Abstract
Ca2+/calmodulin (CaM)-dependent phosphorylation of myosin regulatory light chain (RLC) by myosin light chain kinase (MLCK) initiates smooth muscle contraction and regulates actomyosin-based cytoskeletal functions in nonmuscle cells. The net extent of RLC phosphorylation is controlled by MLCK activity relative to myosin light chain phosphatase activity. We have constructed a CaM-sensor MLCK where Ca2+-dependent CaM binding increases the catalytic activity of the kinase domain, whereas coincident binding to the biosensor domain decreases fluorescence resonance energy transfer between two fluorescent proteins. We have created transgenic mice expressing this construct specifically in smooth muscle cells to perform real-time evaluations of the relationship between smooth muscle contractility and MLCK activation in intact tissues and organs. Measurements in intact bladder smooth muscle demonstrate that MLCK activation increases rapidly during KCl-induced contractions but is not maximal, consistent with a limiting amount of cellular CaM. Carbachol treatment produces the same amount of force development and RLC phosphorylation, with much smaller increases in [Ca2+]i and MLCK activation. A Rho kinase inhibitor suppresses RLC phosphorylation and force but not MLCK activation in carbachol-treated tissues. These observations are consistent with a model in which the magnitude of an agonist-mediated smooth muscle contraction depends on a rapid but limited Ca2+/CaM-dependent activation of MLCK and Rho kinase-mediated inhibition of myosin light chain phosphatase activity. These studies demonstrate the feasibility of producing transgenic biosensor mice for investigations of signaling processes in intact systems.
A primary mechanism for initiating smooth muscle contraction involves an increase in [Ca2+]i, leading to myosin regulatory light chain (RLC) phosphorylation, crossbridge cycling, and force development (1, 2). Phosphorylation of Ser-19 on RLC of myosin II changes the orientation of myosin cross-bridges, allowing actin activation of myosin ATPase activity. A similar mechanism occurs with nonmuscle myosin II with effects on many cellular actomyosin-dependent functions. The Ca2+-dependent phosphorylation of RLC is mediated by Ca2+/calmodulin (CaM)-dependent myosin light chain kinase (MLCK), whereas myosin light chain phosphatase dephosphorylates RLC. In smooth muscles, agonists stimulate greater RLC phosphorylation and force than do depolarizing stimuli at comparable [Ca2+]i because of Ca2+-sensitization mechanisms (2). Although MLCK activity has been inferred from [Ca2+]i measurements, the extent of activation in vivo is not known. Previous investigations have shown that free Ca2+/CaM may be limiting for kinase activation despite the relative abundance of total CaM (40 μM) over MLCK (4 μM) (3). Indeed, recent evidence in nonmuscle cells suggests that there is a limiting pool of available Ca2+/CaM in competition for binding by different CaM targets (4). In addition, CaM may be tethered to MLCK in a way that does not activate the kinase but would promote a rapid and complete activation with increased [Ca2+]i (5).
The general tools for unraveling the qualitative and quantitative complexities in the RLC phosphorylation scheme have been limited to simultaneous measurements of [Ca2+]i and force, as well as RLC phosphorylation in fixed tissue or cell samples. More recently, genetically encoded fluorescent indicators based on GFP and its spectral variants have provided approaches for studying cell biological processes in culture (6, 7). Fluorescence resonance energy transfer (FRET)-based biosensors for Ca2+ and CaM were among the first to exploit GFP for intracellular detection of specific ligands and proteins (8, 9). Recently Chew et al. (10) reported a biosensor MLCK for determination of its localization and relative Ca2+/CaM-binding state in epithelial cells in culture by linking fluorescent indicator proteins to the C terminus of MLCK (10). Images of individual cells showed that MLCK was activated in lamellipodia during migration but not in the retracting tail.
We developed a different CaM-sensor MLCK capable of monitoring MLCK activation to obtain temporal and quantitative information on Ca2+/CaM binding to MLCK where Ca2+-dependent CaM binding increased kinase activity coincident with a decrease in FRET (11). The CaM-sensor MLCK was expressed in smooth muscle tissues of transgenic mice to obtain quantitative insights on CaM activation of MLCK relative to [Ca2+]i and RLC phosphorylation and force development. These results show that genetically encoded biosensors may be used to investigate physiological processes in tissues of transgenic mice.
Methods
Construction of SM8 35 KCS Plasmid. The pSM8 35 KCS construct was prepared by subcloning the 1.6-kb cDNA of Ca2+/CaM-sensor containing the MLCK CaM-binding sequence flanked by enhanced cyan fluorescent protein (ECFP) and enhanced yellow fluorescent protein (EYFP) (12) into the pSM8-CAT vector, which contains the smooth muscle α-actin promoter (13, 14). The 3.1-kb cDNA fragment of short rabbit smooth muscle MLCK was further subcloned into the site between the Ca2+/CaM-sensor gene and the smooth muscle α-actin promoter in pSM8-CAT vector to produce pSM8 35 KCS construct. Correct orientation of the smMLCK gene and fusion site between MLCK and the fragment of short rabbit smooth muscle MLCK was determined by restriction mapping and nucleotide sequencing.
