Abstract
Nutritional or pharmacological perturbations during perinatal growth can cause persistent effects on the function of white adipose tissue, altering susceptibility to obesity later in life. Previous studies have established that saccharin, a nonnutritive sweetener, inhibits lipolysis in mature adipocytes and stimulates adipogenesis. Thus, the current study tested whether neonatal exposure to saccharin via maternal lactation increased susceptibility of mice to diet-induced obesity. Saccharin decreased body weight of female mice beginning postnatal week 3. Decreased liver weights on week 14 corroborated this diminished body weight. Initially, saccharin also reduced male mouse body weight. By week 5, weights transiently rebounded above controls, and by week 14, male body weights did not differ. Body composition analysis revealed that saccharin increased lean and decreased fat mass of male mice, the latter due to decreased adipocyte size and epididymal, perirenal, and sc adipose weights. A mild improvement in glucose tolerance without a change in insulin sensitivity or secretion aligned with this leaner phenotype. Interestingly, microcomputed tomography analysis indicated that saccharin also increased cortical and trabecular bone mass of male mice and modified cortical bone alone in female mice. A modest increase in circulating testosterone may contribute to the leaner phenotype in male mice. Accordingly, the current study established a developmental period in which saccharin at high concentrations reduces adiposity and increases lean and bone mass in male mice while decreasing generalized growth in female mice.
Mammalian development is an intricate and complicated process occurring through sequential events. Each process (eg, organogenesis) is itself governed by a meticulous coordination of complex cellular processes (eg, cell differentiation and migration). Distinct interactions between inherent fetal characteristics (eg, genetics) and environmental influences (eg, gestational nutrition) function in harmony to regulate developmental signaling pathways. Consequently, changes in either component can modify these biological pathways to culminate in abnormal organ development. Abnormal environments do not assure an immediate or deleterious developmental outcome. Changes in the fetal environment and thus organ development may be present at birth or be delayed, presenting as a detrimental or advantageous outcome only later in life. For instance, men exposed to global nutrient restriction during the first 2 trimesters of gestation are twice as likely to become obese by age 19 (1). If exposed to nutrient restriction during the third trimester and first 3–5 postnatal months, men are half as likely to develop obesity compared with control counterparts (1). Similar results have been documented in rodent, ewe, and nonhuman primate models of maternal nutrient and protein restriction (reviewed in Ref. 2).
Nonnutritive or artificial sweeteners (eg, saccharin) are commonly used to decrease the calorie content of food and drink. Artificial sweeteners are therefore part of the arsenal to combat obesity. It was estimated that in 2009, 20% of the United States population aged 2 and above consumed calorie-free or low-calorie drinks containing nonnutritive sweeteners, with 11% consuming more than approximately 500 mL on any given day (3, 4). A large variation in saccharin intake has therefore been reported in adults and children (0.21–3.5 mg/kg−1 · d−1), with as much as 11.7 mg/kg−1 · d−1 reportedly consumed by a population of adult persons with diabetes (5–10). Consumption of artificial sweeteners appears to be largely dependent on the country of origin, year, and availability. Although artificial sweeteners are deemed safe for human consumption in many countries around the world, the safety of artificial sweeteners has often been questioned (11, 12). One recent issue of safety derives from associations made between consumption of artificial sweeteners and a greater risk for overweight and obesity, particularly when consumed in exorbitant amount (13–17). This association remains contentious, because independent studies have been unable to replicate this association (18, 19) or in turn have shown weight loss (20, 21).
The hypothesis that artificial sweeteners increase the propensity for obesity is not unwarranted. Many artificial sweeteners are pharmacologically active. In culture, sucralose produces a dose-dependent release of glucagon-like peptide-1 from enteroendocrine cells (22), whereas saccharin increases insulin section from MIN6 and primary mouse pancreatic β-cells (23, 24). Likewise sucralose, acesulfame potassium and saccharin increase glucose absorption via glucose transporter 2 in perfused mouse intestines (25). Saccharin has also been demonstrated to rapidly increase circulating insulin concentrations in vivo (23). Most recently, our laboratory has described the ability of saccharin and acesulfame potassium to stimulate adipogenesis of mouse and human precursors in vitro. Saccharin induced adipogenesis by stimulating phosphorylation of Akt (protein kinase B) and its downstream effectors, cAMP response element-binding protein and Forkhead box protein O1 and FOXO1, and inducing expression of peroxisome proliferator-activated receptor (PPARγ) and Ccaat-enhancer-binding proteins (C/EBP)α. Furthermore, treatment of mature adipocytes with either sweetener suppressed basal and forskolin-stimulated lipolysis (26). Despite the fact that induction of adipogenesis and inhibition of lipolysis by saccharin was independent of sweet taste receptors (T1R2/T1R3) (26), mice deficient in sweet taste receptors have reduced adiposity and increased bone mass (27). The aforementioned studies together suggest possible mechanisms, through which artificial sweeteners modify adipocyte biology and glucose homeostasis and thus the propensity to develop obesity.
Accordingly, the purpose of the current study was to investigate whether consumption of saccharin during adipose tissue development increased the number and/or size of adipocytes, exacerbated body weight gain on a high-fat diet, or altered glucose homeostasis in adult mice fed a westernized diet. Counter to our hypothesis, neonatal male mice administered saccharin through maternal lactation did not have greater body weight gain. Saccharin instead increased lean and bone mass, reduced white adipose tissue (WAT) mass by decreasing size but not number of adipocytes, and caused a mild improvement in glucose homeostasis. In contrast. female mice treated with saccharin had modified cortical bone and weighed slightly less but were not as affected as male mice.
Materials and Methods
Animals, saccharin administration, weaning, and necropsy
All protocols and procedures were approved by the University of Michigan Committee on the Use and Care of Animals and are in accordance with the Animal Welfare Act. Animals were maintained on a 12-hour light, 12-hour dark cycle and fed either an ad libitum standard chow (lactating females) (catalog number 5008; Purina LabDiet) or westernized diet (offspring) (catalog number D12079B; Research Diets, Inc). Nulliparous C57BL/6J mice (n = 10) (The Jackson Laboratory) at 10 weeks of age were bred with mature C57BL/6J males for 48 hours. Females were individually housed afterwards. Beginning 18 days after breeding and continuing daily afterwards, cages were examined at 8 am, 12 pm, and 5 pm hours to determine which females had given birth. Upon discovery of newborns, each mother was randomly assigned to ad libitum 3% saccharin in water (∼146mM) (catalog number S6047; Sigma-Aldrich) or water alone for 21 days. Lactating mothers were administered saccharin within 15 hours of giving birth. The steady state serum concentration in suckling pups at postnatal day 3, measured by mass spectrometry, was estimated at 280 ± 120μM (n = 4) (for method see Supplemental information, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Three days after birth, all litters were reduced to 5 pups per female to control for litter size effects. On postnatal day 21 (wk 3), all male (n = 13 control and n = 10 saccharin) and female (n = 12 control and n = 15 saccharin) mice were separated and weaned onto a standard westernized diet (41% fat, 43% carbohydrate, and 17% protein) for an additional 11 weeks.
The composition of the litters can be found in Supplemental Table 1. There were no differences in the percentage of males or females between treatment groups. Upon completion of the study, animals were anesthetized with isoflurane before cervical dislocation. After severing the aorta, blood was collected from the thoracic cavity. Liver, inguinal WAT (iWAT), perirenal (prWAT), ovarian (oWAT), or epididymal (eWAT) were dissected, weighed, divided, and snap frozen for RNA and protein analysis, or placed in 10% buffered formalin for histological analysis. At necropsy, 3 control males and 2 control females from the same litter were found to have idiopathic gastrointestinal distress resulting in distended bowels filled with loose and watery stool. These animals were removed from the dataset.
Anthropometric and nuclear magnetic resonance (NMR) analysis of body composition
All mice were weighed daily 1 week after birth until the completion of the study using an analytical scale. NMR (Minispec LF90II; Bruker Biosciences Corp) was used to investigate body composition on postnatal weeks 3, 8, and 13 with the help of the University of Michigan Nutrition Obesity Research Center Animal Phenotyping Core.
Adipocyte histomorphometry
Adipocyte size and number in eWAT were calculated after staining with hematoxylin and eosin using MetaMorph Image Analysis software. Our method is described in detail in Ref. 28. A brief summary is as follows: 100–500 mg of eWAT were fixed in 10% buffered formalin and transferred to 70% ethanol before paraffin embedding. A Leica 2155 rotary paraffin microtome was used to make 5-μm sections at 100-μm intervals across the sample. Samples were stained and representative photos taken using a Zeiss inverted microscope at ×40 objective. The area of individual adipocytes was quantified using MetaMorph Microscopy Automation and Image Analysis software (Molecular Devices).
Blood chemistry
Serum concentrations of insulin (catalog number 90080; Crystal Chem, Inc), IGF-I (catalog number MG100; R&D Systems), and total testosterone (Cayman Chemical) were determined using an ELISA as per the manufacturer's instructions. Random blood glucose was measured between 12 and 5 pm hours using a glucometer (Accu-Chek Aviva Plus, Hofmann-La Roche).
