Abstract
Objectives
To study whether hand osteoarthritis (OA) is associated with increased mortality and cardiovascular events in a large community based cohort (Framingham Heart Study) in which OA, mortality and cardiovascular events have been carefully assessed.
Methods
We examined whether symptomatic (≥1 joint (s) with radiographic OA and pain in the same joint) and radiographic hand OA (≥1 joint(s) with radiographic OA without pain) were associated with mortality and incident cardiovascular events (coronary heart disease, congestive heart failure and/or atherothrombotic brain infarction) using Cox proportional hazards models. In the adjusted models, we included possible confounding factors from baseline (eg, metabolic factors, medication use, smoking/alcohol). We also adjusted for the number of painful joints in the lower limb and physical inactivity.
Results
We evaluated 1348 participants (53.8% women) with mean (SD) age of 62.2 (8.2) years, of whom 540 (40.1%) and 186 (13.8%) had radiographic and symptomatic hand OA, respectively. There was no association between hand OA and mortality. Although there was no significant relation to incident cardiovascular events overall or a relation of radiographic hand OA with events, we found a significant association between symptomatic hand OA and incident coronary heart disease (myocardial infarction/coronary insufficiency syndrome) (HR 2.26, 95% CI 1.22 to 4.18). The association remained after additional adjustment for pain in the lower limb or physical inactivity.
Conclusions
Symptomatic hand OA, but not radiographic hand OA, was associated with an increased risk of coronary heart disease events. The results suggest an effect of pain, which may be a possible marker of inflammation.
INTRODUCTION
While the morbidity of osteoarthritis (OA) has been well characterised, we have limited knowledge of OA-related mortality. A recent review showed moderate evidence for an association between OA and mortality, especially for cardiovascular and gastrointestinal mortality.1 Some but not all studies included adjustment for potential confounding factors.
As cardiovascular disease remains the most common cause of mortality, any reduced survival in OA may be related to a greater risk of cardiovascular events. However, few studies have explored the possible association between OA and cardiovascular disease.2–7 Whereas some studies have suggested an association of hand OA with mortality, cardiovascular mortality or cardiovascular biomarkers, the findings within each study and across studies are inconsistent.2–6 Further, as different types of cardiovascular events may have different aetiologies, it would be valuable to explore whether OA is related to specific types of events. We are not aware of previous explorations of this question.
There may be shared risk factors that are associated with both OA and cardiovascular diseases, including immobilisation, metabolic factors, treatment with non-steroidal anti-inflammatory drugs (NSAIDs) and/or OA-related inflammation.8–10 Unlike knee and hip OA, hand OA is not likely leading to immobilisation, and increased mortality and cardiovascular events may therefore to a larger extent be related to metabolic factors or inflammation. One report suggested a stronger association with imaging biomarkers of cardiovascular disease in those with hand OA compared with those with OA-related knee/hip replacements.11 Alternatively, there may be direct associations between OA and vascular changes.12,13
Hence our aim was to investigate whether participants with hand OA have increased mortality and a greater incidence of cardiovascular events in a community based cohort under continuous surveillance for disease and mortality events, and to explore potential explanations for the observed associations.
METHODS
Participants
We included in the study participants from the Framingham Heart Study (Original and Offspring cohorts), aged 50–75 years, with available radiographs and information about joint pain enabling classification of hand OA, and data on mortality and cardiovascular events.
In the Original cohort, 5209 men and women, aged 30–62 years, from Framingham, Massachusetts, without overt symptoms of cardiovascular disease or previous heart attack or stroke, were examined in 1948–1953. Participants have been examined every second year up to 2008–2011 (examination cycle 31). At examination cycle 22 (1990–1994), the participants were examined for hand OA.
In the Offspring cohort, 5124 men and women, constituting the children of participants in the Original cohort and the spouses of these children, were examined in 1971–1975.14 They have been followed every fourth year (except for 8 years between examination cycle 1 and 2) up to 2011–present (examination cycle 9). At examination cycles 5 (1991–1995) and 7 (1998–2001), participants (whose parents were previously studied for OA) and their spouses were examined for hand OA.
Boston University Medical Center’s institutional review board approved both studies, and written informed consent was obtained.
Assessment of OA and joint pain
Bilateral posteroanterior hand radiographs were obtained at examination cycle 22 (Original cohort), and at examination cycles 5 and 7 (Offspring cohort). Radiographs were read by one of two academically based musculoskeletal radiologists (BS, PA). Bilateral 2nd–5th distal and proximal interphalangeal, thumb interphalangeal, 1st–5th metacarpophalangeal and thumb base (carpometacarpal/scaphotrapezial joint) were graded for OA (30 joints) using a modified Kellgren–Lawrence Scale.15,16
If the participants answered ‘yes’ to the question “On most days, do you have any pain, aching or stiffness in any of your joints?”, they were shown a homunculus and asked to indicate which joint(s) had complaints. We calculated the number of painful joints (range 0–8) in the lower limb, including the bilateral hips, knees, ankles and feet (five toes counted as one joint). The Framingham Physical Activity Index (PAI) was completed at examination cycles 20 (Original cohort) and 4 (Offspring cohort). Participants were divided into tertiles based on their PAI scores.
