Abstract
Endometriosis is a disease defined by the ectopic growth of uterine endometrium. Stem cells contribute to the generation of endometriosis as well as to repair and regeneration of normal endometrium. Here we demonstrate that the selective estrogen receptor modulator bazedoxifene (BZA), administered with conjugated estrogens (CEs), leads to regression of endometriosis lesions as well as reduction in stem cell recruitment to the lesions. Female mice underwent transplantation of male bone marrow. Endometrium was transplanted in the peritoneal cavity of half to create experimental endometriosis. Mice with or without experimental endometriosis were randomized to BZA/CE or vehicle treatment. Endometriosis lesions, bone marrow-derived mesenchymal stem cell engraftment of the lesions, and eutopic endometrium as well as ovarian stimulation were assessed. BZA treatment significantly reduced lesion size, gland number, and expression of proliferation marker proliferating cell nuclear antigen. Ovarian weight was not affected. Stem cells were recruited to the endometriosis lesions, and this recruitment was dramatically reduced by BZA/CE treatment. Stem cell engraftment was reduced in the uterus of animals with endometriosis; however the number of stem cells engrafting the uterus was completely restored by treatment with BZA/CE. Competition between endometriosis and the eutopic endometrium for a limited supply of stem cells and depletion of normal stem cells flux to the uterus is a novel mechanism by which endometriosis interferes with endometrial function and fertility. BZA/CE not only treats lesions of endometriosis, it also dramatically reduces stem cell recruitment to the lesions and restores stem cell engraftment of the uterine endometrium.
Endometriosis is defined by the presence of endometrial glands and stroma in extrauterine locations. It is a common, benign, estrogen-dependent condition affecting women in their reproductive years (1). The prevalence ranges from 6%–12% of asymptomatic women to 35%–50% of those with pelvic pain or infertility (2–5). A conclusive noninvasive diagnostic tool to allow early detection is not currently available. The lack of diagnostic tests leads to a delay in detection, with approximately 7–12 years of latency from the onset of symptoms to definitive diagnosis (6, 7). We likely still do not know the full extent of this disease.
There are several theories to explain the etiology and pathogenesis of endometriosis. The first of proposed etiologies, Sampson's theory of retrograde menstruation, is widely accepted and explains the high incidence of the disease in areas surrounding the fimbriated ends of fallopian tubes (8–11). However, this theory does not account for the discrepancy between the greater than 90% prevalence of retrograde menstruation and the approximately 10% prevalence of the disease; nor does it explain the occurrence of extrapelvic endometriosis. Multiple factors, including a genetic predisposition or deficient immune surveillance, play a role in the growth of the endometriotic implants outside of the uterus (12). Altered cellular phenotypes including an invasive malignant capacity (13), inflammation (14), increased capacity for vasculogenesis (15), altered hormonal environment with autonomous estradiol production, and progesterone resistance (16) have all been implicated in the pathogenesis of endometriosis (17). Multiple genes are expressed differentially in the eutopic endometrium of endometriosis patients compared with normal individuals (18–20). Similarly, several genetic loci have been implicated in this disease (21). A genome-wide association meta-analysis identified new endometriosis risk loci and found a significant overlap in polygenic risk for endometriosis between European and Japanese genome-wide association cohorts (22).
Theories attempting to explain the occurrence of endometriosis in extra pelvic locations include the following: lymphatic or hematogenous dissemination of endometrial cells (23), coelomic metaplasia (24), hormonal or immunologic factors (25), embryonic or Mullerian rests (26), and ectopic differentiation of bone marrow-derived stem cells to endometriosis (27–29). Stem cells not only contribute to endometriosis but also are part of the normal healing process of the endometrium, Stem cells from bone marrow, and perhaps other sources, travel to and engraft the uterus of both humans and other species (27, 28) The flux of stem cells to the uterus is increased by uterine damage or inflammation (30). The availability of stem cells is likely a limiting factor in repair of the endometrium after significant trauma. It is not known whether the presence of endometriosis affects the engraftment of stem cells by the uterus. Similarly, in assessing treatments for endometriosis it is important to evaluate not only the effect on the endometriotic lesion itself; it is also essential to consider the effect on normal uterine endometrium in this reproductive age population, including stem cell recruitment as an essential means of uterine repair.
There are a number of potential interventions for endometriosis, and treatment depends on whether the primary symptom is pain or subfertility (31, 32). The aims of the interventions are reduction or removal of ectopic endometrial implants, reduction of disease progression, pain relief, and treatment of infertility (33).The medical treatments available include oral contraceptives, progestins, danazol, and GnRH agonists. These act by creating either a pseudopregnancy (progestin dominant) or hypoestrogenic (pseudomenopause) state (34). On average, surgery does not provide any greater relief of pain than medical therapy nor does it provide lesser recurrence rates (35–38). Current medical therapies are limited due to significant side effect profiles. Progestins can induce breast tenderness, bloating, and mood changes, whereas danazole is associated with androgenic effects such as acne and hirsuitism, and GnRH agonist use can lead to menopausal symptoms (39–45). There is a need for new targeted and selective medical therapies for endometriosis.