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
GTTs were performed at 9 weeks of age. Mice were weighed 24 hours before the test and after a 6-hour fast, filter-sterilized D-glucose was administered by ip injection (catalog number G5767; Sigma-Aldrich) at 2 mg/g−1. Blood samples were collected from the tail at 0, 15, 30, 60, or 120 minutes after injection, and glucose was measured using a glucometer. Blood (30 μL) was also taken 15 minutes after glucose injection from the saphenous vein for evaluation of insulin concentration. ITTs were performed in mice at 10 weeks of age. Mice were fasted for 6 hours before ip injection of 0.075 U/kg−1 of recombinant mouse insulin. Blood samples were subsequently collected from a tail bleed at 0, 30, 60, and 90 minutes after injection and glucose measured using a glucometer.
Microcomputed tomographic scanning
Mouse tibia were embedded in 1% agarose and scanned using a microcomputed tomographic scanning system (μCT100 Scanco Medical). Agarose-embedded mouse bones were placed in a 19-mm diameter tube before scanning the length of the bones using the following settings: voxel size 12 μm, medium resolution, 70 kVp, 114 μA, 0.5 mm aluminum filter, and integration time 500 milliseconds. Density measurements were calibrated to the manufacturer's hydroxyapatite phantom. Analysis was performed using the manufacturer's evaluation software at a fixed global threshold (on a grayscale of 0–1000) of 28% for cortical bone, 18% for trabecular bone, and 22% for total bone to segment bone from nonbone.
Quantitation of bone marrow adipocytes
Bone marrow adipocytes were quantified using osmium tetroxide staining as described in detail in the following method (29). Briefly, bones were decalcified for 14 days in 14% EDTA, washed with Sorensen's phosphate buffer and placed in 1% osmium tetroxide for an additional 24 hours. The stained bones were subsequently scanned by microcomputed tomographic scanning as described above. A fixed global threshold of 40% (on a grayscale of 0–1000) was used to quantify the marrow adipose tissue between the growth plate and tibia/fibula junction.
Primary islet isolation and ex vivo insulin secretion
Islets were isolated by collagenase digestion following method outlined in Ref. 30. In brief, the pancreas was perfused via the splenic duct with 1-mg/mL collagenase XI (Sigma-Aldrich) in HBSS (Life Technologies). Pancreatic digestion was carried out at 37°C for 15 minutes, after which cold Hank's Balanced Salt Solution with 2.5% fetal bovine serum (FBS) (Life Technologies) was added. The suspension was centrifuged at 1000 rpm for 30 seconds, washed 3 times with HBSS with 2.5% FBS, resuspended in RPMI 1640 with 5mM glucose, 10% FBS,100-IU/mL penicillin, and 100-g/mL streptomycin, and poured onto a 70-μm cell strainer (BD Falcon, BD Biosciences). Islets were rinsed and handpicked. Islets were allowed to recover overnight before experiments were carried out. The next day, islet cells were washed twice with PBS and cultured serum-free and in 2mM glucose for 1 hour before placing in 2mM or 16mM glucose for 15 minutes. Insulin secretion levels were measured ELISA (Mouse-Insulin UltraSensitive, ELISA; ALPCO Immunoassays).
RNA analysis
Flash frozen liver or eWAT (100–500 mg) was ground for 3 minutes in 500 μL of RNA-Stat60 (catalog number CS-110; AMS Biotechnology) using 0.5-mm zirconium oxide beads and a BulletBlender tissue homogenizer (Next Advance, Inc). Samples were spun at 2 × 104g for 5 minutes to pellet any remaining particulate, and total RNA was isolated per manufacturer's instructions. Using random hexamer primers (TaqMan Reverse Transcription kit; Applied Biosystems), 0.5 μg of RNA were reverse transcribed, and quantitative PCR was performed using the StepOnePlus Real-Time PCR system (Life Technologies, Inc) and SYBR green reagents (Bio-Rad Laboratories). Relative gene expression was normalized to TATA-binding protein (TBP) expression and calculated using the cycle threshold method (31). Primer Sequences are listed in Table 1. Western blotting was used to evaluate circulating levels of adiponectin (for method see Supplemental information).
Table 1.
Quantitative Polymerase Chain Reaction Primer Sequences
| Gene Name | Accession Number | Sequence |
|---|---|---|
| Adiponectin | NM_009605 | FW, AAGAAGGACAAGGCCGTTCTCTT |
| RV, GCTATGGGTAGTTGCAGTCAGTT | ||
| Chemerin | NM_027852.2 | FW, TACAGGTGGCTCTGGAGGAGTTC |
| RV, CTTCTCCCGTTTGGTTTGATTG | ||
| Leptin | NM_008493.3 | FW, GACACCAAAACCCTCAT |
| RV, CAGTGTCTGGTCCATCT | ||
| MCP-1 | NM_011333.3 | FW, CTTCTGGGCCTGCTGTTCA |
| RV, CCAGCCTACTCATTGGGATCA | ||
| C/EBPα | NM_007678.3 | FW, TGGACAAGAACAGCAACGAG |
| RV, TCACTGGTCAACTCCAGCAC | ||
| C/EBPβ | NM_009883.3 | FW, GGGACTTGATGCAATCCGG |
| RV, AACCCCGCAGGAACATCTTT | ||
| PPARγ | NM_001127330.1 | FW, GGAAAGACAACGGACAAATCAC |
| RV, TACGGATCGAAACTGGCAC | ||
| FABP4 | CT010390.1 | FW, TGGAAGCTTGTCTCCAGTGA |
| RV, AATCCCCATTTACGCTGATG | ||
| CD68 | BC021637.1 | FW, CTTCCCACAGGCAGCACAG |
| RV, AATGATGAGAGGCAGCAAGAGG | ||
| CD14 | NM_009841.3 | FW, GGCTTGTTGCTGTTGCTTC |
| RV, CAGGGCTCCGAATAGAATCC | ||
| F4/80 | X93328.1 | FW, CTTTGGCTATGGGCTTCCAGTC |
| RV, GCAAGGAGGACAGAGTTTATC | ||
| MUPS-1 | NM_001163011 | FW, AAGGTTTGCACAACTATGTGAGA |
| RV, TCCTGGTGAAAAGTCTCCACTC | ||
| MUPS-3 | BC106100 | FW, GAAAATATCATTGACCTAACCAATGT |
| RV, TCCTGGTGAAAAGTCTCCACTC | ||
| IGF-I | CT010364 | FW, CTACAAAAGCAGCCCGCTCT |
| RV, CTTCTGAGTCTTGGGCATGTCA | ||
| TBP | NM_013684.3 | FW, ACCTTATGCTCAGGGCTTGG |
| RV, GCCATAAGGCATCATTGGAC |
TBP, TATA-binding protein; FW, forward; RV, reverse.
Statistical analysis
All data are expressed as mean ± SD. Statistical analysis was performed using GraphPad Prism. A two-way ANOVA was used for comparing time- or sex-dependent effects of saccharin on variables, including weight, body composition, adipocyte area, glucose tolerance, and MUPS-1 and MUPS-3 mRNA expression. A Fischer's Least Significant Difference test (weight) and Bonferroni (body composition, adipocyte area, glucose tolerance, and litter composition) post hoc analysis were used after the ANOVA to compare individual points. A Student's t test was used to evaluate the influence of saccharin on food intake, tissue weight, area under the curve (AUC)Frequency, fasting glucose, AUC0–30 minutes GTT, bone parameters, IGF-I, testosterone, and expression of adipokines, adipogenic, and immunocyte mRNA markers. A difference in mean values between groups was considered to be significant when *, P ≤ .05.
