3-fold at mRNA levels (Figure 1I) and by 2.5-fold at protein levels (Figure 1J) in FoxO6-tg mice vs control littermates.
FoxO6 augments VLDL-TG production in liver
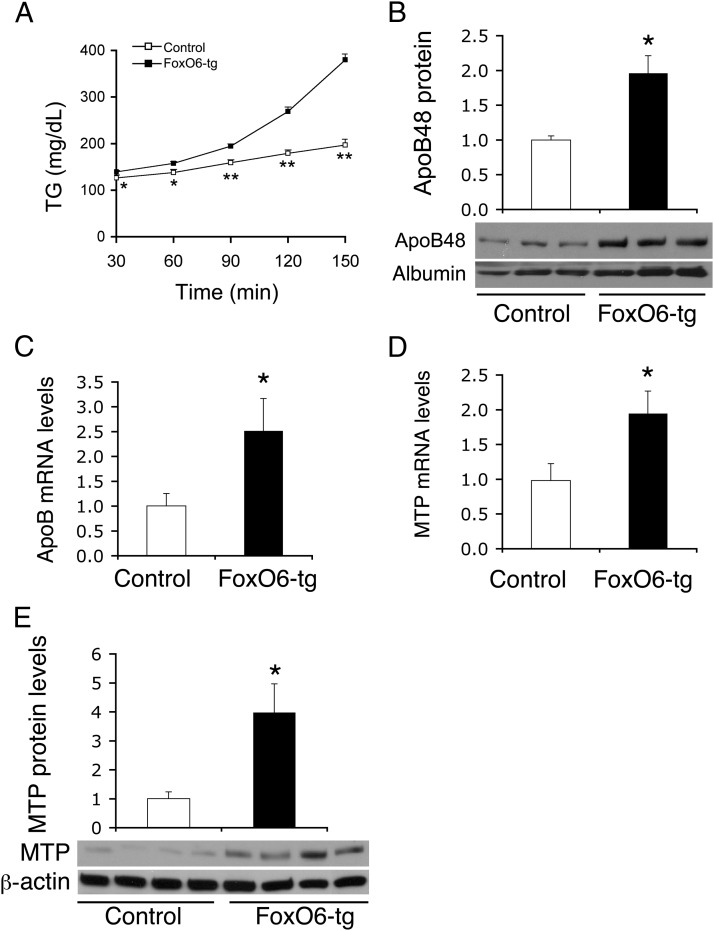
To address the underlying mechanism of FoxO6-mediated impairment in TG metabolism, we determined hepatic VLDL-TG production in FoxO6-tg mice using tyloxapol to inhibit systemic TG clearance, as described elsewhere (23, 31). FoxO6-tg mice, as opposed to control littermates, exhibited significantly higher plasma TG levels at different times following tyloxapol administration (Figure 2A). This effect correlated with significantly elevated plasma ApoB protein levels (Figure 2B) and hepatic ApoB mRNA levels (Figure 2C) in FoxO6-tg vs control littermates. To gain mechanistic insight into FoxO6-mediated induction in hepatic VLDL-TG production, we determined hepatic expression levels of MTP, a molecular chaperone that catalyzes the rate-limiting step in hepatic VLDL-TG production (23, 32–34). Hepatic MTP mRNA levels (Figure 2D) and protein abundance (Figure 2E) were significantly elevated in FoxO6-tg vs control littermates. As control, we determined fecal TG levels. No significant differences in fecal TG content during a 24-hour period were detected between FoxO6-tg and control mice under both fed and fasting conditions (Supplemental Figure 2).
Figure 2.
FoxO6-tg mice exhibit enhanced VLDL-TG production. A, Plasma TG levels after tyloxapol administration. B, Plasma ApoB48 levels relative to albumin. Aliquots of plasma (5-μL) obtained at 2 hours after tyloxapol administration were subjected to immunoblot analysis using anti-ApoB and control antialbumin antibodies. C, Hepatic ApoB mRNA levels. D, Hepatic MTP mRNA levels relative to β-actin mRNA. E, Hepatic MTP protein levels relative to β-actin. Data in panels C and D were obtained from FoxO6-tg (male; 30–32 weeks; n = 6) and age/sex-matched control littermates (n = 4) under fasting conditions. *, P < .05; and **, P < .001 vs control. NS, not significant.
