Abstract
Pain encompasses both a sensory as well as an affective dimension and these are differentially processed in the cortex. Animal models typically use reflexive behaviors to test nociceptive responses; these are thought to reflect the sensory dimension of pain. While several behavioral tests are available for examining the affective dimension of pain it is unclear if these are appropriate in animal models of muscle pain. We therefore tested the utility of existing paradigms as well as new avoidance paradigms in animal models of muscle pain in mice. Specifically we used an escape-avoidance test to noxious mechanical stimuli, a learned avoidance test to noxious mechanical stimuli, and avoidance of physical activity. We used three animal models of muscle pain: carrageenan-induced inflammation, non-inflammatory muscle pain, and exercise-enhanced pain. In the carrageenan model of inflammation mice developed escape-avoidance behaviors to mechanical stimuli, learned avoidance to mechanical stimulation and avoidance of physical activity – these models are associated with unilateral hyperalgesia. When both muscles were inflamed, escape-avoidance behaviors did not develop suggesting equivalent bilateral pain-behaviors cannot be tested with an escape-avoidance test. In the non-inflammatory muscle pain model mice did not show significant changes in escape-avoidance behaviors or learned avoidance, but did avoid physical activity. In the exercise-enhanced pain model, there were no changes in escape avoidance, learned avoidance of noxious or physical activity In conclusion, we developed several testing protocols that assess supraspinal processing of pain-behaviors in models of muscle pain and that are most sensitive in animals with unilateral hyperalgesia.
Keywords: Escape avoidance, Conditioned place preference, inflammation, carrageenan, hyperalgesia, analgesia, pain, muscle
1. Introduction
Animal models of muscle pain are commonly used to gain a better understanding of underlying mechanisms of pain processing. Pain encompasses both a sensory experience as well as an affective experience. Imaging studies in human subjects suggest that the sensory experience of pain is processed in the somatosensory cortex and the affective experience is processed in the cingulate cortex (Rainville et al., 1997; Hofbauer et al., 2001). As such, behavioral tests that assess both the sensory and the emotional aspects of pain are critical to understanding the whole pain experience. The majority of available nociceptive tests depend on the measurement of withdrawal thresholds, which are reflexive responses. Nociceptive withdrawal reflex behaviors are under supraspinal control but can occur in the absence of supraspinal input (for review see Ossipov et al., 2006). Higher order pain behaviors such as escape avoidance and conditioned place preference (CPP) involve the anterior cingulate cortex (LaGraize et al., 2004; Qu et al., 2011), a region involved in the emotional component of pain (Devinsky et al., 1995).
There has been a growing trend toward using behavioral tests that assess higher order cortical processing of pain; these involve having the animal choose to avoid or escape a pain-inducing behavior. The two most commonly used tests for assessing higher order pain related behaviors in animals are the escape avoidance test and conditioned place preference (CPP) tests (Fuchs, 2000; LaBuda and Fuchs, 2000; LaGraize et al., 2004; Ding et al., 2005; Pedersen and Blackburn-Munro, 2006; Betourne et al., 2008; van der Kam et al., 2008; Baastrup et al., 2011; Qu et al., 2011; Fuchs and McNabb, 2012; He et al., 2012; McNabb et al., 2012). In the escape avoidance test, the animal chooses to avoid a noxious stimulus by moving to another chamber of the box. Lesions of the cingulate cortex block the development of escape avoidance pain behaviors (Johansen, 2001; LaGraize et al., 2004; Qu et al., 2011; Uhelski et al., 2012). In contrast, lesions of the somatosensory cortex have no effect on the escape-avoidance task, but they do attenuate withdrawal reflex responses (Uhelski et al., 2012). Thus, the escape-avoidance test is useful to examine the affective or emotional component of pain.
