Abstract
Wnt/β-catenin signaling underlies the pathogenesis of a broad range of human cancers, including the deadly plasma cell cancer multiple myeloma (MM). In this study, we report that downregulation of the tumor suppressor microRNA miR-30-5p is a frequent pathogenetic event in MM. Evidence was developed that miR-30-5p downregulation occurs as a result of interaction between MM cells and bone marrow stromal cells, which in turn enhances expression of BCL9, a transcriptional co-activator of the Wnt signaling pathway known to promote MM cell proliferation, survival, migration, drug resistance and formation of MM cancer stem cells. The potential for clinical translation of strategies to re-express miR-30-5p as a therapeutic approach was further encouraged by the capacity of miR-30c and miR-30mix to reduce tumor burden and metastatic potential in vivo, in three murine xenograft models of human MM, without adversely affecting associated bone disease. Together, our findings offer a preclinical rationale to explore miR-30-5p delivery as an effective therapeutic strategy to eradicate MM cells in vivo.
Keywords: myeloma, miR-30-5p, Wnt pathway, BCL9, replacement therapy
Introduction
MM is a cancer of plasma cells (PCs) that accumulate in the bone marrow (BM). Despite recent advances in understanding the molecular pathogenesis of MM and the unveiling of promising new therapies, it remains incurable; highlighting the need for continual efforts to develop novel and hopefully more effective therapies (1, 2).
Canonical Wnt/β-catenin pathway is implicated in the pathogenesis of a broad range of cancers, and has emerged as a promising target for therapy. Loss-of-function mutations in APC and Axin as well as activating mutations in β-catenin itself, enable β-catenin nuclear translocation, and drive oncogenic Wnt transcription (3). Co-activators for β-catenin transcription have been identified including Pygopus (PYGO), B-cell lymphoma 9 (BCL9), and its homologue B-cell lymphoma 9-like (B9L), among others (4, 5). The formation of a quaternary complex consisting of TCF, β-catenin, BCL9 (or B9L), and PYGO enhances β-catenin-dependent transcriptional activity (6).
Canonical Wnt pathway is constitutively active in MM and promotes tumor cell proliferation, and disease progression (7–11); however, mutations in APC, Axin or β-catenin have not been reported (12). Instead, the mechanism of pathologic Wnt signaling in MM has been linked to post-transcriptional regulation of β-catenin (13) and/or increased levels of BCL9, implicating this β-catenin co-factor as a bona fide oncogene (9). The oncogenic role of BCL9 is further highlighted by the following observations: human BCL9 was first identified by cloning the t(1;14)(q21;q32) translocation from a patient with B-cell acute lymphoblastic leukemia (14); chromosome 1q21 amplifications containing the BCL9 locus are observed in a broad range of human cancers (15), including MM, and is associated with poor clinical outcome (8); shRNA-induced knockdown of BCL9 or treatment with Stabilized Alpha-Helix of BCL9 (SAH-BCL9), which selectively suppress Wnt transcription, elicit mechanism-based anti-tumor responses in colorectal cancer cells and MM (9, 10). Collectively, these data indicate that targeting the BCL9 component of aberrantly activated Wnt signaling in cancer may attenuate invasion, metastasis, and resistance to therapy, highlighting the importance of this pathway and BCL9 for target drug discovery.
In previous studies, we found that BCL9 is over-expressed in a large subset of MM patients (8, 9). However, only a weak correlation was observed between BCL9 DNA copy number gains and BCL9 mRNA expression levels in patient MM cells (Fig. S1A), indicating that mechanisms other than gene dosage due to chromosome1q21 amplification may be involved in regulating expression of BCL9 in MM.
MicroRNAs(miRs) function as negative regulators of gene expression (16) and have been implicated in the pathogenesis of MM (17) and other cancers (18), offering the promise to create novel therapeutic approaches if they can be effectively applied in vivo. However, a functional link between miRs and Wnt pathway and its clinic and pathologic significance has not been established. Here, we document for the first time that expression of BCL9 is regulated by the miR-30s family. MiR-30s are expressed at very low levels in a large subset of the MM samples compared with normal PCs, and there is a reverse relation between miR-30s and BCL9 mRNA expression levels. Bioinformatics analysis revealed that BCL9 mRNA has two different binding sites for miR-30s in the 3’-untranslated region (3’UTR). Enhanced expression of miR-30s in MM cell lines leads to a reduction in cellular proliferation, survival, migration and invasion as well as colony formation and number of side population (SP) cells. These changes were mediated through direct binding of miR-30s to the 3’UTR of BCL9 mRNA, thereby down regulating BCL9 and Wnt transcriptional activity. Overall our studies establish a functional link between miR-30s and BCL9, unveiling their role in MM progression and providing a proof-of-concept for the potential translation of miR-30s as novel therapeutic agent to target oncogenic Wnt/β-catenin/BCL9 complex in MM and other cancers with deregulated Wnt activity.
Materials and Methods
Patients’ tissue preparation and cell lines
BM specimens were obtained from patients with MM and normal donors in accordance with Dana-Farber Cancer Institute Review Board approval, and informed consent performed in compliance with the Declaration of Helsinki. MM and NPCs were purified from BM aspirates using CD138 magnetic beads (Miltenyi Biotec, Auburn, CA) as described (19). CD138 negative mononuclear cells were used to establish long-term BM stem cells (BMSCs). Stable stroma cell line HS-5 was infected with V-ds-red and sorted to generate a stable cell line for co-culture experiments. After a confluent layer of adherent cells was obtained, the cells were ready for co-culture experiments and dexamethasone drug treatment experiments. MM cell lines: H929, MM1S, RPMI8226 were obtained from ATCC, OPM1, MR20 kindly provided by Dr. Teru Hideshima. All of the cells were routinely used and tested using Human Cell Line Genotyping System (Promega) when we frozen and thaw the cells. Cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, in 5% CO2 in humidified air, at 37 °C.