Selective estrogen receptor modulators (SERMS) bind estrogen receptors (ESR1 and ESR2) and can act as either agonists or antagonists, depending on the target tissue. They generally act as ER agonists in the skeleton, on serum lipid metabolism and some coagulation factors but as antagonists (in the presence of an estrogen like estradiol) in the uterus and breast (47). One SERM, TZE-5323, was shown to reduce the volume of endometriosis implants in a rat model of endometriosis without affecting serum estradiol concentration or decreasing bone mineral density (BMD) in intact rats (48).
The SERM raloxifene (RLX) is marketed in the United States for the prevention and treatment of osteoporosis (49) and the prevention of breast cancer in high-risk individuals (ref). A study evaluating the use of RLX to treat endometriosis in an animal model showed a decrease in explant weight by 70% on day 7 and 68% on day 14 (50). In a clinical trial, the use of RLX to treat endometriosis in women with chronic pelvic pain was discontinued when the RLX-treated group experienced greater pain and required a second surgery sooner than the placebo group (51). Unfortunately, the dose used in the human trial was lower on a weight-adjusted basis than the effective dose used in the animal model.
Bazedoxifene (BZA) is another SERM being evaluated for the treatment of menopausal women, both singly and in combination with CEs (conjugated estrogens) (BZA/CE). Alone, BZA does not stimulate the endometrium in postmenopausal women (52, 53). In the murine uterus, BZA, in combination with 17β-estradiol, had the greatest antagonistic effect compared with other SERMs including RLX and lasofenoxifene (54). A tissue-selective estrogen complex (TSEC), which pairs a SERM with one or more estrogens, is currently under investigation for the treatment of menopausal symptoms (55). BZA was chosen due its selective estrogen receptor antagonism and estrogen receptor degradation in the endometrium (56). The TSEC containing BZA/CE has proven effective in treating menopausal symptoms and maintaining BMD without inducing endometrial growth in several clinical trials (57–62).
The efficacy of BZA in a mouse model of endometriosis was evaluated by our laboratory. BZA significantly reduced the mean size of implants, endometrial cell proliferation, and ESR1 expression. The regression of endometriosis likely involved decreased estradiol-mediated cell proliferation (63). In this study we determine the efficacy of the TSEC, BZA/CE, in a mouse model of endometriosis. We also demonstrate an effect of endometriosis on uterine stem cell engraftment and the ability of treatment to restore normal stem cell flux.
Materials and Methods
Animal care and treatment
Female (8–10 weeks of age) and an equal number of male C57BL/6 mice were obtained from Charles River Laboratories and kept under controlled conditions (a 12-hour light, 12-hour dark cycle and 22°C). All mice were allowed to have at least 1 week of acclimation to this environment before surgery. Twenty female C57BL/6 mice were lethally irradiated with 2 doses of 4.8 Gray 3 hours apart and subsequently received 1 × 107 unfractionated bone marrow cells from male donors by jugular vein injection within 1 hour of the second irradiation dose (64). Donor bone marrow was flushed from the femurs, tibias, and humeri of 8- to 10-week-old male mice with cold sterile PBS. The marrow suspension was filtered through sterile 70 μm Nitex mesh (Becton, Dickinson and Co.). Recipient mice were allowed a period of 10 days to recover from the bone marrow transplant after which they were randomly divided into 4 groups. In the nonendometriosis groups 1 and 2, skin and peritoneal incisions were made; however no endometrial tissue was transplanted. In groups 3 and 4, experimental endometriosis was created. Laparotomy was performed by midline incision under anesthesia using xylazine (Lloyd Laboratories) and ketamine (Fort Dodge Animal Health). Whole uterus was removed from C57BL/6 female donor mice (which were used as tissue donors for the ectopic uterine endometrium) washed in PBS, and divided into 2 horns. The lumen of each horn was opened longitudinally and transversely divided into 2 pieces. Two pieces of each uterine horn were sutured to the parietal peritoneum of each recipient mouse to create experimental endometriosis. Finally, the abdominal wall was closed using 4–0 Vicryl sutures. Experimental endometriosis was created in a total of 10 mice, which constituted groups 3 and 4. After surgery, to allow the establishment of endometriotic implants, all 4 groups were initially administered sc estradiol 5 μg/kg/d in dimethylsulfoxide (10%) plus sesame oil (90%), for 10 days (65).