Results
Exposure to saccharin as neonates decreases female body weight and influences male body weight without modifying food intake
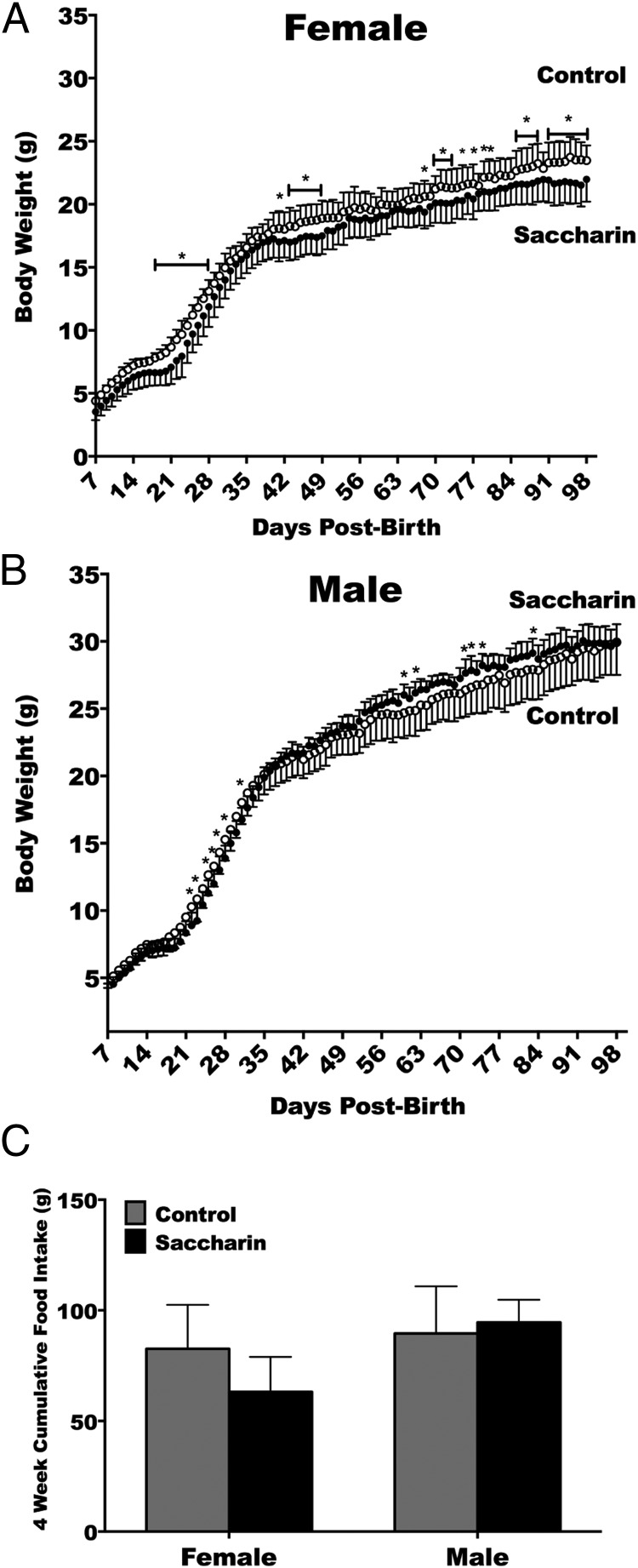
To test the metabolic effects of saccharin treatment on neonates, the first task was to precisely document the growth changes in the mice over the study period. Female body weight increased with age and was affected by saccharin, with an interaction between age and saccharin treatment (Figure 1A). Saccharin therefore led to less weight gain in females, with decreased body weight emerging first during postnatal week 3. Body weight of males increased with age and was affected by an interaction between time and saccharin (Figure 1B). This interaction of saccharin in males was observed as lower body weights for the first week after weaning, mildly higher body weights for a brief period by week 8, and similar body weights thereafter. To determine whether energy consumption accounted for these changes, food intake was measured weekly during postnatal weeks 8–12. The changes in body weight were found to occur without significant cumulative differences in food intake (Figure 1C).
Figure 1.
Saccharin treatment through maternal lactation periodically decreases female but not male body weight without affecting food intake. Female body weight increased with age (P < .0001) and was affected by saccharin (P < .05), with an interaction between age and saccharin treatment (P < .0001) (control n = 10, saccharin n = 15) (A). Body weight of males increased with age (P < .0001) and was affected by an interaction between time and saccharin (P < .0001) (control n = 10, saccharin n = 10) (B). Cumulate food intake (C). Two-way repeated measures ANOVA with Fisher's LSD test (A and B) or Student's t test (C).
Neonatal treatment with saccharin through lactation decreases fat mass and increases lean mass in adult male but not female mice
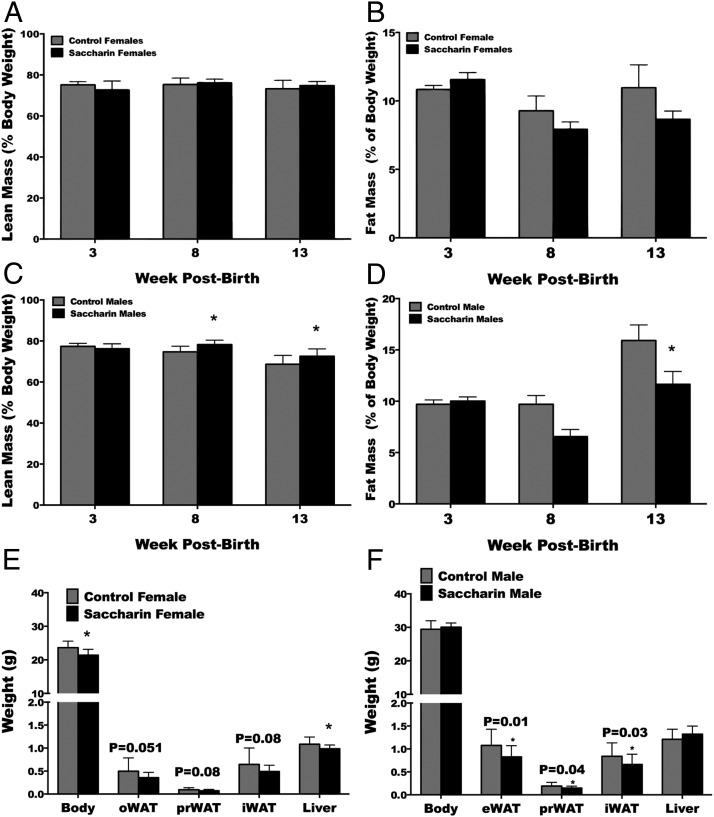
To determine whether the changes in weight associated with saccharin corresponded to changes in body composition, NMR analysis was used to determine changes in lean and fat mass at 3, 8, and 13 weeks of age. Although no effect of saccharin was found on lean (Figure 2A) or fat mass (Figure 2B) of female mice, lean and fat mass in males was affected by time, saccharin, and interaction between time and saccharin treatment. Specifically, male mice exposed to saccharin had elevated lean mass at 8 and 13 weeks (Figure 2C), with a concurrent reduction in fat mass at 13 weeks (Figure 2D). Given the inability of NMR to differentiate between lipids stored in adipose tissues vs those partitioned to other organs, oWAT, eWAT, prWAT, iWAT, and liver were carefully dissected and weighed at necropsy. Female mice exposed to saccharin were found to have significantly smaller livers. Males that consumed saccharin through lactation were found to have significantly reduced eWAT, prWAT, and iWAT, but no change in liver weight.
Figure 2.
Treatment of neonatal mice with saccharin decreases fat mass and increases lean mass in adult male mice. Lean (A) and fat mass (B) for female mice was determined by NMR at 3, 8, and 13 weeks. Male lean (C) and fat mass (D) was affected by time (P < .0001), saccharin (P < .05), and interaction between time and saccharin treatment (P < .05). Body weights and weights of gonadal WAT (oWAT or eWAT), prWAT, and iWAT depot, as well as liver for female (E) and male (F) mice. *, P < .05, two-way repeated measures ANOVA with Bonferroni post hoc test analysis (A–D) or Student's t test (E and F) (control n = 10, saccharin n = 15 for A, B, and E; control n = 10, saccharin n = 10 for C, D, and F).
Saccharin increases the number of small and decreases the number of large adipocytes in adult eWAT
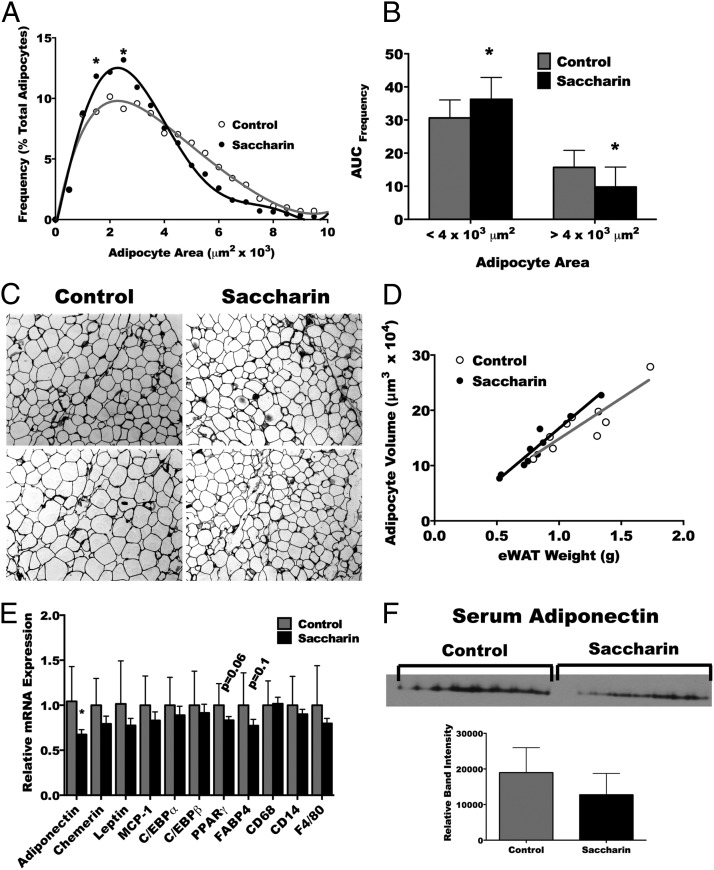
Male but not female mice treated with saccharin were found to have reduced adipose mass without a significant change in body weight. Accordingly, we focused the remainder of the analysis on male mice. To address whether a change in adipocyte size or number occurred in parallel with the reduction in adipose tissue, weight sections of eWAT were analyzed by image analysis software. The interaction between adipocyte size and saccharin treatment resulted from an elevated number of adipocytes with areas between 1500–2000 and 2500–3000 μm2 (Figure 3A). To determine the overall change in adipocyte frequency in male mice, the area under the frequency vs adipocyte area curve for 0–4000 μm2 (smaller adipocytes) and 4000–10 000 μm2 (larger adipocytes) was calculated. Saccharin consumption during lactation elevated the number of smaller adipocytes and decreased the frequency of larger adipocytes (Figure 3B). This difference is clearly visible in the representative photos (Figure 3C). To determine whether the reduced weight of adipose tissue was not only due to increased frequency of small adipocytes but was also a reflection of decreased adipocyte number, the relationship between average adipocyte volume and eWAT weight was compared. As expected (28), the relationship between adipocyte volume and eWAT weight for both treatments was found to be linear. However, the slope and intercept of treatment groups were equal (Figure 3D), indicating that saccharin decreases weight of eWAT by causing a reduction in size but not number of adipocytes. Supplemental analysis of gene expression indicated that saccharin administration decreased the expression of eWAT adiponectin mRNA (Figure 3E), although analysis of total sera adiponectin by Western blotting did not reveal differences between treatment groups (Figure 3F). The expression of the adipokines chemerin, leptin, and MCP-1, the adipogenic genes PPARγ, FABP4, C/EBPα, and C/EBPβ, and the immunocyte markers CD68, CD14, and F4/80 were unaltered (Figure 3E).