We were especially interested in symptomatic hand OA as these participants are more likely to seek medical care. To avoid having participants with non-symptomatic radiographic hand OA in our reference group, we divided participants into three categories based on their hand OA status at examination cycles 22 (Original cohort) and 5 (Offspring cohort); (1) symptomatic hand OA, (2) radiographic hand OA and (3) no symptomatic/ radiographic hand OA. Symptomatic hand OA was defined as ≥1 joint(s) with radiographic hand OA (Kellgren–Lawrence grade ≥2) and pain/aching/stiffness in the same joint(s) (30 joints assessed). Radiographic hand OA was defined as ≥1 joint (s) with radiographic OA (Kellgren–Lawrence grade ≥2) without pain/aching/stiffness in the same joint(s) (30 joints assessed).
Assessment of mortality, cardiovascular events and comorbidities
Participants were carefully followed for occurrence of cardiovascular events and death through hospital admission records, death certificates, death registries, medical records and periodic examinations. The diagnoses were arrived at by a panel of cardiologists and neurologists using published criteria. We had available data on mortality and cardiovascular events at year end 2009 and 2011, respectively.
Mortality
Overall mortality included mortality related to cardiovascular events, cancer and unknown/unspecified causes.
Cardiovascular events
Incident cardiovascular events included incident coronary heart disease (coronary insufficiency syndrome/myocardial infarction), congestive heart failure and/or atherothrombotic brain infarction (table 1).
Table 1.
Detailed description of outcomes of interest and covariates in the analyses
| Mortality | |
| Overall mortality | Mortality related to cardiovascular events, cancer and mortality due to unknown/unspecified causes |
| Cardiovascular events | |
| Coronary insufficiency syndrome | History of ≥15 min chest pain accompanied by transient ischaemic changes in the ECG, but without changes in serum biomarkers of myocardial necrosis |
| Recent/acute myocardial infarction (MI) | ≥2 of 3 findings; (1) typical symptoms, (2) changes in serum biomarkers and/or (3) ECG indicating myocardial infarction. Autopsy showing new/recent MI was also accepted as evidence |
| Congestive heart failure (CHF) | Minimum of two major or one major and two minor criteria present concurrently |
| Major criteria: (1) paroxysmal nocturnal dyspnoea or orthopnoea; (2) distended neck veins (other than supine position); (3) rales; (4) increasing heart size by x-ray; (5) acute pulmonary oedema on x-ray; (6) ventricular S(3) gallop; (7) increased venous pressure>16 cm H2O; (8) hepatojugular reflux; (9) pulmonary oedema, visceral congestion, cardiomegaly shown on autopsy; (10) weight loss on CHF Rx: 10 lbs/5 days | |
| Minor criteria: (1) bilateral ankle oedema; (2) night cough; (3) dyspnoea on ordinary exertion; (4) hepatomegaly; (5) pleural effusion by x-ray; (6) decrease in vital capacity by 1/3 from maximum record; (7) tachycardia (≥120 beats per minute); (8) pulmonary vascular engorgement on x-ray | |
| Atherothrombotic brain infarction | Sudden/rapid onset of focal neurological deficit>24 h without known sources of embolism, intracranial haemorrhage, known hypercoagulable states or other disease processes causing focal neurological deficits |
| Covariates | |
| Body mass index (kg/m2) | Weight (kg) and height (m) measured after standardised procedures |
| Blood glucose | Non-fasting glucose of ≥200 mg/dL (≥11.1 mmol/L) or fasting glucose of ≥126 mg/dL (≥ 7.0 mmol/L) |
| Lipid profile | Total cholesterol:high density lipoprotein ratio (used as continuous scale in the analyses) |
| Hypertension | Elevated blood pressure (systolic ≥160 mmHg and/or diastolic ≥95 mmHg) measured by two physicians |
| Previous cardiovascular events | Previous coronary insufficiency syndrome, MI, CHF and atherothrombotic stroke, as described above (ie, the diagnoses were arrived at by a panel of cardiologists and neurologists) |
| Previous self-reported cancer | Previous cancer reported by the patient in a clinical interview |
| Lipid lowering treatment | Current use of resins, niacin or nicotinic acid, fibrates, statins and/or other anticholesterol drugs assessed in a clinical interview |
| Antihypertensives | Currently receiving medication for the treatment of hypertension assessed in a clinical interview |
| Antidiabetics | Current use of insulin and oral hypoglycaemics assessed in a clinical interview |
| Non-steroidal anti-inflammatory drugs | Current use of non-steroidal anti-inflammatory agents (Motrin, ibuprofen, Indocin, Clinoril) assessed in a clinical interview |
| Aspirin | Daily use of aspirin (≥6 days per week) assessed in a clinical interview |
| Alcohol | Alcohol consumption (yes/no) assessed in a clinical interview |
| Smoking (current and previous) | Regularly smoking of cigarettes the last year and smoking of cigars and pipes assessed in a clinical interview (examination cycles 1–5 for Offspring and examination cycles 17–22 for Original cohort) |
Covariates
Body mass index (BMI), elevated blood glucose,19 lipid profile, hypertension, previous self-reported cancer, medications (lipid lowering treatment, antihypertensives, antidiabetics, NSAIDs and aspirin) and alcohol were assessed at examination cycles 22 (Original cohort) and 5 (Offspring cohort). As covariates, we also included self-reported smoking status at examination cycles 17–22 (Original cohort) and 1–5 (Offspring cohort) in order to capture previous smoking. Previous cardiovascular events had been adjudicated by a panel of physicians (table 1).
Statistical analyses
We calculated mortality/morbidity rates (95% CI) per 1000 person-years. We determined the number of person-years in the analyses by adding the years from assessment of hand OA (examination cycles 22 and 5 for the Original and Offspring cohorts, respectively) until event of interest/death or censoring for all participants.