Cell Culture and Biochemical Characterizations. A7r5 rat thoracic aorta smooth muscle cells (from ATCC, CRL-1444) were transfected with pSM8 35 KCS as described (15, 16). Expression of MLCK biosensor in transfected A7r5 cells was visualized by fluorescence microscopy. Transfected cells were harvested 72 h after transfection by trypsinization, and MLCK was extracted from cell pellets (15, 16).
MLCKs expressed in A7r5 cells were immunoprecipitated with either a mAb against smooth muscle MLCK (K36, Sigma) or anti-GFP Ab (MBL International, Woburn, MA). Briefly, 150 μl of cell lysate was clarified by centrifugation at 13,000 × g for 5 min at 4°C. The supernantant fraction was diluted with 450 μl of 20 mM 4-morpholinepropanesulfonic acid (Mops), pH 7.0/10 mM magnesium acetate, which was precleared with 30 μl (1:1, wt/vol) of protein A-Sepharose and incubated on ice for 1 h. Either K36 or anti-GFP Ab (5 μl) were added and incubated at 4°C for 16 h. Protein A-Sepharose (30 μl) was added to the mixture and incubated 2 h at 4°C after which the protein A-Sepharose beads were washed three times with 20 mM Mops, pH 7.0/10 mM magnesium acetate/100 mM NaCl/0.5 mM DTT. Washed immunoprecipitates were resuspended in 80 μl of 20 mM Mops, pH 7.0/10 mM MgSO4/2 mM DTT/10% glycerol and stored at -80°C. Ca2+/CaM-dependent activity of MLCK immunoprecipitated from transfected A7r5 cells was measured by rates of 32P incorporation into smooth muscle RLC (15).
Production of Transgenic pSM8 35 KCS Mice. The pSM8 35 KCS construct was digested with Sca-1, resulting in a 10-kb linearized construct, and the purified DNA was microinjected into fertilized oocytes, which were subsequently reimplanted into pseudopregnant ICR mice by standard procedures at the Transgenic Mouse Facility at the University of Texas Southwestern Medical Center. All animal protocols were approved by the Institutional Animal Care and Use Committee. Founder animals containing the SM8 35 KCS transgenes were identified by Southern blot analysis of tail genomic DNA by using a 32P-labeled 750-bp cDNA encoding EYFP as a probe. Positive founders were used to establish transgenic mouse lines. Eight different lines were established and characterized further. Three lines showed similar levels of expressed CaM-sensor MLCK that was 30–40% the amount of endogenous MLCK, whereas the other lines showed less or no expression (see below). Mice from the three lines appeared normal, and isolated smooth muscle tissues developed similar amounts of force with KCl or carbachol treatments (see below). To determine expression of CaM-sensor MLCK, mouse tissues were excised from transgenic mice and quick-frozen in liquid nitrogen. Tissues were analyzed by SDS/PAGE (15, 16).
Immunohistochemistry. Heart and bladder tissues were fixed in 2% paraformaldehyde in PBS at 4°C for 2 h, washed three times, and then immersed into 30% sucrose in PBS overnight. After washing the tissues were embedded in OCT compound, cut into 8-μm sections, and stored at -8°C. Subsequently, thawed tissue sections were washed in PBS and incubated in 0.5 M ammonium chloride in PBS at room temperature for 30 min. The sections were blocked in 10% donkey serum in PBS at room temperature for 1 h and then incubated in a 1:100 dilution of rabbit anti-GFP polyclonal Ab in 10% donkey serum/PBS for 1.5 h. After washing, the sections were incubated with donkey anti-rabbit IgG (H+L) Rho-conjugated secondary Ab, washed, dried, and mounted by Fluoroguard Antifade reagent (Bio-Rad). Images were obtained with an LSM 510 confocal laser scanning microscope (Zeiss).
Simultaneous Measurement of Contraction and [Ca2+]i or CaM-Sensor MLCK FRET. Mouse bladder tissue was obtained from 8–12-week-old CaM-sensor MLCK transgenic mice killed with pentobarbital. Simultaneous measurements of mechanical and optical parameters of muscle were made with a fluorescence myograph designed and constructed by K. Güth (Scientific Instruments, Heidelberg) (17), and output was recorded with a TA4000 recorder (Gould, Cleveland). Smooth muscle tissue was dissected into strips (0.5 × 0.5 × 8.0 mm) and mounted and stretched (×1.2 slack length) on a force transducer in a quartz cuvette (180 μl) for simultaneous force and fluorescence measurements. Muscle strips were illuminated by light from a mercury arc lamp filtered at 436 nm (D436/20). Emitted light was distributed to two photomultipliers fitted with D535/25 and D480/40 bandpass filters (Chroma Technology, Brattleboro, VT). The slit was adjusted so that the preparation filled the entire aperture. The ratio of emission intensities 480 to 535 nm and isometric force were recorded simultaneously.