Groups 2 and 4 received BZA (Pfizer) at a dose of 3 mg/kg/d by ip injection in dimethylsulfoxide (10%) plus sesame oil (90%), in combination with CE at 3 mg/kg/d administered orally using a gavage tube (54, 65). Groups 1 and 3 received CE (3 mg/kg/d) only, administered orally using a gavage tube. BZA used in this study was provided by Pfizer. After 2 months of treatment, mice were subsequently euthanized, and uteri and ectopic endometrial lesions were collected from all groups. Ectopic implants were measured (length and width) and then fixed in 10% formalin and embedded in paraffin for histologic and immunohistochemical analysis, as well as Y chromosome fluorescent in situ hybridization (Y FISH). Harvested uteri were processed in the same manner. Histology was examined by 2 observers blinded to the treatment group. Five high-power fields were examined from each of 5 slides obtained from each specimen (eutopic endometrium or endometrosis) This study was approved by Yale University's Institutional Animal Care and Use Committee, conforming to the US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training.
To determine whether there was a direct effect of BZA on the bone marrow cells, 2 additional male mice were treated with BZA (3 mg/kg/d) for 2 weeks prior to bone marrow harvest. Marrow was transplanted into 4 females treated identically to those in group 1 above.
Immunohistochemistry
Formalin-fixed paraffin-embedded biopsy specimens were cut into serial sections 5 μm thick, placed on coated slides, and deparaffinized through a series of xylene and ethanol washes. Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA) expression was performed as previously described (54). A 5-minute rinse in distilled water was followed by steaming slides in 0.01 M sodium citrate buffer for 20 minutes and cooled for 20 minutes. Slides were rinsed for 5 minutes in PBS with 0.1% Tween 20 (PBST), and sections were circumscribed using a hydrophobic pap pen. Endogenous peroxidase was inactivated using 3% hydrogen peroxide for 5 minutes followed by a 5-minute PBST wash. After a preincubation with 2% horse serum in PBST for 1 hour at room temperature to block nonspecific sites, slides were incubated with the primary antibody for PCNA (Fl-2b1; 1:400), purchased from Santa Cruz Biotechnology overnight at 4°C in a humidified chamber. Normal goat IgG (Santa Cruz) was used as a negative control. Horse antigoat biotinylated secondary antibody was used for PCNA and applied for 1 hour at 4°C. Slides were washed in 1×PBST, incubated in ABC Elite (Vector Laboratories) for 15 minutes at room temperature, washed in 1×PBST, and incubated for 5 minutes in diaminobenzidine (Vector). A 20-second exposure to hematoxylin was used as a counterstain. All slides were processed simultaneously for each primary antibody. Slides were rehydrated through 3-minute ethanol and xylene washes and mounted with Permount (Fisher Scientific). For PCNA labeling index, nuclei from more than 1000 cells were counted from at least 10 random fields in each sample. The PCNA labeling index was calculated as the percentage of positive cell nuclei. Student's t test was used to compare the labeling index between CE/BZA and CE-only treated mice.
Y FISH and immunofluorescence
Formalin-fixed paraffin-embedded biopsy specimens were cut into serial sections 3 μm thick, placed on coated slides, and deparaffinized through a series of xylene and ethanol washes. Sections were treated with Retrievagen A solution (BD Biosciences) for 30 minutes at 100°C and then 20 minutes at room temperature. The Y chromosome probe was generated following our previously published method (27). Y-FISH was performed using a digoxigenin-labeled Y chromosome probe and antidigoxigeninrhodamine antibody (Roche Diagnostics). After Y FISH, slides were incubated simultaneously with both 1:20 rat antimouse CD45 (BD Biosciences) and 1:100 rat antimouse F4/80 (eBioscience, Inc.) at 4°C overnight followed by 1:500 anti-rat-Alexa 488 (Molecular Probes) for 1 hour at 37°C. All slides were coverslipped using Vectashield Mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories). Positive controls for Y chromosome consisted of sections of the testis. These were processed simultaneously in each staining run. Cells staining positive for Y chromosome and CD45 were counted in 3 sections per animal. At least 150 000 cells were counted for each cell type. Results were compared using Student's t test.