Q-RT-PCR, Western blot, immunofluorescence, immunohistochemistry and miRs locked nucleic acid (LNA) in situ hybridization (ISH)
MiRs quantitative reverse transcriptase-PCR (Q-RT-PCR) was performed according to manufacturer’s instruction (Applied Biosystem). U44 primer from ABI was used as an internal control. Q-RT-PCR was performed for evaluation of BCL9 mRNA levels as previously described (9, 20), and GAPDH severed as an internal control. The primers of BCL9, Axin 2, and CD44 are listed in Table S1. Western blot, miRs-LNA ISH, immunofluorescence (IF), and immunohistochemistry (IHC) were carried out as previously described (21). Antibodies included: BCL9 (6109) antibody for western blotting and IF (10); Alexa Fluor 546 goat anti-rabbit IgG (A-11010, Invitrogen) as secondary antibody for IF; as well as actin-HRP (C-11, Santa Cruz) and anti-Rabbit IgG HRP conjugated secondary antibodies (W401b, Promega) for western blotting; as well as BCL9 (ab37305, Abcam), Caspase-3 (#9662, Cell Signaling), Ki-67 (NB110-90592, Novus), Axin-2 (#2151, Cell Signaling), CD44 (#5640s, Cell Signaling), and BCL6 (ab9479, Abcam) were used for IHC.
Lentiviral infection of miRs and transient transduction of mature miRs
Expression plasmids of miR-30s were purchased from SBI and referred to as V-miR-30s. The construct expresses the pre-miR-30s which is processed into mature miRs in infected cells. Lentiviral packaging and infection of MM cells were done according the protocol from manufacturer (SBI). MM cells were infected with viral supernatant containing polybrene, and GFP-expressing cells sorted by FACS. RPMI 8226, H929 and MM1S Luc-neo cells were transiently transduced with either mature miR-30a/b/c/d/e, miR-30c only, or control cel-miR-67 (IDT) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cel-miR-67 has minimal sequence identity with miRs in human, mouse, and rat (sequence is listed in Table S1). Following transduction of cells, BCL9 immunoblots were performed.
2'-O-methyl oligoribonucleotides for knockdown of miR-30s and Bcl9 3’UTR luciferase constructs
The knockdown of miR-30s family members in MM cells was achieved by transfection with antisense 2'-O-methyl oligoribonucleotides (ASO) against miR-30s using Lipofectamine 2000. Transfection complexes were prepared according to manufacturer’s instructions and added directly to the cells at a final oligonucleotide concentration of 10 nmol/liter. The sequences of ASO against miR-30s and scrambled miR-30s are listed in Table S1. Following 48 h incubation, cells were assayed for BCL9 mRNA and protein expression as described (9). PmiR-Report- Bcl9 were created by cloning Bcl9 3’UTR 329–335bp and 486–492bp, which match the seed sequence of miR-30s, into downstream luciferase gene of pmiR reporter plasmid (pmiR-0), as we previously described (20). Their mutants (e.g., pmiR-Report-BCL9-mut-1 and pmiR-Report-BCL9-mut-2) were obtained by deletion of the matching seed sequence to indicated nucleotides (Fig. 2A). The sequences used to create these plasmids are listed in Table S1.
Figure 2. MiR-30s target BCL9 mRNA.
(A) Sequence alignment of miR-30a/b/c/d/e with the seed binding sequences on the 3'-UTR regions of BCL9 mRNA; wt-1 (orange), wt-2 (yellow) and the corresponding mutants (mut-1 and mut-2). (B) Q-RT-PCR verification of induced ectopic expression of miR-30s members in H929 cells after transduction of V-GFP or each miR-30s member. Ectopic expression of each miR-30s member is associated with a reduction in BCL9 mRNA (C) and protein (D) levels. * p<0.05. (E) Luciferase reporter assays in HEK cells transduced with GFP or each miR-30s member. pmiR-0 (empty reporter plasmid), pmiR-BCL9-30-wt-1 (reporter plasmid containing wt-1), pmiR-BCL9-30-wt-2 (reporter plasmid containing wt-2), pmiR-BCL9-30-mut-1 (reporter plasmid containing mut-1), or pmiR-BCL9-30-mut-2 (reporter plasmid containing the mut-2). * p<0.05.
BCL9 3’UTR luciferase reporter assay and TOP/FOP luciferase assay
Luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega). 293T cells were co-transfected with miRs virus (V-miR-30s or V-GFP), reporter plasmids, and Renilla luciferase using lipofectamine 2000 (Invitrogen). 24h postransfection, cells were subjected to luciferase reporter assay using Dual-Luciferase Reporter Assay System (Promega). Luciferase activities were analyzed as activity of firefly relative to Renilla. To measure Wnt reporter activity, H929 and MM1S cells were transfected with TOP-FLASH, FOP-FLASH plasmid (Millipore Corporation), along with an internal Renilla control plasmid (hRL-null), according to the manufacturer’s protocol. The results were normalized to control Renilla activity. The reported data represent the average of three independent transfection experiments performed in triplicate.
The MM1s-Luc-neo tumor dissemination mouse model
NOD/SCID mice were injected i.v. with 5×106 MM1S-Luc-neo cells, and one week after injection, groups of 8 mice were randomly separated and treated by i.p. injection once a week with RNA-LANCErII (BioScience) (vehicle) or 100 pmol total of an echymolar mixture of miR-30a/b/c/d/e (miR-30mix) pre-mixed in RNA-LANCErII. Tumor development was monitored by whole body imaging using Xenogen system. Mice were evaluated every week after initiation of treatment, and the tumor ratio of fluorescence intensity was compared among the three groups. Fluorescent tissues were harvested for histological and IHC evaluation. To exclude toxic effects of miR-30s therapy non fluorescent tissue were also evaluated by histopathology. At day 21 of treatment two mice from control and treated groups were euthanized and their spines subjected to micro-computed tomographic analysis.
Results
MiR-30s is the only predicted miR binding to the 3’UTR of BCL9 mRNA
We investigated whether BCL9 mRNA expression is regulated by miRs. By searching databases TargetScan (27), PicTar (28), miRDB, and microCosm, we found that the 3’UTR of BCL9 mRNA contains 2 sequence motifs designated wt-1 (9129–9135bp) and wt-2 (9880–9886bp) (Fig. S1B), which perfectly match with the “seed” sequence of the miR-30s family members (Figs. S1C and 2A).