Higher order processing using the escape avoidance paradigm has been tested in animal models of neuropathic and inflammatory pain, primarily in rats (LaBuda and Fuchs, 2000; LaGraize et al., 2004; Pedersen and Blackburn-Munro, 2006; van der Kam et al., 2008; Baastrup et al., 2011; Qu et al., 2011; McNabb et al., 2012). The purpose of the current study was to develop higher order behavioral tests that would be useful for mice with both acute and chronic muscle pain. Specifically, we examined the utility of escape-avoidance, learned avoidance, and physical activity avoidance in an acute inflammatory muscle pain model that presents with unilateral decreases in withdrawal thresholds, in a model of non-inflammatory muscle pain that presents with bilateral decreases in withdrawal thresholds and a model of exercise- enhanced pain that also results in bilateral decreases in withdrawal thresholds.
2. Experimental Procedures
2.1. Animals
All experiments in mice (male, C57BL/6) were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the National Institute of Health. Mice were acclimated to their environment for one week before any experimental procedures were initiated. All mice were housed five per cage and kept in a temperature controlled environment with available food and water.
2.2. Models of muscle pain
2.2.1. Muscle inflammation
To generate acute inflammation mice were anesthetized with isoflurane (4%) and injected with 20 μl of carrageenan (3%, sterile saline) into the left gastrocnemius muscle as previously described (Radhakrishnan et al., 2003; Yokoyama et al., 2007a). Controls were injected with 20 μl of pH 7.2 saline into the left gastrocnemius muscle. An additional group was tested with bilateral carrageenan injection and compared to controls with bilateral pH 7.2 saline injections.
2.2.2. Repeated acid injections
To generate non-inflammatory hyperalgesia, mice received two injections of pH 4.0 saline five days apart into the left gastrocnemius muscle as previously described (Sluka et al., 2001; Sluka et al., 2003; Yokoyama et al., 2007a). Mice were anesthetized with isoflurane (4%) and 20 μl of pH 4.0 saline was injected each time. Controls were injected with 20 μl of pH 7.2 saline.
2.2.3. Exercise-enhanced pain
To generate exercise-enhanced pain mice were given two injections of 20 μl of pH 5.0 saline into the left gastrocnemius muscle five days apart in combination with a 2h run in a running wheel immediately before the second injection of pH 5.0, as previously described (Yokoyama et al., 2007b; Sluka et al, 2012). Controls were exercised similarly but were injected with 20 μl of pH 7.2 saline. Mice were acclimated to the running wheel for two days, ten minutes each time, three times a day. To ensure the mice continued running in the wheel, the top of the cage was tapped when mice stopped running for more than five seconds.
2.3. Behavioral protocols
2.3.1. Escape avoidance experiment
We used a modified escape avoidance paradigm as previously published (LaBuda and Fuchs, 2000). The testing box was made of Plexiglas with dimensions 16cm × 7cm × 13cm and placed on top of a wire mesh screen. The box was divided into two chambers. So that there was no preference in animals without injury one chamber was white with vertical black lines while the other chamber was solid white. Mice were randomly placed on either the left or the right side of the box to start and an equal number of animals in each group started on either the left or the right side of the box. During a subsequent test, the side of the box was switched to the opposite side. During behavioral testing, the mice were allowed to move unrestricted to either side of the box for thirty minutes. Mechanical stimulation was initiated with an 0.4 mN von Frey filament to the plantar surface of either the right or left hind paw. The right side was stimulated when the animal was on one side of the box and the left side was stimulated when the animal was on the opposite side of the box. Stimuli were given to the hind-paw once per second. All three models of muscle pain were tested in the escape avoidance protocol. Measurements were taken before, twenty-four hours, and one week after initiation of the model. The time the animal spent on each side of the box was recorded using a stopwatch. Four different groups of experiments were performed 1) Unilateral carrageenan (n=6) compared to unilateral saline control (n=6), 2) bilateral carrageenan injection (n=6) compared to bilateral saline injections (n=6), 3) 2, pH 4.0 injections (n=10) compared to 2, pH 7.2 saline control injections (n=10), 4) 2, pH 5.0 injections plus exercise (n=10) compared to 2, pH 7.2 saline control injections plus exercise (n=10).