Effect of FoxO6 on hepatic lipid metabolism
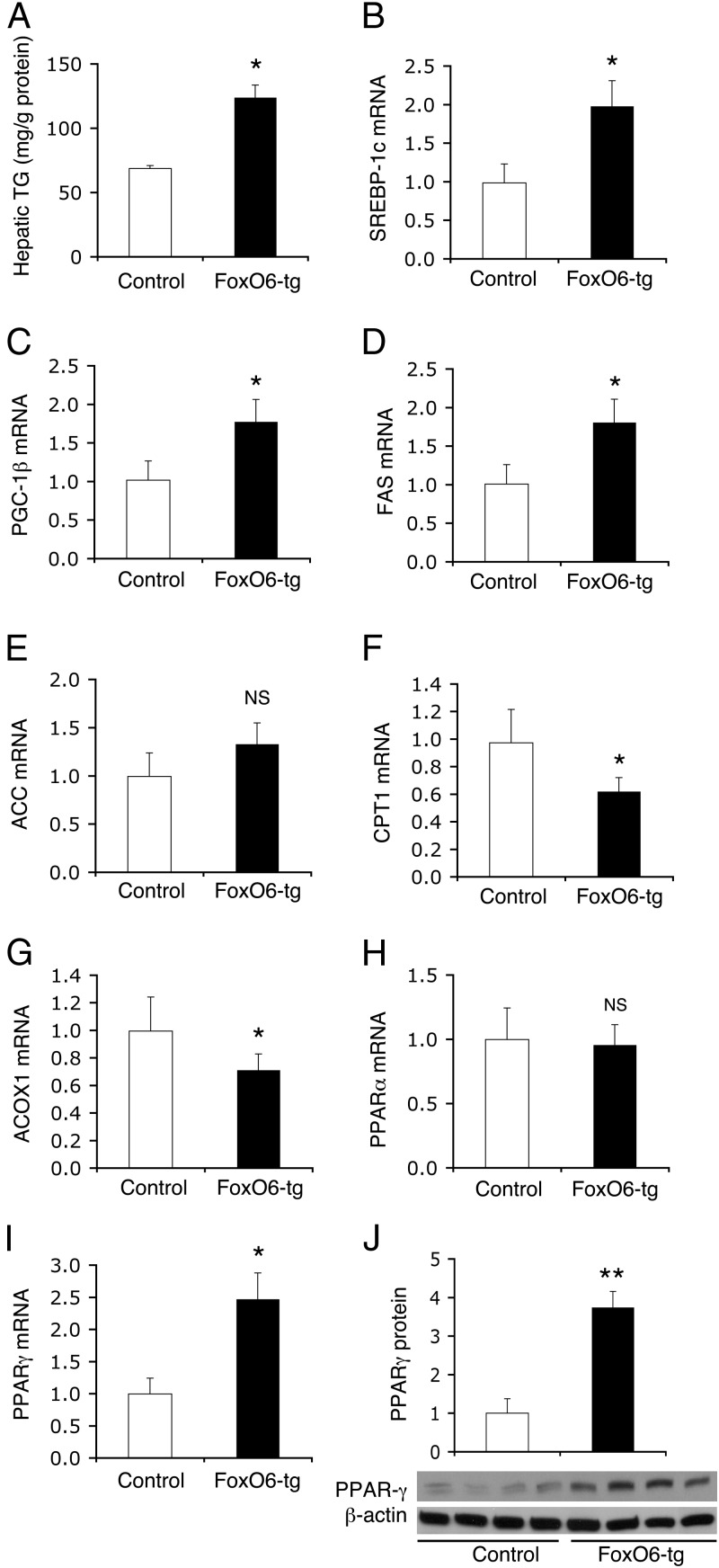
To determine the effect of FoxO6 on hepatic lipid metabolism, we measured hepatic TG content. FoxO6-tg mice, as opposed to control littermates, were associated with increased fat content in the liver (Figure 3A), consistent with the idea that FoxO6 promotes hepatic lipogenesis and augments hepatic VLDL-TG secretion. To characterize the underlying mechanism, we determined hepatic expression of genes in lipogenesis and fatty acid oxidation, 2 opposing pathways in hepatic lipid metabolism. We performed these studies in fasted mice to remove the confounding factor that is associated with variable food intake, accompanied by variable insulin secretion in individual mice under fed conditions. Indeed, hepatic FoxO6 activity is suppressed to basal levels in response to postprandial insulin release (20). Transgenic FoxO6-CA production resulted in significant induction in lipogenesis, as reflected in significantly increased expression of sterol regulatory element-binding protein (SREBP)-1c (Figure 3B), its coactivator PGC-1β (Figure 3C), and fatty acid synthase (FAS) (Figure 3D) mRNA levels, and a nonsignificant induction of acetyl-coenzyme A carboxylase (ACC) mRNA (Figure 3E) in the liver of FoxO6-tg vs control mice. This effect was accompanied by decreased expression of carnitine palmitoyltransferase (CPT)1 (Figure 3F) and acyl-coenzyme A oxidase 1(ACOX1) (Figure 3G), two key enzymes in fatty acid oxidation. Hepatic PPAR-α expression remained unchanged in FoxO6-tg vs control littermates (Figure 3H). Instead, we detected a significant induction in hepatic expression of PPAR-γ (Figure 3I), a nuclear receptor the activation of which is linked to enhanced lipid synthesis and increased fat storage in the liver (35–37). We confirmed this finding by immunoblot analysis, showing that hepatic PPAR-γ protein levels were markedly up-regulated in FoxO6-tg mice (Figure 3J).
Figure 3.
FoxO6-tg mice display increased hepatic lipogenesis. Male FoxO6-tg (n = 6; 32 weeks) and age/sex-matched control littermates (n = 4) were humanely destroyed after 16 hours of fasting. Liver tissues were retrieved for the determination of hepatic TG content (A). Aliquots of liver tissues were subjected to real-time qRT-PCR analysis for determining hepatic levels of SREBP-1c mRNA (B), PGC-1β mRNA (C), FAS mRNA (D), ACC mRNA (E), CPT1 mRNA (F), ACOX1 mRNA (G), PPAR-α mRNA (H), PPAR-γ mRNA (I), using β-actin mRNA as control. Additional aliquots of liver tissues were subjected to immunoblot assay for determining hepatic PPAR-γ protein levels using β-actin protein as control (J). *, P < .05; and **, P < .001 vs control. NS, not significant.
FoxO6 regulates hepatic PPAR-γ expression
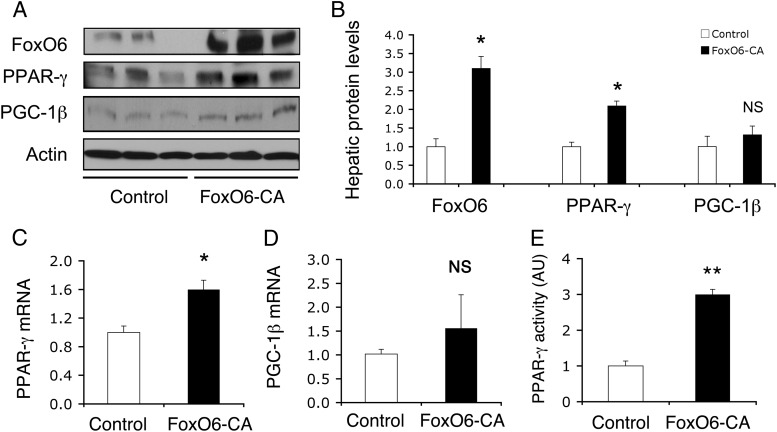
To understand the mechanism of FoxO6-elicited induction in lipogenesis, we hypothesized that FoxO6 might target key lipogenic genes for trans-activation. Because the SREBP-1c gene is not a direct target of the FoxO subfamily (28, 38), we centered our studies on the impact of FoxO6 on hepatic PGC-1β and PPAR-γ expression. To address the above hypothesis, we examined the effect of FoxO6-CA on PGC-1β and PPAR-γ expression in HepG2 cells. As shown in Figure 4, hepatic FoxO6-CA production resulted in a significant induction in PPAR-γ at both mRNA and protein levels, without significant alterations in hepatic PGC-1β mRNA and protein expression in HepG2 cells.
