Abstract
The human pregnane X receptor (hPXR), a major chemical toxin sensor, is a ligand-induced transcription factor activated by various xenobiotics and toxins, resulting in the transcriptional upregulation of detoxifying enzymes. To date, little is known about the upstream regulation of hPXR. Using mass spectrometry analysis and kinome-wide siRNA screen, we report that the E3 ligase UBR5 and DYRK2 regulates hPXR stability. UBR5 knockdown resulted in accumulation of cellular hPXR and a concomitant increase in hPXR activity, whereas the rescue of UBR5 knockdown decreased the cellular hPXR level and activity. Importantly, UBR5 exerted its effect in concert with the serine/threonine kinase DYRK2, as the knockdown of DYRK2 phenocopied UBR5 knockdown. hPXR was a substrate for DYRK2, and DYRK2-dependent phosphorylation on hPXR facilitated its subsequent ubiquitination by UBR5. This is the first report of the posttranslational regulation of hPXR via phosphorylation-facilitated ubiquitination by DYRK2 and UBR5. Our results reveal the role of the ubiquitin-proteasomal pathway in modulating hPXR activity and indicate that pharmacological inhibitors to the ubiquitin-proteasomal pathway that regulates hPXR stability may negatively affect treatment outcome from unintended hPXR-mediated drug-drug interactions.
Keywords: phosphorylation, ubiquitination, nuclear receptor
INTRODUCTION
The human liver detoxification system comprises transporters and drug-metabolizing enzymes and is tightly governed by the human pregnane receptor (hPXR), a member of the nuclear receptor family [1]. hPXR has been shown to be activated by a wide array of compounds both endogenous and exogenous, including bile acids, xenobiotics, herbal supplements, and prescription medications [2–4]. Ligand-induced activation of hPXR leads to the transcriptional upregulation of many drug-metabolizing enzymes and transporters, namely cytochrome p450 3A4 (CYP3A4) [5, 6], efflux pump protein MDR1 [7], and UDP-glucuronosyl transferase 1A1 (UGT1A1) [8], which can lead to xenobiotic detoxification and pharmacoresistance. The ligand promiscuity of hPXR often leads to undesirable cross-reactivity with many prescription pharmaceuticals, prompting heavy investments in eliminating the PXR-activating effect of drug candidates. As a result, the downstream regulation of hPXR and its target genes is well characterized. However, little is known about the upstream regulation of hPXR, which can occur through the modulation of its protein level and activity. Some posttranslational modifications of hPXR have been reported, such as phosphorylation [9] and more recently ubiquitination and SUMOylation [10, 11].
Phosphorylation commonly occurs on tyrosine by tyrosine kinases, or serine/threonine residues by serine/threonine kinases. Phosphorylation on hPXR mainly occurs on serine and threonine residues, and this phosphorylation is mainly associated with the negative regulation of its activity [12]. Experiments using phosphomimetic mutants further show that phosphorylation on hPXR alters its subcellular localization [13] or abrogates the recruitments of co-activators [14]. Ubiquitination is a process by which ubiquitin moieties are added onto lysine residues of a target protein. Ubiquitination is a multistep process involving the sequential transfer of a ubiquitin molecule between an E1 ubiquitin activating enzyme, E2 ubiquitin conjugating enzyme and E3 ubiquitin ligase [15]. Depending on how the ubiquitin moieties are attached, the target protein may follow different fates, ranging from proteasomal mediated degradation to relocalization into various cellular compartments [15]. In addition to phosphorylation, ubiquitination on hPXR further serves to modulate its activity. hPXR was shown to interact with suppressor for gal1 (SUG1), a component of the proteasome, in a progesterone-dependent manner to result in the degradation of PXR [16, 17]. More recently, ubiquitination on hPXR has been reported and ring-B-box-coiled-coil protein interacting with protein kinase C-1 (RBCK1) was identified as an E3 ligase responsible for ubiquitinating hPXR [10, 11]. Thus, post-translational modifications of nuclear receptors, including hPXR, contribute to regulating their activity and function. Understanding how hPXR is regulated is an important yet less explored avenue in modulating drug–drug interactions and drug resistance.
In this study, we sought to identify other modulators of hPXR activity and function. We used a small interfering RNA (siRNA)–based screen of human kinases and mass spectrometry (MS) analysis to show that the ubiquitin protein ligase E3 component n-recognin 5 (UBR5) in conjunction with the dual specificity tyrosine-phosphorylation-regulated kinase 2 (DYRK2) negatively regulates hPXR stability and activity. We show that hPXR can be phosphorylated by DYRK2 and this phosphorylation facilitates the ubiquitination of hPXR by UBR5. We further determined that DYRK2 and UBR5 exert their function as a multiprotein complex to regulate hPXR homeostasis. Our findings provide the molecular mechanisms by which hPXR stability is regulated.
MATERIALS AND METHODS
Cell culture, plasmids, and antibodies
HepG2 human liver carcinoma and HEK 293T cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cells were grown in Minimum Essential Media or Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin, 2 mM L-glutamine, and 1 mM sodium pyruvate. Primary human hepatocytes were obtained through the Liver Tissue Cell Distribution System (Pittsburgh, Pennsylvania) or Triangle Research Laboratories (Ashville, NC). The donors were 13-004, HUM4046 and GC4003. The primary hepatocytes were maintained in Williams Medium E media (Sigma-Aldrich, St Louis, MO) supplemented cell maintenance supplement (Life Technologies, Carlsbad, CA). HEK 293T cells stably expressing FLAG-hPXR (fPXR) were used to establish stable knockdown of UBR5, DYRK2, or non-targeting control. Briefly, HEK 293T/fPXR cells were transduced with a short hairpin (sh) RNA against UBR5 (shUBR5) that targets the 3’-UTR of UBR5, shDYRK2 that targets the 3’-UTR of DYRK2, or non-targeting shRNA (NT). Non-targeting shRNA, shUBR5, and shDYRK2 were from Sigma-Aldrich (St. Louis, MO), purchased as transfection-ready lentiviral particles. The knock down of UBR5 or DYRK2 was confirmed by Western blotting analysis. Cells were cultured in an incubator with a humidified atmosphere maintained at 5% CO2 at 37°C. FLAG-DYRK2 [18] were purchased from Addgene (Cambridge, MA). pcDNA3-FLAG-hPXR and CYP3A4 luciferase reporter (CYP3A4-luc) plasmids were generated as described previously [19]. SF2-FLAG-hPXR was constructed by using standard molecular biology methods. The SF2 hybrid promoter uses the spleen focus forming virus LTR enhancer fused to the CMV TATA box (provided by Dr. John Gray). GFP-WT UBR5 and GFP-CA UBR5 (C2768A) were generated in the laboratory of Darren Saunders. The following antibodies were used: anti-GFP, anti-UBR5, anti- PXR (H-11; sc-48340) and anti-ubiquitin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-DYRK2 (Abcam, Cambridge, MA), anti-DDB1, anti-Cul4a, anti-VprBP and anti-RbBP7 (Bethyl Laboratories, Montgomery, TX), anti-GAPDH (Ambion, Austin, TX), and anti-FLAG (Sigma-Aldrich, St. Louis, MO). Anti-CYP3A4 (K03) was previously described [20].