2.3.2. Learned avoidance experiment
To determine whether mice learn to avoid a painful stimulus, the following protocol was tested in a separate group of animals. The pain model was induced and twenty-four hours after injection, mice were stimulated with a 0.4 mN von Frey filament at a rate of once per second. When mice were on one side of the box, their left hindpaw was stimulated. When mice were on the opposite side of the box, their right hindpaw was stimulated. An hour later, mice were tested again in escape avoidance box without mechanical stimulation of the paw, and the time they spent on each side of the box was calculated. Mice were randomly placed on either the left or the right side of the box to start and an equal number of animals in each group started on either the left or the right side of the box. Three different groups of experiments were performed 1) Unilateral carrageenan (n=6) compared to unilateral saline control (n=6), 2) 2, pH 4.0 injections (n=6) compared to 2, pH 7.2 saline control injections (n=6), 3) 2, pH 5.0 injections plus exercise (n=6) compared to 2, pH 7.2 saline control injections plus exercise (n=6).
2.3.4. Avoidance of voluntary physical activity
To determine if there is a decrease in physical activity levels a separate group of mice were placed in an exercise running wheel for one hour and videotaped. Mice were allowed to voluntarily run. Off-line analysis examined the time spent running, distance run, and number of bouts. Tests were performed before and twenty-four hours after the initiation of the pain model. Three different groups of experiments were performed 1) Unilateral carrageenan (n=6) compared to unilateral saline control (n=6), 2) 2, pH 4.0 injections (n=10) compared to 2, pH 7.2 saline control injections (n=10), 3) 2, pH 5.0 injections plus exercise (n=5) compared to 2, pH 7.2 saline control injections plus exercise (n=7).
2.4. Statistical Analysis
A repeated measures analysis of variance (ANOVA) quantified differences in escape avoidance behaviors and physical activity between groups. Individual differences at each testing time were analyzed with a Tukey’s test. A one-way ANOVA quantified differences in learned avoidance behaviors between groups (*, p<0.05).
3. Results
3.1 Inflammatory muscle pain
3.1.1. Escape avoidance experiment
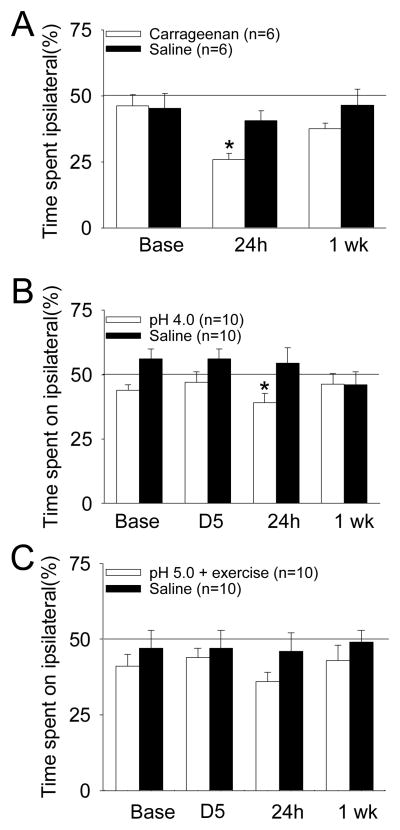
To test if mice would choose to avoid a painful stimulus, mice were timed for side preference while receiving repetitive noxious mechanical stimulation. Prior to injection of 3% carrageenan into the muscle, mice spend approximately 50% of the thirty minutes on either side of the escape avoidance box. After muscle inflammation, mice significantly decrease their time on the side of the box they received noxious stimuli to the side with the muscle insult (n=6) when compared to mice that received pH 7.2 saline injection as a control (n=6) (*, P<0.05) (Figure 1A). One week after injury this response returns toward baseline. We next tested if animals with bilateral inflammatory hyperalgesia could show a change in the escape-avoidance test by injecting 3% carrageenan into the left and right gastrocnemius muscle. The side preference after induction of inflammation remained unchanged from before induction of inflammation with the inflammation group showing 44 ± 13% of their time in the left chamber before inflammation and 39 ± 10% on the left 24h after inflammation. Controls spent 48 ±11 % of their time on the left before injection, and 45 ± 13% 24h after injection.
Figure 1.