Intact muscle strips were contracted with 65 mM KCl or 10 μM carbachol, and ratios of fluorescence values were expressed as a percentage of the maximal Ca2+-induced values obtained for each strip at the end of the experiment. Strips were incubated at 26°C in relaxing solution (RS) containing 20 mM Pipes, 5 mM magnesium methanesulfonate, 90 mM potassium methanesulfonate, 4 mM ATP, 4 mM EGTA, 1 mM DTT, 0.1 mM diisopropyl fluorophosphate, and 50 mM transepoxysuccinyl-l-leucylamido (4-guanidino) butane (pH 7.4), and they were then skinned for 20 min with RS containing 100 μM β-escin. The minimum ratio of fluorescence (Rmin) was obtained by superfusion with RS containing EGTA, and the maximum ratio of fluorescence (Rmax) was obtained at pCa 3.7 (pCa = -log10 Ca concentration) with 0.5 μM CaM. Rmin and Rmax values were 0.62 ± 0.04 and 1.19 ± 0.07, respectively.
To characterize Ca2+ sensitivity of the fluorescence ratio and force, as well as the spectral properties of bladder tissue from transgenic mice, strips were skinned by treatment with 0.5% Triton X-100 in RS with 0.5 μM CaM. The dependence of both force and fluorescence in response to different [Ca2+] was determined in the fluorescence myograph. The minimum ratio of fluorescence (Rmin) was obtained by superfusion with RS and the maximum ratio (Rmax) was obtained at pCa 3.7 with 0.5 μM CaM. Maximum force (Fmax) was obtained also at pCa 3.7. For measurements of emission spectra in transgenic tissues, bladder strips (2.0 × 0.5 × 8.0 mm) were attached to a coverslip at their ends by cyanoacrylate glue and skinned as described above, and the emission spectrum determined with an excitation wavelength of 430 nm in a 650–10S fluorescence spectrophotometer (Perkin–Elmer) (11).
Intracellular calcium concentrations were estimated by measures of Indo-1 fluorescence ratio in intact bladder strips. Bladder tissue was dissected into small strips (0.5 × 0.5 × 8.0 mm), stretched (×1.2 slack length), and incubated in the dark with physiological salt solution (PSS) containing 10 μM Indo-1 AM, 0.01% pluronic F-127, and 0.02% cremophor for 4 h at room temperature. After incubation, tissues were mounted in the fluorescence myograph and washed with fresh PSS for 30 min at 36°C. Strips were illuminated at 365 nm (D365/10X), and emission intensities were measured at 405 nm (D405/30) and 485 nm (D485/25). The ratio of fluorescence (R405/485) was determined and used to calculate [Ca 2+]i. Maximal fluorescence was obtained by superfusion with 50 μM ionomycin in the presence of 5 mM Ca2+; minimal fluorescence was obtained with Ca2+-free PSS containing 2 mM EGTA. After each experiment, autofluorescence was determined by superfusing with 20 mM Mn2+.
Results
We have constructed a biosensor MLCK capable of monitoring Ca2+/CaM binding and activation of the kinase in cells (11). This CaM-sensor MLCK contains short smooth muscle MLCK fused to two GFPs, ECFP and EYFP, linked by the MLCK CaM-binding sequence yielding FRET from the donor ECFP (480 nm emission) to the acceptor EYFP (525 nm emission) as shown in Fig. 1A. Ca2+-dependent CaM binding increases kinase activity coincident with a disruption in FRET measured as an increased ratio of emission at 480–525 nm (11). To examine the physiological properties of Ca2+/CaM regulation of MLCK in smooth muscle tissues, we used a smooth muscle α-actin promoter (18, 19).
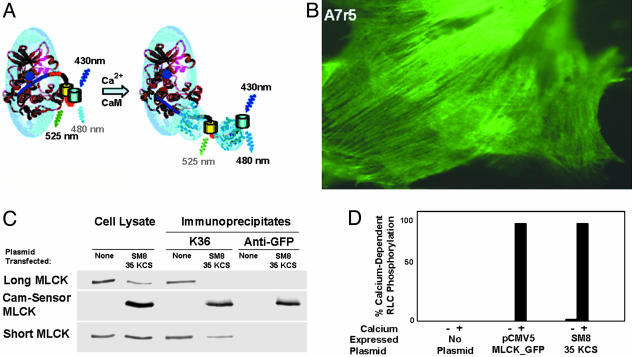
Fig. 1.