Figure 4.
FoxO6 regulates hepatic PPAR-γ expression. HepG2 cells were transduced with Adv-FoxO6-CA and control Adv-Empty vectors (200 pfu/cell). After a 24-hour incubation, cells were collected for immunoblot analysis (A). Hepatic protein levels of FoxO6, PPAR-γ, and PGC-1β were determined using actin protein as control (B). Aliquots of cells were used for preparing total RNA, which was subjected to real-time qRT-PCR analysis for determining PPAR-γ mRNA (C) and PGC-1β mRNA levels (D), using β-actin mRNA as control. Furthermore, HepG2 cells were cotransfected with PPREx3-TK-Luc and control pGL4.75 plasmids in the presence of Adv-FoxO6-CA or Adv-Empty control vector. After a 24-hour incubation, cells were subjected to dual luciferase activity assay. PPAR-γ activity was defined as the ratio of Firefly vs Renilla luciferase activity (E). *, P < .05; and **, P < .001 vs control. NS, not significant.
To bolster the idea that FoxO6 regulates hepatic PPAR-γ expression, we determined hepatic PPAR-γ activity in HepG2 cells with elevated FoxO6 production. HepG2 cells were transduced with Adv-FoxO6-CA and control Adv-Empty vectors, followed by determination of hepatic PPAR-γ activity, using the PPRE (PPAR-γ responsive element)-directed luciferase reporter system. PPAR-γ binds to PPRE and stimulates PPRE-directed luciferase activity, which is used as an index of PPAR-γ transcriptional activity. We detected a 3-fold induction of PPAR-γ activity in FoxO6-expressing HepG2 cells (Figure 4E).
FoxO6 stimulates TG production in cultured hepatocytes
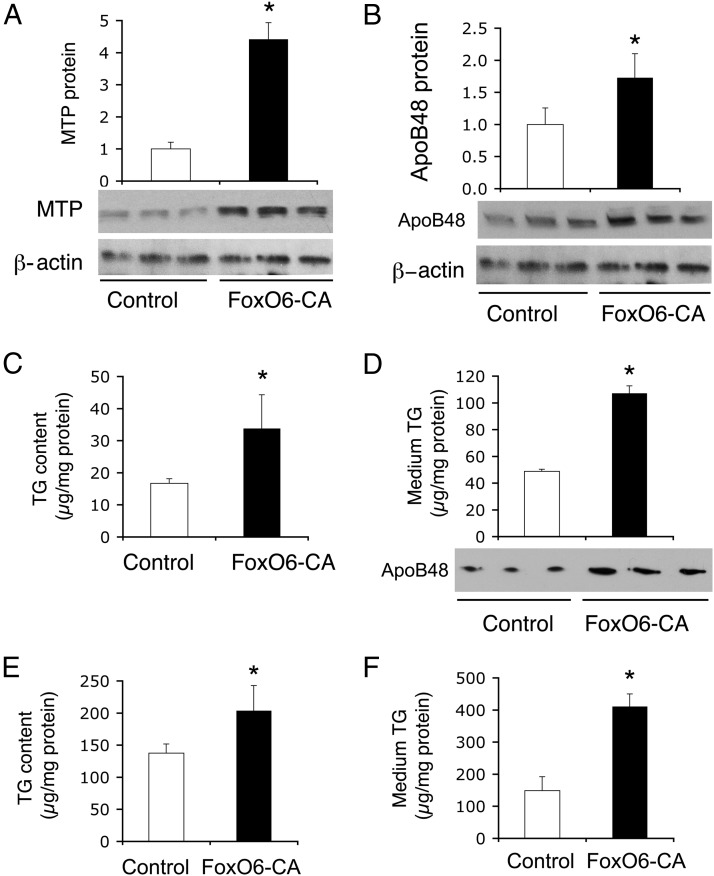
To corroborate the above findings, we determined the effect of FoxO6 on hepatic TG production in HepG2 cells. Adenovirus-mediated FoxO6-CA production resulted in significant induction in hepatic MTP (Figure 5A) and ApoB (Figure 5B) protein levels in HepG2 cells. This effect translated into a marked induction in hepatic TG production, as evidenced by significantly increased TG content (Figure 5C), and elevated medium TG and ApoB levels in conditioned medium (Figure 5D) obtained from FoxO6-CA vector-treated HepG2 cells.
Figure 5.
FoxO6 stimulates TG production in HepG2 and human hepatocytes. HepG2 cells were transduced with control Adv-Empty and FoxO6-CA vectors (200 pfu/cell). After a 24-hour incubation, cells were collected for determination of hepatic MTP (A) and ApoB48 (B) protein levels, and intracellular TG levels (C). Conditioned medium was collected for determination of medium TG and ApoB48 levels (D). Likewise, human primary hepatocytes were transduced with control Adv-Empty and FoxO6-CA vectors (200 pfu/cell). After a 24-hour incubation, TG levels were determined in human hepatocytes (E) and conditioned medium (F). *, P < .05 vs control.
To recapitulate the above findings, we replicated our studies in human primary hepatocytes. We treated human primary hepatocytes in culture with Adv-FoxO6-CA and control Adv-Empty vectors, followed by determination of TG in hepatocytes and conditioned medium. Hepatic FoxO6-CA production resulted in a significant elevation of TG concentrations in both hepatocytes (Figure 5E) and conditioned medium (Figure 5F) in FoxO6-CA vs control vector-treated human primary hepatocytes.