Transient transfections, immunoprecipitation, and immunoblotting
Transient transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Cell lysates were prepared by using 0.5% Triton X-100 lysis buffer (0.5% Triton X-100, 20 mM Tris-HCl, pH 7.4, 5 mM EDTA, 150 mM NaCl) supplemented with 10 mM NaF, 10 mM sodium orthovanadate, and Halt protease inhibitors (Pierce Biotechnology, Rockford, IL). Immunoprecipitation was performed on lysates by using 1 μg of specific antibodies conjugated to Protein A/G Plus beads (Santa Cruz Biotechnology) for 2 h at 4°C. Immunoblotting was performed using standard protocols. One representative Western blot was shown for each study. The intensity of the protein band was quantified using LiCor Odyssey (LI-COR Biosciences, NE).
siRNA screen
The human kinome collection (Dharmacon, Inc., Lafayette, CO) was reverse transfected into HepG2 cells stably expressing ectopic hPXR and CYP3A4-luc by using Lipofectamine RNAiMAX (Life Technologies, Carlsbad, CA) at a final concentration of 25 nM. Briefly, reverse transfection is a process by which cells are overlaid onto lipid:siRNA mix. Reverse transfection was employed since this process is more amenable to high throughput assays. Cells were treated with rifampicin at a final concentration of 1.25 μM using pin-tool transfer 72 h post-transfection. Luciferase readout was measured 24 h post-rifampicin treatment. A robust strictly standardized mean difference (SSMD)-based algorithm on GUItars (A GUI tool for analysis of high-throughput RNA interference screening) was used for hit selection [21]. Briefly, SSMD is defined as the ratio of mean to standard deviation of the random variable representing the difference between two independent populations. The bigger the magnitude of SSMD between two populations is, the more the two populations separate from each other. An siRNA with SSMD ≥ 1 is considered a fairly moderate hit. In high throughput assays, the data analysis methods should be robust to outliers. The robust version of SSMD (robust SSMD, or SSMD*) can be obtained by replacing mean with median, and standard deviation with median absolute deviation [22]. The higher the SSMD score the higher possibility that the siRNA is a hit [23, 24]. Putative negative regulators of hPXR activity were identified as having a robust SSMD value of ≥ 1. siDYRK2 was from Dharmacon (Lafayette, CO), Qiagen (Valencia, CA) and Novus Biologicals (Littleton, CO).
Luciferase assay
Cells either transiently transfected with CYP3A4-luc (the activity of CYP3A4-luc is regulated by hPXR and used to measure the transcription activity of hPXR) and TK-Renilla (used as a transfection control) plasmids or stably expressing the CYP3A4-luc plasmid were used to measure PXR activity, using rifampicin (Sigma-Aldrich) as the PXR agonist. Cells were plated on 96-well plates in phenol red–free media supplemented with 5% charcoal-dextran–treated fetal bovine serum. After 24h, luciferase activity was measured by the SteadyLite Plus substrate (PerkinElmer, Waltham, MA) for cells stably expressing the CYP3A4-luc plasmid or the Dual-Glo substrate system (Promega) for transiently transfected cells. Luminescence was read on the EnVision plate reader (PerkinElmer). CYP3A4 promoter activity was expressed as relative luciferase unit (RLU), which was determined by normalizing the firefly luciferase activity (from CYP3A4-luc) to the Renilla luciferase activity (from TK-Renilla) in the Dual-Glo assay, or represented by the total counts from the SteadyLite assay. The values represent the mean of 3 independent experiments, and the bars denote the standard deviation.
RNA isolation and real-time reverse transcription–polymerase chain reaction
RNA was isolated using the Maxwell 16 LEV simplyRNA purification kits (Promega, Madison, WI). Real-time reverse transcription–polymerase chain reaction (RT-PCR) was used to measure the levels of mRNA, and was performed on Applied Biosystems 7900HT Fast Real-Time PCR System (Life Technologies, Carlsbad, CA). Taqman probes were obtained from Life Technologies (Carlsbad, CA) (Cat # 4331182). Briefly, the cycle threshold (Ct) values of each gene of interest and of GAPDH were calculated for each sample, and then the normalized value was derived by subtracting the Ct value of GAPDH from that of the gene of interest (ΔCt). Data are shown as mRNA fold change (2–ΔΔCt) relative to the mRNA level of the corresponding transcript in the control samples as indicated. The values represent the mean of 3 independent experiments, and the bars denote the standard deviation.
Mass spectrometric analysis
HEK 293T cells were transiently transfected with pcDNA3.1 FLAG-PXR or pcDNA3.1-FLAG vector using Lipofectamine2000 (Invitrogen), according to manufacturer’s protocol. Cells were lysed with 1% Triton X-100 lysis buffer (1% Triton X-100, 20 mM Tris-HCl, pH 7.4, 5 mM EDTA, 150 mM NaCl) supplemented with 10 mM NaF, 10 mM sodium orthovanadate, and Halt protease inhibitors (Pierce Biotechnology, Rockford, IL). After centrifugation at 10,000g for 20 min, lysates were incubated with 10 μl of EZview red anti-FLAG M2 affinity gel (Sigma-Aldrich) for 2 h at 4°C. The M2 beads with the bound proteins were washed twice with the lysis buffer, twice with Tris-buffered saline (TBS), and eluted with 100 ng/μl FLAG peptide (Sigma-Aldrich) in TBS for 1 h at 4°C. The eluted protein solution was mixed with ice-cold ethanol and incubated overnight at −20°C to precipitate the proteins. The solution was then centrifuged at 6000g for 30 min and dried using a vacuum evaporator. The protein pellet was then submitted for processing and protein identification by the St. Jude Proteomics & Mass Spectrometry Shared Resource using an LTQ-Orbitrap mass spectrometer (ThermoFisher, San Jose, CA). Briefly, a solution phase digestion of the protein samples was performed using Lys-C and Trypsin. Mass spectrometric analysis was performed using an LTQ-Orbitrap mass spectrometer, which employs electrospray ionization (ESI), in conjunction with an Orbitrap mass analyzer. The digest was introduced into the instrument via on line chromatography using reverse phase (C18) ultra-high pressure liquid chromatography on the nanoAcquity (Waters, Milford, MA). The column used was a Waters BEHC18 with an I.D. of 75μm and bed length of 10cm. The particle size was 1.7μm. Peptides were gradient eluted into the linear ion trap through a non-coated spray needle with voltage applied to the liquid by increasing the concentration of acetonitrile. Data acquisition involved acquiring the peptide mass spectra followed by fragmentation of the peptide to produce MS/MS spectra that provides information about the peptide sequence. Protein/peptide assignments are made on the basis of MS/MS spectra. The data were processed using the Mascot searching algorithm (Matrix Science Inc., Boston, MA) and Scaffold (Proteome Software, Portland, OR) [25].