Expression of CaM-sensor MLCK in A7r5 smooth muscle cells. (A) Schematic representation illustrating change in structure of CaM-sensor MLCK upon Ca2+/CaM binding. Ellipsoids, with solved (CaM, light blue) and modeled (MLCK, magenta) ribbon structures inserted, represent structures determined previously by neutron scattering (27). EYFP and ECFP are shown as yellow and cyan cylinders linked by the CaM-binding sequence (red) and located C-terminal to the native CaM-binding sequence (red). When Ca2+/CaM binds to the two CaM-binding sequences, it displaces the autoinhibitory sequence (dark blue line) to expose the catalytic cleft (dark blue circle) and separates the EYFP/ECFP dimer, resulting in diminished FRET. (B) Rat thoracic aortic A7r5 cells transfected with pSM8 35 KCS plasmid. CaM-sensor MLCK bound to stress fibers was visualized 72 h after transfection by fluorescence microscopy. (C) Western blots of lysates and immunoprecipitates from A7r5 cells. Cells were transfected with pSM8 35 KCS plasmid and lysates collected after 72 h. Proteins were immunoprecipitated with either K36 or anti-GFP Abs and analyzed by Western blotting using K36 mAb to detect MLCK (short and long isoforms) endogenous to A7r5 cells and CaM-sensor MLCK. (D) MLCKs immunoprecipitated with anti-GFP Abs from lysates of A7r5 cells expressing CaM-sensor MLCK or MLCK fused to GFP show Ca2+-dependent activity in the presence of CaM.
Expression of CaM-Sensor MLCK in A7r5 Smooth Muscle Cells. A7r5 cells transfected with pSM8 35 KCS plasmid expressed CaM-sensor MLCK, as visualized by fluorescence microscopy (Fig. 1B). The expressed MLCK was localized to cellular filaments similar to the localization of a GFP-tagged MLCK expressed in A7r5 cells and consistent with its established binding to F-actin filaments (15, 16). Vertebrate smooth muscle MLCK exists in two isoforms, which are products of a single gene (20). The high-molecular-weight isoform (long MLCK) has a larger molecular mass because of an extended N terminus. Western blot analysis of cell lysates with a mAb against smooth muscle MLCK showed expression of both the long (220 kDa) and short (150 kDa) MLCK in nontransfected A7r5 cells (Fig. 1C). In A7r5 cells transfected with pSM8 35 KCS plasmid, both long MLCK and short MLCK were present, in addition to the 175-kDa CaM-sensor MLCK. The K36 Ab immunoprecipitated the long and short MLCKs in nontransfected cell lysates and, additionally, the CaM-sensor MLCK in cells transfected with pSM8 35 KCS plasmid (Fig. 1C). The anti-GFP Ab immunoprecipitated only CaM-sensor MLCK from A7r5 cell lysates transfected with pSM8 35 KCS plasmid. MLCK activity was found in immunoprecipitates obtained from A7r5 cells transfected with either pCMV5 MLCK-GFP (positive control) or pSM8 35 KCS (Fig. 1D). Both MLCK activities immunoprecipitated with the anti-GFP Ab were at least 98% Ca2+/CaM-dependent. Furthermore, changes in the emission spectra in the presence and absence of Ca2+/CaM were similar to the CaM-sensor MLCK expressed in HEK 293 cells with the CMV promoter (ref. 11 and data not shown). Thus, the plasmid containing the smooth muscle α-actin promoter expresses CaM-sensor MLCK that shows predicted biochemical and cellular properties.
Production and Characterization of Transgenic Mice Expressing CaM-Sensor MLCK. Transgenic SM8 35 KCS mice were screened for the presence of the inserted transgene by Southern blot analysis using a 750-bp EYFP cDNA probe. A total of eight positive founder mice (A–H) were identified. Expression of CaM-sensor MLCK was determined by Western blotting of bladder extracts prepared from mouse lines established from the founders. Based on densitometric measurements of Western blots of bladder smooth muscle tissues, CaM-sensor MLCK expression was always less than the endogenous short MLCK and ranged from 5–10% in lines E and H, 20% in line D, 25–30% in line B, and 30–40% in line F (data not shown). The F-line transgenic mice were used for subsequent studies. There was no obvious phenotype in any of the transgenic mouse lines. Furthermore, bladder tissues from the F line showed concentration-dependent and maximal contractile responses to KCl and carbachol similar to tissues from wild-type animals (data not shown).
Various tissues were analyzed for expression of CaM-sensor MLCK by Western blotting. Fig. 2A shows the expression of CaM-sensor MLCK in tissues abundant with smooth muscle cells with the highest expression in the bladder. No CaM-sensor MLCK was detected in extensor digitorum longus skeletal muscle, soleus skeletal muscle, kidney, liver, heart, or brain. Expression of the CaM-sensor MLCK transgene in smooth muscle tissues did not appear to change the expression of endogenous short MLCK when compared with control non-transgenic mouse smooth muscle tissues (Fig. 2 A).
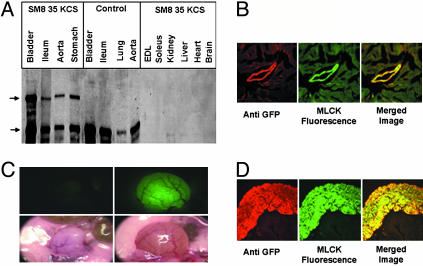
Fig. 2.