Results
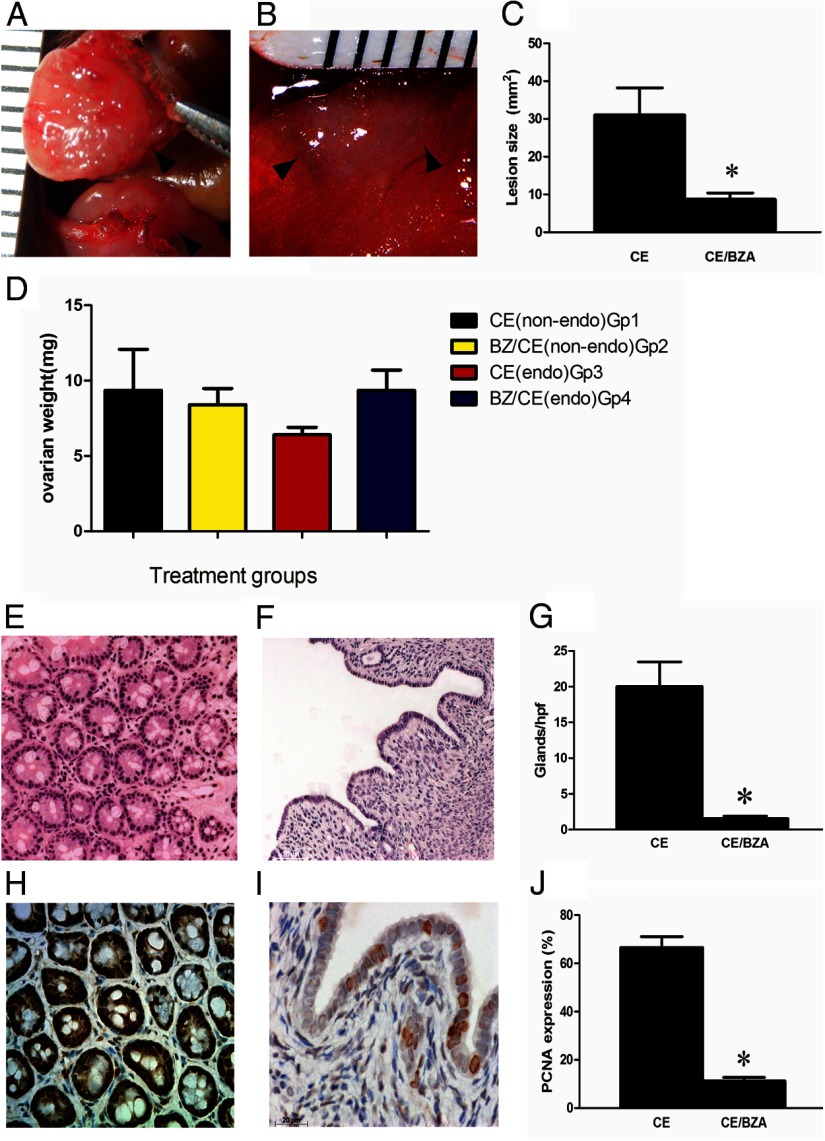
All mice appeared healthy after 2 months of treatment. Vaginal cytology was used to assess estrous cyclicity. All lesions, in both groups, were identified at the sites of initial suturing (n = 10 mice with a total of 20 lesions). Adhesions were seen between the lesions and the peritoneum. BZA/CE-treated mice (group 4) had inactive cystic lesions whereas the CE treated group (group 3) had large red lesions. The surface area of the endometriotic lesions was calculated by multiplying the length (millimeters) by the width (millimeters). There was a significant reduction in the mean endometriosis lesion size in the BZA/CE-treated group compared with the CE treated group, 8.7 and 31 mm2, respectively (P = .001) (Figure 1). There was no evidence of ovarian enlargement/cyst formation in any of the treatment groups. There was no statistically significant difference in ovarian weight between the BZA/CE- and CE-treated groups (Figure 1D).
Figure 1.
Endometriotic lesions and ovarian weight, 8 weeks after treatment A, Representative image of the CE-treated group; B, the CE/BZA-treated group; and C, mean lesion size; n = 20 (10 lesions per group in 5 mice). *, P = .001. D, No statistically significant difference in the ovarian weight in the 4 treatment groups. Photomicrographs of endometriosis lesions stained with hematoxylin and eosin are demonstrated in panel E (CE treated) and panel F (CE/BZA treated) with a significant reduction in the number of glands/high-power field as demonstrated in panel G, *, P = .0007. Panels H (CE treated) and I (CE/BZA treated) demonstrate PCNA expression as a marker of proliferation in the ectopic endometrial glands and stroma. A significant reduction in PCNA expression is observed after treatment. J, *, P < .0001. endo, endometriotic.
Histologic examination showed a significant reduction in gland number/high-power field in the ectopic endometrium in response to BZA treatment (20.0 ± 3.7 vs 1.6 ± 0.4, respectively) P = .0007 (Figure 1). The eutopic endometrium (Figure 2) showed a similar decrease in gland number/high-power field in response to BZA treatment (16.3 ± 0.7 vs 2.9 ± 0.02, respectively) P = .0001. Ectopic and eutopic endometrium treated with CE showed significant gland crowding when compared with the CE/BZA-treated groups. The epithelial cell height in the ectopic and eutopic endometrium of the CE/BZA-treated mice was significantly decreased compared with CE-treated mice. The luminal height in the eutopic lesions in response to BZA treatment measured 48.3 ± 7.2 compared with 19.1 ± 4.7 μm in the BZA-treated animals, P = .001.