We examined whether symptomatic/radiographic hand OA were associated with mortality and incident cardiovascular events using Cox proportional hazards models (IBM SPSS Statistics V.20, SAS V.9.2). Participants with previous events of interest were excluded from the analyses. Analyses were adjusted for age, sex, cohort, body mass index, comorbidities and lifestyle factors. We also performed analyses with additional adjustment for the number of painful joints in the lower limb and tertiles of the PAI score. In the main analyses, those with incident hand OA at examination cycle 7 were characterised as no OA (based on examination cycle 5 status). We repeated the analyses excluding participants with incident hand OA at examination cycle 7 from the no OA group (n=244), and also carried out analyses using age as the primary time scale.20 Appropriateness of the multivariable models was checked by testing the assumption of proportionality of hazards.
To explore possible dose–response relationships, we examined whether bilateral OA showed stronger associations than unilateral OA, and whether the associations got stronger with increasing number of affected joints and with increasing Kellgren– Lawrence sum score.
We excluded participants below 50 or above 75 years of age. Those below 50 years had few events and the majority had no hand OA. Among those over 75 years of age, hand OA was almost universal (see online supplementary table S1).
RESULTS
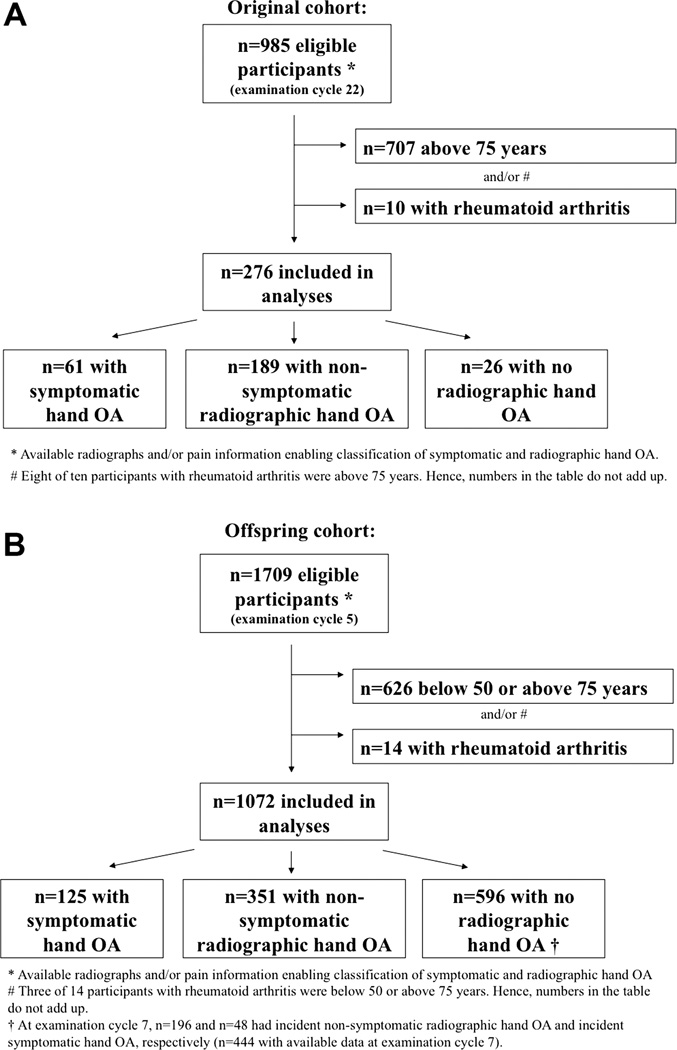
In the Original cohort, 5209 participants were enrolled at examination cycle 1, of whom 1166 (22.4%) attended examination cycle 22. Hand OA status at examination cycle 22 was available for 985 participants, and 276 met our inclusion criteria (figure 1). In the Offspring cohort, 5124 participants were enrolled at examination cycle 1, of whom 3799 (74.1%) and 3539 (69.1%) attended examination cycles 5 and 7, respectively. Hand OA status at examination cycle 5 was available for 1709 participants, and 1072 met our inclusion criteria (figure 1).
Figure 1.
Flowcharts for the Original (A) and Offspring (B) cohorts.
OA, osteoarthritis.
Participants with hand OA were older and there was a higher proportion of women. Further, previous cancer, hypertension, elevated blood glucose, use of antihypertensives, antidiabetic treatment, NSAIDs and aspirin were more frequent in participants with hand OA (table 2).
Table 2.