Expression of CaM-sensor MLCK in smooth muscle tissues of pSM8 35 KCS transgenic mice. (A) Western blot analysis of tissue extracts from transgenic and control mice with anti-MLCK Ab K36. Upper arrow, CaM-sensor MLCK; lower arrow, short MLCK. (B) Confocal images of a region of myocardium from transgenic mice containing a blood vessel. GFP was detected with Rho-labeled secondary Ab, whereas GFP fluorescence was detected in the fluorescein channel. (C) Images of control (Left) and CaM-sensor MLCK transgenic (Right) urinary bladder in situ. Photographs were taken with a stereo microscope equipped with MAA-03 Universal Light Source (BLS, Budapest) for GFP visualization (Upper) and standard illumination (Lower). (D) Confocal images of bladder cross-section from CaM-sensor transgenic mice. Tissue sections were treated as in B.
Although the CaM-sensor MLCK was not detected by Western blotting in heart, a careful examination of cardiac tissue by fluorescence microscopy of the sensor itself or with Abs to GFP showed its expression in blood vessels but not cardiac myocytes (Fig. 2B). Furthermore, CaM-sensor MLCK fluorescence was obvious in whole bladder (Fig. 2C) as well as bladder smooth muscle cells but not epithelial cells (Fig. 2D).
CaM-Sensor MLCK FRET in Permeable Smooth Muscle Tissues. Bladder smooth muscle strips from CaM-sensor MLCK transgenic mice were treated with Triton X-100 in RS containing EGTA to maintain low [Ca2+] (pCa >8.0). Measurements of the emission spectra of skinned bladder smooth muscle strips showed a high emission peak at 525 nm at pCa 8.0, which decreased with increasing [Ca2+] (Fig. 3A). A distinct peak at 480 was not obvious, probably because of tissue contributing factors; however, emission intensity did exhibit an increase with elevated [Ca2+]. The overall tissue fluorescence intensity from the transgenic animals was substantially greater than that obtained from wild-type tissues.
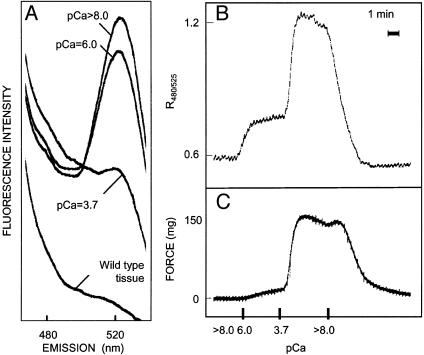
Fig. 3.
Fluorescent properties of permeabilized bladder strips. (A) Emission spectrum of Triton X-100-permeabilized bladder smooth muscle from CaM-sensor MLCK transgenic mice at pCa 8 to 3.7. Measurement of CaM-sensor MLCK FRET ratio (B) and force (C) in Triton X-100-permeabilized strip.
Skinned strips exhibited a Ca2+-dependent increase in the ratio of fluorescence emission at 480–525 nm increased with increased [Ca2+] and then returned to a low value at pCa 8.0 (Fig. 3B). There was a similar Ca2+-dependent increase in force that returned to a low level with relaxing conditions (Fig. 3C). The reason for the minor transient increase in force when the solution was changed from 3.7 to 8.0 is not known. It is not due to a delay in inactivation of the kinase because the FRET response declines immediately. Maximal contractile and FRET responses were obtained at pCa 3.7 and did not increase with greater concentrations of CaM (data not shown). Simultaneous measurements in skinned bladder smooth muscle showed that at pCa 6.0 FRET ratio increased 28%, whereas force increased 8% of the respective maximal responses measured at pCa 3.7. The greater extent of MLCK activation at pCa 6.0 relative to force is probably necessary for kinase activity to exceed myosin light chain phosphatase activity, thereby allowing significant RLC phosphorylation and force development.
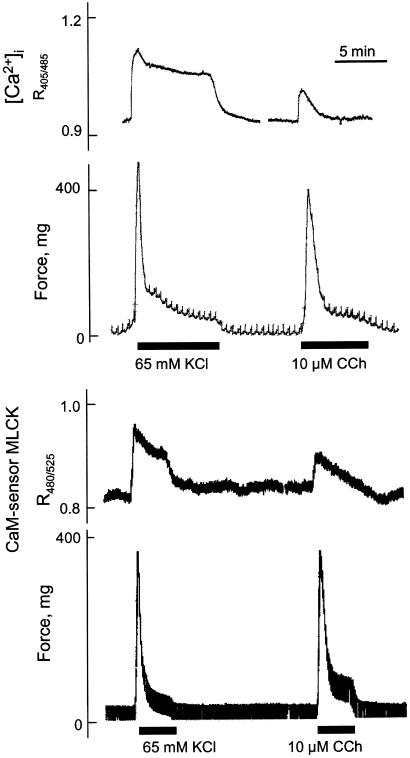
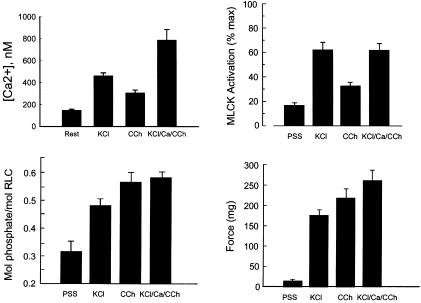
Ca2+/CaM Activation of MLCK in Intact Smooth Muscle Tissues. Representative temporal measurements of [Ca2+]i, CaM-sensor MLCK FRET and force responses in intact tissue strips are shown in Fig. 4. [Ca2+]i measurements were made in tissues without urothelium to obtain smooth muscle cell specific measurements. As expected, depolarization of smooth muscle cells with KCl showed a greater maximal increase in [Ca2+]i than that obtained with the agonist carbachol, although the force responses were comparable. In this phasic smooth muscle tissue, there is a rapid and marked increase in force associated with the increase in [Ca2+]i, both reaching maximal values at 1 min. The force then declines more rapidly than [Ca2+]i. The [Ca2+]i increased from 148 nM to 461 nM and 306 nM at 1 min for KCl and carbachol treatments respectively (Fig. 5). Measurements of CaM-sensor MLCK FRET showed temporal responses similar to changes in [Ca2+]i with a greater response in the presence of KCl than carbachol.