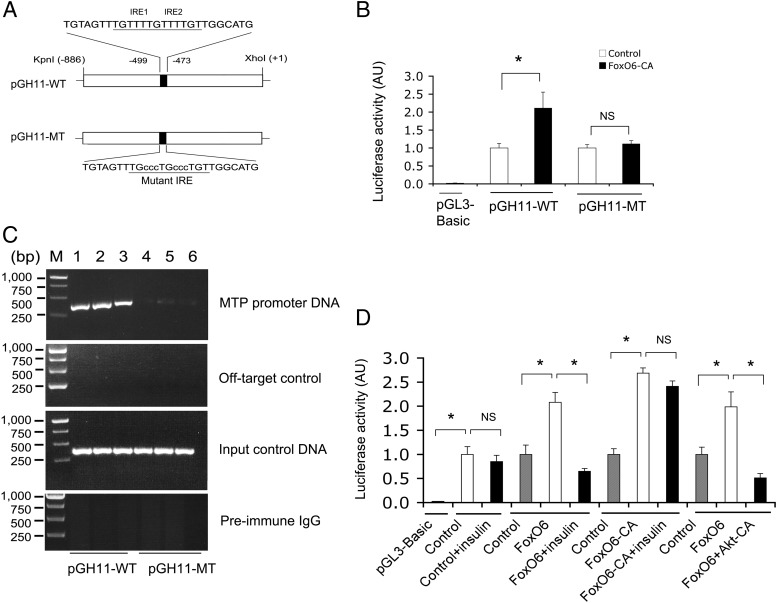
FoxO6 transactivates the MTP promoter activity
To probe the mechanism of FoxO6-mediated induction of VLDL-TG production, we hypothesized that FoxO6 targets the MTP gene for trans-activation. Implicit in this hypothesis is the presence of two tandem copies of FoxO6 binding motifs, known as IREs, within the MTP promoter (Figure 6A). To address this hypothesis, we tested the ability of FoxO6 to bind and enhance MTP promoter activity in HepG2 cells, using the MTP promoter-directed luciferase reporter assay. Adenovirus-mediated FoxO6-CA production resulted in two-fold induction of luciferase activity (Figure 6B). This effect correlated with FoxO6 binding to the MTP promoter, as determined by ChIP assay (Figure 6C). To substantiate these findings, we mutagenized the consensus FoxO6 binding sites, followed by analysis of the mutant MTP promoter activity in response to FoxO6 production in HepG2 cells. Mutations in the IRE motifs resulted in abolition of FoxO6-mediated induction of MTP promoter activity (Figure 6D), consistent with the disability of FoxO6 to bind to the mutant MTP promoter (Figure 6C).
Figure 6.
FoxO6 binds and stimulates the MTP promoter activity. A, Schematic depiction of wild-type (WT) and mutant versions (MT) of the mouse MTP promoter. Nucleotides of wild-type and mutant IRE motifs were underlined. B, MTP promoter activity. HepG2 cells were transfected with pGH11-WT or pGH11-MT, followed by transduction with Adv-FoxO6-CA or Adv-Empty control vectors. Plasmid pGL3-basic encoding promoter-less luciferase was used as control for determining baseline luciferase activity. Plasmid pGL4.75 expressing Renilla luciferase was included for normalizing transfection efficiency. After a 24-hour incubation, cells were subjected to dual luciferase activity assay for determining the MTP promoter activity. C, ChIP analysis of FoxO6 binding to MTP promoter. HepG2 cells pretransduced with Adv-FoxO6-CA vector were transfected with pGH11-WT (lanes 1–3) or pGH11-MT (lanes 4–6). After a 24-hour incubation, cells were subjected to ChIP assay using rabbit anti-FoxO6 antibody or preimmune IgG. Immunoprecipitates were analyzed by PCR assay using primers flanking the IRE DNA motif (−767/−369 nt) in the MTP promoter. This assay produced a 400-bp DNA from immunoprecipitates by anti-FoxO6, but not by preimmune IgG. As input control, aliquots of cell lysates prior to immunoprecipitation were subjected to PCR analysis. As off-target control, primers flanking a distal MTP promoter region (−3528/−3045 nt) devoid of IRE motifs were included in PCR analysis. D, MTP promoter activity in response to insulin or Akt. HepG2 cells were transfected with pGH11-WT, followed by transduction with Adv-FoxO6 or Adv-FoxO6-CA vector using Adv-Empty vector as control in the absence or presence of insulin (100 nM) or presence of Adv-Akt-CA vector (100 pfu/cell) in culture medium. After a 24-hour incubation, MTP promoter activity in cells was determined using the dual luciferase reporter assay. Data were from 3–5 independent experiments. *, P < .05 vs control. NS, not significant.
To address the hypothesis that FoxO6 mediates insulin action on hepatic MTP expression, we determined the ability of FoxO6 to stimulate MTP promoter activity in response to insulin, using the dual luciferase assay system. We showed that FoxO6 stimulated MTP promoter activity, and this effect was inhibited by insulin (Figure 6D). Likewise, FoxO6-CA also stimulated MTP promoter activity, but its stimulatory effect is insensitive to insulin (Figure 6D), consistent with the inability of FoxO6-CA to undergo insulin-dependent phosphorylation and inhibition (20).
Furthermore, we determined the ability of FoxO6 to stimulate MTP promoter activity in the absence or presence of Akt-CA, a constitutively active Akt that has been shown to phosphorylate its targets independently of insulin (39). In response to adenovirus-mediated Akt-CA production, FoxO6-mediated induction of MTP promoter activity was reversed to basal levels in HepG2 cells in the absence of insulin (Figure 6D).