Percentage of total time spent on the side of the escape avoidance box in which the noxious stimulation was applied to the side of muscle insult before and after induction of the model. A. Effects in inflammatory muscle pain induced by injection of 3% carrageenan (*, P<0.05) B. Effects in a model of non-inflammatory muscle pain induced by two injections of pH 4.0 saline (*, P=0.058) C. Effects in a model of exercise-enhanced muscle pain induced by two injections of pH 5.0 saline in combination with a novel exercise task. Data are mean ± S.E.M.
3.1.2. Learned avoidance experiment
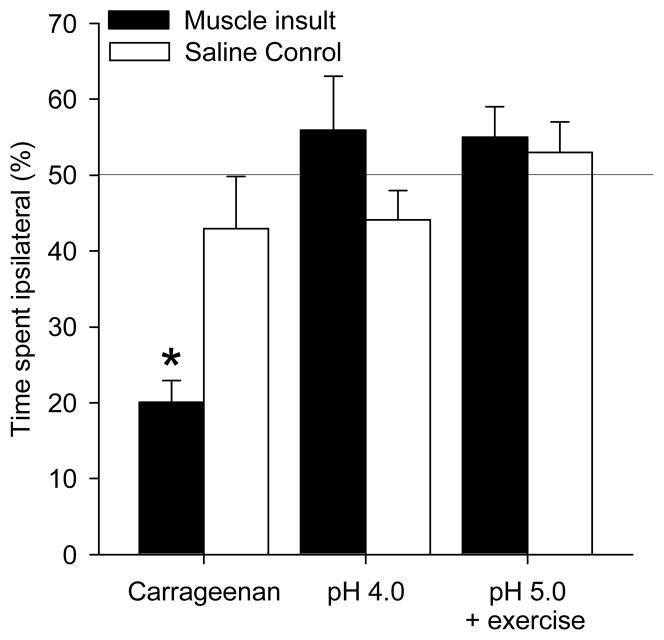
To test if mice learned to avoid a noxious environment, mice were tested for a side preference one hour after thirty minutes of mechanical stimulation. Mice that received 3% carrageenan injection showed a significant preference for the side of the box in which they received noxious stimulation contralateral to the muscle insult when compared to pH 7.2 saline (*, P<0.05)(Figure 2).
Figure 2.
Time spent on the side of the box in which rats received noxious stimulation to paw on the side of muscle insult. In the inflammatory pain model, mice injected with carrageenan spent significantly less time on the side of the box where noxious stimulation was applied to the side of muscle insult (*, P<0.05). In the non-inflammatory muscle pain model (pH 4.0) and the exercise-enhanced pain model (pH 5.0 + exercise) there were no preferences in chamber. Data are mean ± S.E.M.
3.1.3. Avoidance of voluntary physical activity
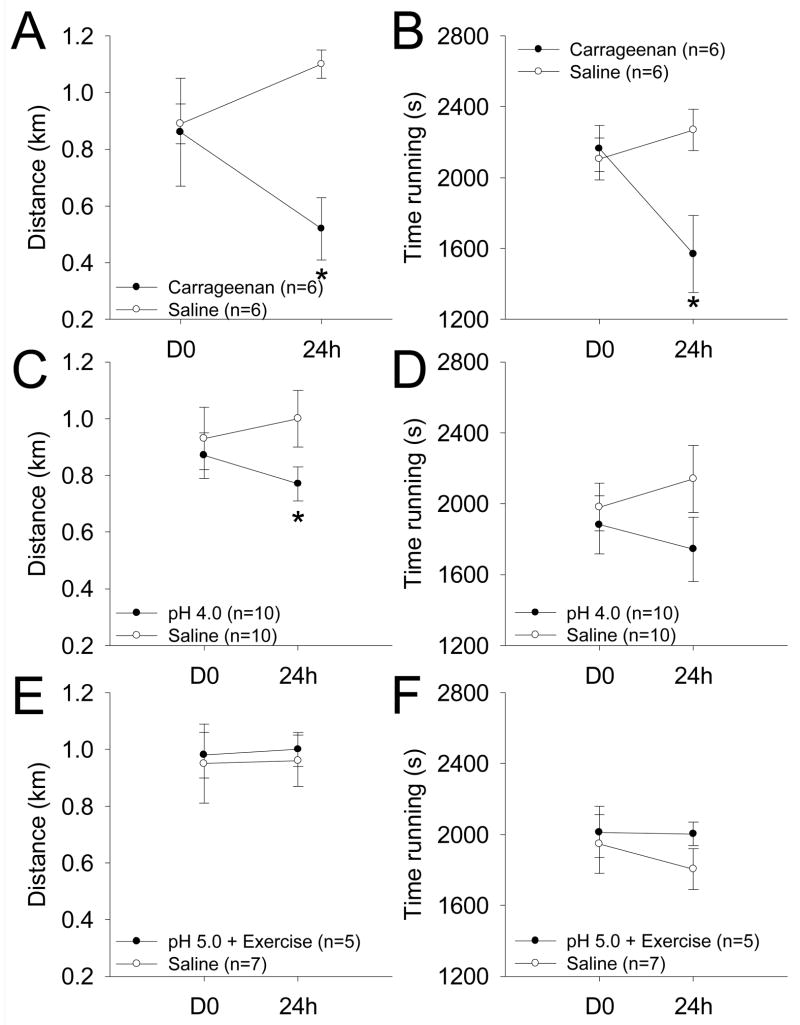
We next tested if mice would demonstrate avoidance of voluntary physical activity by placing mice in a running wheel for one hour. Mice that received an intramuscular injection of 3% carrageenan to one side showed a significant decrease in the amount of time spent running as well as total distance traveled compared to controls (*, p<0.05) (Figure 3A and B). The number of bouts of exercise was not different between groups or across time before (56 ± 5.9 3% carrageenan vs. 67 ± 5.4 for saline), or 24h after inflammation (61 ± 5.8 3% carrageenan vs. 72 ± 6.3 saline).
Figure 3.