In vitro kinase assays
His-PXR (OriGene, Rockville, MD) immobilized on nickel beads (Qiagen, Valencia, CA) was used as substrate. 100 ng of GST-WT DYRK2 (SignalChem, Richmond, BC, Canada) was incubated with 1 μg of His-PXR or 1 μg of histone H1 (as a positive substrate control) in kinase buffer [25 mM Tris-HCl (pH 7.5), 5 mM beta-glycerophosphate, 2 mM dithiothreitol (DTT), 0.1 mM Na3VO4, 10 mM MgCl2, 5 μM ATP, 10 μCi [γ-32P] ATP] for 30 min at 30°C. Reactions were terminated by adding SDS sample loading buffer, followed by boiling the samples at 95°C for 5 min. Samples were resolved on SDS-PAGE, transferred onto polyvinylidene fluoride (PVDF) membrane, and analyzed by autoradiography, or Western blotting.
In vitro ubiquitination assays
Reactions were carried out using 1 μg of purified His-PXR as substrate in 50 μl ubiquitination buffer containing 100 nM E1, 2.5 μM UbcH5a, 2.5 μM biotinylated ubiquitin, 5 mM Mg-ATP, and 1 mM DTT (ubiquitinylation kit, Cat # BML-UW9920-0001, Enzo Life Sciences, Farmingdale, NY). GFP, GFP-WT UBR5, or GFP-CA UBR5 immunoprecipitated by using anti-GFP antibody from transiently transfected HEK 293T cells was used as a source of E3. The reaction mix was incubated for 90 min at 37°C followed by 3 washes of RIPA lysis buffer.
Statistical analysis
Results are expressed as the mean ± SD of at least 3 independent experiments. The Student’s t-test was used to determine the statistical significance of the difference between the paired samples. For grouped comparison in Figure 4B, Kruskal-Wallis test was employed, followed by Wilcoxon-Mann Whitney post hoc test for pairwise comparison. Differences were considered significant if p < 0.05 (*).
Figure 4. hPXR is a substrate for DYRK2 and UBR5.
(A) In vitro kinase assay using purified GST-WT DYRK2 (DYRK2) and His-tagged hPXR (His-PXR). Histone was used as a positive control for the kinase activity of DYRK2. (B) In vitro ubiquitination assay using purified His-PXR in the presence of GFP (as a negative control) or GFP-WT UBR5 (GFP-UBR5) as a source of E3 ligase. Ubiquitinated His-hPXR was detected using streptavidin, which recognizes biotinylated ubiquitin. The bar graph below the representative image displays the average relative intensity of the area representing the ubiquitinated hPXR from 3 independent experiments. * denotes statistical significance with p < 0.05 (for grouped comparison among lanes 2, 3, 4, and 5, Kruskal-Wallis test was employed, followed by Wilcoxon-Mann Whitney post hoc test for pairwise comparison). Lanes 1, 2, 3, 4, and 5 are all from the same gel. (C) (Left panel) In vitro ubiquitination assay using purified His-hPXR in the presence of either GFP-WT UBR5 or GFP-CA UBR5 as a source of E3 ligase. Neg. Crtl, EDTA was added to the sample to inhibit the reaction process and serves as a negative control. The amounts of input His-PXR are shown in the bottom panel, as revealed in a paralleled Western blotting analysis using anti-PXR antibodies. (Right panel) Western blotting analysis showing the amount of WT UBR5 and CA UBR5 used in the in vitro ubiquitination reaction. Molecular weight markers are indicated on the left of the Western blot images.
RESULTS
DYRK2 negatively regulates hPXR activity
hPXR activity is known to be negatively regulated by phosphorylation [12]. To identify the kinases that regulate hPXR activity, we performed an siRNA screen against the human kinase collection using liver-derived HepG2 cells stably expressing ectopic hPXR and the CYP3A4 promoter luciferase plasmid (CYP3A4-luc); the activity of the CYP3A4 promoter is regulated by hPXR. As seen from the waterfall plot (Fig. 1A), the top hits identified as negative regulators of hPXR activity, including CDK4, CDK18, and DGKA (denoted as dots on the waterfall plot; SSMD > 1; see materials and methods for more details), are involved in cell cycle regulation and cellular proliferation, which is consistent with what we and other groups have previously reported [26, 27]. Interestingly, DYRK2, which is involved in cell cycle regulation and cellular growth, also emerged as one of the top hits from the screen (Fig. 1A; denoted with an “X”; see the magnified view on the right). DYRK2 was previously reported to be present in a complex containing E3 ubiquitin protein ligase UBR5 and mediates the stability of katanin [28].
Figure 1. DYRK2 is a negative regulator of hPXR activity.
(A) Waterfall plot of hits generated from a kinome-wide siRNA screen performed on HepG2 cells stably expressing hPXR and CYP3A4 promoter–driven luciferase plasmid (CYP3A4-luc). Y-axis displays the value of robust SSMD for each siRNA. Robust SSMD is a parameter used to select hit (see “Materials and Methods” section). (Inset) Magnified view of the waterfall plot showing DYRK2 (denoted with an “X”) as one of the top hits (robust SSMD > 1). Cell cycle regulated genes (denoted with a “♦”) shown as top hits. (B) Rifampicin [RIF] dose response of endogenous PXR activity following a non-targeting (NT) or DYRK2 knockdown (siDYRK2) in HepG2 cells stably expressing CYP3A4-luc. * denotes statistical significance with p < 0.05 (comparison was made between siDYRK2 and NT). (C) RT-PCR of endogenous CYP3A4 transcript level after NT or siDYRK2 knockdown. The value from untreated (−RIF) NT was set as “1”. * denotes statistical significance with p < 0.05 (comparison was made between siDYRK2 and NT).