Hepatic FoxO6 depletion ameliorates hypertriglyceridemia in db/db mice
To provide further physiological underpinning for FoxO6 regulation of hepatic lipid metabolism, we used siRNA-mediated gene-silencing approach to ablate FoxO6 expression in the liver of obese and diabetic db/db mice. Based on the observations that hepatic FoxO6 expression becomes deregulated in insulin-resistant liver (20), contributing in part to abnormalities in lipid metabolism in db/db mice, we hypothesized that selective FoxO6 inhibition in the liver would improve hepatic lipid metabolism and ameliorate hypertriglyceridemia in db/db mice.
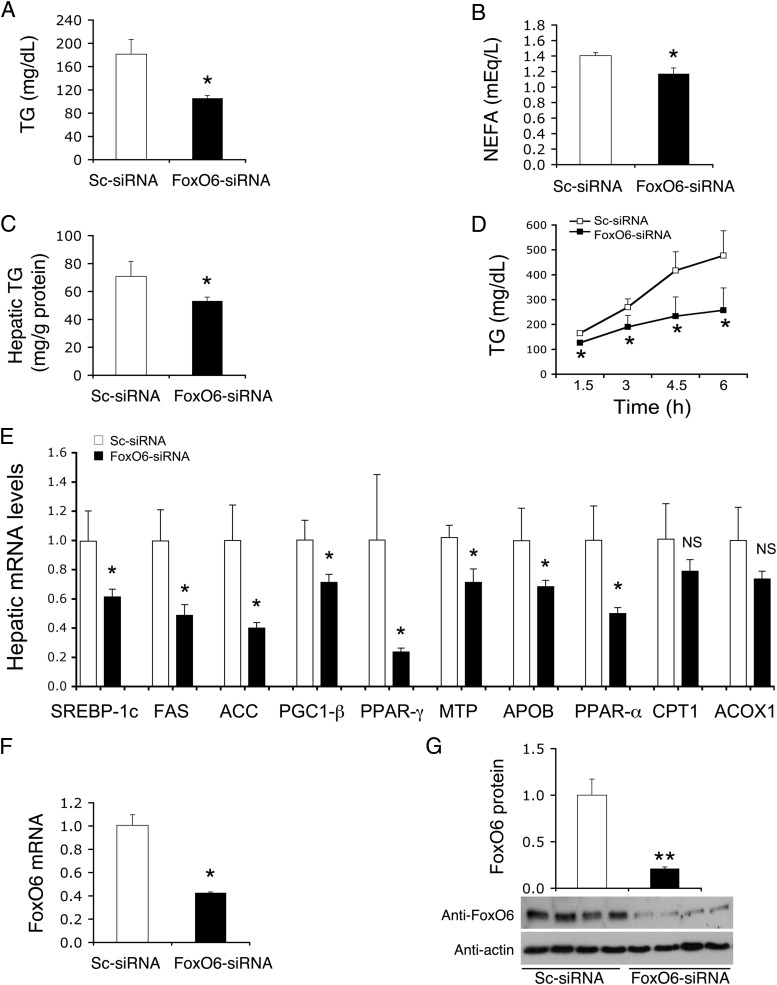
Diabetic db/db mice were iv injected with Adv-FoxO6-siRNA or scrambled Adv-Sc-siRNA vector, followed by the determination of lipid metabolism. We have validated the FoxO6-siRNA vector for its specificity in selective FoxO6 knockdown without affecting hepatic expression of other FoxO members in the liver (20). We detected a significant reduction in plasma TG (Figure 7A) and NEFA (Figure 7B) levels, accompanied by mitigation of hepatic fat deposition in FoxO6-siRNA vector-treated db/db mice (Figure 7C). Furthermore, hepatic FoxO6 knockdown resulted in significant reduction in hepatic VLDL-TG production in db/db mice (Fig, 7D).
Figure 7.
Effect of hepatic FoxO6 knockdown on TG metabolism in db/db mice. A, Plasma TG levels. B, Plasma NEFA levels. C, Hepatic TG content. D, Hepatic VLDL-TG production. Plasma TG levels were determined at different times after tyloxapol administration. E, Hepatic mRNA levels of key enzymes in lipogenesis, VLDL-TG production and fatty acid oxidation. F, FoxO6 mRNA levels. G, FoxO6 protein levels. Diabetic db/db mice (male, 12 weeks old) were randomly assigned to two groups (n = 7), which were treated with 1.5 × 1011 pfu/kg of FoxO6-siRNA or Sc-siRNA control vector. Plasma TG and NEFA levels were determined at day 10 after vector administration. VLDL production was determined at day 11 after vector administration. Mice were humanely destroyed after 16 hours of fasting at day 12, and liver tissues were collected for the determination of hepatic TG content and hepatic mRNA levels by real-time qRT-PCR assay using β-actin mRNA as control. Hepatic FoxO6 protein levels were determined by anti-FoxO6 immunoblot assay using anti-actin antibody as control. *, P < .05; and **, P < .001 vs control. NS, not significant.
To address the underlying mechanism, we determined the effect of FoxO6 knockdown on hepatic expression of key enzymes in lipogenesis vs fatty acid oxidation. Hepatic mRNA levels of lipogenic factors including SREBP-1c, FAS, ACC, PGC-1β, and PPAR-γ (Figure 7E) were significantly reduced in response to hepatic FoxO6 knockdown in db/db mice. Furthermore, we detected a significant reduction in hepatic MTP and ApoB mRNA levels (Figure 7E), consistent with the reduction of hepatic VLDL-TG production in FoxO6-siRNA-treated db/db mice (Figure 7D). As control, we determined hepatic FoxO6 expression levels, demonstrating that FoxO6 mRNA (Figure 7F) and FoxO6 protein (Figure 7G) levels were significantly decreased in FoxO6-siRNA vector-treated db/db mice.
In addition, we detected a significant reduction in PPAR-α mRNA levels in the liver of FoxO6-siRNA vector-treated db/db mice (Figure 7E). In contrast, hepatic mRNA levels of CPT1 and ACOX1 were unchanged in FoxO6-siRNA vector-treated db/db mice (Figure 7E). Likewise, no significant differences were detected in body weight between control Sc-siRNA (38.7 ± 0.33 g) and FoxO6-siRNA (39.5 ± 0.43 g) groups. These data indicate that FoxO6 knockdown curbed lipogenesis and suppressed hepatic VLDL production, contributing to the improvement of plasma TG metabolism and amelioration of hepatic steatosis in db/db mice.