Measure of physical activity with respect to distance run in km and time spent running over the course of one hour. A. Mice run less distance than controls 24h after injection of 3% carrageenan (*, P<0.05). B. Mice spend less time running than controls 24h after injection of 3% carrageenan (*, P<0.05). C. Mice run less distance than the controls 24h after the second pH 4.0 injection (*, P<0.05). D. Mice show no significant differences in the time spent running when compared to controls 24h after the second pH 4.0 injection. E, F. In the exercise-enhanced pain model (pH 5.0 + exercise) no significant differences were observed for the distance run (E) or in the time spent running (F). Data are mean ± S.E.M.
3.2. Non-inflammatory muscle pain
To test if these behavioral responses found with acute inflammatory pain also occur in models of non-inflammatory muscle pain we used our model of repeated intramuscular acid injections as previously published and characterized (Sluka et al., 2001; Sluka et al., 2003). This model produces bilateral mechanical hyperalgesia of the paw and muscle (Sluka et al., 2003; Yokoyama et al., 2007a; DaSilva et al., 2010).
3.2.1. Escape avoidance experiment
Before the first pH 4.0 injection and before the second pH 4.0 injection mice spend approximately 50% of their time on either side of the escape/avoidance box. However, 24h after the second intramuscular injection of acidic saline there is a small decrease in the time spent on the side in which the rats received noxious stimulation ipsilateral to the muscle insult when compared to controls injected with pH 7.2 (p=0.058). This effect is normalized by one week without significant differences between groups (Figure 1B).
3.2.2. Learned avoidance experiment
Mice that received the pH 4.0 saline injection did not show a significant preference for either side of the box when compared to control mice that received pH 7.2 saline (Figure 2).
3.2.3. Avoidance of voluntary physical activity
Mice that received an intramuscular injection of pH 4.0 saline showed a significant decrease in the distance traveled during the physical activity avoidance task when compared to those that received control injections of pH 7.2 saline (P<0.05)(Fig. 3C and D). However, there was no difference in the time spent running between the pH 4.0 and the pH 7.2 groups. The number of bouts of exercise was not different between groups or across time before (63 ± 4.3 pH 4.0 vs. 73 ± 3.2 for saline), or 24h after the second acid injection (60 ± 4.5 pH 4.0 vs. 60 ± 3.7 saline).