Fig. 4.
Simultaneous measurements of force and intracellular calcium (Upper) or force and FRET (Lower) in intact bladder strips stimulated by depolarization (KCl) or carbachol (CCh).
Fig. 5.
Measurements of [Ca2+]i, MLCK activation, RLC phosphorylation and force in intact bladder strips. Average values are shown for control (PSS), or contracted strips treated for 1 min with 65 mM KCl (KCl), 10 μM carbachol (CCh), or a mixture of KCl and CCh in PSS containing 10 mM calcium (KCl/Ca/CCh). Values represent mean ± SEM (n ≥ 5). *, P < 0.05, compared with PSS group; #, P < 0.5, compared with KCl group.
Under nonstimulating conditions, the phasic bladder smooth muscle was partially contracted with RLC phosphorylation at 0.32 mol phosphate per mol RLC and 18% MLCK activation (Fig. 5). With the KCl treatment for 1 min, the extent of MLCK activation increased to 62%, whereas RLC phosphorylation increased to 0.48 mol phosphate per mol RLC. The responses to carbachol for RLC phosphorylation and force were similar, however the extent of MLCK activation increased to only 32%.
To induce a maximal contractile response, tissues were treated simultaneously with 65 mM KCl/10 μM carbachol/10 mM CaCl2 (Fig. 5). With this treatment [Ca2+]i increased to a concentration (786 nM) significantly greater than KCl alone (461 nM). However, the extent of MLCK activation was similar with both treatments, suggesting that the limited MLCK activation may be due to an insufficient amount of CaM, not [Ca2+]i (Fig. 5). The RLC phosphorylation and force responses increased significantly compared with KCl alone and are likely to be due to activation of a Ca2+-sensitization mechanism mediated by Rho kinase and inhibition of myosin light chain phosphatase activity (2). Consistent with this scheme, Y27632, a Rho kinase inhibitor, had no effect on the extent of MLCK activation but significantly inhibited force development in smooth muscle tissues treated with carbachol (Table 1).
Table 1. Y27632 inhibits force but not MLCK activation in carbachol-stimulated smooth muscle.
| Treatment | % MLCK activation | Force, mg |
|---|---|---|
| Control | 10 ± 3 | 18 ± 3 |
| Carbachol | 36 ± 7 | 235 ± 30 |
| Carbachol + Y27632 | 30 ± 4 | 50 ± 10* |
Mouse bladder strips from transgenic mice were incubated for 15 min with or without 10 μM Y27632, followed by 1 min with or without 10 μM carbachol. Values represent mean ± SEM (n ≥ 5). *, P < 0.5, compared with carbachol alone.
Discussion
Results from previous investigations have led to the proposal that there may be insufficient free Ca2+/CaM to maximally activate target enzymes (3, 21). If this proposal is true, it has a significant impact on our understanding of the RLC signaling network and the relative contributions of factors that affect both RLC phosphorylation and dephosphorylation, particularly in smooth muscle tissues. We, therefore, developed a biosensor with short MLCK that was capable of monitoring Ca2+/CaM activation of the kinase in vivo. This Ca2+/CaM-dependent MLCK binds to stress fibers, thus exhibiting actin-binding properties similar to native smooth muscle MLCK (15, 16). Previous data showed the Ca2+-dependence of FRET changes and activation of this CaM-sensor MLCK were coincident and the catalytic properties (Km, Vmax) were not different from wild-type kinase (11).