MiR-30s are downregulated in MM cells, and their expression is inversely related with BCL9 expression
Our expression profiling data from 78 MM patient samples showed that the levels of each miR-30 family member (miR-30a/b/c/d/e) were variable, and that 60% (45/78) of samples expressed low levels of miR-30s compared with 9 normal PCs (Fig. 1A). We next asked whether low levels of miR-30s were associated with high BCL9 mRNA expression levels, and vice versa using Q-RT-PCR analysis. A total of 6 normal PCs (N1–N6) and 6 patient MM cells (T1–T6) were examined in parallel for expression of miR-30s and BCL9 mRNA (Fig. 1B). We observed that normal PCs with undetectable levels of BCL9 mRNA display high levels of miR-30s expression, while patient MM cells with variable levels of BCL9 mRNA were almost devoid of miR-30s expression. This inverse relation was also detected in MM cell lines (Fig. 1C). For example, the H929 cell line that expresses relatively low levels of miR-30s showed high levels of BCL9 mRNA expression, while the MM1S cell line that expresses high levels of miR-30s showed relatively low levels of BCL9 mRNA expression. Although miR-30s members are located at three different chromosomal regions: 1p34.2 (miR-30e and miR-30c-1), 6q13 (miR-30c-2 and miR-30a) and 8q24.22 (miR-30b and miR-30d) they all have similar expression patterns among different MM patient samples (Fig. 1A), suggesting that they share a similar regulatory network that is independent of chromosomal copy number alterations. Indeed miR-30c, the only member with two copies in the human genome, does have the most abundant expression levels in normal PCs, and show the lowest levels of expression in MM patient samples, which frequently have chromosomal 1p34 and 6q13 deletions (29).
Figure 1. MiR-30s are downregulated in MM.
(A) Heatmap of hierarchical cluster analysis of miR-30s expression in patient’s MM cells (MM) and normal PCs (NPCs). Q-RT-PCR analysis of miR-30s and BCL9 mRNA expression in normal PCs (N1–N6), patient’s MM cells (T1–T6) (B), and MM cell lines (C). (D) Analysis of miR-30s and BCL9 mRNA expression levels in patient’s MM cells from published dataset GSE27306 (miR-30a, p= 0.030; miR-30b, p=0.007; miR-30c, p=0.001; miR-30d, p=0.028; miR-30e, p=0.050). (E) Two of six representative cases of ISH (left) and IHC (right) analysis of miR-30s and BCL9 expression levels in MM patient’s BM.
To further investigate the relation between BCL9 mRNA and miR-30s levels, we analyzed published data set GSE17306 (30) in which information for both mRNA and miR expression in MM patient samples was available. We found that BCL9 mRNA is highly expressed in late stage MM patient samples, and that its expression inversely associated with expression of miR-30a(also known as miR-30a-5p), miR-30b, miR-30c, miR-30d and miR-30e (also known as miR-30e-5p) (p<0.05) (Fig. 1D), but not with miR-30a-3p and miR-30e-3p, two miRs functionally unrelated to the miR-30s family (p>0.05, data not shown). In addition, we examined the relation between miR-30s and BCL9 protein levels on BM biopsies. Since miR-30c showed the most significant changes above (Fig. 1D), we selected this family member for this and further studies. MiR-LNA in situ hybridization (ISH) of miR-30c and IHC staining of BCL9 protein on 6 BM biopsies from MM patients also showed an inverse association between miR-30s expression and BCL9 protein levels (Fig. 1E).
BCL9 mRNA is a direct target of miR-30s
In order to establish a functional relationship between miR-30s and BCL9 mRNA regulation, we first transduced individual pre-miR-30s into HEK293T cells using lentiviral vectors expressing GFP as a marker for flow sorting of stably transduction of cells. Cells transduced with vector alone (V-GFP) were used as a control. Ectopic expression of each pre-miR-30s member (Fig. 2B) was associated with down regulated expression of BCL9 mRNA and protein levels, as evaluated by Q-RT-PCR (Fig. 2C) and immunoblot analysis (Fig. 2D) respectively. To further demonstrate that miR-30s directly regulates expression of BCL9 mRNA through binding to the 3’UTR, two wild type-(pmiR-BCL9-30-wt-1 and pmiR-BCL9-30-wt-2) and two mutant- (pmiR-BCL9-30-Mut-1 and pmiR-BCL9-30-Mut-2) BCL9-3'UTR reporter vectors were co-transfected into HEK293T cells, together with each individual V-miR-30s member or V-GFP. Luciferase activity of wild type, but not mutant, was significantly decreased with each V-miR-30s compared to V-GFP (Fig. 2E), confirming the specificity of the interaction between miR-30s and BCL9-3’UTR mRNA.
MiR-30s regulates BCL9 mRNA expression in MM cells
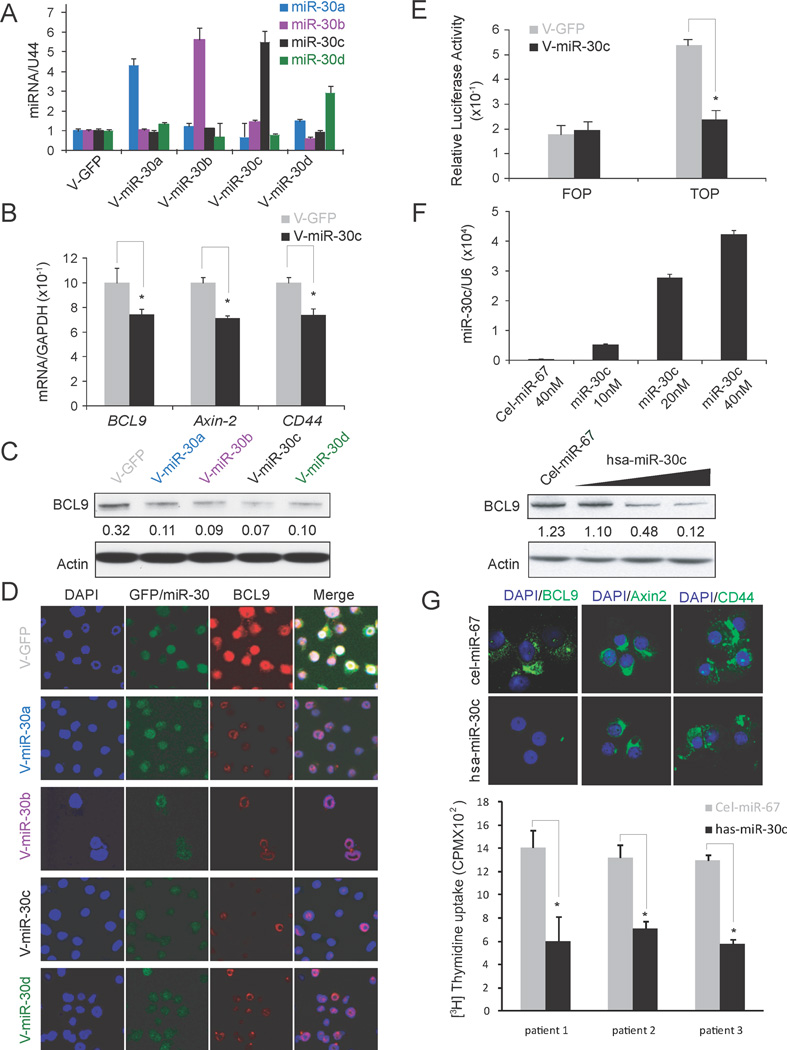