To further confirm that DYRK2 is a negative regulator of hPXR activity, the activity of endogenous hPXR was measured in HepG2 cells stably expressing CYP3A4-luc against siRNA for DYRK2 (siDYRK2) obtained from different sources. This approach of measuring low expression levels of endogenous hPXR in HepG2 cells eliminates the possibility of an off-target effect of siRNA on the luciferase reporter gene. All siRNAs efficiently knocked down the DYRK2 transcript level (Fig. S1A). Knockdown of DYRK2 consistently led to an increase in hPXR activity, as measured by luciferase readout (Fig. 1B and Fig. S1C) and the endogenous CYP3A4 transcript level (Fig. 1C and Fig. S1A). The increase in hPXR activity was not accompanied by an increase in the hPXR transcript level (Fig. S1A), ruling out the possibility of transcriptional regulation of hPXR. These results indicate that DYRK2 negatively regulates hPXR, most likely at a post-translational level.
hPXR associates with DYRK2 in a complex
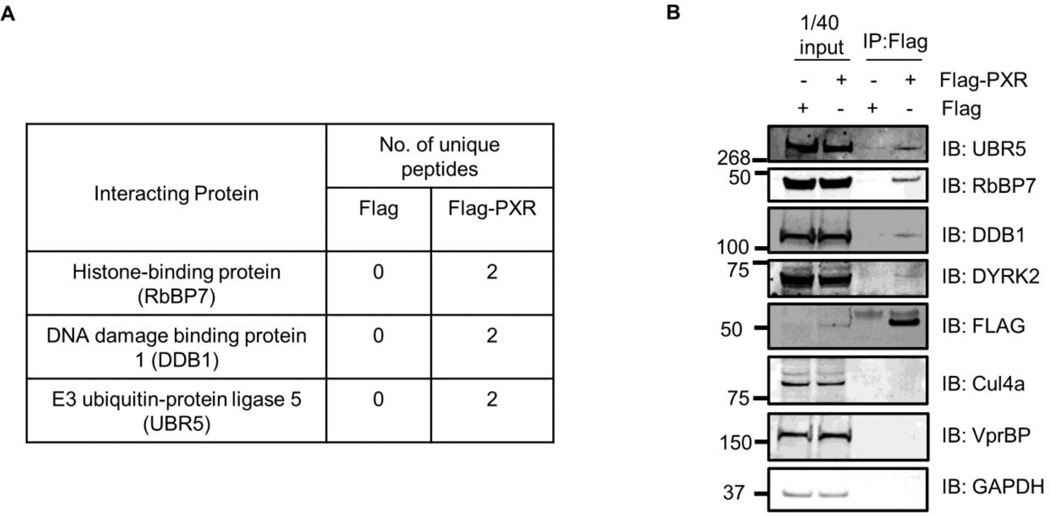
The identification of DYRK2 as a negative regulator of hPXR, and the previous report that DYRK2 associated with UBR5 in a complex to regulate the stability of katanin [28], prompted us to determine whether hPXR interacts with DYRK2 and UBR5 in a multiprotein complex. To identify the binding partners of hPXR, HEK 293T cells transiently transfected with either an empty vector containing the FLAG epitope (as a negative control to eliminate non-specific binding proteins), or FLAG-hPXR, were immunoprecipitated using M2-agarose beads and then analyzed by mass spectrometry (MS). HEK 293T cells were chosen because they were used in the study that identified the interaction between DYRK2 and UBR5 [28]. HEK 293T cells do not express hPXR endogenously. UBR5, DNA damage binding protein 1 (DDB1), and histone binding protein 7 (RbBP7) were among the putative binding partners identified (Fig. 2A). UBR5 is a HECT domain–containing E3 ligase that targets proteins for proteasomal-mediated degradation. DDB1 functions in nucleotide excision repair and is also a component of the Cul4-X-box E3 ligase complex. DDB1 is also found in a complex containing UBR5 [28]. RbBP7 is a WD40 domain–containing protein also found in a complex with the DDB1-Cul4A complex [29]. To confirm the results from the MS analysis, we transiently overexpressed FLAG or FLAG-hPXR in HEK 293T cells and performed co-immunoprecipitation assays. UBR5 interacted with hPXR in cells, and Western blot analysis indicated that this interaction occurs in a complex containing DDB1 and RbBP7, as suggested by data obtained from MS analysis (Fig. 2B). DYRK2 was also detected in this complex (Fig. 2B), although it was not identified from the MS analysis. Since VprBP1 is the substrate-recognition component of the E3 ligase complex containing UBR5 and DDB1 reported by Maddika et al. [28], we checked whether VprBP1 was present in this complex. We found that VprBP1 was not an interacting protein (Fig. 2B). Since both VprBP and RbBP7 contain WD40 domains, we reasoned that RbBP7 may be the substrate-recognition protein that recruits PXR to the complex. Cul4a was also not detected in this complex, which precludes the possibility that hPXR interacted with RbBP7 in a complex containing Cul4A. Taken together, these results indicate that hPXR associates with DYRK2 in a multiprotein complex that also contains UBR5, DDB1 and RbBP7, and suggest that UBR5 might contribute to the negative regulation of hPXR by DYRK2.
Figure 2. hPXR interacts with DYRK2 in a multiprotein complex containing UBR5.
(A) Table showing hPXR-associated proteins and the corresponding number of peptides identified from mass spectrometric (MS) analysis. (B) Co-immunoprecipitation using anti-FLAG antibodies (IP: FLAG) in HEK 293T cells expressing FLAG-hPXR to confirm the presence of associated proteins identified by MS. The association was confirmed by antibodies against UBR5, DDB1, DYRK2, and RbBP7 in a Western blot analysis (IB). “1/40 input”: 2.5% of input lysates used to show the expression levels of proteins. GAPDH was used as a loading control. Molecular weight markers are indicated on the left of the Western blot images.
hPXR is ubiquitinated
Because hPXR occurred in a complex that contained E3 ubiquitin ligase UBR5, we investigated whether hPXR was degraded through the ubiquitin proteasomal pathway. Protein levels of hPXR increased by 60% in HEK 293T cells stably expressing FLAG-hPXR treated with the proteasomal inhibitor MG132 (Fig. 3A, 3rd and 4th lanes), suggesting that hPXR is a substrate for the proteasome. To determine whether hPXR was ubiquitinated, we immunoprecipitated FLAG-hPXR from the MG132-treated cells by using anti-FLAG antibodies, followed by Western blotting analysis using antibodies against the FLAG epitope (Fig. 3B, top panel) or ubiquitin (Fig. 3B, bottom panel). hPXR underwent significant ubiquitination (Fig. 3B), as evidenced by the higher-molecular-weight laddering of hPXR (Fig. 3B, 4th lane, top panel) and the corresponding ubiquitin immunoblot (Fig. 3B, 4th lane, bottom panel). These results indicated that hPXR is ubiquitinated and degraded through the ubiquitin proteasomal pathway. Next, we sought to determine whether UBR5 also functions as a negative regulator of hPXR activity. We used HepG2 stably expressing the CYP3A4-luc, the same cellular model in which we demonstrated DYRK2 as a negative regulator of hPXR activity (Fig. 1B). As shown in Fig. 3C, knockdown of UBR5 using siRNA against UBR5 (siUBR5) led to higher activity of hPXR, as revealed by the increased activity of the hPXR-regulated CYP3A4 promoter. These results indicate that UBR5 is a negative regulator of hPXR.
Figure 3. hPXR undergoes UBR5 dependent polyubiquitination.