Discussion
In this study, we characterized the role of FoxO6 in regulating hepatic MTP expression and VLDL-TG production in vivo and in vitro. MTP is an endoplasmic reticulum-resident protein that acts to transport neutral lipids to nascent ApoB polypeptide chains, the rate-limiting step in VLDL-TG assembly and secretion (1, 15, 40). We showed that FoxO6 targeted the MTP promoter for trans-activation, accounting for increased MTP production in the liver. This action contributed to augmented VLDL-TG production and elevated plasma TG levels in FoxO6-tg mice. We recapitulated this finding in HepG2 cells, in which FoxO6 stimulated hepatic MTP production, contributing to a significant induction in VLDL-TG and ApoB secretion. Furthermore, we showed that FoxO6-mediated induction of hepatic MTP expression was inhibited by insulin (or Akt-CA), correlating with the ability of insulin to promote FoxO6 phosphorylation and disable FoxO6 binding to target promoter DNA (20). In contrast, FoxO6-CA-mediated induction of MTP expression became refractory to insulin inhibition, due to the inability of FoxO6-CA to undergo insulin-dependent phosphorylation (20). Furthermore, we showed that siRNA-mediated FoxO6 knockdown in insulin-resistant liver decreased hepatic MTP expression, contributing to a significant reduction of hepatic VLDL-TG output in diabetic db/db mice. Together these data underscore the importance of FoxO6 in integrating insulin signaling to hepatic MTP expression for modulating VLDL-TG production in the liver.
In addition, transgenic FoxO6 expression was associated with augmented lipogenesis, contributing to increased fat accumulation in the liver of FoxO6-CA transgenic mice. To account for the underlying mechanism, we showed that hepatic expression of SREBP-1c, FAS, and ACC genes was significantly up-regulated in the liver of FoxO6-tg mice. Furthermore, FoxO6 was shown to stimulate hepatic PPAR-γ expression. PPAR-γ is a nuclear receptor that acts in complex with retinoid X receptor to promote lipogenesis in the liver (35, 41–43). Liver-specific PPAR-γ depletion attenuates lipogenesis and diminishes hepatic fat infiltration, protecting against fat-induced steatosis in mice (35, 42). PPAR-γ is markedly increased in the liver of mice with overt steatosis (37, 44) or dietary obesity (45–47) and in the liver of obese patients with clinical manifestation of hepatosteatosis (48). These data underscore the importance of PPAR-γ in promoting hepatic fat synthesis and storage in response to insulin resistance. Nevertheless, the mechanism underlying hepatic up-regulation of PPAR-γ activity in response to insulin resistance remains elusive. Our studies characterize FoxO6 as an important transcription factor for linking insulin resistance to PPAR-γ up-regulation in the liver. FoxO6 is subject to insulin inhibition. Loss of insulin inhibition consequently results in unchecked FoxO6 activity in insulin-resistant states (20). It is anticipated that this effect promotes PPAR-γ expression and enhances lipogenesis, contributing to abnormal fat accumulation in the liver in obesity and type 2 diabetes. In support of this notion, we showed that hepatic PPAR-γ expression was significantly reduced in response to hepatic FoxO6 knockdown in insulin-resistant liver, resulting in the diminution of hepatic lipogenesis and reduction of hepatic fat content in diabetic db/db mice.
Whereas insulin resistance is closely associated with hypertriglyceridemia, factors that mechanistically link insulin resistance to the pathogenesis of hypertriglyceridemia are incompletely characterized (1, 49, 50). We showed that FoxO6 stimulated hepatic MTP and ApoB expression, contributing to increased VLDL-TG secretion. This finding was reproduced in FoxO6-tg mice as well as in cultured HepG2 cells and human primary hepatocytes, raising the possibility that FoxO6-mediated induction of VLDL-TG secretion serves as a potential compensatory mechanism for protecting against excessive fat accumulation in the liver. Consistent with this view, Kamagate et al (23) show that FoxO1 mediates insulin-dependent regulation of MTP in regulating hepatic VLDL-TG secretion. Like FoxO1, FoxO6 activity is enhanced in the liver in response to fasting and inhibited by insulin in fed states (20, 23). FoxO6 expression becomes deregulated, accounting for its increased activity in insulin-resistant liver of mice with obesity and type 2 diabetes (20, 23). Unlike FoxO1 that undergoes insulin-dependent nuclear export, FoxO6 mediates insulin action on target gene expression in a distinct mechanism. Insulin inhibits FoxO6 activity by inducing FoxO6 phosphorylation and disabling its transcriptional activity without altering its subcellular distribution in hepatocytes (20). It follows that insulin signaling bifurcates downstream of Akt/PKB to two parallel checkpoints, FoxO1 and FoxO6, for adjusting the rate of hepatic VLDL-TG production in response to metabolic shift from fasting to feeding states.