We next investigated whether BCL9 mRNA expression is regulated by miR-30s in MM cells using both gain- and loss- of function studies. For gain-of-function studies we used H929 cells that express the lowest levels of miR-30s among the MM cell lines examined (Fig. 1C). We first induced ectopic expression of individual V-miR-30s family members in H929 cells using lentiviral infection. After flow sorting of GFP positive cells, levels of miR-30s were verified by Q-RT-PCR (Fig. 3A) and then used in all of the following experiments. As shown in HEK293T cells (Figs. 2B, C), ectopic expression of miR-30c in MM cells was also associated with a significant reduction in the expression of BCL9 mRNA (Fig. 3B). Ectopic expression of miR-30s was also associated with a reduction in the expression of BCL9 protein, as evaluated by immunoblot (Fig. 3C) and IF (Fig. 3D) studies. Consistent with the role of BCL9 as a transcriptional co-activator of Wnt signaling pathway, ectopic expression of miR-30c was also associated with reduced expression of “bonafide” Wnt pathway downstream targets CD44 and Axin2 (Fig. 3B)(9, 10) as well as Wnt reporter FOP/TOP activity (Fig. 3E). We next focused on evaluating the effect of the miR-30s family member miR-30c and validated its effect on BCL9 down regulation in other MM cell lines using immunoblot (Fig. S1D). Wild type TOP reporter activity was inhibited in V-miR-30c stable H929 cells compared with V-GFP stable H929 cells, while the mutant FOP activity was not changed (Fig. 3E). To exclude the possibility that the observed changes in BCL9 expression may be due to non specific and/or secondary effects of stable transfection of V-miR-30c, we also performed transient transfection with increasing amounts of mature miR-30c into RPMI8226 cells, using cel-miR-67 as a control (Fig. 3F). To confirm transfection efficiency, cells were collected 72 hours after transfection, and miR-30c expression levels were checked by Q-RT-PCR (Fig. 3F, top). We found an inverse association between downregulation of BCL9 protein expression and increasing amounts of transfected mature miR-30c (Fig. 3F bottom). In order to verify miR-30’s potential for therapy in MM patients, CD138+ primary cells (n=3) were transfected with 40 pmol of mature miR-30c or same amount of cel-miR-67 as control. Expression of BCL9, CD44 and Axin2 were downregulated as evaluated by IF in all three patient samples. One representative case is shown in Fig. 3G, top. Furthermore, MM cell proliferation was dramatically inhibited by miR-30c (Fig. 3G, bottom).
Figure 3. MiR-30s inhibit BCL9 and Wnt target gene expression in MM.
(A) Q-RT-PCR verification of induced ectopic expression of miR-30 members in H929 cells after transduction of V-GFP or each miR-30s member. (B) Ectopic expression of miR-30c reduces mRNA levels of BCL9, as well as the Wnt target genes Axin-2 and CD44 but not GAPDH. Ectopic expression of miR-30s member reduces protein levels of BCL9 as evaluated by immunoblot (C) and IF (D) studies * p<0.05. (E) Wnt reporter activity in H929 cells stably transduced with V-miR-30c or control V-GFP. * P<0.01. (F) RPMI8226 cells were transduced with Cel-miR-67 control or increased concentrations of mature miR-30c, and levels of miR-30c and BCL9 protein were measured by Q-RT-PCR (top) or immunoblot analysis (bottom) respectively. (G) IF analysis of BCL9, CD44 and Axin-2 expression (top) as well as [3H] thymidine uptake (bottom), of MM patient CD138+ cells transduced with Cel-miR-67 or has-miR-30c. * p<0.05.
For loss-of-function studies we used the MM1S cell line, which expresses relatively high levels of miR-30s and relatively low levels of BCL9 mRNA (Fig. 1C). MM1S cells were transfected with a pool of 2’O-me anti-miR-30a/b/c/d/e cocktail (anti-miR-30mix) or scrambled oligonucleotides as a control. Q-RT-PCR analysis revealed a significant reduction of all members of miR-30s in cells treated with individual anti-miR-30s compared with cells transfected with scrambled oligonucleotides (Fig. S2A). Levels of BCL9 mRNA (Fig. S2B) and protein (Fig. S2C) were increased in 2’O-me anti-miR-30 treated cells compared with cells transfected with scrambled nucleotides. Furthermore, expression of the Wnt downstream targets Axin-2 and CD44 was upregulated (Fig. S2B), as it was Wnt reporter activity (Fig. S2D), in MM1S cells treated with anti-miR-30mix, but not with scrambled, oligonucleotides.
MiR-30c inhibits MM cell proliferation, invasion, and migration and induces apoptosis
Then we examined if miR-30s can mimic the functional consequences of BCL9 deregulation in MM (9, 10). Based on results shown in Figs. 1D and E, we focused on miR-30c. A consistent pattern emerged whereby H929 and OPM1 cells overexpressing miR-30c (V-miR-30c), but not control cells (V-GFP), showed significantly reduced proliferation (Fig. 4A), colony formation (Fig. 4B), invasion, and migration (Fig. 4C). In addition, we found that miR-30c induces a modest increase in apoptosis, from 10.1 ± 1.1% in H929 cells expressing GFP (V-GFP) to 14.2 ± 1.9% in H929 cells expressing miR-30c (V-miR-30c) (n=3, p<0.05) (Fig. 4D). Taken together, these data demonstrate that miR-30c specifically disrupts a series of physiologic processes regulated by the Wnt/BCL9/β-catenin transcriptional complex, and highlighting the potential therapeutic role of miR-30c in MM.
Figure 4. MiR-30c inhibits cell proliferation, invasion and migration, and induces apoptosis of MM.
(A) [3H] thymidine uptake of H929 and OPM1 cells transduced with V-GFP or V-miR-30c. * p<0.05. (B) Representative images of colony formation assay of H929 cells transduced with V-GFP or V-miR-30c). Insets: morphology of the spheres under light and florescence microscopy. Numbers of colonies per well are expressed as means. * p<0.01. (C) Invasion and migration ability of H929 cells stably transduced with V-GFP or V-miR-30C. * p<0.01. (D) Flow cytometry analysis of Annexin V and propidium iodine (PI) staining of H929 cells transduced with V-GFP or V-miR-30C. The y axis represents PI staining (10,000 cells) and the x axis represents Annexin V staining (right). Data as percentages from triplicate experiments are also shown (left). * p<0.05.