CaM-sensor MLCK was expressed in fully differentiated smooth muscle cells in tissues of transgenic mice. Expression was limited to smooth muscle-containing tissues, including smooth muscle cells in blood vessels of the heart and bladder with no significant CaM-sensor MLCK in cardiac or skeletal myocytes, liver, kidney, or brain. By restricting expression of the CaM-sensor MLCK, we specifically monitored its activity in smooth muscle cells in intact tissues. Smooth muscle tissues express an abundance of the short MLCK relative to other cell types, and our selective transgene expression resulted in CaM-sensor MLCK that did not exceed 40% of the endogenous short MLCK. This relatively low level of selective expression may account for the lack of differences in contraction properties of isolated smooth muscles from wild-type and transgenic animals or any obvious phenotypic properties in live animals.
The signaling cascade involved in the regulation of smooth muscle contraction by means of RLC phosphorylation has evolved into more complicated schemes. For example, biochemical studies have suggested a scheme in which CaM with two Ca2+ bound to its C-terminal domain would be tethered to MLCK under resting conditions in cells in a way that does not activate the kinase but would lead to a rapid and complete activation upon two Ca2+ binding in the CaM N-terminal domain with increased [Ca2+]i (5). Recent structural studies showed that (Ca2+)2/CaM has a similar collapsed structure to (Ca2+)4/CaM when bound to MLCK, although they are at different positions relative to the catalytic cleft of the kinase (22). Thus, both (Ca2+)2/CaM and (Ca2+)4/CaM would be predicted to decrease FRET similarly. Our measurements showed no apparent dissociation between changes in FRET and kinase activity with increasing Ca2+/CaM concentrations in cell lysates or in permeable cells (11). In isolated bladder tissues from transgenic mice, there was a small extent of kinase activation associated with significant RLC phosphorylation and force in the nonstimulated muscles, probably because of constant tonus of the phasic bladder smooth muscles. However, the extent of MLCK activation under these conditions was small, indicating that most of the kinase did not have bound Ca2+/CaM.
The results also show that MLCK is not activated maximally at high [Ca2+]i. These physiological results with smooth muscle tissues are consistent with recent observations on cells in culture, in which a limited amount of CaM is available to activate MLCK (4, 11, 21). This limitation may arise from competition with other target proteins that bind CaM with high affinity in Ca2+-independent and Ca2+-dependent manners.
Additional complexities in the RLC phosphorylation scheme were shown with the introduction of Ca2+-dependent fluorophores for measurements of [Ca2+]i in cells and tissues (6). They revealed a general discrepancy in the Ca2+-dependence of RLC phosphorylation and force (23, 24). Agonists typically produce small increases in [Ca2+]i in smooth muscle tissues relative to depolarization with high concentrations of KCl, yet the extent of RLC phosphorylation and force are similar. The mechanisms responsible for this apparent increase in sensitivity to [Ca2+]i with agonists were examined subsequently by many investigators where it was found to involve primarily inhibition of myosin light chain phosphatase activity by means of coupling among ligand receptors, G proteins, and guanine nucleotide-binding factors (2). A major pathway involves RhoA activation of Rho kinase, which phosphorylates a myosin phosphatase-targeting subunit of protein phosphatase type 1 (MYPT1) leading to inhibition of myosin light chain phosphatase activity (25, 26). Consistent with these observations, we found that a Rho kinase inhibitor decreased force developed with carbachol while not significantly affecting MLCK activation. How these mechanisms interact with the MLCK activation mechanism is not clear, but one important consideration is how much MLCK is activated because the extent of RLC phosphorylation depends on the ratio of kinase to phosphatase activities. An unexpected observation from our studies was the small extent of CaM-sensor MLCK activation with an agonist yielding a net maximal increase of only 14%. Thus, a rapid and coordinated inhibition of myosin light chain phosphatase activity with carbachol treatment may enhance the effect of the small extent of Ca2+/CaM-dependent MLCK activation.
Genetically encoded indicators that measure protein–protein interactions offer opportunities for investigations on physiological processes when combined with molecular genetics for transgene expression. Expression of our CaM-sensor MLCK in smooth muscle cells in tissues of mice provides an example of this approach.
Acknowledgments
We thank Arthur Strauch for the generous gift of the smooth muscle α-actin promoter and Pam Silver (Dana–Farber Institute) for the generous gift of the anti-GFP Ab. This work was supported by National Institutes of Health Grant HL29043, the Moss Heart Fund, the Fouad A. and Val Imm Bashour Distinguished Chair in Physiology (to J.T.S.), and National Institutes of Health Grant DK53863 (to A.P.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CaM, calmodulin; ECFP, enhanced cyan fluorescent protein; EYFP, enhanced yellow fluorescent protein; FRET, fluorescence resonance energy transfer; MLCK, myosin light chain kinase; Mops, 4-morpholinepropanesulfonic acid; PSS, physiological salt solution; RLC, regulatory light chain; RS, relaxing solution.