Figure 3.
Saccharin treatment increases frequency of small adipocytes and decreases number of large adipocytes in the eWAT. Quantitative histomorphometry for size of adipocytes in the eWAT depot of mice treated with saccharin as neonates (A). AUC for small and large adipocytes (B). Representative hematoxylin and eosin (H&E)-stained adipose tissue photomicrographs (C). Relationship between calculated adipocyte volume and eWAT weight for control and saccharin treatments (D). Expression of adipokines, adipogenic and immunocyte markers (E). Western blotting and quantitation of band intensity of circulating adiponectin in sera (F). *, P < .05, two-way ANOVA followed by a Bonferroni (A and F), linear regression with a two-tailed comparison of slope and intercept (D), or Student's t test (B and E) (control n = 9 for A–C, n = 10 for E, and n = 8 for F; saccharin n = 10 for A–C and E and n = 7 for F).
Administration of saccharin through maternal lactation improves glucose tolerance without altering insulin secretion or sensitivity in adult male mice
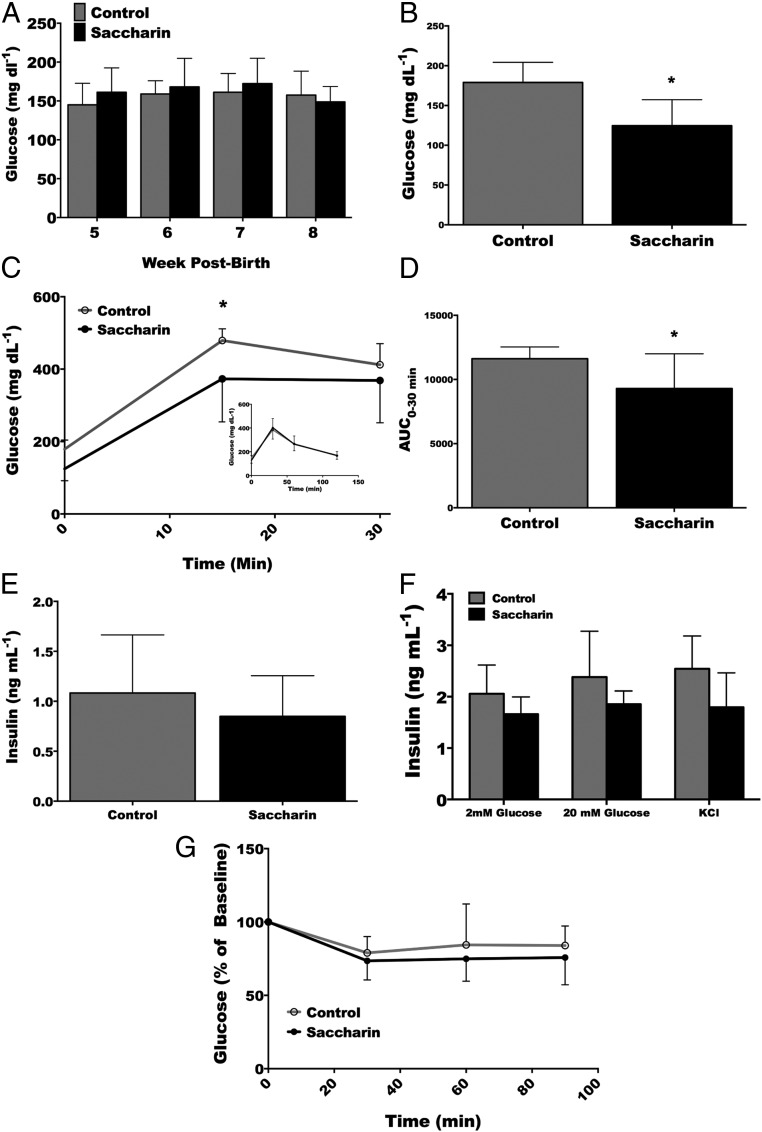
We next evaluated whether saccharin exposure also influenced glucose homeostasis. Accordingly, random blood glucose levels were measured on weeks 5–8. No difference was found between treatment groups of male (Figure 4A) or female mice (data not shown). After a 6-hour fast, however, saccharin-treated males were found to have significantly reduced blood glucose levels (Figure 4B). When challenged with an ip bolus of glucose, this reduction in circulating glucose was still observed at the 15-minute time point but was not different from control glucose concentrations at 30 minutes or thereafter (Figure 4C). To determine the effect of saccharin on total circulating glucose concentration, the AUC was calculated from the GTT. Saccharin was found to significantly reduce total serum glucose of adult male mice for 30 minutes after glucose administration (Figure 4D). Glucose concentrations during fasting or with a GTT were not different in female mice (data not shown). To evaluate whether elevated insulin could explain the decrease in serum glucose in male mice, insulin was measured in serum drawn 15 minutes after the glucose injection. No difference was found in circulating insulin between treatment groups (Figure 4E). Given the possibility that saccharin improves glucose homeostasis by increasing insulin secretion, primary β-cells were evaluated for secreted insulin in response to glucose and KCl, but differences between treatment groups were not observed (Figure 4F). Improved insulin sensitivity could also explain the decrease in serum glucose observed in the male mice during the GTT. However, differences in glucose concentrations were not observed during the ITT (Figure 4G).
Figure 4.
Saccharin administration improves glucose intolerance without altering insulin secretion or insulin sensitivity in adult male mice. Random blood glucose at weeks 5, 6, 7, and 8 (A). Glucose concentration in blood after a 6 hours fast at 9 weeks of age (B). GTT after a 6-hour fast (C). AUC for glucose concentration over the 30-minute test. (D). Serum insulin concentrations 15 minutes after glucose injection (E). Insulin secretion from isolated islets in response to 2mM or 20mM insulin, or KCl (F). ITT (G). Blood glucose concentrations at the indicated times in response to ip insulin administration at 10 weeks of age. *, P < .05 two-way repeated measures ANOVA with Bonferroni post hoc analysis (A and F), Student's t test (B–E) (control n = 10, saccharin n = 10 for A–C, E, and G; control n = 10, saccharin n = 9 for D; and control n = 5, saccharin n = 4 for F).