MiR-30c inhibits MM cancer stem cell formation
We next investigated whether miR-30c is involved in regulating behavior of CSCs in MM (Fig. 5). Functional Hoechst22234 staining assay was employed to define the SP in V-miR-30c stably infected cells, and V-GFP cells were used as a control (Fig. 5A). The stem cell SP was significantly reduced from 0.698% ± 0.04% in H929 V-GFP cells to 0.068% ± 0.05% in H929 cells expressing V-miR-30c (n=3, p<0.05) (Fig. 5B). Moreover, in experiments using stem cell medium to culture sorted SP cells (Fig. 5C), the sphere numbers (Fig. 5D) and size of spheres as evaluated by cell number per sphere (Fig. 5E), were significantly decreased in H929 V-miR-30c SP compared with H929 V-GFP control CSCs. These results highlight the potential role of miR-30c in blocking Wnt signaling pathway in CSC, further confirming the relevance of this pathway for target drug discovery in MM.
Figure 5. MiR-30c decreases population of MM cancer stem cells.
(A, B) SP fraction of H929 cells transduced with V-GFP or V-miR-30c, as detected by functional Hoechst 33342 stem cell staining assay. Verapamil is used as an inhibitor of SP cells. (C) Representative phase contrast (left) and fluorescence microscopy images (right) of cell spheres formed after culture of SP cells isolated from H929 cells transduced with either V-GFP or V-miR-30c in stem cell medium. Sphere numbers per 1000 sorted SP cells (D) and numbers of cells per sphere (E) in cells transduced with V-GFP or V-miR-30c.
MiR-30c restores drug sensitivity in BM stroma cell-induced drug resistance of MM
BMSCs promote migration, homing, proliferation, survival, and drug resistance in MM (31). The relatively high levels of miR-30s in MM cell lines compared with patient MM cells (Figs. 1B and 1C) prompted us to next investigate the possible role of BMSCs in regulating miR-30s expression in MM cells. GFP-labeled H929 cells were co-cultured with HS-5 dsRed stable BMSCs. After 48 h of co-culture, GFP-positive H929 cells were flow sorted and total RNA was isolated for Q-RT-PCR analysis. As shown in Fig. S3A, co-culture with HS-5 dsRed downregulates expression of miR-30s in H929 cells, associated with enhanced expression of BCL9 mRNA and the Wnt downstream targets Axin-2 and CD44, but not of GAPDH, a non Wnt target gene used as a control (Fig. S3B).
Since CD44 is a downstream target of Wnt/β-catenin/BCL9 transcriptional complex (9, 10), miR-30c downregulates expression of CD44 (Fig. 3B) in MM cells, and CD44 is a functional component of cell adhesion-mediated drug resistance (CAM-DR) (31), we investigated the possible role of miR-30s in MM drug resistance in the context of BM microenvironment. After ectopic overexpression of miR-30c or miR-30a/b/c/d/e cocktail (miR-30mix) or a negative control (cel-miR–67) in H929 cells (Fig. S3C), we co-cultured these cells alone or in the presence of HS-5 cells for 48h and treated them with 200 nM dexamethasone. Interestingly, we found that both miR-30c and miR-30mix can resensitize the H929 cells to dexamethasone treatment with miR-30mix apparently being more effective than miR-30c (Fig. S3D). Overall these findings indicate that expression of miR-30s in MM cells can be modulated by the BM microenvironment and further support its therapeutic potential to overcome CAM-DR.
MiR-30c inhibits tumor progression in murine xenograft models of human MM
To further explore the therapeutic potential of miR-30c, we next examined its capacity to suppress tumor growth and metastatic potential in vivo using two established murine xenograft models of human MM (9). In the first model (i.e. subcutaneous), H929 V-GFP control and H929 V-miR-30c stably transduced cells were injected subcutaneously into opposite flanks of SCID mice; tumor volume was evaluated over time up to day 25, when mice were sacrificed and whole body imaging was performed. As shown in Fig. 6A (top and bottom), tumor growth was significantly decreased in mice injected with H929 V-miR-30c (green arrows) as compared with H929 V-GFP control cells (red arrows). In the second model (i.e. intravascular), H929 V-GFP control or H929 V-miR-30c stably transduced cells were injected by tail vein into SCID mice; survival, tumor burden and spreading were assessed (Fig. 6B). Tumor involvement was observed in the intestine, spine, and skull, which was similar in V-miR-30c and V-GFP control group (122.5±33.0 days vs. 162.2± 21.7 days, n=6, p=0.03) (Fig. 6B, top). However, tumor burden was decreased and survival was significantly increased in mice injected with H929 V-miR-30c compared with H929 V-GFP control cells (Fig. 6B, bottom). In agreement with our in vitro studies, tumors developing in mice injected with stable V-miR-30c H929 cells showed decreased expression levels of BCL9, Ki-67, CD44 and Axin2 proteins, as well as increased levels of Caspase 3 expression compared with V-GFP control tumors evidenced by IHC analysis (Fig. 6C). BCL6 that is a target of miR-30 in diffuse large B-cell lymphomas (32) was not identified in MM (Fig. S1E). The miR-30c over-expression and downregulation of BCL9 in GFP-positive harvested tumors was further verified by Q-RT-PCR (Fig. 6D, upper panel) and immunoblot (Fig. 6D, bottom panel).
Figure 6. MiR-30c inhibits cell proliferation, invasion and migration, and induces apoptosis, in mouse xenograft models of MM.
(A) Representative image of tumors (top) and tumor growth curves (bottom) of NOD/SCID mice (n=8) subcutaneously injected with 5×106 H929 cells transduced with V-GFP or V-miR-30c. Tumor size was evaluated over time by fluorescence whole body imaging. P<0.01. (B) Tumor burden and metastasis (top) and survival (bottom) of NOD/SCID mice (n=6) intravenously injected with 1×106 H929 cells transduced with V-GFP or V-miR-30c. Tumor burden and spread were evaluated over time by fluorescence whole body imaging. p=0.03. (C) IHC analysis of BCL9, Ki-67, Caspase 3, CD44, and Axin2 expression on tissue sections of GFP-labeled tumor isolated from mice injected with H929 cells transduced with V-GFP or V-miR-30c. (D) Q-RT-PCR of miR-30c (top), and immunoblot of BCL9 protein (bottom) expression levels in H929 cells transduced with V-GFP or V-miR-30c and isolated from mice injected subcutaneously (#1 and #2) or intravenously (#3 to #6).