We noted that FoxO6-tg mice also exhibited significantly higher plasma NEFA levels. This effect promoted NEFA influx into the liver, which in turn fueled VLDL-TG overproduction in FoxO6-tg mice. What is the source of elevated plasma NEFA levels in FoxO6-tg mice? It is plausible that this effect derived from FoxO6-mediated inhibition of fatty acid oxidation. Consistent with this explanation is that hepatic expression of CPT1 and ACOX1, two key enzymes in the fatty acid oxidation pathway, was significantly down-regulated in the liver of FoxO6-tg mice. However, adenovirus-mediated FoxO6 production did not result in significant alterations in fatty acid oxidation in HepG2 cells (Supplemental Figure 3), arguing against the possibility that FoxO6 directly inhibited hepatic fatty acid oxidation. Alternatively, elevated NEFA levels might result from increased lipolysis of TG stores in adipose tissues due to attenuated insulin action. Indeed, FoxO6-tg mice displayed insulin resistance, as evidenced by the development of glucose intolerance, hyperinsulinemia, and impaired insulin tolerance (20). Conversely, siRNA-mediated FoxO6 depletion in insulin-resistant liver improved insulin sensitivity, culminating in the reduction of hyperinsulinemia and improvement in glucose and insulin tolerance in diabetic db/db mice (20). This effect, which helped suppress VLDL-TG overproduction and mitigate steatosis in insulin-resistant liver, contributed to the reduction of plasma NEFA levels in FoxO6-siRNA-treated db/db mice. Thus, aside from its effect on hepatic VLDL-TG production, FoxO6 exerts an impact on NEFA metabolism. Further studies are warranted to characterize the role of FoxO6 in fatty acid oxidation under physiological and pathological conditions.
Although our FoxO6-tg mice expressed FoxO6-CA specifically in the liver, we could not preclude the possibility that intestinal production of TG-rich lipoproteins was a confounding factor for the induction of hypertriglyceridemia in FoxO6-tg mice. Indeed, intestinal overproduction of TG-rich particles contributes to the pathogenesis of hypertriglyceridemia in obesity and type 2 diabetes (51–54). FoxO6 is also expressed in the intestine (20). Further investigation is needed to determine the functional contribution of FoxO6 to intestinal lipoprotein production in mice with FoxO6 transgenic production conditionally in enterocytes.
In conclusion, our studies characterize FoxO6 as a significant transcription factor for mediating insulin action on hepatic MTP expression in regulating VLDL-TG production in the liver. Unchecked FoxO6 activity, resulting from insulin resistance, promoted hepatic MTP and ApoB overproduction, contributing to excessive VLDL-TG production and the pathogenesis of hypertriglyceridemia in type 2 diabetes. Hepatic FoxO6 knockdown suppressed hepatic MTP and ApoB expression, and curbed VLDL-TG production in insulin-resistant liver, resulting in the amelioration of hypertriglyceridemia in diabetic db/db mice. Our data characterize FoxO6 as a potential target, portending that selective inhibition of FoxO6 activity in insulin-resistant liver will improve lipid metabolism by suppressing both hepatic lipogenesis and VLDL-TG overproduction in type 2 diabetes.
Acknowledgments
We thank Sandra Slusher for technical assistance, and Dr. Steven Ringquist for critical proofreading of the manuscript.
This work was supported by National Institutes of Health Grant R01 DK087764. J.Y. was the recipient of Grant-in-Aid Scholarship 24–3549 from the Japan Society for the Promotion of Science.
Authors' contributions: D.H.K., T.Z., S.L., and H.H.D. conducted all studies in FoxO6-tg mice and diabetic db/db mice. V.C.N. and A.P. conducted studies in female FoxO6-tg mice. J.Y. performed in vitro studies in HepG2 cells and human primary hepatocytes. Y.F. conducted VLDL-TG production assay in db/db mice. R.H. and E.G. performed studies for NEFA metabolism. H.H.D. designed the entire study and wrote the manuscript.
Disclosure Summary: None of the authors has a conflict of interest to declare in this manuscript. H.H.D. is the guarantor of this study, taking full responsibility for the integrity of data and accuracy of data analysis.
Footnotes
- ACC
- acetyl-coenzyme A carboxylase
- ACOX1
- acyl-coenzyme A oxidase 1
- ApoB
- apolipoprotein B
- CA
- constitutively active
- ChIP
- chromatin immunoprecipitation
- CPT1
- carnitine palmitoyltransferase 1
- FAS
- fatty acid synthase
- FoxO6
- forkhead box O6
- HL
- hepatic lipase
- LPL
- lipoprotein lipase
- MTP
- microsomal triglyceride transfer protein
- NEFA
- nonesterified fatty acids
- nt
- nucleotides
- PGC-1β
- PPAR-γ coactivator 1-β
- PPAR
- peroxisome proliferator-activated receptor
- PPRE
- PPAR response element
- qRT-PCR
- quantitative RT-PCR
- SREBP-1c
- sterol regulatory element-binding protein 1c
- TG
- triglyceride
- VLDL
- very low-density lipoprotein
- VLDL-TG
- triglyceride-rich very low-density lipoprotein.