Total, trabecular, and cortical bone are increased, and cortical bone modified, respectively, in male and female mice administered saccharin
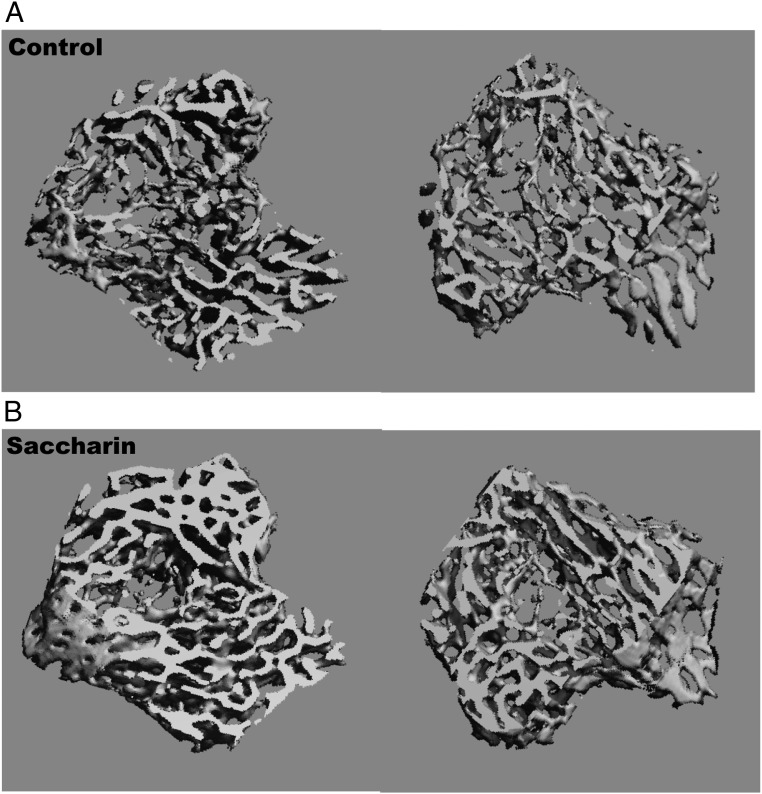
Fat and lean mass have both been correlated with bone mineral content and bone mass (32); accordingly, we next evaluated tibial bone variables by μCT. Although marrow adiposity was not different in male (Table 2), the male tibial length, bone volume, and bone volume fraction were all increased by saccharin treatment. Specifically, analyses of the male trabecular bone in proximal tibiae revealed that saccharin increased the total volume, bone volume, bone volume fraction, structure model index, trabecular thickness, and bone mineral content (Table 2). Elevations in tibial trabecular bone of saccharin-treated male mice are clearly visible in the representative 3-dimensional reconstructions when compared with controls (Figure 5). Cortical bone volume, bone volume fraction, and bone mineral content were also increased in males after saccharin consumption (Table 2). In contrast, female mice showed no difference in trabecular bone length or any trabecular bone parameters, although saccharin treatment decreased cortical total volume and increased cortical bone volume fraction and bone mineral content (data not shown). Together, these results indicate that neonatal consumption of saccharin increases cortical and trabecular bone mass in adult male mice and altered cortical bone variables in female mice.
Table 2.
Total, Trabecular, and Cortical Bone Is Increased in the Proximal Tibiae of Male Mice Exposed to Saccharin Through Maternal Lactation
| Bone Variable | Control | Saccharin | P Value |
|---|---|---|---|
| Total bone | |||
| Length of tibiae | 9.814 (0.128) | 9.978 (0.163) | .022a |
| Total volume (mm3) | 18.782 (1.837) | 20.506 (1.352) | .028a |
| Bone volume (mm3) | 8.751 (1.295) | 10.170 (1.049) | .015a |
| Bone volume fraction (%) | 0.464 (0.023) | 0.495 (0.028) | .015a |
| Marrow volume (mm3) | 10.031 (0.576) | 10.337 (0.592) | .257 |
| Marrow adipose tissue (mm3) | 0.204 (0.281) | 0.078 (0.165) | .238 |
| Marrow adipose tissue fraction (%) | 0.021 (0.029) | 0.008 (0.016) | .227 |
| Marrow adipose fraction per bone (%) | 0.011 (0.015) | 0.004 (0.009) | .237 |
| Trabecular bone | |||
| Total volume (mm3) | 1.900 (0.129) | 2.012 (0.096) | .041a |
| Bone volume (mm3) | 0.521 (0.141) | 0.705 (0.144) | .010a |
| Bone volume fraction (%) | 0.272 (0.059) | 0.349 (0.064) | .012a |
| Connectivity density (relative units) | 180.552 (23.169) | 193.129 (30.365) | .312 |
| Structure model index (relative units) | 1.582 (0.521) | 0.979 (0.568) | .023a |
| Trabecular number (mm−1) | 6.140 (0.348) | 6.412 (0.357) | .101 |
| Trabecular thickness (mm) | 0.060 (0.005) | 0.068 (0.006) | .003a |
| Trabecular separation (mm) | 0.146 (0.011) | 0.134 (0.014) | .056 |
| Bone mineral content (mg HA) | 226.928 (42.105) | 285.092 (48.134) | .010a |
| Cortical bone | |||
| Total volume (mm3) | 0.528 (0.055) | 0.552 (0.030) | .251 |
| Bone volume (mm3) | 0.274 (0.034) | 0.303 (0.025) | .045a |
| Bone volume fraction (%) | 0.518 (0.015) | 0.549 (0.024) | .003a |
| Structure model index (relative units) | 0.946 (0.241) | 0.926 (0.274) | .864 |
| Bone mineral content (mg HA) | 639.576 (21.127) | 674.614 (30.596) | .008a |
CT analysis of tibial bone from 14-week-old C57BL6/J mice exposed to saccharin (n = 10) or a control (n = 10) for 21 days through lactation.
Significance (P < .05) between the control and the saccharin-treated mice by Student's t-test.
Figure 5.
Three-dimensional reconstruction of tibial trabecular bone from male control (A) or saccharin (B)-treated mice at 13 weeks of age.
Trend towards higher circulating serum concentrations of testosterone but not IGF-I in male mice administered saccharin through lactation
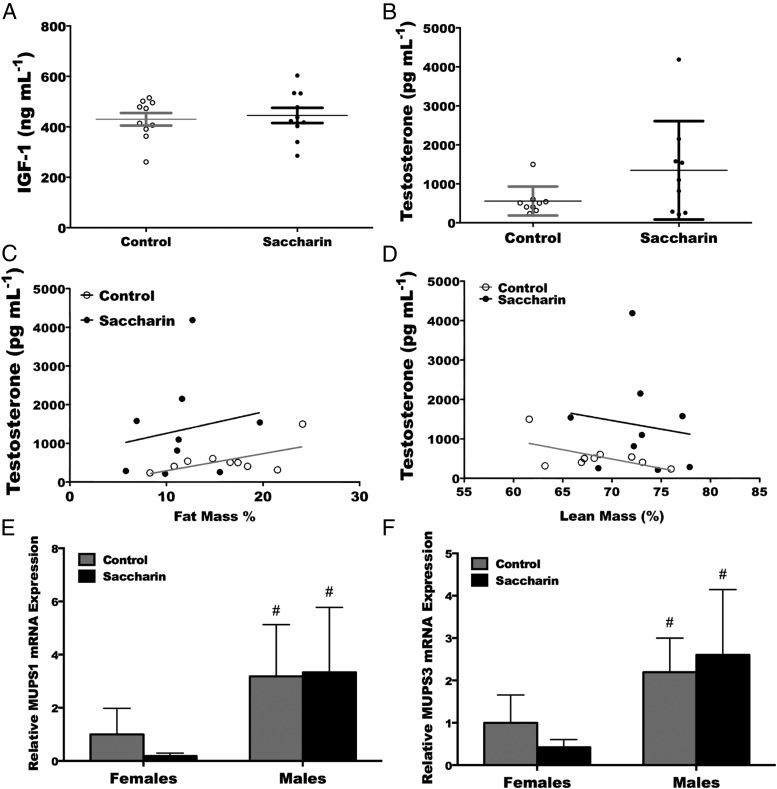
To investigate the underlying mechanism responsible for the “lean” phenotype in male mice administered saccharin, a number of known anabolic hormones were evaluated. No difference was found in liver IGF-I expression in males or females (data not shown), nor were there changes in serum IGF-I (Figure 6A) or total testosterone concentrations (P = .09) (Figure 6B). Although the relationships between serum testosterone and fat mass (Figure 6C) or serum testosterone and lean mass (Figure 6D) did not differ, there was a strong trend for saccharin to increase the intercepts in both cases. To evaluate gene expression downstream of testosterone, we measured expression of hepatic major urinary protein 1 and 3, both of which are positively regulated by testosterone (33). Although no differences in expression were found with saccharin treatment, as expected, males did express higher amounts of MUPS-1 and MUPS-3 when compared with females (Figure 6, E and F). Thus, neither IGF-I nor testosterone can explain in totality the phenotype observed in male mice that consumed saccharin during the suckling period.
Figure 6.
Trend towards higher serum concentrations of testosterone but not IGF-I in male mice exposed to saccharin during lactation. ELISA of serum IGF-I (A) and testosterone (B) from 13-week-old male mice. Saccharin tends to increase the average concentration of circulating testosterone (P = .09). There was no relationship between serum testosterone and percent fat mass (C) (control r2 = 0.36, saccharin r2 = 0.03) and percent lean mass (D) (control r2 = 0.35, saccharin r2 = 0.02) in either treatment group. There was, however, a strong trend for saccharin to increase the intercept of the relationship between testosterone and percent fat mass (P = .059) and percent lean mass (P = .068). Expression of MUPS-1 (E) and MUPS-3 (F) mRNAs in liver of female and male mice. *, P < .05 Student's t test (A and B), linear regression with a two-tailed comparison of slope and intercept (C and D), or #, P < .05 (comparison between males and females) two-way ANOVA with Bonferroni post hoc analysis (E and F) (control n = 10, saccharin n = 10 for A–D; control n = 9, saccharin n = 15 for females in E and F; and control n = 7, saccharin n = 7 for males in E and F).