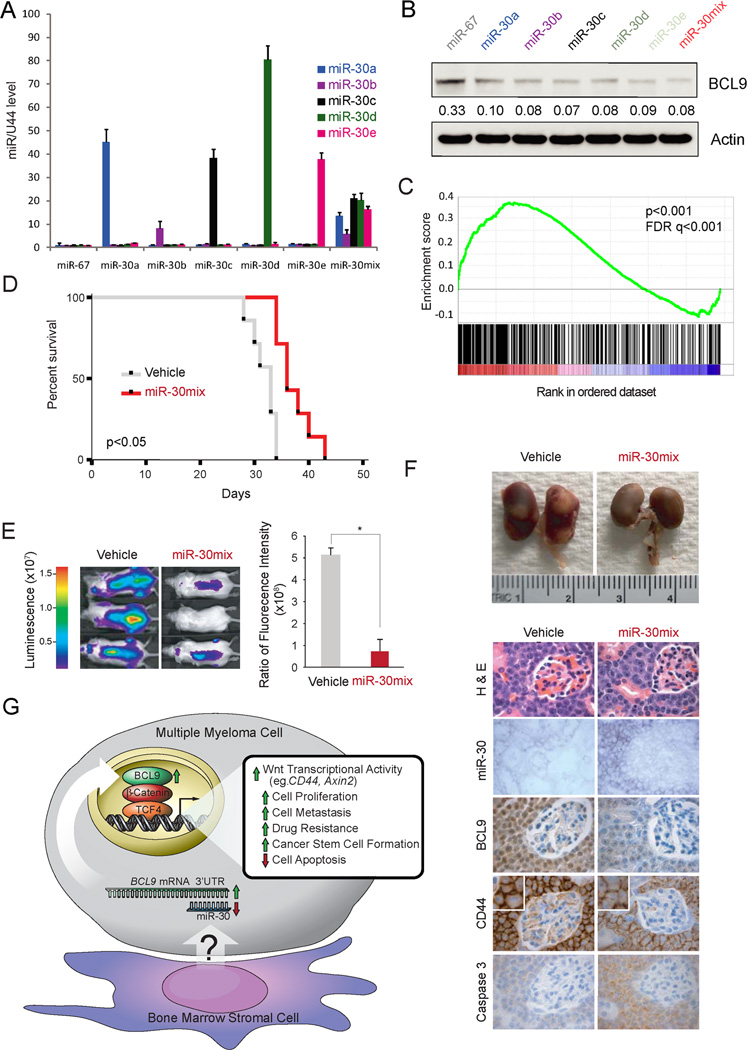
To further evaluate the possibility of translating this biological proof-of-concept into a pharmacologic strategy for inhibiting oncogenic Wnt/β-catenin/BCL9 complex using miR-30s, we next determined whether intraperitoneal delivery of miR-30s could inhibit MM tumor growth in vivo. In order to achieve therapeutic benefits in vivo, miR-based drugs require trans-membrane delivery systems to protect encapsulated miRs from degradation in the circulation, promote accumulation in target tissue, facilitate intracellular delivery into target cells and must also be relatively non-toxic. Lipid nanoparticles are currently the most favored delivery system for satisfying these needs; therefore, we used miR-30s premixed with RNA-LANCErII, consisting of a mixture of neutral lipid, non-ionic detergent, and oil (33) to perform this in vivo experiment. We first evaluated if lipid nanoparticles could deliver miR-30s in to H929 MM cells and if there was preferential delivery and inhibition of BCL9 expression among individual members of the miR30s family. Q-RT-PCR analysis revealed that all miR-30 members were taken up by the cells, although at different extent when added to the medium individually or as a miR-30a/b/c/d/e echymolar cocktail (miR-30mix) (Fig. 7A). In addition, all individual miR-30 members or the echymolar cocktail decreased expression of BCL9 to similar extent, evaluated by immunoblot analysis (Fig. 7B). Reasoning that treatment with a cocktail mixture containing lower amounts of each individual member could be better tolerated by the mice we performed this in vivo experiment with miR-30mix. Therefore, we first investigated the specificity of miR-30mix treatment in inhibiting expression of Wnt/β-catenin/BCL9 transcriptional targets by performing comparative genome-wide expression analyses (Fig. 7C). We generated triplicate gene expression profiling data sets from H929 cells treated with miR-30mix or Cel-miR-67 (control) as well as from H929 cells lentivirally transduced with previously validated shRNA hairpins against BCL9 (sh-BCL9) or scrambled sequences (control) (9) using Affimetrix oligonucleotides microarrays. Gene set enrichment analysis (GSEA) revealed a statistically significant correlation between the genes downregulated by miR-30mix and by sh-BCL9 (family-wise error (FWER) p-value <0.001; false discovery rate (FDR) q-value<0.001), documenting the specificity of miR-30mix in blocking expression of Wnt/β-catenin/BCL9 transcriptional targets (Fig. 7C).
Figure 7. MiR-30s treatment decreases tumor burden in MM1S-Luc-Neo bearing mice.
Q-RT-PCR expression analysis of miR-30s (A), and immunoblot analysis of BCL9 expression (B) in H929 cells transduced with control mature cel-miR-67 (miR–67), each individual miR-30 family member (miR-30s) or an echymolar mixture (miR-30mix) using RNA-LANCErII. (C) GSE analysis of genes down regulated by miR-30mix and shBCL9 treatment in H929 cells. (D) Kaplan-Meier survival plots of mice treated with control or miR-30mix after injection of MM1s-Luc-Neo cells. (E) Representative Xenogen images of control and miR-30mix treated mice. (F) Top: Gross image of tumor metastasis in kidneys. Bottom: H&E, ISH, and IHC analysis of miR-30s, BCL9 and CD44 expression levels in MM1s-Luc-Neo cells after i.p. administration of vehicle or miR-30mix using RNA-LANCErII. (G) Proposed model for regulation of BCL9 oncogene and therefore Wnt/β-catenin signaling pathway by miR-30s in MM.