Figure 2.
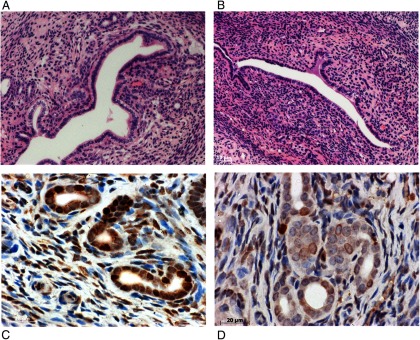
Representative photomicrographs of the uterus in the CE-treated (panel A) and CE/BZA-treated (panel B) groups Note the significant reduction in the number of glands in the CE/BZA treated group as was observed in the endometriotic lesions. Panels C, (CE treated) and D (CE/BZA treated) show PCNA expression in the eutopic endometrial glands and stroma. A significant reduction in PCNA expression was noted, P < .0001.
PCNA was used as a marker of proliferation. A labeling index was used to assess the number of cells expressing PCNA. The labeling index decreased in the group treated with BZA/CE compared with CE: 66.56 vs 11.22% in the ectopic endometrium and 52.75 vs 18.07% in the eutopic endometrium (P < .0001 in both groups) (Figures 1 and 2).
FISH was used to identify the Y chromosome (red) indicating a bone marrow-derived stem cell origin. Immunofluorescence for CD45 (green) was used to identify leukocytes and distinguish them from endometrial cells. 4′,6-Diamidino-2-phenylindole identified nuclei (blue). We compared the differential recruitment of the Y chromosome-positive stem cells to the ectopic and eutopic endometrium. CD45-stained cells (white blood cells) were excluded from all counts (Figure 3). A significant reduction in the percentage of stem cells recruited to the ectopic endometrium (from 4.48% to 0.91%, P < .0001) was seen after the addition of BZA to CE (Figure 3, A and B). The opposite effect was seen in the eutopic endometrium in which a significantly higher percentage of stem cells were recruited to the eutopic endometrium with the addition of BZA to CE (0.41 to 1.31%, P = .006) (Figure 3 D).
Figure 3.
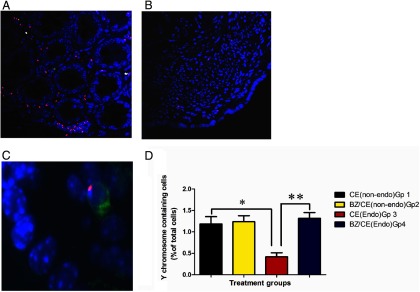
Y FISH/immunoflourescent images of the ectopic lesions and the uterus Y chromosome (red), CD45 (green), nuclei (blue). Panel A (CE) and panel B (CE/BZA) show Y chromosome signals in the ectopic lesions demonstrating engraftment of male bone marrow-derived stem cells. C, An example of a Y +/CD45+ leukocyte that was excluded from the analysis. Note the significantly lower number of Y chromosome signals recruited to the ectopic lesions under the influence of BZA, P < .0001. Panel D compares the number of Y+/CD45− cells recruited to the uteri (endometrial glands and stroma) in each treatment group. *, P = .04; **, P = .0006 The presence of endometriois results in reduced uterine stem cell engraftment whereas treatment restores stem cell engraftment to control levels. endo, endometriotic.
Figure 3D illustrates the differences in the percentage of endometrial cells of bone marrow stem cell origin recruited to the eutopic endometrium in the 4 treatment groups. Group 1 (CE treated; no endometriosis) is representative of normal stem cell recruitment to a well-estrogenized eutopic endometrium. In group 2 (BZA/CE treated; no endometriosis), the addition of BZA does not alter stem cell recruitment to the eutopic endometrium in the absence of endometriosis. In group 3 (CE treated; endometriosis), a significant reduction in the percentage of stem cell recruited is seen (P = .004), suggesting that the presence of endometriosis recruits these cells away from the uterus. However, the addition of BZA to CE (group 4: CE/BZA treated; endometriosis) prevents this flux of stem cells from the uterus to the endometriosis, allowing recruitment of a similar number of stem cells to the uterus as is normally seen in the absence of endometriosis.
To assure that BZA/CE did not have a direct effect on the stem cells' ability to migrate, engraft, or survive in the endometrium, 4 additional female mice were transplanted with bone marrow obtained from BZA/CE-pretreated bone marrow donors. Transplant into CE-treated females showed similar engraftment when compared with bone marrow stem cells derived from untreated mice. BZA/CE treatment of the bone marrow donor did not affect the ability of the cells to populate the endometrium.