Discussion
Obesity is one of the greatest health problems in the industrialized world (34). Epidemiological and animal studies have indicated exposure to an aberrant nutritional environment during fetal development increases the susceptible to obesity in later life by modifying underlying mechanisms (eg, leptin resistance), increasing food intake, and decreasing energy output (28). Based on an in vitro study from our group, in which saccharin stimulated preadipocyte differentiation and repressed lipolysis (26), we tested whether administration of saccharin to neonatal mice would increase their susceptibility to diet-induced obesity. Contrary to our hypothesis, our experiments identified a developmental period in which saccharin reprograms male mice towards a phenotype characterized by elevated lean and bone mass, and reduced adiposity, while only mildly decreasing body weight and modifying cortical bone in females.
Saccharin is readily absorbed through the gut (35), reaches maximal blood concentrations quickly (35), binds serum proteins (36), and is eliminated in the urine without being metabolized (37). The limited tissue distribution and high renal clearance of saccharin results in a short elimination half-life of approximately 30 minutes in rats (38–40). To determine whether saccharin administration to neonatal mice regulates adipocyte development and/or metabolism, a 3% sodium saccharin solution was provided to lactating mothers. Saccharin easily distributes into serum and milk. Administration of a 333-mg (5.5 mg/kg−1 · d−1, US Food and Drug Administration-suggested maximal dose) bolus to human males results in a peak circulating concentration of approximately 70μM (41), whereas consumption of 252 mg of saccharin by a lactating mother results in milk concentrations that range from approximately 0.97μM to 5.14μM (42). In previous work, approximately 450μM saccharin was required to induce expression of fatty acid-binding protein 4 (FABP4) in preadipocyte models (26). Consequently, a 3% saccharin solution was chosen based on the distribution of saccharin into sera and breast milk, the peak blood concentrations observed in high-dosage consumers, and the need to maximize the likelihood of effects on adipogenesis. Congruous with these calculations and previous literature, a 3% solution consumed by a nursing mouse resulted in a circulating concentration of approximately 280μM saccharin in 3-day-old neonatal pups.
The timing of administration was likewise important. In rodents, sc adipocyte commitment and differentiation are initiated in utero. Subcutaneous adipocytes are therefore present at birth and continue to grow postnatally (43, 44). In contrast, gonadal adipose tissue is first detectable on postnatal day 5 and will undergo commitment, differentiation, and growth thereafter (43, 44). Administering saccharin through lactation targets the specific period of adipose tissue commitment, development, and growth while the natural pulsatility of suckling and the short half-life of saccharin models a repeated bolus dose, without saturating blood or tissues.
Over the course of the study, saccharin decreased female body weights, which was reflected by a decrease in liver weight at the conclusion of the study. This is consistent with studies in rats fed 1%–7.5% saccharin during gestation and/or lactation, as well as postweaning. The difference in weight loss between the current (∼10%) and previous (∼40%) studies may be a consequence of different administration routes or dosage (45–47). In male mice, however, saccharin had mild and temporary effects on body weight, with weight initially decreasing 1 week after weaning but rebounding quickly and transiently thereafter. Surprisingly saccharin caused male mice alone to have elevated lean body mass and decreased fat mass. The decreased fat mass was a reflection of diminished iWAT, prWAT, and eWAT weight and adipocyte size. Hence, saccharin had divergent effects between sexes; producing a leaner phenotype in males and a generalized smaller phenotype in females.
Blood glucose concentrations rise and peak 15 minutes after an ip injection of glucose in rats. This rise results from absorbed glucose and endogenous glucose production (ie, gluconeogenesis, glycogenolysis, and futile glucose-glucose-6-phosphate cycling) (48). In an insulin-resistant state, the elevated peak and sustained blood glucose concentrations during a GTT are largely due to diminished inhibition of endogenous glucose production rather than glucose disposal. Mice lacking liver insulin receptors (liver insulin receptor knockout mice) exhibit hyperglycemia due to this decreased suppression of hepatic glucose production (49), whereas mice deficient in skeletal muscle insulin receptors (muscle insulin receptor knockout mice), which contribute to glucose disposal, have normal blood glucose and response to GTT (50). Saccharin-treated male mice had lower fasting blood glucose concentrations and at the 15-minute time point of the GTT. The principle effect of saccharin exposure was therefore likely due to decreased endogenous glucose production rather than increased glucose disposal (48). This improvement in glucose tolerance, although consistent with independent studies in adult mice (51), was not associated with increased insulin sensitivity or elevated insulin secretion. It is possible that dose of insulin used during the ITT, or the insensitivity of the method, was insufficient to detect differences in insulin responsiveness. A more sensitive method, such as the hyperinsulinemic euglycemic clamp, will need to be used in the future.
In humans, both fat and lean mass have been correlated to bone mineral density and bone mass (32). Fat mass appears especially important for determining and maintaining bone composition and density (52–54). Surprisingly, male mice administered saccharin have longer tibiae with higher bone mass in trabecular and cortical bone without any change in marrow adipose tissue volume. Saccharin-treated female mice in contrast have altered cortical bone variables alone. To the best of our knowledge, this is the first reported effect of saccharin on bone mass. There is, however, some precedent for effects of nonnutritive sweeteners on bone. Aspartame is documented to slow the onset of lean and bone deterioration in a mouse model of osteoarthritis (55). Although aspartame is hypothesized to mediate these effects by decreasing inflammation and increasing calcium bioavailability, saccharin is unlikely to increase bone mass through these mechanisms. Further investigations pertaining to saccharin and bone mass are therefore needed but are beyond the scope of the current manuscript.
Development in rodents is dependent on the quality and amount of food consumed and digested in addition to the energy the animal exerts. Consequently, during the suckling period, rodents are dependent on the quantity and quality of their mother's milk. The composition and volume of milk can be modified by maternal diet. For example, global nutrient restriction decreases milk production (56–60) and reduces lactose concentrations (58, 61), leading to delayed growth and smaller mice at weaning (62–64). Changes in rodent milk production and composition occur when maternal dietary intake is 70% or less of ad libitum (65). Previous experiments found consumption of a 5% saccharin solution modestly increased food intake in females (66). Saccharin is therefore unlikely to have caused sufficient nutrient restricted to modify milk volume or composition leading to the modified body weight or composition noted in saccharin-treated mice. This does not eliminate the possibility, however, that saccharin is directly affecting the mammary gland influencing protein or fat secretion into the milk.
The mechanism responsible for saccharin-associated weight loss in previous studies was in part through reduction in food intake (46, 47). Although no difference in food intake was found in our study, it is possible saccharin modestly decreased food intake. The seemingly minor changes in food consumption may have cumulated over the longer study period decreasing weight gain in saccharin-treated females. Although we evaluated food intake from 8 to 12 weeks of age, it is conceivable that saccharin may have also impaired food intake earlier in the study. Nevertheless, food intake would not explain the change in body composition in male mice administered saccharin. Elevated energy expenditure, including adaptive thermogenesis, may also explain the decreased weight in female mice administered saccharin. However, although independent randomized control trials in humans indicate exercise can modify body composition decreasing fat and increasing lean mass (67, 68), this relationship does not appear consistent in rodents. In wild-type mice, or those bred for high voluntary running, long-term access to running wheels results in decreased body fat without any significant change in lean mass (69, 70). In both mouse models, an increase in food intake also accompanied the change in body composition. Accordingly, although it is possible that elevated energy output plays a role in the altered body composition, it is unlikely the dominant mechanism in male mice administered saccharin.
Thus, the final aim of the study was to explore potential mechanisms responsible for the change in male body composition. IGF-I (71) and testosterone (72, 73) are important regulators of male development. Mice haploinsufficient for the IGF-I receptor have severely restricted adipose tissue weight and adipocyte number (71), whereas low serum total testosterone is associated with accumulation of adipose tissue and obesity (74, 75) and decreased lean and bone mass (76, 77). No change was found in hepatic IGF-I expression in males or females (data not shown), and circulating concentrations of IGF-I or total testosterone did not differ in males. However, there was a strong trend for saccharin to modify the relationship between serum testosterone and fat mass (P = .059) and lean mass (P = .068). The change in intercept but not slope suggests that saccharin may increase basal circulating testosterone but not its relation to body composition. In support of this concept, transgenic male mice that overexpress androgen receptor in mesenchymal stromal cells have increased lean and trabecular bone mass and reduced adipose mass and fasting blood glucose (72, 73). Elevated testosterone may also explain the decrease in eWAT adiponectin expression, because testosterone has been shown to decrease circulating adiponectin in humans (78). Saccharin administration did not phenocopy the androgen receptor-transgenic mice perfectly, and elevated serum testosterone was not reflected by increased hepatic MUPS-1 or MUPS-3 expression. Equally the linear relationship between serum testosterone and lean or fat mass is not strong in either control or saccharin-treated mice. It is reasonable to infer that testosterone may contribute to, but is not the driving force behind, the phenotype in saccharin-treated males.