Baseline characteristics of participants included in the study
| Hand OA status |
||||
|---|---|---|---|---|
| Overall (n=1348) | No hand OA (n=622) |
Radiographic OA (n=540) |
Symptomatic OA (n=186) |
|
| Age (years) (mean (SD)) | 62.2 (8.2) | 57.6 (6.4) | 66.1 (7.6) | 66.6 (7.1) |
| Sex (n (%) female) | 725/1348 (53.8) | 303/622 (48.7) | 284/540 (74.2) | 138/186 (74.2) |
| BMI (kg/m2) (mean (SD)) | 27.6 (4.6) (n=1347) | 27.4 (4.5) | 27.8 (4.9) | 28.0 (4.4) |
| No of symptomatic hand OA joints (median (IQR)) (range 0–30) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 2 (1, 4) |
| No of joints with radiographic OA (range 0–30) | 1 (0, 5) | 0 (0, 0) | 4 (2, 7) | 8 (3, 13) |
| No of painful finger joints (range 0–30) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) | 6 (2, 11) |
| No of painful joints in lower limb (median (IQR)) (range 0–8) | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) |
| Comorbidities and risk factors | ||||
| Increased blood pressure (n (%)) | 98/1346 (7.3) | 31/622 (5.0) | 48/538 (8.9) | 19/186 (10.2) |
| Total cholesterol:HDL ratio, mean (SD) | 4.6 (1.5) | 4.6 (1.6) | 4.5 (1.4) | 4.5 (1.4) |
| Increased fasting/non-fasting blood glucose (n (%)) | 101/1338 (7.5) | 38/619 (6.1) | 49/536 (9.1) | 14/183 (7.7) |
| Self-reported previous cancer (n (%)) | 282/1342 (21.0) | 114/622 (18.3) | 120/536 (22.4) | 48/184 (26.1) |
| Smoking (current/previous) (n (%)) | 605/1348 (44.9) | 316/622 (50.8) | 219/540 (40.6) | 70/186 (37.6) |
| Alcohol consumption (n (%)) | 887/1347 (65.7) | 425/621 (68.4) | 343/540 (63.5) | 119/186 (64.0) |
| Low physical activity (n (%)) | 414/1252 (33.3) | 177/577 (30.7) | 174/496 (35.1) | 63/171 (36.8) |
| Previous events (before examination cycle 22 in the Original cohort and before examination cycle 5 in the Offspring cohort) | ||||
| Cardiovascular events (n (%)) | 92/1348 (6.8) | 37/622 (5.9) | 40/540 (7.4) | 15/186 (8.1) |
| Coronary heart disease (n (%)) | 75/1348 (5.6) | 31/622 (5.0) | 32/540 (5.9) | 12/186 (6.5) |
| Congestive heart failure (n (%)) | 19/1348 (1.4) | 5/622 (0.8) | 10/540 (1.9) | 4/186 (2.2) |
| Atherothrombotic brain infarction (n (%)) | 13/1348 (1.0) | 6/622 (1.0) | 5/540 (0.9) | 2/186 (1.1) |
| Medication | ||||
| Current antihypertensive treatment (n (%)) | 371/1338 (27.7) | 123/621 (19.8) | 174/534 (33.3) | 70/183 (38.3) |
| Current lipid lowering treatment (n (%)) | 139/1348 (10.3) | 59/622 (9.5) | 53/540 (9.8) | 27/186 (14.5) |
| Current antidiabetic treatment (oral/insulin) (n (%)) | 52/1348 (3.9) | 16/622 (2.6) | 25/540 (4.6) | 11/186 (5.9) |
| Current NSAIDs (n (%)) | 163/1347 (12.1) | 66/621 (10.6) | 62/540 (11.5) | 35/186 (18.8) |
| Daily aspirin (n (%)) | 258/1333 (19.4) | 87/616 (14.1) | 126/533 (23.6) | 45/184 (24.5) |
BMI, body mass index; HDL, high density lipoprotein; NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis.
Mortality
We observed 454 deaths, of which 243 and 75 occurred in participants with radiographic and symptomatic hand OA, respectively. Deaths per 1000 person-years were higher in participants with hand OA (table 3). However, those with hand OA were older than hand OA free participants. In the adjusted analyses (in which age was the main confounder), there was no significant association between hand OA and mortality (table 4). Male sex, age, elevated blood glucose, antihypertensive treatment, smoking, cancer and previous cardiovascular events were associated with higher mortality, whereas alcohol was associated with lower mortality (data not shown). When we excluded Offspring participants with incident hand OA at examination cycle 7 from our reference group (figure 1), we found a statistically significant lower risk of mortality associated with hand OA (table 5). Similar results in adjusted analyses were found using age as the primary time scale (data not shown).
Table 3.
Deaths and events per 1000 person-years
| Hand OA status |
||||
|---|---|---|---|---|
| Overall (n=1348) | No hand OA (n=622) |
Radiographic hand OA (n=540) |
Symptomatic hand OA (n=186) |
|
| Survival time (time to death or censoring) (years) (median (IQR)) | 16.0 (14.4, 16.9) | 16.2 (14.8, 17.0) | 15.7 (13.1, 16.8) | 15.7 (14.0, 16.6) |
| Deaths per 1000 person-years (95% CI) | ||||
| Overall deaths | 23 (21,25) | 14 (12, 17) | 32 (28, 36) | 28 (22, 34) |
| Cardiovascular deaths (coronary heart disease, cerebrovascular accidents, other) | 6 (5, 7) | 3 (2, 5) | 8 (6, 10) | 9 (5, 12) |
| Deaths due to cancer | 7 (6, 8) | 6 (4, 8) | 8 (6, 10) | 7 (4, 10) |
| Deaths due to unknown/other causes | 10 (9, 11) | 5 (4, 6) | 15 (13, 18) | 12 (8, 16) |
| Events in those with no previous events per 1000 person-years (95% CI) | ||||
| Cardiovascular events | 14 (12, 16) | 8 (6, 10) | 18 (15, 21) | 20 (15, 27) |
| Coronary heart disease | 6 (5, 8) | 4 (3, 5) | 8 (6, 11) | 10 (6, 14) |
| Congestive heart failure | 8 (7, 9) | 4 (3, 6) | 11 (9, 13) | 13 (9, 18) |
| Atherothrombotic brain infarction | 3 (2, 4) | 2 (1, 3) | 4 (2, 5) | 5 (2, 7) |
OA, osteoarthritis.