Discussion
SERMs have a spectrum of ER agonistic/antagonistic activity depending on the target tissue and the type of SERM. BZA does not stimulate the endometrium in postmenopausal women (52, 53). Compared with other SERMs, BZA is best able to counteract the effect of estrogens on endometrial proliferation (54). BZA has been proven to significantly reduce the mean size of endometriotic implants and effectively treat endometriosis in animal models (62). The effect on endometriosis appears to be mediated through a decrease in ER expression and suppression of estrogen-mediated cell proliferation (62).
TSECs, which pair a SERM with one or more estrogens, are currently under investigation for the treatment of menopausal symptoms (55). BZA has a better tissue-selective profile than other SERMS; it has a greater ER antagonistic effect on the endometrium and a lower antagonist effect in the central nervous system, thus not fully inhibiting the positive effect of the CE on the central nervous system (ie, reducing hot flash frequency and severity). BZA/CE, the first developed TSEC tested in large clinical trials, significantly reduced the frequency and severity of vasomotor symptoms, improved vulvovaginal atrophy, and significantly increased BMD, without inducing proliferation of the breast or endometrium (56–61).
The uterine-selective antagonist effect also makes BZA an ideal candidate for the treatment of endometriosis. In this study, the TSEC, BZA/CE, proved effective in treating endometriosis in a mouse model. Despite the addition of CE, BZA maintained the ability to shrink the ectopic implants. This effect of BZA was seen in the eutopic and ectopic endometrium, confirming the antagonistic effect of BZA on both.
We have previously demonstrated that stem cells from bone marrow and other sources can contribute to the generation of endometriosis (27–29). Here we demonstrate that BZA treatment leads to decreased stem cell engraftment in endometriosis. Decreased stem cell recruitment to the lesions is a novel mechanism by which BZA and perhaps other treatments affect endometriosis.
Stem cells also engraft the uterus and play a role in uterine repair after injury. An estrous cycle and endogenous hormone production are not required for stem cell recruitment to the uterus; uterine stem cell flux is increased in response to injury or inflammation rather than in response to the hormonal changes (30). Similarly, in this study stem cell recruitment to the uterus was not significantly different between treatment groups in the absence of endometriois. BZA did not seem to have an influence on the migration of stem cells to the uterus in the absence of disease. However, BZA not only reduced endometriosis lesion size, it also reduced stem cell recruitment to the ectopic sites. The decrease in stem cell contribution to the endometriotic lesions may have prevented their continued growth and contributed to lesion regression. Inhibition of stem cell recruitment is a novel mechanism by which this and perhaps other drugs treat endometriosis.
Further, we demonstrate that a limited supply of circulating stem cells is recruited away from the uterus to lesions of endometriosis, a novel mechanism explaining how endometriosis affects the eutopic endometrium. Smaller inactive lesions recruited fewer stem cells and allowed a greater number of stem cells to engraft the uterus. Ectopic endometriosis implants seem to function as a “sponge,” attracting stem cells and leading to their migration away from the uterus. In this model BZA treatment restored a normal level of stem cell recruitment to the uterus, comparable to that of control groups without endometriosis.
Stem cell engraftment is believed to be a mechanism that enables uterine repair and regeneration after injury. Migration of stem cells away from the uterus may adversely affect endometrial receptivity and fertility in patients with endometriosis. The ability of stem cells to enhance endometrial receptivity has reported benefits in a murine model of Asherman's syndrome (66). Several recent studies have suggested that treating endometriosis with surgery or long-term suppressive therapy may improve endometrial receptivity and implantation (46, 67–69). These therapies may also restore stem cell flux to the uterus and result in improved endometrial function and fertility.
Acknowledgments
This work was supported by Pfizer and National Institutes of Health Grant HD076422 (to H.S.T.). S.S. was supported by the Partnership and Ownership Initiative (ParOwn), Egyptian Ministry of Higher Education and Scientific Research.
Present address for S.S.: Wayne State University, Detroit, Michigan, and University of Alexandria, Alexandria, Egypt.
Disclosure Summary: S.S. and H.N have nothing to declare. B.K is an employee of Pfizer. H.T has received grant support from Pfizer.
Footnotes
- BMD
- bone mineral density
- BZA
- bazedoxifene
- CE
- conjugated estrogen
- PBST
- PBS with 0.1% Tween 20
- PCNA
- proliferating cell nuclear antigen
- RLX
- raloxifene
- SERM
- selective estrogen receptor modulator
- TSEC
- tissue-selective estrogen complex
- Y FISH
- Y chromosome fluorescent in situ hybridization.