Then, we performed an in vivo miR-30mix delivery experiment to determine whether tumor growth is antagonized in a well established MM1S Luc-neo MM murine xenograft model of human MM (Fig. 7D–F) after i.p. delivery using lipid nanoparticles. As shown in Fig. 7D, survival was increased in mice treated with miR-30mix (vehicle group, 31.9±2.3 days vs miR-30mix group, 35.7±4.4 days, n=8, p<0.05.), and associated with decreased tumor burden (Fig. 7E), metastatasis to the kidney (Fig. 7F, top panel), as well as decreased expression of BCL9 and CD44 proteins (Fig. 7F, bottom panel). In vivo delivery of miR-30s to target cells was confirmed using miR LNA-ISH (Fig. 7F second row, bottom panel).
Due to the documented role of Wnt activity in bone metabolism (34), and the potential side effect of worsening osteolytic bone disease in myeloma patients treated with Wnt inhibitors (35), we evaluated the effect of miR-30s therapy in our murine xenograft model using micro-computed tomography (µCT) of bones. We first evaluated the effect of miR-30c in SCID mice no transplanted with myeloma cells. No apparent development of bone osteolytic lesions was observed in mice treated with miR-30c as compared with mice treated with vehicle (Fig. S4A, B). We also evaluated by µCT analysis the spines of two vehicle-treated and two miR-30mix-treated SCID mice transplanted with MM1S-Luc-Neo cells at day 21 of treatment (Fig. 7D). As shown in Figs. S4C, D, no major differences in trabecular bone volume and cortical void fraction was observed between mice treated with vehicle or miR-30mix. In addition, the cortical void fraction of mice transplanted with MM cells and treated with miR-30mix was similar to mice not transplanted with MM cells (Figs. S4B, D). Furthermore, no evidence of bone lytic lesions as evaluated histologically was observed in long bones which were not involved by MM cells (data not shown). Overall these results suggest that miR-30s treatment does not have a negative impact on bone, an issue that need to be further explored. Thus, miR-30s treatment effectively inhibited BCL9-driven Wnt transcriptional activity in vivo, thereby suppressing tumor growth, invasion, and enhancing survival, highlighting the potential role of miR-30s as a novel therapeutic approach in MM. The lack of improvement in MM associated bone disease in miR-30s treatment suggest that this approach should be implemented in association with therapies that reduce osteoclast-mediated bone resorption such as bisphosphonates (34).
Discussion
Here we provide evidence for a novel functional link between miR-30s and oncogenic Wnt/β-catenin/BCL9 transcriptional activity. Using MM as a model cancer system with deregulated Wnt activity (7–11), we proved that miR-30s as a tumor suppressor and novel therapeutic tool by targeting BCL9, a critical co-activator of canonical Wnt/β-catenin signaling pathway. Deregulated Wnt/β-catenin transcriptional activity underlies the pathogenesis of a wide variety of human carcinomas (36, 37), and hematologic malignancies including MM (7–11). Although the Wnt/β-catenin transcriptional complex is a promising target for cancer therapy (38), a major limitation to therapies targeting this pathway is the importance of β-catenin activity in normal adult tissue homeostasis (39). Thus, further investigation of novel and more selective Wnt signaling components are needed.
The BCL9 is an essential co-activator of Wnt/β-catenin transcriptional activity, and studies from our lab and others have unveiled its role as novel therapeutic target (9, 10). The lack of detectable phenotypic alterations in the intestinal tract of mice with conditional deletion of BCL9 and B9L (40) suggests that BCL9/BL9 proteins do not play an essential homeostatic role in mammalian Wnt signaling, although BCL9/B9L regulates Wnt target genes that control epithelial mesenchymal transition(EMT) and stem cell-like behavior (40). This data indicates that targeting the BCL9/B9L component of aberrantly activated Wnt signaling in cancer may attenuate tumor spread, and resistance to therapy, while leaving normal tissues relatively undisturbed. Indeed, treatment with SAH-BCL9 peptide elicits mechanism-based anti-tumor responses in vitro as well as in mouse xenograft models of colorectal cancer and MM without detectable alterations in host tissues (10).
A link between miRNA-30c* and BCL9 was recently documented in ovarian carcinoma (41). MiR-30c* (also called miR-30c-3p) and miR-30c (also called miR-30c-5p) are related miRs processed from the same precursor but from different regions denoted 3p and 5p, respectively, with almost complementary sequences indicating they have different target genes (42). Using four different target prediction software algorithms we found that the 5p miR-30 family, but not the 3p complementary family, matched with the 3’UTR of BCL9 mRNA. Furthermore, our analysis of miRs and mRNA expression arrays in MM patient datasets showed that expression of the miR-30s-5p family, but not the miR-30s-3p family, is inversely associated with BCL9 mRNA levels.
MiR-30s also function as tumor suppressor genes in other cancers: miR-30s are downregulated in diffuse large B cell lymphoma where BCL6 gene was identified as a target (32); ectopic expression of miR-30s inhibits the self-renewal capacity of breast tumor-initiating cells by reducing Ubc9, and induces apoptosis by silencing integrin beta 3 (43); miR-30s regulate B-Myb expression, highlighting the pivotal role of miR-30s in Rb-driven cellular senescence (44). Interestingly, we didn’t find changes in the expression of BCL6, Ubc9, and B-Myb in MM cells overexpressing miR-30c or miR-30mix, in accordance with the notion that the target of miRs are tumor cell specific (45).
The small size of miRs makes them very attractive for drug development in multiple myeloma (46–48): miRs are natural antisense interactors and will not induce immune response (49), and the expression levels of specific miRs responds to physiological stimuli (50) compare with other Wnt inhitors (38). However, the success of miRs therapy has hold upon the development of suitable in vivo delivery systems. Because miR-30s plays its role as a tumor suppressor, and is expressed in all normal tissues, it is expected that replacement therapy will not affect normal cell function. In agreement with this scenario, we have observed that intraperitoneal delivery of miR-30mix using lipid nanoparticles increased survival in a murine xenograft model of human MM with lack of negative impact on normal tissues as it has been observed with other Wnt inhibitors (19, 38).
In conclusion, we have documented that downregulation of the tumor suppressor miR-30s is a frequent pathogenetic event in MM, and provide evidence for a model in which the interaction between MM cells and BMSCs decreases miR-30s levels in MM cells, which in turn enhances expression of BCL9, as a transcriptional co-activator of Wnt signaling pathway, promotes expression of downstream target genes involved in MM tumor cell proliferation, survival, migration, drug resistance and MM CSC (Fig. 7G). The potential for clinical translation of strategies using miR-30s as a novel therapeutic tool is further confirmed by the capacity of miR-30c and miR-30mix to reduce tumor burden and metastatic potential in vivo in three murine xenograft models of human MM.