References
- 1. Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Betteridge DJ. Diabetic dyslipidaemia. Diabetes Obes Metab. 2000;2 (Suppl 1):S31–S36 [DOI] [PubMed] [Google Scholar]
- 3. Marcoux C, Tremblay M, Fredenrich A, Davignon J, Cohn JS. Lipoprotein distribution of apolipoprotein C-III and its relationship to the presence in plasma of triglyceride-rich remnant lipoproteins. Metabolism. 2001;50:112–119 [DOI] [PubMed] [Google Scholar]
- 4. Knopp RH, Retzlaff B, Aikawa K, Kahn SE. Management of patients with diabetic hyperlipidemia. Am J Cardiol. 2003;91:24E–28E [DOI] [PubMed] [Google Scholar]
- 5. Stewart MW, Laker MF, Alberti KG. The contribution of lipids to coronary heart disease in diabetes mellitus. J Intern Med Suppl. 1994;736:41–46 [PubMed] [Google Scholar]
- 6. DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med. 1997;50:191–197 [DOI] [PubMed] [Google Scholar]
- 7. Krauss RM. Atherogenicity of triglyceride-rich lipoproteins. Am J Cardiol. 1998;81(4A):13B–17B [DOI] [PubMed] [Google Scholar]
- 8. Brewer HB., Jr Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. Am J Cardiol. 1999;83(9B):3F–12F [DOI] [PubMed] [Google Scholar]
- 9. Bard JM, Charles MA, Juhan-Vague I, et al. Accumulation of triglyceride-rich lipoprotein in subjects with abdominal obesity. Arterioscher Thromb Vasc Biol. 2001;21:407–414 [DOI] [PubMed] [Google Scholar]
- 10. Olivieri O, Stranieri C, Bassi A, et al. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res. 2002;43:1450–1457 [DOI] [PubMed] [Google Scholar]
- 11. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229 [DOI] [PubMed] [Google Scholar]
- 12. Chan DC, Watts GF, Barrett PH, Mamo JC, Redgrave TG. Markers of triglyceride-rich lipoprotein remnant metabolism in visceral obesity. Clin Chem. 2002;48:278–283 [PubMed] [Google Scholar]
- 13. Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52:172–179 [DOI] [PubMed] [Google Scholar]
- 14. Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamagate A, Dong HH. FoxO1 integrates insulin signaling to VLDL production. Cell Cycle. 2008;7:3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426 [DOI] [PubMed] [Google Scholar]
- 17. Hwangbo DS, Gershman B, Gersham B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566 [DOI] [PubMed] [Google Scholar]
- 18. Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300:644–647 [DOI] [PubMed] [Google Scholar]
- 19. Kim DH, Zhang T, Lee S, Dong HH. 2013 FoxO6 in glucose metabolism. J Diabetes. 2013;5:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim DH, Perdomo G, Zhang T, et al. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60:2763–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278:35959–35967 [DOI] [PubMed] [Google Scholar]
- 22. van der Heide LP, Jacobs FM, Burbach JP, Hoekman MF, Smidt MP. FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem J. 2005;391:623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamagate A, Qu S, Perdomo G, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altomonte J, Cong L, Harbaran S, et al. Foxo1 mediates insulin action on ApoC-III and triglyceride metabolism. J Clin Invest. 2004;114:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamagate A, Kim DH, Zhang T, et al. FoxO1 links hepatic insulin action to endoplasmic reticulum stress. Endocrinology. 2010;151:3521–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su D, Coudriet GM, Hyun Kim D, et al. FoxO1 links insulin resistance to proinflammatory cytokine IL-1β production in macrophages. Diabetes. 2009;58:2624–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qu S, Perdomo G, Su D, D'Souza FM, Shachter NS, Dong HH. Effects of apoA-V on HDL and VLDL metabolism in APOC3 transgenic mice. J Lipid Res. 2007;48:1476–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qu S, Altomonte J, Perdomo G, et al. Aberrant forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jansen H, Breedveld B, Schoonderwoerd K. Role of lipoprotein lipases in postprandial lipid metabolism. Atherosclerosis. 1998; 141(Suppl 1):S31–S34 [DOI] [PubMed] [Google Scholar]
- 30. Chatterjee C, Sparks DL. Hepatic lipase, high density lipoproteins, and hypertriglyceridemia. Am J Pathol. 2011;178:1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raabe M, Véniant MM, Sullivan MA, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussain MM, Nijstad N, Franceschini L. Regulation of microsomal triglyceride transfer protein. Clin Lipidol. 2011;6:293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adeli K, Taghibiglou C, Van Iderstine SC, Lewis GF. Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc Med. 2001;11:170–176 [DOI] [PubMed] [Google Scholar]
- 35. Morán-Salvador E, López-Parra M, García-Alonso V, et al. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550 [DOI] [PubMed] [Google Scholar]
- 36. Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARγ2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288:E1195–E1205 [DOI] [PubMed] [Google Scholar]
- 37. Zhang YL, Hernandez-Ono A, Siri P, et al. Aberrant hepatic expression of PPARγ2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J Biol Chem. 2006;281:37603–37615 [DOI] [PubMed] [Google Scholar]
- 38. Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res. 2009;50(Suppl):S162–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276 [DOI] [PubMed] [Google Scholar]
- 42. Matsusue K, Haluzik M, Lambert G, et al. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARγ in promoting steatosis. Gastroenterology. 2008;134:556–567 [DOI] [PubMed] [Google Scholar]
- 44. Bedoucha M, Atzpodien E, Boelsterli UA. Diabetic KKAy mice exhibit increased hepatic PPARγ1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J Hepatol. 2001;35:17–23 [DOI] [PubMed] [Google Scholar]
- 45. Memon RA, Tecott LH, Nonogaki K, et al. Up-regulation of peroxisome proliferator-activated receptors (PPAR-α) and PPAR-γ messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPAR-γ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141:4021–4031 [DOI] [PubMed] [Google Scholar]
- 46. Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, Dube GP. Hepatic over-expression of peroxisome proliferator activated receptor γ2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem. 2001;224:29–37 [DOI] [PubMed] [Google Scholar]
- 47. Inoue M, Ohtake T, Motomura W, et al. Increased expression of PPARγ in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222 [DOI] [PubMed] [Google Scholar]
- 48. Pettinelli P, Videla LA. Up-regulation of PPAR-γ mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011;96:1424–1430 [DOI] [PubMed] [Google Scholar]
- 49. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32:2104–2112 [DOI] [PubMed] [Google Scholar]
- 50. Sparks JD, Dong HH. FoxO1 and hepatic lipid metabolism. Curr Opin Lipidol. 2009;20:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Risser TR. Intestinal very low density lipoprotein secretion in insulin-deficient rats. Diabetes. 1978;27:902–908 [DOI] [PubMed] [Google Scholar]
- 52. Haidari M, Leung N, Mahbub F, et al. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem. 2002;277:31646–31655 [DOI] [PubMed] [Google Scholar]
- 53. Avramoglu RK, Qiu W, Adeli K. Mechanisms of metabolic dyslipidemia in insulin resistant states: deregulation of hepatic and intestinal lipoprotein secretion. Front Biosci. 2003;8:d464–476 [DOI] [PubMed] [Google Scholar]
- 54. Lewis GF, Uffelman K, Naples M, Szeto L, Haidari M, Adeli K. Intestinal lipoprotein overproduction, a newly recognized component of insulin resistance, is ameliorated by the insulin sensitizer rosiglitazone: studies in the fructose-fed Syrian golden hamster. Endocrinology. 2005;146:247–255 [DOI] [PubMed] [Google Scholar]