References
- 1. Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83(1–5):149–155 [DOI] [PubMed] [Google Scholar]
- 2. Houston DE, Noller KL, Melton LJ, 3rd, Selwyn BJ, Hardy RJ. Incidence of pelvic endometriosis in Rochester, Minnesota, 1970–1979. Am J Epidemiol. 1987;125(6):959–969 [DOI] [PubMed] [Google Scholar]
- 3. Surrey ES, Schoolcraft WB. Management of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2003;30(1):193–208 [DOI] [PubMed] [Google Scholar]
- 4. Houston DE. Evidence for the risk of pelvic endometriosis by age, race and socioeconomic status. Epidemiol Rev. 1984;6:167–191 [DOI] [PubMed] [Google Scholar]
- 5. Kuohung W, Jones GL, Vitonis AF, et al. Characteristics of patients with endometriosis in the United States and the United Kingdom. Fertil Steril. 2002;78(4):767–772 [DOI] [PubMed] [Google Scholar]
- 6. Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod,. 1996;11(4):878–880 [DOI] [PubMed] [Google Scholar]
- 7. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–373.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–154 [PubMed] [Google Scholar]
- 9. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154(1):39–43 [DOI] [PubMed] [Google Scholar]
- 10. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21(2):542–544 [DOI] [PubMed] [Google Scholar]
- 11. Barbieri RL. Stenosis of the external cervical os: an association with endometriosis in women with chronic pelvic pain. Fertil Steril. 1998;70(3):571–573 [DOI] [PubMed] [Google Scholar]
- 12. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17(5):637–653 [DOI] [PubMed] [Google Scholar]
- 13. Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84(1):16–21 [DOI] [PubMed] [Google Scholar]
- 14. Rana N, Braun DP, House R, Gebel H, Rotman C., Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65(5):925–930 [PubMed] [Google Scholar]
- 15. McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11(1):220–223 [DOI] [PubMed] [Google Scholar]
- 16. Zeitoun K, Takayama K, Sasano H, et al. Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinol Metab. 1998;83(12):4474–4480 [DOI] [PubMed] [Google Scholar]
- 17. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):93–110.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999; 14(5):1328–1331 [DOI] [PubMed] [Google Scholar]
- 19. Penna I, Du H, Ferriani R, Taylor HS. Calpain5 expression is decreased in endometriosis and regulated by HOXA10 in human endometrial cells. Mol Hum Reprod. 2008;14(10):613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penna IA, Hongling Du, Kallen AN, Taylor HS. Endothelin type A receptor (ETA) expression is regulated by HOXA10 in human endometrial stromal cells. Reprod Sci. 2010;17(5):471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grechukhina O, Petracco R, Popkhadze S, et al. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med. 2012;4(3):206–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nyholt DR, Low SK, Anderson CA, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44(12):1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927; 3(2):93–110 43 [PMC free article] [PubMed] [Google Scholar]
- 24. Gruenwald P. Origin of endometriosis from the mesenchyme of the coelomic walls. Am J Obstet Gynecol. 1942;44:474 [Google Scholar]
- 25. Levander G, Normann P. The pathogenesis of endometriosis; an experimental study. Acta Obstet Gynecol Scand. 1955;34(4):366–398 [DOI] [PubMed] [Google Scholar]
- 26. Von Recklinghausen F. Adenomyomas and cystadenomas of the wall of the uterus and tube: their origin as remnants of the wolffian bosy. Wien Klin Wochenschr. 1896;8:530 [Google Scholar]
- 27. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–2086 [DOI] [PubMed] [Google Scholar]
- 28. Taylor H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85 [DOI] [PubMed] [Google Scholar]
- 29. Figueira PG, Abräo MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann NY Acad Sci. 2011;1221: 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21(18):3324–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ACOG Committee on Practice Bulletins ACOG Practice Bulletin No. 11: medical management of endometriosis. Obstet Gynecol. 1999;94(6):1–14 [PubMed] [Google Scholar]
- 32. D'Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21(2): 243–254 [DOI] [PubMed] [Google Scholar]
- 33. Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann NY Acad Sci. 2008;1127:92–100 [DOI] [PubMed] [Google Scholar]
- 34. Crosignani P, Olive D, Bergqvizt A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update. 2006;12(2): 179–189 [DOI] [PubMed] [Google Scholar]
- 35. Winkel CA. A cost-effective approach to the management of endometriosis. Curr Opin Obstet Gynecol. 2000;12(4):317–320 [DOI] [PubMed] [Google Scholar]
- 36. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864 [DOI] [PubMed] [Google Scholar]