Supplementary Material
Acknowledgments
We thank the animal, flow cytometry, and microscopy facilities at the Dana-Farber Cancer Institute for technical assistance.
Financial support:
J.J.Z was supported by a Multiple Myeloma Research Foundation (MMRF) research fellow award. J.L was supported by a SPORE in Multiple Myeloma Career Development Award. Z.B.C was supported by a China Scholarship Council (CSC) award. W.P.K was supported by Harvard Catalyst, The Harvard Clinical and Translational Science Center. K.C.A. is an American Cancer Society Clinical Research Professor. D.R.C. is supported by a MMRF senior award and a 1R01CA151391.
Footnotes
Disclosure of Potential Conflicts of Interest:
No Conflicts of Interest.
Reference
- 1.Rajkumar SV. Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. American journal of hematology. 2011;86:57–65. doi: 10.1002/ajh.21913. [DOI] [PubMed] [Google Scholar]
- 2.Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012;122:3456–3463. doi: 10.1172/JCI61188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 5.Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 6.Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Mol Cell. 2006;24:293–300. doi: 10.1016/j.molcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9:313–325. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Mani M, Carrasco DE, Zhang Y, Takada K, Gatt ME, Dutta-Simmons J, et al. BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res. 2009;69:7577–7586. doi: 10.1158/0008-5472.CAN-09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao JJ, Mani M, et al. Targeted disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med. 2012;4:148ra17. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiang YW, Endo Y, Rubin JS, Rudikoff S. Wnt signaling in B-cell neoplasia. Oncogene. 2003;22:1536–1545. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 12.Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta-Simmons J, Zhang Y, Gorgun G, Gatt M, Mani M, Hideshima T, et al. Aurora kinase A is a target of Wnt/beta-catenin involved in multiple myeloma disease progression. Blood. 2009;114:2699–2708. doi: 10.1182/blood-2008-12-194290. [DOI] [PubMed] [Google Scholar]
- 14.Willis TG, Zalcberg IR, Coignet LJ, Wlodarska I, Stul M, Jadayel DM, et al. Molecular cloning of translocation t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21. Blood. 1998;91:1873–1881. [PubMed] [Google Scholar]
- 15.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichiorri F, Suh SS, Rocci A, De Luca L, Taccioli C, Santhanam R, et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358:502–511. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 19.Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–2639. doi: 10.1182/blood-2009-09-243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, et al. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, et al. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 25.Smyth GK, Yang YH, Speed T. Statistical issues in cDNA microarray data analysis. Methods Mol Biol. 2003;224:111–136. doi: 10.1385/1-59259-364-X:111. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nature genetics. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 29.Walker BA, Leone PE, Chiecchio L, Dickens NJ, Jenner MW, Boyd KD, et al. A compendium of myeloma-associated chromosomal copy number abnormalities and their prognostic value. Blood. 2010;116:e56–e65. doi: 10.1182/blood-2010-04-279596. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Chen L, Barlogie B, Stephens O, Wu X, Williams DR, et al. High-risk myeloma is associated with global elevation of miRNAs and overexpression of EIF2C2/AGO2. Proc Natl Acad Sci U S A. 2010;107:7904–7909. doi: 10.1073/pnas.0908441107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Lwin T, Zhao JJ, Tam W, Choi YS, Moscinski LC, et al. Follicular dendritic cell-induced microRNA-mediated upregulation of PRDM1 and downregulation of BCL-6 in non-Hodgkin's B-cell lymphomas. Leukemia. 2011;25:145–152. doi: 10.1038/leu.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 35.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 36.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rask K, Nilsson A, Brannstrom M, Carlsson P, Hellberg P, Janson PO, et al. Wnt-signalling pathway in ovarian epithelial tumours: increased expression of beta-catenin and GSK3beta. British journal of cancer. 2003;89:1298–1304. doi: 10.1038/sj.bjc.6601265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polakis P. Drugging Wnt signalling in cancer. The EMBO journal. 2012;31:2737–2746. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 40.Deka J, Wiedemann N, Anderle P, Murphy-Seiler F, Bultinck J, Eyckerman S, et al. Bcl9/Bcl9l are critical for Wnt-mediated regulation of stem cell traits in colon epithelium and adenocarcinomas. Cancer Res. 2010;70:6619–6628. doi: 10.1158/0008-5472.CAN-10-0148. [DOI] [PubMed] [Google Scholar]
- 41.Jia W, Eneh JO, Ratnaparkhe S, Altman MK, Murph MM. MicroRNA-30c-2* expressed in ovarian cancer cells suppresses growth factor-induced cellular proliferation and downregulates the oncogene BCL9. Molecular cancer research : MCR. 2011;9:1732–1745. doi: 10.1158/1541-7786.MCR-11-0245. [DOI] [PubMed] [Google Scholar]
- 42.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29:4194–4204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 44.Martinez I, Cazalla D, Almstead LL, Steitz JA, DiMaio D. miR-29 and miR-30 regulate B-Myb expression during cellular senescence. Proc Natl Acad Sci U S A. 2011;108:522–527. doi: 10.1073/pnas.1017346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci U S A. 2006;103:2746–2751. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Martino MT, Gulla A, Cantafio ME, Lionetti M, Leone E, Amodio N, et al. In vitro and in vivo anti-tumor activity of miR-221/222 inhibitors in multiple myeloma. Oncotarget. 2013;4:242–255. doi: 10.18632/oncotarget.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leone E, Morelli E, Di Martino MT, Amodio N, Foresta U, Gulla A, et al. Targeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growth. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:2096–2106. doi: 10.1158/1078-0432.CCR-12-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Martino MT, Leone E, Amodio N, Foresta U, Lionetti M, Pitari MR, et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6260–6270. doi: 10.1158/1078-0432.CCR-12-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tili E, Michaille JJ, Gandhi V, Plunkett W, Sampath D, Calin GA. miRNAs and their potential for use against cancer and other diseases. Future Oncol. 2007;3:521–537. doi: 10.2217/14796694.3.5.521. [DOI] [PubMed] [Google Scholar]
- 50.Gu J, Iyer VR. PI3K signaling and miRNA expression during the response of quiescent human fibroblasts to distinct proliferative stimuli. Genome Biol. 2006;7:R42. doi: 10.1186/gb-2006-7-5-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.