It should be noted that at the circulating concentrations achieved in the current study, saccharin binds both the sweet (EC50 = ∼0.1mM) (79) and bitter (EC50 = ∼1.1mM–1.7mM) (80) taste receptors. Sweet and bitter receptors are expressed in numerous nongustatory tissues (eg, pancreatic β-cells and testes), where they mediate diverse biological functions, including insulin secretion, airway constriction, spermatogenesis, and nutrient absorption (22, 23, 25, 81–91). We previously described that saccharin robustly induced adipogenesis in vitro. In contrast, the current study indicates administration of saccharin to neonatal mice slows female growth and reprograms male body composition. Although food intake and testosterone may play ancillary roles in the resulting phenotypes, the divergence between our in vitro and in vivo studies may result from the complex interplay of sweet and bitter taste receptor activation in multiple metabolic tissues. The fact that the male mice administered saccharin largely phenocopied mice deficient in sweet taste receptors points towards the possibility that saccharin is down-regulating T1R2/T1R3 during development (27). In addition, one estimate suggests that 1%–1.5% of adipocytes are turned-over daily in rodents (92). Thus, it is formally possible that saccharin increases adipogenesis, but increased number of adipocytes is masked by an increased rate of adipocyte turnover. Future studies will need to clarify the role of sweet and bitter receptors in isolated cells and how they function together in vivo if the mechanisms used by saccharin to metabolically program mice are to be identified. Finally, although the results of the current study seem superficially favorable in male mice and unfavorable in female mice, the long-term consequences of saccharin at clinical concentrations on lean, fat, bone, and glucose homeostasis will need to be determined.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 DK95705 (to O.A.M.) and RO1 DK073716 and RO1 DK084236 (to E.B.-M.) and by the University of Michigan Training Program in Organogenesis Training Grant T32-HD007505 (to S.D.P. and B.R.S.). B.R.S. was also supported by the Cell and Molecular Biology Training Grant T32-MG007315 and a by Rackham Merit Fellowship. E.U.A. is supported by the Pediatric Endocrinology Training Program T32-DK071212. O.A.M. is the recipient of a Fulbright Scholar's Award. This work used the University of Michigan Animal Phenotyping, and Morphology and Imaging Core Services, supported by National Institutes of Health Grants P30-DK089503 and P30-DK020572, and Metabolomic Core Services supported by DK097153.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- C/EBP
- Ccaat-enhancer-binding proteins
- eWAT
- epididymal WAT
- FABP4
- fatty acid-binding protein 4
- FBS
- fetal bovine serum
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- iWAT
- inguinal WAT
- NMR
- nuclear magnetic resonance
- oWAT
- ovarian WAT
- PPARγ
- peroxisome proliferator-activated receptor
- prWAT
- perirenal prWAT
- T1R
- taste receptor type 1
- WAT
- white adipose tissue.
References
- 1. Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353 [DOI] [PubMed] [Google Scholar]
- 2. Parlee SD, Macdougald OA. Maternal nutrition and risk of obesity in offspring: the trojan horse of developmental plasticity. Biochim Biophys Acta. 2014;1842(3):495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fakhouri TH, Kit BK, Ogden CL. Consumption of diet drinks in the United States, 2009–2010. NCHS Data Brief. 2012;(109):1–8 [PubMed] [Google Scholar]
- 4. Benton D. Can artificial sweeteners help control body weight and prevent obesity? Nutr Res Rev. 2005;18(1):63–76 [DOI] [PubMed] [Google Scholar]
- 5. Renwick AG. The intake of intense sweeteners - an update review. Food Addit Contam. 2006;23(4):327–338 [DOI] [PubMed] [Google Scholar]
- 6. Renwick AG. Intake of intense sweeteners. World Rev Nutr Diet. 1999;85:178–200 [DOI] [PubMed] [Google Scholar]
- 7. Huvaere K, Vandevijvere S, Hasni M, Vinkx C, Van Loco J. Dietary intake of artificial sweeteners by the Belgian population. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29(1):54–65 [DOI] [PubMed] [Google Scholar]
- 8. Diogo JS, Liliana Oliveira S, Pena A, Lino CM. Risk assessment of additives through soft drinks and nectars consumption on Portuguese population: a 2010 survey. Food Chem Toxicol. 2013;62:548–553 [DOI] [PubMed] [Google Scholar]
- 9. Leclercq C, Berardi D, Sorbillo MR, Lambe J. Intake of saccharin, aspartame, acesulfame K and cyclamate in Italian teenagers: present levels and projections. Food Addit Contam. 1999;16(3):99–109 [DOI] [PubMed] [Google Scholar]
- 10. Morgan KJ, Stults VJ, Zabik ME. Amount and dietary sources of caffeine and saccharin intake by individuals ages 5 to 18 years. Regul Toxicol Pharmacol. 1982;2(4):296–307 [DOI] [PubMed] [Google Scholar]
- 11. Bell W, Clapp R, Davis D, et al. Carcinogenicity of saccharin in laboratory animals and humans: letter to Dr. Harry Conacher of Health Canada. Int J Occup Environ Health. 2002;8(4):387–393 [DOI] [PubMed] [Google Scholar]
- 12. Whitehouse CR, Boullata J, McCauley LA. The potential toxicity of artificial sweeteners. AAOHN J. 2008;56(6):251–259; quiz 260–261 [DOI] [PubMed] [Google Scholar]
- 13. Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring). 2008;16(8):1894–1900 [DOI] [PubMed] [Google Scholar]
- 14. Stellman SD, Garfinkel L. Artificial sweetener use and one-year weight change among women. Prev Med. 1986;15(2):195–202 [DOI] [PubMed] [Google Scholar]
- 15. Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clin Nutr. 1990;51(6):1100–1105 [DOI] [PubMed] [Google Scholar]
- 16. Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the atherosclerosis risk in communities study. Circulation. 2008;117(6):754–761 [DOI] [PubMed] [Google Scholar]
- 17. Blum JW, Jacobsen DJ, Donnelly JE. Beverage consumption patterns in elementary school aged children across a two-year period. J Am Coll Nutr. 2005;24(2):93–98 [DOI] [PubMed] [Google Scholar]
- 18. Anderson GH, Foreyt J, Sigman-Grant M, Allison DB. The use of low-calorie sweeteners by adults: impact on weight management. J Nutr. 2012;142(6):1163S–1169S [DOI] [PubMed] [Google Scholar]
- 19. Kral TV, Stunkard AJ, Berkowitz RI, Stallings VA, Moore RH, Faith MS. Beverage consumption patterns of children born at different risk of obesity. Obesity (Silver Spring). 2008;16(8):1802–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raben A, Vasilaras TH, Møller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–729 [DOI] [PubMed] [Google Scholar]
- 21. Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997;65(2):409–418 [DOI] [PubMed] [Google Scholar]
- 22. Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104(38):15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakagawa Y, Nagasawa M, Yamada S, et al. Sweet taste receptor expressed in pancreatic β-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4(4):e5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in β cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 2012;109(8):E524–E532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582(pt 1):379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon BR, Parlee SD, Learman BS, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independent of sweet taste receptors. J Biol Chem. 2013;288(45):32475–32489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simon BR, Learman BS, Parlee SD, et al. Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PLoS One. 2014;9(1):e86454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Adipose Tissue Biol. 2014;537:93–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scheller EL, Troiano N, VanHoutan JN, et al. Use of osmium tetroxide staining with micro-computerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Adipose Tissue Biol. 2014;537:123–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alejandro EU, Lim GE, Mehran AE, et al. Pancreatic β-cell Raf-1 is required for glucose tolerance, insulin secretion, and insulin 2 transcription. FASEB J. 2011;25(11):3884–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−[Delta][Delta]C(T)) method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 32. Gjesdal CG, Halse JI, Eide GE, Brun JG, Tell GS. Impact of lean mass and fat mass on bone mineral density: the Hordaland Health Study. Maturitas. 2008;59(2):191–200 [DOI] [PubMed] [Google Scholar]
- 33. Szoka PR, Paigen K. Genetic regulation of mup production in recombinant inbred mice. Genetics. 1979;93(1):173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8 [PubMed] [Google Scholar]
- 35. Matthews HB, Fields M, Fishbein L. Saccharin: distribution and excretion of a limited dose in the rat. J Agric Food Chem. 1973;21(5):916–919 [DOI] [PubMed] [Google Scholar]
- 36. Bekersky I, Poynor WJ, Colburn WA. Pharmacokinetics of saccharin in the rat. Renal clearance in vivo and in the isolated perfused kidney. Drug Metab Dispos. 1980;8(2):64–67 [PubMed] [Google Scholar]
- 37. Minegishi KI, Asahina M, Yamaha T. The metabolism of saccharin and the related compounds in rats and guinea pigs. Chem Pharm Bull (Tokyo). 1972;20(7):1351–1356 [DOI] [PubMed] [Google Scholar]