Table 4.
HR (95% CI) of mortality and cardiovascular events in participants with radiographic and symptomatic hand osteoarthritis compared with those with no hand osteoarthritis as reference
| Crude model | Adjusted model 1* | Adjusted model 2† | |
|---|---|---|---|
| Mortality | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 2.32 (1.88–2.86); p<0.001 | 0.79 (0.61–1.01); p=0.06 | 0.82 (0.63–1.07); p=0.14 |
| Symptomatic hand OA | 2.07 (1.56–2.75); p<0.001 | 0.75 (0.55–1.03); p=0.08 | 0.79 (0.57–1.10); p=0.16 |
| Crude model | Adjusted model 1* | Adjusted model 2‡ | |
| Events (in participants with no previous events of interest) | |||
| Cardiovascular events | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 2.25 (1.68–3–02); p<0.001 | 1.04 (0.74–1.46); p=0.83 | 1.02 (0.72–1.45); p=0.90 |
| Symptomatic hand OA | 2.59 (1.80–3.73); p<0.001 | 1.25 (0.83–1.89); p=0.28 | 1.32 (0.87–2.03); p=0.19 |
| Coronary heart disease | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 2.19 (1.44–3.34); p<0.001 | 1.44 (0.89–2.35); p=0.14 | 1.60 (0.96–2.66); p=0.07 |
| Symptomatic hand OA | 2.56 (1.52–4.32); p<0.001 | 1.89 (1.04–3.41); p=0.04 | 2.26 (1.22–4.18); p=0.009 |
| Congestive heart failure | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 2.66 (1.82–3.89); p<0.001 | 0.92 (0.59–1.44); p=0.72 | 0.89 (0.56–1.41); p=0.63 |
| Symptomatic hand OA | 3.25 (2.06–5.14); p<0.001 | 1.18 (0.71–1.98); p=0.52 | 1.23 (0.71–2.13); p=0.45 |
| Atherothrombotic stroke | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 1.84 (1.03–3.30); p=0.04 | 0.82 (0.43–1.58); p=0.56 | 0.73 (0.37–1.43); p=0.36 |
| Symptomatic hand OA | 2.28 (1.11–4.71); p=0.03 | 1.04 (0.47–2.31); p=0.91 | 0.99 (0.44–2.26); p=0.99 |
Adjusted for age, sex, cohort and BMI.
Adjusted for age, sex, cohort, BMI, total cholesterol:HDL ratio, current lipid lowering treatment, increased blood pressure, current antihypertensive treatment, elevated fasting or non-fasting blood glucose, current antidiabetic treatment (oral or insulin), previous cardiovascular events (coronary heart disease, congestive heart failure, atherothrombotic stroke), previous cancer, current use of NSAIDs, daily use of aspirin, current/previous smoking, alcohol use.
Adjusted for age, sex, cohort, BMI, total cholesterol:HDL ratio, current lipid lowering treatment, increased blood pressure, current antihypertensive treatment, elevated fasting or non-fasting blood glucose, current antidiabetic treatment (oral or insulin), current use of NSAIDs, daily use of aspirin, current/previous smoking, alcohol use.
BMI, body mass index; HDL, high density lipoprotein; NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis.
Table 5.
HR (95% CI) of mortality and cardiovascular events in those with radiographic and symptomatic hand osteoarthritis compared with those without hand osteoarthritis
| Crude model | Adjusted model 1* | Adjusted model 2† | |
|---|---|---|---|
| Mortality | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 1.53 (1.22–1.90); p<0.001 | 0.58 (0.45–0.74); p<0.001 | 0.62 (0.48–0.81); p<0.001 |
| Symptomatic hand OA | 1.36 (1.02–1.82); p=0.04 | 0.55 (0.40–0.76); p<0.001 | 0.60 (0.43–0.84); p=0.003 |
| Crude model | Adjusted model one* | Adjusted model two‡ | |
| Events (in participants with no previous events of interest) | |||
| Cardiovascular events | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 1.85 (1.33–2.58); p<0.001 | 0.86 (0.59–1.24); p=0.42 | 0.85 (0.58–1.25); p=0.42 |
| Symptomatic hand OA | 2.12 (1.42–3.16); p<0.001 | 1.01 (0.65–1.58); p=0.95 | 1.08 (0.68–1.71); p=0.76 |
| Coronary heart disease | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 2.02 (1.23–3.33); p<0.001 | 1.31 (0.75–2.29); p=0.34 | 1.42 (0.79–2.52); p=0.24 |
| Symptomatic hand OA | 2.36 (1.31–4.24); p<0.001 | 1.69 (0.88–3.25); p=0.12 | 1.98 (1.00–3.89); p=0.05 |
| Congestive heart failure | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 2.29 (1.47–3.56); p<0.001 | 0.88 (0.54–1.44); p=0.61 | 0.85 (0.51–1.42); p=0.54 |
| Symptomatic hand OA | 2.79 (1.67–4.65); p<0.001 | 1.12 (0.63–1.96); p=0.70 | 1.16 (0.64–2.09); p=0.63 |
| Atherothrombotic stroke | |||
| No hand OA | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Radiographic hand OA | 1.33 (0.71–2.49); p=0.37 | 0.61 (0.30–1.20); p=0.15 | 0.56 (0.28–1.14); p=0.56 |
| Symptomatic hand OA | 1.65 (0.77–3.52); p=0.20 | 0.75 (0.33–1.72); p=0.50 | 0.74 (0.32–1.74); p=0.49 |
| Offspring participants with incident radiographic and symptomatic hand OA identified at examination 7 were excluded from the reference group (n=244). | |||
Adjusted for age, sex, cohort and BMI.