- 37. Shaw RW. Evaluation of the role of laser treatment for the treatment of pain in endometriosis. Ann NY Acad Sci. 2003;997:240–246 [DOI] [PubMed] [Google Scholar]
- 38. Waller KG, Shaw RW. Gonadotropin-releasing hormone analogues for the treatment of endometriosis: long-term follow-up. Fertil Steril. 1993;59(3): 511–515 [DOI] [PubMed] [Google Scholar]
- 39. Allen C, Hopewell S, Prentice A, Gregory D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753. [DOI] [PubMed] [Google Scholar]
- 40. Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst Rev. 2009(1):CD005997. [DOI] [PubMed] [Google Scholar]
- 41. Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG. 2008;115(7):818–822 [DOI] [PubMed] [Google Scholar]
- 42. Rodgers AK, Falcone T. Treatment strategies for endometriosis. Expert Opin Pharmacother. 2008;9:243–255 [DOI] [PubMed] [Google Scholar]
- 43. Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(3):CD001019. [DOI] [PubMed] [Google Scholar]
- 44. Surrey ES. Gonadotropin-releasing hormone agonist and add-back therapy: what do the data show? Curr Opin Obstet Gynecol. 2010;22(4):283–288 [DOI] [PubMed] [Google Scholar]
- 45. DiVasta AD, Laufer MR. The use of gonadotropin releasing hormone analogues in adolescent and young patients with endometriosis. Curr Opin Obstet Gynecol. 2013;25(4):287–292 [DOI] [PubMed] [Google Scholar]
- 46. Sallan HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006(1): CD004635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337(23):1641–1647 [DOI] [PubMed] [Google Scholar]
- 48. Saito T, Yoshizawa M, Yamauchi Y, et al. Effects of the novel orally active antiestrogen TZE-5323 on experimental endometriosis. Arzneimittelforschung,. 2003;53(7):507–514 [DOI] [PubMed] [Google Scholar]
- 49. Fuchs-Young R, Glasebrook AL, Short LL, et al. Raloxifene is a tissue-selective agonist/antagonist that functions through the estrogen receptor. Ann NY Acad Sci. 1995;761:355–360 [DOI] [PubMed] [Google Scholar]
- 50. Yao Z, Shen X, Capodanno I., et al. Validation of rat endometriosis model by using raloxifene as a positive control for the evaluation of novel SERM compounds. J Invest Surg. 2005;18(4):177–183 [DOI] [PubMed] [Google Scholar]
- 51. Stratton P, Sinaii N, Segars J, et al. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(1):88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pinkerton JV, Archer DF, Utian WH, et al. Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause. 2009;16(6):1102–1108 [DOI] [PubMed] [Google Scholar]
- 53. Archer DF, Pinkerton JV, Utian WH, et al. Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause. 2009;16(6): 1109–1115 [DOI] [PubMed] [Google Scholar]
- 54. Crabtree JS, Peano B, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008; 287(1–2):40–46 [DOI] [PubMed] [Google Scholar]
- 55. Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149(12):6084–6091 [DOI] [PubMed] [Google Scholar]
- 56. Ethun KF, Wood CE, Cline JM, Register TC, Appt SE, Clarkson TB. Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model. Menopause. 2013;20(7):777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038 [DOI] [PubMed] [Google Scholar]
- 58. Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril. 2009;92(3):1039–1044 [DOI] [PubMed] [Google Scholar]
- 59. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92(3):1045–1052 [DOI] [PubMed] [Google Scholar]
- 60. Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009;92(3):1018–1024 [DOI] [PubMed] [Google Scholar]
- 61. Pinkerton JV, Utian WH, Constantine G, Oliver S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 2009;16(6): 1116–1124 [DOI] [PubMed] [Google Scholar]
- 62. Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010;17(2):281–289 [DOI] [PubMed] [Google Scholar]
- 63. Kulak J, Jr, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology. 2011;152(8):3226–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weimann JM, Johansson CB, Trejo A, Blau H. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5(11):959–966 [DOI] [PubMed] [Google Scholar]
- 65. Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology. 2009;150(4):1897–1903 [DOI] [PubMed] [Google Scholar]
- 66. Du H, Alawadhi H, Cakmak H, Taylor HS. Bone marrow-derived stem cells transplantation improves fertility in a murine model of Asherman's syndrome. Reprod Sci. 2013;20(3):Abstract 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Surrey ES, Silverberg KM, Surrey MW, Schoolcraft WB. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril. 2002;78(4):699–704 [DOI] [PubMed] [Google Scholar]
- 68. de Ziegler D, Gayet V, Aubriot FX, et al. , Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil Steril. 2010;94(7):2796–2799 [DOI] [PubMed] [Google Scholar]
- 69. Ma C, Qiao J, Liu P, Chen G. Ovarian suppression treatment prior to in-vitro fertilization and embryo transfer in Chinese women with stage III or IV endometriosis. Int J Gynaecol Obstet. 2008;100(2):167–170 [DOI] [PubMed] [Google Scholar]