(A) HEK 293T cells stably expressing FLAG-hPXR was treated either with vehicle control (DMSO, “−”) or 10 μM MG132 (“+”) for 6 hours followed by Western blotting analysis to determine cellular hPXR level. SF2, empty vector control; fPXR, SF2-FLAG-hPXR. The numbers at the bottom indicate the relative fold change in the FLAG-PXR bands, with the DMSO treated sample set as “1” (B) HEK 293T cells stably expressing FLAG-hPXR or empty vector control (Flag) was treated either with vehicle control (DMSO, “−”) or 10 μM MG132 (“+”) for 6 hours followed by immunoprecipitation using anti-FLAG antibodies and Western blotting analysis using antibodies against ubiquitin and FLAG epitope. (C) Knockdown of UBR5 (siUBR5) was performed in HepG2 cells stably expressing CYP3A4-luc. Seventy-two hours posttransfection with either siUBR5 or non-targeting (NT) control, the transfected cells were treated with either RIF or DMSO as indicated. CYP3A4-luc activity was measured 24 hours post RIF or DMSO treatment. Molecular weight markers are indicated on the left of the Western blot images. * denotes statistical significance with p < 0.05 (comparison was made between siUBR5 and NT).
hPXR is a substrate for DYRK2 and UBR5
We then sought to determine whether hPXR is a substrate for DYRK2 and UBR5, and whether DYRK2 exerts its function by phosphorylating hPXR and facilitate its ubiquitination by UBR5. In an in vitro kinase assay we demonstrated that purified wild-type DYRK2 phosphorylates purified hPXR (Fig. 4A, 7th lane, top panel), indicating that hPXR is a substrate for DYRK2. Histone H1 was used as a positive substrate control in the assays (Fig. 4A, 4th lane, top panel). Next, we tested whether PXR was the substrate for UBR5 in vitro. We used GFP-UBR5, or GFP, transiently expressed and immunoprecipitated from HEK 293T cells as a source of E3, or a negative control, respectively. As shown in Fig. 4B in the in vitro ubiquitination assay, GFP-UBR5 but not GFP, mediated the ubiquitination of purified His-PXR in the presence of purified DYRK2. As an additional negative control, we showed that a catalytically inactive UBR5 (CA UBR5) failed to mediate hPXR ubiquitination (Fig. 4C, left panel). Taken together, these results indicate that hPXR is a substrate for DYRK2 and UBR5, and the kinase activity of DYRK2 facilitates the efficient ubiquitination of hPXR.
UBR5 and DYRK2 regulate the protein level and activity of hPXR
To further study the effect of UBR5 and DYRK2 on hPXR, we determined whether modulation of the cellular levels of UBR5 and DYRK2 affected the ubiquitination status, protein level, and activity of hPXR.
We first transiently knocked down UBR5 in HEK 293T cells stably expressing either FLAG vector (SF2) or FLAG-hPXR (fPXR). Transient UBR5 knockdown led to a decrease in hPXR ubiquitination (Fig. 5A, 3rd and 4th lanes, top panel) and a concomitant increase in hPXR protein level (Fig. 5A, 3rd and 4th lanes, second panel from top). This increase in protein level was not accompanied by an increase in the hPXR transcript level (data not shown). To further show that the increase in cellular level of hPXR is mediated through the knockdown of UBR5, we used HEK 293T/fPXR cells to stably and efficiently knocked down UBR5 by using a short hairpin (sh) RNA against UBR5 (shUBR5) that targets the 3’-UTR of UBR5; Non-targeting shRNA (NT) was used in a control cells (Fig. 5B, 1st and 4th lanes, top panel). Stable knockdown of UBR5 increased the protein level of hPXR (Fig. 5B, 1st and 4th lanes, middle panel), which was partially rescued by reintroducing WT UBR5 but not CA UBR5 (neither plasmid contains a 3’-UTR of UBR5 so they are insensitive to the regulation of shUBR5) (Fig. 5B, lanes 4–6). Fig. 5C quantified the levels of hPXR observed in Fig. 5B. These results indicate that the level of hPXR is inversely correlated with those of wild-type UBR5.
Figure 5. hPXR level is modulated by UBR5 and DYRK2.
(A) siRNA knockdown of UBR5 (“+siUBR5”) or non-targeting control (“−”) was performed on HEK 293T cells stably expressing an empty vector (SF2) or SF2-FLAG-hPXR (fPXR). Seventy-two hours post-transfection, the cells were treated with 10 μM MG132 for 6 hours, followed by an immunoprecipitation using anti-FLAG antibodies and immunoblotting using antibodies as indicated. (B) A stable knockdown of UBR5 was performed on HEK 293T cells stably expressing FLAG-hPXR using shRNA targeting the 3’-UTR of UBR5 (shUBR5). A rescue experiment was done by transiently overexpressing knockdown-resistant WT UBR5 (WT) or CA UBR5 (CA). Total hPXR was determined using antibodies against FLAG epitope. (C) Quantification of (B) by normalizing hPXR level to GAPDH level. * denotes statistical significance with p < 0.05, from at least 3 independent experiments. (D) Stable knockdown of DYRK2 on HEK 293T cells stably expressing FLAG-hPXR was performed using shRNA against the 3’-UTR of DYRK2 (shDYRK2). FLAG-hPXR was immunoprecipitated from cells pretreated with 10 μM of MG 132 for 6 hours. Ubiquitinated hPXR was visualized using antibodies against ubiquitin. (E) Rescue experiment was performed by transiently overexpressing knockdown-resistant WT DYRK2 (WT) or KD DYRK2 (KD). Total hPXR level was determined by antibodies against FLAG epitope. (F) Quantification of (E) by normalizing hPXR level to GAPDH level. * denotes statistical significance with p < 0.05 from at least 3 independent experiments. NT, non-targeting shRNA control; ø, empty vector control. Molecular weight markers are indicated on the left of the Western blot images.
Since DYRK2 and UBR5 exerted their functions on hPXR in a complex, we next determined whether modulation of DYRK2 had the same effect as UBR5 modulation did. Stable knockdown of DYRK2 using shDYRK2 that targets the 3’-UTR of DYRK2 in HEK 293T/fPXR cells led to an increase in protein level of hPXR and a concomitant decrease in hPXR ubiquitination (Fig. 5D). Importantly, the level of hPXR ubiquitination (Fig. 5D, top panel) was inversely correlated with the total hPXR level (Fig. 5D, second panel from top). To determine whether the change in hPXR was dependent on the DYRK2 level, a rescue experiment was performed in this stable knockdown cell system. The HEK 293T/fPXR cells stably expressing shRNA against the 3’-UTR of DYRK2 were transiently transfected with WT DYRK2 or the kinase-dead mutant of DYRK2 (KD DYRK2). The increase in hPXR protein level was partially rescued by reintroduction of WT DYRK2 but not KD DYRK2, confirming that the kinase activity of DYRK2 is required for its function (Fig. 5E). Fig. 5F quantified the levels of hPXR observed in Fig. 5E. These results indicate that the level of hPXR is inversely correlated with those of wild-type DYRK2.