Adjusted for age, sex, cohort, BMI, total cholesterol:HDL ratio, current lipid lowering treatment, increased blood pressure, current antihypertensive treatment, elevated fasting or non-fasting blood glucose, current antidiabetic treatment (oral or insulin), previous cardiovascular events (coronary heart disease, congestive heart failure, atherothrombotic stroke), previous cancer, current use of NSAIDs, daily use of aspirin, current/previous smoking, alcohol use.
Adjusted for age, sex, cohort, BMI, total cholesterol:HDL ratio, current lipid lowering treatment, increased blood pressure, current antihypertensive treatment, elevated fasting or non-fasting blood glucose, current antidiabetic treatment (oral or insulin), current use of NSAIDs, daily use of aspirin, current/previous smoking, alcohol use.
BMI, body mass index; HDL, high density lipoprotein; NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis.
Cardiovascular events
Among those with no previous cardiovascular events, 242 incident events were observed, of which 122 and 48 occurred in participants with radiographic and symptomatic hand OA, respectively. Incident coronary heart disease (n=117) and congestive heart failure (n=155) occurred more frequently than atherothrombotic brain infarction (n=59).
Cardiovascular events per 1000 person-years were more frequent in participants with hand OA (table 3). There was a trend, although not statistically significant in multivariable analyses, towards an association between symptomatic hand OA and cardiovascular events (table 4). However, a statistically significant association was found between symptomatic hand OA and coronary heart disease, whereas the association was borderline statistically significant for radiographic hand OA (table 4). Male sex, age, antidiabetic treatment and smoking were also associated with coronary heart disease (smoking borderline statistically significant) (data not shown). When we excluded Offspring participants with incident hand OA at examination cycle 7 from the analyses (figure 1), we found similar results (table 5). No significant associations were found between hand OA and congestive heart failure or atherothrombotic stroke (tables 4 and 5). Similar results were found when using age as the primary time scale (data not shown).
In multivariable analyses, we found similar strengths of associations with coronary heart disease after additional adjustment for PAI score (radiographic hand OA: HR 1.51, 95% CI 0.88 to 2.60, symptomatic hand OA: HR 2.35, 95% CI 1.24 to 4.46). The association with coronary heart disease was stronger after additional adjustment for painful joints in the lower limbs (radiographic hand OA: HR 2.00, 95% CI 0.96 to 4.15, symptomatic hand OA: HR 3.25, 95% CI 1.42 to 7.43). Neither PAI score nor painful joints in the lower limb were associated with coronary heart disease in the multivariable model (data not shown).
Similar magnitude of associations with coronary heart disease was found for participants with bilateral and unilateral hand OA, and the association did not get stronger with increasing number of affected joints or Kellgren–Lawrence sum score (data not shown). When we repeated our analyses including all participants (those below 50 and above 75 years), we found similar results with a significant association between symptomatic hand OA and coronary heart disease (data not shown). We found similar trends of associations to coronary heart disease in those below 50 years, but not above 75 years (see online supplementary table S1).
The validity of the multivariable models in table 4 was found to be satisfactory (data not shown).
DISCUSSION
In the community based study of Framingham, hand OA was not associated with increased mortality. However, participants with symptomatic hand OA were more likely to experience coronary heart disease.
We had the advantage of a long follow-up period, assessment of survival status and a thorough clinical examination, allowing us to study the association to mortality with adjustment for possible confounding/mediating factors. In adjusted analyses, we found a trend towards reduced mortality associated with hand OA (table 4), in contrast with a previous study by Nüesch et al investigating mortality in participants with knee/hip OA.21 Contrasting findings may be due to different study designs. Participants in the study by Nüesch et al were selected from general practices in the UK, and only those with pain were invited for a clinical examination and knee/hip radiography. Mortality of the study participants was compared against mortality of the general population. In the Framingham Study, both participants with and without OA were recruited from the general population, limiting the risk of selection bias. There may also be differences in mortality between participants with OA in the lower limb and hand OA, although the diseases may overlap. We did not investigate cardiovascular mortality separately as we have the impression that this is a specific but not a sensitive outcome in the Framingham Study. Specifically, in case of sudden death, participants were classified with ‘unknown cause’, although cardiovascular causes were most likely. We therefore used cardiovascular events instead of cardiovascular death as the outcome of interest.
Previous studies have shown associations between hand OA and imaging markers of atherosclerosis,36 which was confirmed in the current study examining cardiovascular events. Among cardiovascular events being adjudicated in the Framingham Study, we included ischemic events that were most objectively measured. More subjective diagnoses such as angina pectoris and intermittent claudication were not included. We observed a statistically significant increased risk of coronary heart disease in participants with symptomatic hand OA whereas no associations were found for atherothrombotic stroke or congestive heart failure (table 4). The conflicting results may be due to a variable contribution of different risk factors to individual cardiovascular disease outcomes. As an example, hypertension seemed to be a stronger risk factor for atherothrombotic stroke and congestive heart failure than for coronary heart disease (data not shown). These results are consistent with previous studies showing that hypertension is the single most important risk factor for stroke and a major risk factor for congestive heart failure together with coronary and valvular diseases.22,23 Approximately two-thirds of participants with incident congestive heart failure did not have coronary heart disease events (data not shown), suggesting different pathogenetic processes in the two diseases.