3.3. Exercise-enhanced pain
Finally, we tested if exercise-enhanced pain results in significant changes in these behavioral tests using our model of two injections of pH 5.0 saline combined with a two hour fatiguing exercise task. In the exercise-enhanced pain model, mice ran 2.6 km (± 0.11) during the escape avoidance experiment, 3.0 km (± 0.21) during the learned avoidance experiment, and 2.8 km (±0.20) during the physical activity avoidance task. This model results in bilateral mechanical hyperalgesia of the paw and muscle (Yokoyama et al., 2007b; Sluka et al., 2012).
3.3.1. Escape avoidance experiment
Before the first injection of pH 5.0 or before the second injection of pH 5.0 saline mice spend approximately 50% of their time on each side of the escape avoidance box and are similar to controls injected with pH 7.2. After the second injection and two hour exercise task, 24h and 1 week, mice show no difference in time spent on either side of the box. Mice that received pH 7.2 saline injection as a control show no difference in time spent on either side of the box (Figure 1C).
3.3.2. Learned avoidance experiment
Mice that received the pH 4.0 saline injection did not show a significant preference for either side of the box when compared to control mice that received pH 7.2 saline (Figure 2).
3.3.3. Avoidance of voluntary physical activity
Mice that received an intramuscular injection of pH 5.0 saline with the exercise task showed no significant change in amount of time spent running or total distance traveled when compared to controls (Figure 3E and F). The number of bouts of exercise was not different between groups or across time before (61 ± 6.1 pH 5.0 + exercise vs. 72 ± 2.3 for saline), or 24h after the second acid injection and exercise task (64 ± 5.0 pH 5.0 + exercise vs. 57 ± 4.7 saline).
4. Discussion
These results show for the first time that induction of inflammatory muscle pain results in escape avoidance behaviors. We further demonstrate that after muscle inflammation, mice learn to avoid noxious stimuli - both cutaneous mechanical stimuli and that associated with physical activity. While prior studies have tested the escape avoidance paradigm in rats with paw inflammation and neuropathic pain (LaBuda and Fuchs, 2000; LaGraize et al., 2004; LaBuda and Fuchs, 2005; Pedersen et al., 2007; Baastrup et al., 2011; Uhelski et al., 2012; McNabb et al., 2012), we were able to translate these behaviors to mice with inflammatory muscle pain where mice have unilateral hyperalgesia.
In the current study, we were not able to show the escape avoidance or learned avoidance tests in models of non-inflammatory muscle pain (p=0.058) or exercise-enhanced pain models. This may be a result of the bilateral nature of the hyperalgesia that develops in these models (Sluka et al., 2001; Yokoyama et al., 2007a,b; DaSilva et al., 2010, Sluka et al., 2012). While there was a trend in the escape avoidance behavior for an effect on the injected side this was only observed at 24h and not at 1 week. One possibility for this is that initially there is a greater hyperalgesia on the injected side and as the hyperalgesia progresses the loss of effect is due to the fact that the hyperalgesia is equivalent bilaterally. In fact prior work shows rats with unilateral paw inflammation on one side and a nerve injury on the other show similar decreases in mechanical withdrawal thresholds bilaterally when compared to sham or controls but do show place preference (McNabb et al., 2012), and the current study shows rats with bilateral inflammation do not show place preference. Previous studies in rats demonstrate the escape avoidance test shows side preference in models of unilateral neuropathic and inflammatory pain (LaBuda and Fuchs, 2000; LaBuda and Fuchs, 2005; Perdersen and Blackburn-Munro, 2006; Bassturp et al., 2010; Boyce-Rustay et al., 2010; Uhelski et al., 2012). Thus, the escape-avoidance and learned avoidance tests may be useful as an additional behavioral measure for assessing the multidimensional pain experience in mice with muscle inflammation or those with unilateral injury.
We developed a test to examine if animals would learn to avoid a painful stimulus. Human subjects with chronic muscle pain learn to avoid physical activity that enhances pain responses (Crombez et al., 1998, 1999; Vlaeyen and Linton, 2000). It has been hypothesized that the escape avoidance test is an assessment of both the initial escape from the noxious stimulus and the avoidance of the noxious stimulus after presentation (LaBuda and Fuchs, 2000). The learned avoidance test shows that after an hour after delivering a noxious stimulus, mice spend significantly less time on the side of the box ipsilateral to their injury. This data suggest that mice have learned to avoid a potential noxious stimulus by associating a physical environment to that in which they were given a prior noxious stimulus.