- 38. Renwick AG, Sweatman TW. The absorption of saccharin from the rat urinary bladder. J Pharm Pharmacol. 1979;31(9):650–652 [DOI] [PubMed] [Google Scholar]
- 39. Sweatman TW, Renwick AG, Burgess CD. The pharmacokinetics of saccharin in man. Xenobiotica. 1981;11(8):531–540 [DOI] [PubMed] [Google Scholar]
- 40. Colburn WA, Bekersky I, Blumenthal HP. A preliminary report on the pharmacokinetics of saccharin in man: single oral dose administration. J Clin Pharmacol. 1981;21(4):147–151 [DOI] [PubMed] [Google Scholar]
- 41. Pantarotto C, Salmona M, Garattini S. Plasma kinetics and urinary elimination of saccharin in man. Toxicol Lett. 1981;9(4):367–371 [DOI] [PubMed] [Google Scholar]
- 42. Egan PC, Marx CM, Heyl PS, Popick A., Bekersky I. Saccharin excretion in mature human milk. Drug Intell Clin Pharm. 1984;18:511 (Abstract) [Google Scholar]
- 43. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han J, Lee JE, Jin J, et al. The spatiotemporal development of adipose tissue. Development. 2011;138(22):5027–5037 [DOI] [PubMed] [Google Scholar]
- 45. Anderson RL, Lefever FR, Maurer JK. Comparison of the responses of male rats to dietary sodium saccharin exposure initiated during nursing with responses to exposure initiated at weaning. Food Chem Toxicol. 1988;26(11–12):899–907 [DOI] [PubMed] [Google Scholar]
- 46. Schoenig GP, Goldenthal EI, Geil RG, Frith CH, Richter WR, Carlborg FW. Evaluation of the dose response and in utero exposure to saccharin in the rat. Food Chem Toxicol. 1985;23(4–5):475–490 [DOI] [PubMed] [Google Scholar]
- 47. Garland EM, Kraft PL, Shapiro R, et al. Effects of in utero and postnatal sodium saccharin exposure on the nutritional status of the young rat. I. Effects at 30 days post-birth. Food Chem Toxicol. 1991;29(10):657–667 [DOI] [PubMed] [Google Scholar]
- 48. Delgado TC, Barosa C, Nunes PM, Cerdan S, Geraldes CF, Jones JG. Resolving the sources of plasma glucose excursions following a glucose tolerance test in the rat with deuterated water and [U-13C]glucose. PLoS One. 2012;7(3):e34042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97 [PubMed] [Google Scholar]
- 50. Brüning JC, Michael MD, Winnay JN, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2(5):559–569 [DOI] [PubMed] [Google Scholar]
- 51. Bailey CJ, Day C, Knapper JM, Turner SL, Flatt PR. Antihyperglycaemic effect of saccharin in diabetic ob/ob mice. Br J Pharmacol. 1997;120(1):74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–1338 [DOI] [PubMed] [Google Scholar]
- 53. Lau EM, Chan YH, Chan M, et al. Vertebral deformity in chinese men: prevalence, risk factors, bone mineral density, and body composition measurements. Calcif Tissue Int. 2000;66(1):47–52 [DOI] [PubMed] [Google Scholar]
- 54. Reid IR, Evans MC, Ames RW. Volumetric bone density of the lumbar spine is related to fat mass but not lean mass in normal postmenopausal women. Osteoporos Int. 1994;4(6):362–367 [DOI] [PubMed] [Google Scholar]
- 55. Manion CV, Hochgeschwender U, Edmundson AB, Hugli TE, Gabaglia CR. Dietary aspartyl-phenylalanine-1-methyl ester delays osteoarthritis and prevents associated bone loss in STR/ORT mice. Rheumatology. 2011;50(7):1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grigor MR, Allan JE, Carrington JM, et al. Effect of dietary protein and food restriction on milk production and composition, maternal tissues and enzymes in lactating rats. J Nutr. 1987;117(7):1247–1258 [DOI] [PubMed] [Google Scholar]
- 57. Mueller AJ, Cox JWM. The effect of changes in diet on the volume and composition of rat milk. J Nutr. 1946;31:249. [DOI] [PubMed] [Google Scholar]
- 58. Warman NL, Rasmussen KM. Effects of malnutrition during the reproductive cycle on nutritional status and lactational performance of rat dams. Nutr Res. 1983;3(4):527 [Google Scholar]
- 59. Sturman JA, Devine E, Resnick O, Morgane PJ. Maternal protein malnutrition in the rat: effect on protein and two enzymes in the milk. Nutr Res. 1986;6(4):437 [Google Scholar]
- 60. Bautista CJ, Boeck L, Larrea F, Nathanielsz PW, Zambrano E. Effects of a maternal low protein isocaloric diet on milk leptin and progeny serum leptin concentration and appetitive behavior in the first 21 days of neonatal life in the rat. Pediatr Res. 2008;63(4):358–363 [DOI] [PubMed] [Google Scholar]
- 61. Pine AP, Jessop NS, Oldham JD. Maternal protein reserves and their influence on lactational performance in rats. 3. The effects of dietary protein restriction and stage of lactation on milk composition. Br J Nutr. 1994;72(6):815–830 [DOI] [PubMed] [Google Scholar]
- 62. Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, et al. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49(8):1974–1984 [DOI] [PubMed] [Google Scholar]
- 63. Desai M, Babu J, Ross MG. Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2306–R2314 [DOI] [PubMed] [Google Scholar]
- 64. Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol. 2007;196(6):555 ( e551–e557). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rasmussen KM. The influence of maternal nutrition on lactation. Annu Rev Nutr. 1992;12:103–117 [DOI] [PubMed] [Google Scholar]
- 66. Arnold DL, Moodie CA, Grice HC, et al. Long-term toxicity of ortho-touenesulfonamide and sodium saccharin in the rat. Toxicol Appl Pharmacol. 1980;52(1):113–152 [DOI] [PubMed] [Google Scholar]
- 67. Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE–a randomized controlled study. Arch Intern Med. 2004;164(1):31–39 [DOI] [PubMed] [Google Scholar]
- 68. Marandi SM, Abadi NG, Esfarjani F, Mojtahedi H, Ghasemi G. Effects of intensity of aerobics on body composition and blood lipid profile in obese/overweight females. Int J Prev Med. 2013;4(suppl 1):S118–S125 [PMC free article] [PubMed] [Google Scholar]
- 69. Bell RR, McGill TJ. Body composition and brown adipose tissue in sedentary and active mice. Nutr Res. 1991;11(6):633–642 [Google Scholar]
- 70. Swallow JG, Koteja P, Carter PA, Garland T., Jr Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol B. 2001;171(8):651–659 [DOI] [PubMed] [Google Scholar]
- 71. Holzenberger M, Hamard G, Zaoui R, et al. Experimental IGF-I receptor deficiency generates a sexually dimorphic pattern of organ-specific growth deficits in mice, affecting fat tissue in particular. Endocrinology. 2001;142(10):4469–4478 [DOI] [PubMed] [Google Scholar]
- 72. Semirale AA, Zhang XW, Wiren KM. Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J Cell Biochem. 2011;112(7):1773–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wiren KM, Zhang XW, Toombs AR, et al. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology. 2004;145(7):3507–3522 [DOI] [PubMed] [Google Scholar]
- 74. Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):224–232 [DOI] [PubMed] [Google Scholar]
- 75. Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40(1):189–207 [DOI] [PubMed] [Google Scholar]
- 76. Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab. 2003;88(11):5240–5247 [DOI] [PubMed] [Google Scholar]
- 77. Kung AW. Androgen and bone mass in men. Asian J Androl. 2003;5(2):148–154 [PubMed] [Google Scholar]
- 78. Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26(1):85–92 [PubMed] [Google Scholar]
- 79. Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99(7):4692–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kuhn C, Bufe B, Winnig M, et al. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 2004;24(45):10260–10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol. 2000;10(4):519–527 [DOI] [PubMed] [Google Scholar]
- 82. Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104(38):15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Le Gall M, Tobin V, Stolarczyk E, Dalet V, Leturque A, Brot-Laroche E. Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism. J Cell Physiol. 2007;213(3):834–843 [DOI] [PubMed] [Google Scholar]
- 84. Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996;490(pt 2):325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Raybould HE. Does Your Gut Taste? Sensory Transduction in the Gastrointestinal Tract. News Physiol Sci. 1998;13:275–280 [DOI] [PubMed] [Google Scholar]
- 86. Tan X, Sanderson MJ. Bitter-taste compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br J Pharmacol. 2014;171(3):646–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Deshpande DA, Wang WC, McIlmoyle EL, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325(5944):1131–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kaji I, Karaki S, Fukami Y, Terasaki M, Kuwahara A. Secretory effects of a luminal bitter tastant and expressions of bitter taste receptors, T2Rs, in the human and rat large intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G971–G981 [DOI] [PubMed] [Google Scholar]
- 91. Xu J, Cao J, Iguchi N, Riethmacher D, Huang L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod. 2013;19(1):17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Neese RA, Misell LM, Turner S, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci USA. 2002;99(24):15345–15350 [DOI] [PMC free article] [PubMed] [Google Scholar]