To further confirm our observations, we examined the effect of knocking down or overexpressing UBR5 or DYRK2 on the level and activity of PXR in primary human hepatocytes. As shown in Fig. 6A and B, shRNA against UBR5 and DYRK2 knocked down UBR5 and DYRK2, respectively, which are accompanied by a concomitant increase in hPXR protein level. Conversely, the ectopic expression of DYRK2 and UBR5 led to a decrease in hPXR protein level (Fig. 6C and D). Importantly, modulation of hPXR protein levels led to corresponding changes in CYP3A4 protein levels.
Figure 6. hPXR level and activity are modulated by UBR5 and DYRK2 in human primary hepatocytes.
(A) shRNA knockdown of UBR5 (shUBR5) and DYRK2 (shDYRK2) was performed on primary hepatocytes. Seventy-two hours post-transduction, the cells were harvested and subjected to Western blot analysis using antibodies as indicated. Molecular weight markers are indicated on the left of the Western blot images. The images show results from one donor (HUM4046). (B) RT-PCR of the samples in (A) using probes specific to UBR5 and DYRK2. The value from shNT was set as “1”. The Student’s t-test was used to determine the statistical significance of the difference between shUBR5 or shDYRK2 and shNT (B), DYRK2 or UBR5 and mock (D). * denotes statistical significance with p < 0.05, from 3 different donors. (C) Transient over-expression of UBR5 or DYRK2 into primary hepatocytes followed by Western blotting analysis 48-hour post-transfection. (D) RT-PCR of the samples in (C) using probes specific to UBR5 and DYRK2. The value from mock transfection was set as “1”.
To further determine the effect of reintroducing UBR5 and DYRK2 on the activity of hPXR, we used HEK 293T/fPXR cells stably expressing either shUBR5 or shDYRK2, and transfected with CYP3A4-luc. Reintroduction of WT UBR5, but not CA UBR5 or empty vector (GFP) to HEK 293T/fPXR/shUBR5 cells, led to a decrease in PXR activity (Fig. 7A). Similarly, reintroduction of WT DYRK2, but not KD DYRK2 or empty vector (FLAG) to HEK 293T/fPXR/shDYRK2 cells, led to a decrease in PXR activity (Fig. 7B). Taken together, our results indicate that UBR5 and DYRK2 play a role in regulating hPXR protein level and activity.
Figure 7. hPXR activity is modulated by UBR5 and DYRK2.
(A) Either GFP vector (GFP), GFPtagged WT-UBR5 (GFP-WT UBR5) or CA-UBR5 was reconstituted into HEK 293T cells stably expressing shRNA against the 3’-UTR of UBR5. hPXR activity was measured by luciferase-based CYP3A4 promoter activity following treatment with DMSO, or a dose response of RIF for 24 hours. (B) Either, FLAG vector (FLAG), FLAG-tagged WT-DYRK2 or KD-DYRK2 were reconstituted into HEK 293T cells stably expressing shRNA against the 3’-UTR of DYRK2. hPXR activity was measured by luciferase-based CYP3A4 promoter activity following treatment with DMSO, or a dose response of RIF for 24 hours. * denotes statistical significance with p < 0.05. Otherwise, unlabeled points are statistically insignificant.
DISCUSSION
hPXR is a master transcriptional regulator of xenobiotic detoxification enzymes that is predominantly expressed in the liver and intestines. In the resting state, hPXR is transcriptionally repressed by binding to corepressors [30]. Ligand binding to hPXR leads to the dissociation of corepressors and the recruitment of coactivators, resulting in a transcriptionally active hPXR. Surprisingly, we observed that the increase in cellular hPXR level can increase its basal activity, seemingly independent of ligand engagement. Since we cannot preclude the possibility of the presence of endogenous ligands of hPXR in our experimental model, and because of the ligand promiscuity of hPXR, elevated levels of hPXR may enhance the effect of low-potency hPXR agonists. Our findings suggest that an increase in the cellular hPXR level is sufficient to induce transcriptional activation of its target genes. A positive correlation between hPXR level and its target gene levels has been seen in colorectal cancer [31]. An increase in hPXR level was observed with the decrease in chemosensitivity of irinotecan due to inactivation of the drug and its active metabolite SN-38 by CYP3A4-mediated oxidation and UGT1A1-mediated glucuronidation, respectively [31]. hPXR can also form a transcriptionally active homodimer [32], which may explain the increase in basal hPXR activity in the absence of ligand. The increase in hPXR level (2 fold increase following MG132 treatment) is relatively modest in this study suggesting that hPXR stability may be governed by other factors. Indeed, PXR stability has been shown to be modulated via the ubiquitin proteasomal system. In a yeast two-hybrid protein interaction assay, Masuyama et al. showed that PXR interacts with SUG1, a component of the proteasome, in a progesterone-dependent manner [16]. Interestingly, progesterone enhanced PXR degradation, and overexpression of SUG1 led to proteolytic PXR fragments, which was blocked by a proteasome inhibitor [16, 17]. These data provided the first evidence that PXR may be degraded through a proteasome-mediated pathway. More recently, in another yeast two-hybrid screening, Rana et al. identified E3 ubiquitin ligase RBCK1 as an interacting partner of hPXR [10]. They further demonstrated that RBCK1 ubiquitinates hPXR, thus targeting hPXR for degradation by the ubiquitin-proteasome pathways. Our studies, by using mass spectrometry analysis and kinome-wide siRNA screen, identified a pathway that regulates hPXR stability via phosphorylation-facilitated ubiquitination by serine/threonine kinase DYRK2 and E3 ubiquitin ligase UBR5, further confirming the role of the ubiquitin-proteasomal pathway in modulating hPXR levels. Since multiple E3 ligases can act on the same substrate, it is possible that hPXR is a substrate for multiple E3 ligases.
In this study, we found that the E3 ligase UBR5 works in concert with the serine/threonine kinase DYRK2, possibly in a complex containing the adaptor proteins DDB1 and RbBP7, to mediate hPXR stability. UBR5, DYRK2, DDB1, and VprBP were previously reported to be in a multiprotein complex that regulates the stability of katanin, where VprBP serves as a substrate recognition protein for katanin [28]. However, the substrate recognition adaptor in our study appears to be RbBP7 and not VprBP. Since both RbBP7 and VprBP contain WD40 domains, which are one of the most abundant domains in eukaryotes [33], it is exciting to speculate that the WD40 domain–containing adaptors confer substrate specificity to the UBR5-DYRK2 complex. Although our findings do not preclude the existence of other WD40 domain–containing proteins in the UBR5-DYRK2-DDB1 complex, they suggest that RbBP7 may act as the substrate-recognition component that recruits hPXR to the complex. We further showed that the kinase activity of DYRK2 is needed for its effect on facilitating UBR5-mediated ubiquitination of hPXR. However, we cannot rule out the role of DYRK2 as scaffold in the multiprotein complex. Both the kinase activity and the scaffold function of DYRK2 are needed for the UBR5-DYRK2 complex to regulate the stability of katanin [28]. It is possible that DYRK2 is acting in the same manner here in exerting its function on hPXR. Further efforts to delineate the residues on hPXR that are phosphorylated by DYRK2 would be of interest. It is noteworthy that hPXR contains a consensus phosphorylation site for DYRK2 [34]. It is possible that the phosphorylation of this residue abrogates the ligand binding capacity of hPXR.