We hypothesized that if a muscle was painful, mice would voluntarily perform less physical activity associated with that muscle’s use. Indeed we previously show that the inflammatory muscle pain, non-inflammatory muscle pain and the exercise-enhanced pain models all show enhanced muscle hyperalgesia as measured by decreases in withdrawal thresholds (Yokoyama et al., 2007a; Radhakrishnan et al., 2003; Sluka and Rasmussen, 2010, daSilva et al., 2010). This study demonstrates the inflammatory and the non-inflammatory muscle pain models resulted in significantly decreased measures of voluntary physical activity when examined over a one hour time period as compared to saline injections. This is in agreement with our prior results that measured voluntary physical activity over a twenty-four hour time period with a running wheel placed in their home cages and were able to show approximately a 36% reduction in running wheel activity in mice with carrageenan injection into the muscle (Sluka and Rasmussen, 2010). Similarly, reductions in running wheel activity were observed after induction of hindpaw inflammation with complete Freund’s adjuvant. This decrease was reduced by anti-inflammatory and analgesic drugs (Cobos et al., 2012). Locomotor activity is also reduced in animals with inflammatory pain and in the first week after nerve injury (Urban et al., 2011; Cobos et al., 2012). However, animals with nerve injury showed normal physical activity levels by 2 weeks despite enhanced mechanical sensitivity (Urban et al., 2011). Further, feeding, drinking and rearing were unchanged after nerve injury (Urban et al., 2011). In contrast, in the open field test 14–21 days after nerve injury or 28 days after CFA-induced inflammation showed reductions in time spent in the inner zone of the elevated-plus maze demonstrating a reduction of locomotor activity (Gregoire et al., 2012; Parent et al., 2012). While we interpret this decrease in physical activity as a pain-related behavior we cannot rule out that the decrease is fatigue-related.
In the current study, we did not see a reduction in voluntary physical activity in the exercise-enhanced pain model. These tests were done in the middle of the day when physical activity is lowest and might not be sensitive enough to account for small changes. We believe that the inflammatory model is a more severe and robust pain model with observable limping and guarding of the limb for 24–48 hours, and the repeated acid injections produces a robust hyperalgesia of the muscle and paw. On the other hand, the exercise-enhanced pain model shows no overt behavioral deficits, suggesting the hyperalgesia, while long-lasting, is less debilitating to the animal. Future studies will need to develop behavioral assays that are capable of examining effects of bilateral hyperalgesia, perhaps examining physical activity levels over a longer period of time.
In summary, we developed several tests of higher order processing in animals with unilateral muscle inflammation. These included the escape avoidance test, learned avoidance to noxious mechanical stimulation, and reductions in voluntary physical activity. It should be pointed out that these tests are indirect measures of nociception (in contrast to reflexes for example) that require diffuse and complex interactions of multiple neural systems for manifestation of the behaviors. The underlying neural circuitry for these behaviors is only now being elucidated. These tests provide valuable information regarding the consequences of pain in an animal and may prove useful for evaluating future treatments. However, despite the potential usefulness of these methods in aspects of preclinical pain research, their value in delineating the precise neural circuitry of pain is limited. We suggest that muscle pain models could use tests that examine aversive behaviors to assess the affective dimension of pain in rodents.
Acknowledgments
FUNDING SOURCES: Funded by National Institutes of Health AR053509, AR052316 and AR061371.
The authors would like to thank Francis Jareczek and Nicholas Gregory for the insightful discussions and aid in editing. Lynn Rasmussen provided technical assistance.