The underlying mechanisms behind the observed association between symptomatic hand OA and coronary heart disease remain speculative. Obesity, hyperlipidaemia, diabetes and hypertension are well known risk factors for coronary heart disease.24 Several studies have demonstrated an association between obesity and hand OA, suggesting that metabolic factors (adipokines) are involved in the OA pathophysiology.25 Adipokines, lipids and glucose may have direct negative effects on cartilage26,27 but clinical studies have shown conflicting results.8,9,28–30 One longitudinal study found that accumulation of metabolic syndrome components was significantly related to occurrence and progression of knee OA.31 A recent study by Hoeven et al found significant associations between imaging markers of atherosclerosis and progression of metacarpophalangeal OA, but the results were neither consistent for different markers of atherosclerosis nor significant for OA in other finger joints.3 NSAIDs are commonly used as pain management for OA and have been associated with cardiovascular disease.32,33 Hence both metabolic factors and specific OA treatment could potentially confound or mediate the association between symptomatic hand OA and coronary heart disease. However, the observed association remained statistically significant after adjustment for several metabolic factors and the use of NSAIDs (table 4). Whether the association is mediated through other metabolic factors not adjusted for in our analyses is unknown.
Alternatively, OA-related inflammation may be a risk factor for coronary heart disease. Systemic inflammation, like in rheumatoid arthritis, is associated with development of athero-sclerosis.34 A recent systematic review concluded that OA was not associated with C reactive protein serving as a proxy for systemic inflammation, but they did not specifically evaluate symptomatic hand OA.35 OA finger joints with pain may exhibit more synovitis than non-painful joints36 although the systemic effect is not yet known.
As hand OA is often a marker of generalised OA, the observed association between symptomatic hand OA and coronary heart disease could have been due to a more sedentary lifestyle associated with knee/hip OA. Nüesch et al found that walking disability was a major risk factor for mortality in patients with knee/hip OA,21 supporting this hypothesis. However, in the current study, neither pain in the lower limb nor low physical activity levels could explain the observed association between symptomatic hand OA and coronary heart disease, rather suggesting the importance of metabolic/systemic factors. These findings, which may provide important insights into shared disease mechanisms, need confirmation in other investigations.
We can only speculate why we observed an association between symptomatic hand OA and coronary heart disease on the one hand, but a trend towards lower mortality on the other. One reason may be the use of overall mortality as the outcome and not cardiovascular mortality specifically. Hence other diseases causing death such as cancer may drive the association to reduced overall mortality. Although this was not the aim of the study, we found an association between hand OA and reduced cancer-related mortality, which was statistically significant for radiographic hand OA and borderline statistically significant for symptomatic hand OA (data not shown).
Some limitations are worth mentioning. Even though there was no evidence for a dose dependent relationship (ie, number of OA joints, total OA score and bilateral vs unilateral disease), we showed that participants with symptomatic hand OA had a higher risk of coronary heart disease than those with radio-graphic (non-symptomatic) hand OA (table 4). We believe that hand OA severity does not necessarily depend on the number of joints affected. Symptomatic hand OA may represent a more severe stage than non-symptomatic hand OA, giving us some support for a dose dependent relationship. The PAI score was not completed at the examination cycle on which hand OA was diagnosed. Due to the explorative study design, we did not adjust for multiple testing. However, using the Bonferroni method, the association between symptomatic hand OA and coronary heart disease would remain statistically significant.
In conclusion, we found that symptomatic hand OA was associated with more coronary heart disease events. However, the link behind the observed association is not completely understood. Additional studies are needed to confirm these findings. Established risk factors for coronary heart disease, such as unfavourable metabolic profile, NSAIDs, pain in the lower limb or low physical activity, could not fully explain the observed association.
Supplementary Material
Acknowledgements
We wish to thank Piran Aliabadi and Burton Sack for reading the hand radiographs, and the study participants for their willingness to participate in the study.
Funding Supported by NIH AR47785 and HL-NO-025195 (NHLBI). IKH received funding from South-Eastern Norway Regional Health Authority, Anders Jahre′s fund and Nathalia and Knut Juul Christiansen′s foundation.
Footnotes
Contributors IKH: study design, analyses, interpretation of the data, drafting the article and final approval. VSR and DM: study design, critical revision of the article and final approval. TN, YZ and DTF: study design, interpretation of the data, critical revision of the article and final approval. JN and TY: analyses, critical revision of the article and final approval.
Competing interestsNone.