References
- 1.Kamm, K. E. & Stull, J. T. (2001) J. Biol. Chem. 276, 4527-4530. [DOI] [PubMed] [Google Scholar]
- 2.Somlyo, A. P. & Somlyo, A. V. (2003) Physiol. Rev. 83, 1325-1358. [DOI] [PubMed] [Google Scholar]
- 3.Tansey, M. G., Luby-Phelps, K., Kamm, K. E. & Stull, J. T. (1994) J. Biol. Chem. 269, 9912-9920. [PubMed] [Google Scholar]
- 4.Tran, Q. K., Black, D. J. & Persechini, A. (2003) J. Biol. Chem. 278, 24247-24250. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, J. D., Snyder, C., Walsh, M. & Flynn, M. (1996) J. Biol. Chem. 271, 761-767. [DOI] [PubMed] [Google Scholar]
- 6.Tsien, R. Y. (1998) Annu. Rev. Biochem. 67, 509-544. [DOI] [PubMed] [Google Scholar]
- 7.Zhang, J., Campbell, R. E., Ting, A. Y. & Tsien, R. Y. (2002) Nat. Rev. Mol. Cell Biol. 3, 906-918. [DOI] [PubMed] [Google Scholar]
- 8.Miyawaki, A., Llopis, J., Heim, R., McCaffery, J. M., Adams, J. A., Ikura, M. & Tsien, R. Y. (1997) Nature 388, 882-887. [DOI] [PubMed] [Google Scholar]
- 9.Persechini, A., Lynch, J. A. & Romoser, V. A. (1997) Cell Calcium 22, 209-216. [DOI] [PubMed] [Google Scholar]
- 10.Chew, T. L., Wolf, W. A., Gallagher, P. J., Matsumura, F. & Chisholm, R. L. (2002) J. Cell Biol. 156, 543-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geguchadze, R., Zhi, G., Lau, K. S., Isotani, E., Persechini, A., Kamm, K. E. & Stull, J. T. (2004) FEBS Lett. 557, 121-124. [DOI] [PubMed] [Google Scholar]
- 12.Persechini, A. & Cronk, B. (1999) J. Biol. Chem. 274, 6827-6830. [DOI] [PubMed] [Google Scholar]
- 13.Foster, D. N., Min, B., Foster, L. K., Stoflet, E. S., Sun, S., Getz, M. J. & Strauch, A. R. (1992) J. Biol. Chem. 267, 11995-12003. [PubMed] [Google Scholar]
- 14.Min, B. H., Foster, D. N. & Strauch, A. R. (1990) J. Biol. Chem. 265, 16667-16675. [PubMed] [Google Scholar]
- 15.Lin, P., Luby-Phelps, K. & Stull, J. T. (1999) J. Biol. Chem. 274, 5987-5994. [DOI] [PubMed] [Google Scholar]
- 16.Smith, L., Su, X., Lin, P., Zhi, G. & Stull, J. T. (1999) J. Biol. Chem. 274, 29433-29438. [DOI] [PubMed] [Google Scholar]
- 17.Guth, K. & Wojciechowski, R. (1986) Pflügers Arch. 407, 552-557. [DOI] [PubMed] [Google Scholar]
- 18.Wang, J., Niu, W., Witte, D. P., Chernausek, S. D., Nikiforov, Y. E., Clemens, T. L., Sharifi, B., Strauch, A. R. & Fagin, J. A. (1998) Endocrinology 139, 2605-2614. [DOI] [PubMed] [Google Scholar]
- 19.Maeda, S., Sutliff, R. L., Qian, J., Lorenz, J. N., Wang, J., Tang, H., Nakayama, T., Weber, C., Witte, D., Strauch, A. R., Paul, R. J., Fagin, J. A. & Clemens, T. L. (1999) Endocrinology 140, 1815-1825. [DOI] [PubMed] [Google Scholar]
- 20.Watterson, D. M., Schavocky, J. P., Guo, L., Weiss, C., Chlenski, A., Shirinsky, V. P., Van Eldik, L. J. & Haiech, J. (1999) J. Cell Biochem. 75, 481-491. [PubMed] [Google Scholar]
- 21.Persechini, A. & Stemmer, P. M. (2002) Trends Cardiovasc. Med. 12, 32-37. [DOI] [PubMed] [Google Scholar]
- 22.Heller, W. T., Krueger, J. K. & Trewhella, J. (2003) Biochemistry 42, 10579-10588. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, M. J. & Morgan, K. G. (1989) Pflügers Arch. 413, 637-643. [DOI] [PubMed] [Google Scholar]
- 24.Himpens, B. & Casteels, R. (1990) Pflügers Arch. 416, 28-35. [DOI] [PubMed] [Google Scholar]
- 25.Feng, J., Ito, M., Ichikawa, K., Isaka, N., Nishikawa, M., Hartshorne, D. J. & Nakano, T. (1999) J. Biol. Chem. 274, 37385-37390. [DOI] [PubMed] [Google Scholar]
- 26.Murthy, K. S., Zhou, H., Grider, J. R. & Makhlouf, G. M. (2003) Am. J. Physiol. Gastrointest. Liver Physiol. 284, G1006-G1016. [DOI] [PubMed] [Google Scholar]
- 27.Krueger, J. K., Zhi, G., Stull, J. T. & Trewhella, J. (1998) Biochemistry 37, 13997-14004. [DOI] [PubMed] [Google Scholar]