References
- Basstrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB. Spinal-brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain like behavior. Pain. 2010;151:670–679. doi: 10.1016/j.pain.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res. 2011;1370:129–135. doi: 10.1016/j.brainres.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Betourne A, Familiades J, Lacassagne L, Halley H, Cazales M, Ducommun B, Lassalle JM, Zajac JM, Frances B. Decreased motivational properties of morphine in mouse models of cancerous- or inflammatory-chronic pain: implication of supraspinal neuropeptide FF(2) receptors. Neurosci. 2008;157:12–21. doi: 10.1016/j.neuroscience.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Zhong C, Kohnken R, Baker SJ, Simler GH, Wensink EJ, Decker MW, Honore P. Comparison of mechanical allodynia and the affective component of inflammatory pain in rats. Neuropharmacol. 2010;58:537–543. doi: 10.1016/j.neuropharm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombez G, Vervaet L, Lysens R, Baeyens F, Eelen P. Avoidance and confrontation of painful, back-straining movements in chronic back pain patients. Behav Modif. 1998;22:62. doi: 10.1177/01454455980221004. [DOI] [PubMed] [Google Scholar]
- Crombez G, Vlaeyen VWS, Heuts PHTG, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–339. doi: 10.1016/s0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- Da Silva LF, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010;151(1):155–61. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Ding HK, Shum FWF, Ko SW, Zhuo M. A new assay of thermal-based avoidance test in freely moving mice. Neurosci. 2005;6(7):411–416. doi: 10.1016/j.jpain.2005.01.361. [DOI] [PubMed] [Google Scholar]
- Uhelski ML, Davis MA, Fuchs PN. Pain effect in the absence of pain sensation: Evidence of asomaesthesia after somatosensory cortex lesions in the rat. Pain. 2012;153:885–892. doi: 10.1016/j.pain.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Fuchs PN. Beyond reflexive measures to examine higher order pain processing in rats. Pain Res Management. 2000;5:215–219. [Google Scholar]
- Fuchs PN, McNabb MT. The place escape/avoidance paradigm: a novel method to assess nociceptive processing. J Integ Neurosci. 2012;11:61–72. doi: 10.1142/S0219635212500045. [DOI] [PubMed] [Google Scholar]
- Gregoire S, Michaud V, Chapuy E, Eschalier A, Ardid D. Study of emotional and cognitive impairments in mononeuropathic rats: effect of duloxetine and gabapentin. Pain. 2012;153:1657–1663. doi: 10.1016/j.pain.2012.04.023. [DOI] [PubMed] [Google Scholar]
- He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. Pain. 2012;13(6):598–607. doi: 10.1016/j.jpain.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86(1):402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98(14):8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. Attenuation of negative pain affect produced by unilateral spinal nerve injury in the rat following anterior cingulate cortex activation. Neurosci. 2005;136(1):311–322. doi: 10.1016/j.neuroscience.2005.07.010. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol. 2004;188:139–148. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- McNabb CT, Uhelski ML, Fuchs PN. A direct comparison of affective pain processing underlying two traditional pain modalities in rodents. Neurosci Lett. 2012;507(1):57–61. doi: 10.1016/j.neulet.2011.11.051. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Spinal and Supraspinal Mechanisms of Neuropathic Pain. Ann NY Acad Sci. 2006;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Parent AJ, Beaudet N, Beaudry H, Bergeron J, Bérubé P, Drolet G, Sarret P, Gendron L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res. 2012;229:160–167. doi: 10.1016/j.bbr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LH, Blackburn-Munro G. Pharmacological characterization of place escape/avoidance behavior in the rat chronic constriction injury model of neuropathic pain. Psychopharmacol. 2006;185:208–217. doi: 10.1007/s00213-005-0281-3. [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Scheel-Krüger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain. 2007;127(7):17–26. doi: 10.1016/j.pain.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152(7):1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104(3):567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Danielson J, Rasmussen L, DaSilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc. 2012;44(3):420–7. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & Nerve. 2001;24(1):37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106(3):229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148(2):188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhelski ML, Davis MA, Fuchs PN. Pain affect in the absence of pain sensation: evidence of asomaesthesia after somatosensory cortex lesions in the rat. Pain. 2012;153:885–892. doi: 10.1016/j.pain.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152(5):990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136(3):373–379. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Maeda Y, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007a;8(5):422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain. 2007b;8(9):692–699. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]