In the experiments using primary hepatocytes, we noticed that the endogenous levels of UBR5 and DYRK2 were low. It is important to note that UBR5 expression [35] and DYRK2 expression [36] are downregulated in the liver (by comparing mRNA levels of UBR5 and DYRK2 in human liver to those in other human tissues in these studies) whereas hPXR level is highest in the liver, further suggesting an interplay among UBR5, DYRK2, and hPXR.
Since UBR5 [37, 38] and DYRK2 [39, 40] are often overexpressed in cancers, they are potential therapeutic targets. However, caution must be exercised while modulating UBR5 and DYRK2 at the hPXR protein level due to the possible resulting undesired drug–drug interactions and drug resistance. Future efforts in eliminating the PXR-activating effect of drug candidates in drug development should focus not only on determining whether the drug candidates bind to PXR but also on studying how they affect the protein stability of PXR, since both factors affect the net activity of PXR and can cause adverse drug effects.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Vani Shanker for editing the manuscript, Dr. John Gray for providing the SF2 expression vector , St. Jude Vector Core Laboratory and St. Jude Proteomics & Mass Spectrometry Shared Resource for technical assistance, Ta Chi Ong for assistance with statistical analysis, Yueming Wang and other members of the Chen Research Laboratory for valuable discussions. Normal human hepatocytes were obtained through the Liver Tissue Cell Distribution System (Pittsburgh, Pennsylvania), which was funded by NIH Contract #N01-DK-7-0004 / HHSN267200700004C.
FUNDING
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital, and grants from National Institutes of Health National Institute of General Medical Sciences [Grant GM086415; to T.C.], and National Institutes of Health National Cancer Institute [Grant P30-CA21765].
Abbreviations used
- hPXR
human Pregnane X Receptor
- CYP3A4
cytochrome p450 3A4
- MDR1
Multidrug resistance protein 1
- UGT1A1
UDP-glucuronosyl transferase 1A1
- siRNA
small interfering RNA
- MS
mass spectrometry
- UBR5
ubiquitin protein ligase E3 component n-recognin 5
- DYRK2
dual specificity tyrosine-phosphorylation-regulated kinase 2
- SSMD
strictly standardized mean difference
- GUItars
GUI tool for analysis of high-throughput RNA interference screening
- WT
wild-type
- KD
kinase-dead
- CA
catalytically inactive
- RIF
rifampicin
- DDB1
DNA damage binding protein 1
- RbBP7
histone binding protein 7
Footnotes
AUTHOR CONTRIBUTION
A.N.G. participated in the siRNA screen and data analysis. A.E. performed the mass spectrometric experiments. J.W. constructed and tested SF2-FLAG-hPXR. D.S. generated and tested GFP-WT UBR5 and GFP-CA UBR5. S.S.O. performed all other experiments. S.S.O. and T.C. designed the experiments, analyzed the data and wrote the manuscript.
REFERENCES
- 1.Wang YM, Ong SS, Chai SC, Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin. Drug Metab. Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kliewer SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J. Nutr. 2003;133:2444S–2447S. doi: 10.1093/jn/133.7.2444S. [DOI] [PubMed] [Google Scholar]
- 3.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. U S A. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. U S A. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 6.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc. Natl. Acad. Sci. U S A. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat. Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 8.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDPglucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38:62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichti-Kaiser K, Brobst D, Xu C, Staudinger JL. A systematic analysis of predicted phosphorylation sites within the human pregnane X receptor protein. J. Pharmacol Exp. Ther. 2009;331:65–76. doi: 10.1124/jpet.109.157180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rana R, Coulter S, Kinyamu H, Goldstein JA. RBCK1, an E3 ubiquitin lLigase, interacts with and ubiquinates the human pregnane X receptor (hPXR) Drug Metab. Dispos. 2012;41:398–405. doi: 10.1124/dmd.112.048728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staudinger JL, Xu C, Biswas A, Mani S. Post-translational modification of pregnane X receptor. Pharmacol. Res. 2011;64:4–10. doi: 10.1016/j.phrs.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pondugula SR, Dong H, Chen T. Phosphorylation and protein-protein interactions in PXRmediated CYP3A repression. Expert Opin. Drug Metab. Toxicol. 2009;5:861–873. doi: 10.1517/17425250903012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pondugula SR, Brimer-Cline C, Wu J, Schuetz EG, Tyagi RK, Chen T. A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane x receptor. Drug Metab. Dispos. 2009;37:719–730. doi: 10.1124/dmd.108.024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugatani J, Uchida T, Kurosawa M, Yamaguchi M, Yamazaki Y, Ikari A, Miwa M. Regulation of pregnane X receptor (PXR) function and UGT1A1 gene expression by posttranslational modification of PXR protein. Drug Metab. Dispos. 2012;40:2031–2040. doi: 10.1124/dmd.112.046748. [DOI] [PubMed] [Google Scholar]
- 15.Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat. Rev. Cancer. 2006;6:776–788. doi: 10.1038/nrc1994. [DOI] [PubMed] [Google Scholar]
- 16.Masuyama H, Hiramatsu Y, Mizutani Y, Inoshita H, Kudo T. The expression of pregnane X receptor and its target gene, cytochrome P450 3A1, in perinatal mouse. Mol. Cell. Endocrinol. 2001;172:47–56. doi: 10.1016/s0303-7207(00)00395-6. [DOI] [PubMed] [Google Scholar]
- 17.Masuyama H, Inoshita H, Hiramatsu Y, Kudo T. Ligands have various potential effects on the degradation of pregnane X receptor by proteasome. Endocrinology. 2002;143:55–61. doi: 10.1210/endo.143.1.8578. [DOI] [PubMed] [Google Scholar]