Ethics approval Boston University Medical Center′s institutional review board approved the studies.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Hochberg MC. Mortality in osteoarthritis. Clin Exp Rheumatol. 2008;26:120–124. [PubMed] [Google Scholar]
- 2.Cerhan JR, Wallace RB, el-Khoury GY, et al. Decreased survival with increasing prevalence of full-body, radiographically defined osteoarthritis in women. Am J Epidemiol. 1995;141:225–234. doi: 10.1093/oxfordjournals.aje.a117424. [DOI] [PubMed] [Google Scholar]
- 3.Hoeven TA, Kavousi M, Clockaerts S, et al. Association of atherosclerosis with presence and progression of osteoarthritis: the Rotterdam Study. Ann Rheum Dis. 2013;72:646–651. doi: 10.1136/annrheumdis-2011-201178. [DOI] [PubMed] [Google Scholar]
- 4.Haara MM, Heliövaara M, Kröger H, et al. Osteoarthritis in the carpometacarpal joint of the thumb Prevalence and associations with disability and mortality. J Bone Joint Surg Am. 2004;86:1452–1457. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Haara MM, Manninen P, Kröger H, et al. Osteoarthritis of finger joints in Finns aged 30 or over: prevalence, determinants, and association with mortality. Ann Rheum Dis. 2003;62:151–158. doi: 10.1136/ard.62.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson H, Helgadottir GP, Aspelund T, et al. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik study. Ann Rheum Dis. 2009;68:1696–1700. doi: 10.1136/ard.2008.096289. [DOI] [PubMed] [Google Scholar]
- 7.Nielen MM, van Sijl AM, Peters MJ, et al. Cardiovascular disease prevalence in patients with inflammatory arthritis, diabetes mellitus and osteoarthritis: a cross-sectional study in primary care. BMC Musculoskelet Disord. 2012;13:150. doi: 10.1186/1471-2474-13-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stürmer T, Sun Y, Sauerland S, et al. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J Rheumatol. 1998;25:1827–1832. [PubMed] [Google Scholar]
- 9.Stürmer T, Brenner H, Brenner RE, et al. Non-insulin dependent diabetes mellitus (NIDDM) and patterns of osteoarthritis The Ulm Osteoarthritis Study. Scand J Rheumatol. 2001;30:169–171. doi: 10.1080/030097401300162969. [DOI] [PubMed] [Google Scholar]
- 10.Kerola AM, Kauppi MJ, Kerola T, et al. How early in the course of rheumatoid arthritis does the excess cardiovascular risk appear? Ann Rheum Dis. 2012;71:1606–1615. doi: 10.1136/annrheumdis-2012-201334. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson H, Helgadottir GP, Aspelund T, et al. The presence of total knee or hip replacements due to osteoarthritis enhances the positive association between hand osteoarthritis and atherosclerosis in women: the AGES-Reykjavik study. Ann Rheum Dis. 2011;70:1087–1090. doi: 10.1136/ard.2010.144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conaghan PG, Vanharanta H, Dieppe PA. Is progressive osteoarthritis an atheromatous vascular disease? Ann Rheum Dis. 2005;64:1539–1541. doi: 10.1136/ard.2005.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh P, Cheras PA. Vascular mechanisms in osteoarthritis. Best Pract Res Clin Rheumatol Vol. 2001;15:693–709. doi: 10.1053/berh.2001.0188. [DOI] [PubMed] [Google Scholar]
- 14.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Niu J, Kelly-Hayes M, et al. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol. 2002;156:1021–1027. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 16.Haugen IK, Englund M, Aliabadi P, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: The Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70:1581–1586. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson PW, Paffenbarger RS, Jr, Morris JN, et al. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J. 1986;111:1177–1192. doi: 10.1016/0002-8703(86)90022-0. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Kannel WB, Dawber TR, et al. An evaluation of follow-up methods in the Framingham Heart Study. Am J Public Health Nations Health. 1967;57:1015–1024. doi: 10.2105/ajph.57.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 21.Nüesch E, Dieppe P, Reichenbach S, et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:1165. doi: 10.1136/bmj.d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubow J, Fink ME. Impact of hypertension on stroke. Curr Atheroscler Rep. 2011;13:298–305. doi: 10.1007/s11883-011-0187-y. [DOI] [PubMed] [Google Scholar]
- 23.Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev. 2012;17:581–588. doi: 10.1007/s10741-011-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jousilahti P, Vartiainen E, Tuomilehto J, et al. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–1172. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 26.Masuko K, Murata M, Suematsu N, et al. A metabolic aspect of osteoarthritis: lipid as a possible contributor to the pathogenesis of cartilage degradation. Clin Exp Rheumatol. 2009;27:347–353. [PubMed] [Google Scholar]
- 27.Rosa SC, Gonçalves J, Judas F, et al. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther. 2009;11:R80–R80. doi: 10.1186/ar2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuo Q, Yang W, Chen J, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8:729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 29.Frey MI, Barrett-Connor E, Sledge PA, et al. The effect of noninsulin dependent diabetes mellitus on the prevalence of clinical osteoarthritis A population based study. J Rheumatol. 1996;23:716–722. [PubMed] [Google Scholar]
- 30.Al-Arfaj AS. Radiographic osteoarthritis and serum cholesterol. Saudi Med J. 2003;24:745–747. [PubMed] [Google Scholar]
- 31.Yoshimura N, Muraki S, Oka H, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage. 2012;11:1217–1226. doi: 10.1016/j.joca.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH, Avorn J, Stürmer T, et al. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006;54:1378–1389. doi: 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- 33.Farkouh ME, Greenberg JD, Jeger RV, et al. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66:764–770. doi: 10.1136/ard.2006.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John H, Kitas G. Inflammatory arthritis as a novel risk factor for cardiovascular disease. Eur J Intern Med. 2012;23:575–579. doi: 10.1016/j.ejim.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Vlad SC, Neogi T, Aliabadi P, et al. No association between markers of inflammation and osteoarthritis of the hands and knees. J Rheumatol. 2011;38:1665–1670. doi: 10.3899/jrheum.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haugen IK, Bøyesen P, Slatkowsky-Christensen B, et al. Associations between MRI-defined synovitis, bone marrow lesions and structural features and measures of pain and physical function in hand osteoarthritis. Ann Rheum Dis. 2012;71:899–904. doi: 10.1136/annrheumdis-2011-200341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.