- 18.Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 19.Lin W, Wu J, Dong H, Bouck D, Zeng FY, Chen T. Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. J. Biol. Chem. 2008;283:30650–30657. doi: 10.1074/jbc.M806132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YM, Lin W, Chai SC, Wu J, Ong SS, Schuetz EG, Chen T. Piperine activates human pregnane X receptor to induce the expression of cytochrome P450 3A4 and multidrug resistance protein 1. Toxicol. Appl. Pharmacol. 2013;272:96–107. doi: 10.1016/j.taap.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goktug AN, Ong SS, Chen T. GUItars: a GUI tool for analysis of high-throughput RNA interference screening data. PLoS One. 2012;7:e49386. doi: 10.1371/journal.pone.0049386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XD. Illustration of SSMD, z score, SSMD*, z* score, and t statistic for hit selection in RNAi high-throughput screens. J. Biomol. Screen. 2011;16:775–785. doi: 10.1177/1087057111405851. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XD. A new method with flexible and balanced control of false negatives and false positives for hit selection in RNA interference high-throughput screening assays. J. Biomol. Screen. 2007;12:645–655. doi: 10.1177/1087057107300645. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XD, Yang XC, Chung N, Gates A, Stec E, Kunapuli P, Holder DJ, Ferrer M, Espeseth AS. Robust statistical methods for hit selection in RNA interference high-throughput screening experiments. Pharmacogenomics. 2006;7:299–309. doi: 10.2217/14622416.7.3.299. [DOI] [PubMed] [Google Scholar]
- 25.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Dong H, Lin W, Wu J, Chen T. Flavonoids activate pregnane x receptor-mediated CYP3A4 gene expression by inhibiting cyclin-dependent kinases in HepG2 liver carcinoma cells. BMC Biochem. 2010;11:23. doi: 10.1186/1471-2091-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugatani J, Osabe M, Kurosawa M, Kitamura N, Ikari A, Miwa M. Induction of UGT1A1 and CYP2B6 by an antimitogenic factor in HepG2 cells is mediated through suppression of cyclin-dependent kinase 2 activity: cell cycle-dependent expression. Drug Metab. Dispos. 2010;38:177–186. doi: 10.1124/dmd.109.029785. [DOI] [PubMed] [Google Scholar]
- 28.Maddika S, Chen J. Protein kinase DYRK2 is a scaffold that facilitates assembly of an E3 ligase. Nat. Cell. Biol. 2009;11:409–419. doi: 10.1038/ncb1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He YJ, McCall CM, Hu J, Zeng Y, Xiong Y. DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev. 2006;20:2949–2954. doi: 10.1101/gad.1483206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DR, Li CW, Chen LY, Ghosh JC, Chen JD. Regulation and binding of pregnane X receptor by nuclear receptor corepressor silencing mediator of retinoid and thyroid hormone receptors (SMRT) Mol. Pharmacol. 2006;69:99–108. doi: 10.1124/mol.105.013375. [DOI] [PubMed] [Google Scholar]
- 31.Raynal C, Pascussi JM, Leguelinel G, Breuker C, Kantar J, Lallemant B, Poujol S, Bonnans C, Joubert D, Hollande F, Lumbroso S, Brouillet JP, Evrard A. Pregnane X Receptor (PXR) expression in colorectal cancer cells restricts irinotecan chemosensitivity through enhanced SN-38 glucuronidation. Mol. Cancer. 2010;9:46. doi: 10.1186/1476-4598-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble SM, Carnahan VE, Moore LB, Luntz T, Wang H, Ittoop OR, Stimmel JB, Davis-Searles PR, Watkins RE, Wisely GB, LeCluyse E, Tripathy A, McDonnell DP, Redinbo MR. Human PXR forms a tryptophan zipper-mediated homodimer. Biochemistry. 2006;45:8579–8589. doi: 10.1021/bi0602821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stirnimann CU, Petsalaki E, Russell RB, Muller CW. WD40 proteins propel cellular networks. Trends Biochem. Sci. 2010;35:565–574. doi: 10.1016/j.tibs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Campbell LE, Proud CG. Differing substrate specificities of members of the DYRK family of arginine-directed protein kinases. FEBS Lett. 2002;510:31–36. doi: 10.1016/s0014-5793(01)03221-5. [DOI] [PubMed] [Google Scholar]
- 35. http://www.ebi.ac.uk/s4/jump?from=aHR0cDovL3d3dy5lYmkuYWMudWsvczQvc3VtbWFyeS9tb2xlY3VsYXIvZXhwcmVzc2lvbj90ZXJtPXVicjUmY2xhc3NpZmljYXRpb249OTYwNiZ0aWQ9bmFtZU9yZ0VOU01VU0cwMDAwMDAzNzQ4Nw%3D%3D&hash=14B06&url=http://www.ebi.ac.uk/gxa/das/../gene/ENSG00000104517.
- 36. http://www.ebi.ac.uk/s4/jump?from=aHR0cDovL3d3dy5lYmkuYWMudWsvczQvc3VtbWFyeS9tb2xlY3VsYXIvZXhwcmVzc2lvbj90ZXJtPWR5cmsyJmNsYXNzaWZpY2F0aW9uPTk2MDYmdGlkPW5hbWVPcmdFTlNHMDAwMDAxMjczMzQ%3D&hash=30625&url=http://www.ebi.ac.uk/gxa/das/../gene/ENSG00000127334.
- 37.Clancy JL, Henderson MJ, Russell AJ, Anderson DW, Bova RJ, Campbell IG, Choong DY, Macdonald GA, Mann GJ, Nolan T, Brady G, Olopade OI, Woollatt E, Davies MJ, Segara D, Hacker NF, Henshall SM, Sutherland RL, Watts CK. EDD, the human orthologue of the hyperplastic discs tumour suppressor gene, is amplified and overexpressed in cancer. Oncogene. 2003;22:5070–5081. doi: 10.1038/sj.onc.1206775. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien PM, Davies MJ, Scurry JP, Smith AN, Barton CA, Henderson MJ, Saunders DN, Gloss BS, Patterson KI, Clancy JL, Heinzelmann-Schwarz VA, Murali R, Scolyer RA, Zeng Y, Williams ED, Scurr L, Defazio A, Quinn DI, Watts CK, Hacker NF, Henshall SM, Sutherland RL. The E3 ubiquitin ligase EDD is an adverse prognostic factor for serous epithelial ovarian cancer and modulates cisplatin resistance in vitro. Br. J. Cancer. 2008;98:1085–1093. doi: 10.1038/sj.bjc.6604281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorringe KL, Boussioutas A, Bowtell DD. Novel regions of chromosomal amplification at 6p21, 5p13, and 12q14 in gastric cancer identified by array comparative genomic hybridization. Genes Chromosomes Cancer. 2005;42:247–259. doi: 10.1002/gcc.20136. [DOI] [PubMed] [Google Scholar]
- 40.Miller CT, Aggarwal S, Lin TK, Dagenais SL, Contreras JI, Orringer MB, Glover TW, Beer DG, Lin L. Amplification and overexpression of the dual-specificity tyrosine-(Y)- phosphorylation regulated kinase 2 (DYRK2) gene in esophageal and lung adenocarcinomas. Cancer Res. 2003;63:4136–4143. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.