Abstract
Nicotiana benthamiana often displays more intense symptoms after infection by RNA viruses than do other Nicotiana species. Here, we examined the role of RNA-dependent RNA polymerases (RdRPs) in N. benthamiana antiviral defense. cDNAs representing only two genes encoding RdRPs were identified in N. benthamiana. One RdRP was similar in sequence to SDE1/SGS2 required for maintenance of transgene silencing, whereas the second, named NbRdRP1m, was >90% identical in sequence to the salicylic acid (SA)-inducible RdRP from Nicotiana tabacum required for defense against viruses. NbRdRP1m expression was induced by SA treatment or challenge with Tobacco mosaic virus, but the gene and transcript sequences differed from those of other SA-inducible RdRPs in that they contained a 72-nt insert with tandem in-frame stop codons in the 5′ portion of the ORF. N. benthamiana plants transformed with an SA-inducible RdRP gene from Medicago truncatula were more resistant to infection by Tobacco mosaic virus, Turnip vein-clearing virus, and Sunn hemp mosaic virus (members of Tobamovirus genus), but not to Cucumber mosaic virus and Potato virus X (members of different genera than the tobamoviruses). Our results indicate that N. benthamiana lacks an active SA- and virus-inducible RdRP and thus is hypersusceptible to viruses normally limited in their accumulation by this RdRP. These findings are significant for those studying virus-induced gene silencing, the hypersensitive response and systemic acquired resistance.
RNA silencing constitutes an important host response against the inappropriate expression of foreign RNAs and transposable elements (1). Host RNA-dependent RNA polymerases (RdRPs) were among the first enzymes to be identified in plant, nematode, and fungal RNA-silencing pathways (2–4). Genetic screens identified RdRP homologs necessary for RNA silencing in Neurospora crassa (QDE-1) (5), Caenorhabditis elegans (EGO-1 and RRF-1) (6, 7), and Dictyostelium (RrpA) (8). Mutant Arabidopsis plants deficient in RNA silencing contain a modified gene (sde1/sgs2) (9, 10) with sequence similarity to an RdRP previously identified and cloned from Lycopersicon esculentum (11). However, the RdRP from L. esculentum has not been shown to influence RNA silencing in vivo. Evidence of polymerase activity from an RdRP known to influence RNA silencing was reported recently for QDE-1 from N. crassa (12).
RdRPs were first implicated in regulating virus accumulation in plants through the analysis of the sgs2 mutant Arabidopsis line (10). Loss of SGS2 function correlates with the greater susceptibility of these plants to infection by Cucumber mosaic virus (CMV). However, the loss of SGS2 or SDE1, which is identical to SGS2, does not alter the susceptibility of Arabidopsis to Turnip vein-clearing virus (TVCV), Turnip mosaic virus,or Tobacco rattle virus (TRV) (9,10). It was speculated that some viruses such as CMV only partially defeat RNA silencing, whereas other viruses such as TVCV and TRV fully defeat the silencing system in Arabidopsis (10). Antisense-mediated inhibition of a salicylic acid (SA)-inducible RdRP in Nicotiana tabacum (NtRdRP1) yielded plants more susceptible to Tobacco mosaic virus (TMV) and Potato virus X (PVX) infection (13). In other studies with N. tabacum, local accumulation of these viruses was inhibited by SA treatment (14, 15). More recently, an Arabidopsis thaliana mutant containing a defective SA-inducible RdRP similar in sequence to NtRdRP1 (i.e. AtRdRP1) was shown to be more susceptible to a crucifer tobamovirus related to TMV (TMV-cg, a strain of TVCV) and TRV than were wild-type plants (16). The greater susceptibility of plants lacking SA-inducible RdRPs to viruses unaffected in accumulation in plants lacking the SDE1/SGS2 RdRP suggested that there are important mechanistic differences for RNA silencing of different viruses (16). In addition, the SA-inducible character of some of the RdRPs points to a possible relationship between RNA silencing and the SA-inducible defense system active against some viruses (17–19). The identification of additional mutants defective in genes associated with RNA silencing would be helpful to further determine the function of individual RdRPs in RNA silencing and the relationship between the RNA silencing and SA-induced defense pathways in plants.
Nicotiana benthamiana is one of 64 species within the genus Nicotiana and is remarkable, when compared with other members of the genus, because of its extreme susceptibility to certain viruses, which can lead to the death of the plant (20, 21). The cause of the hypersusceptibility to virus infection by this species compared with other Nicotiana species has not been determined.
Here, we report characteristics of RdRPs from N. benthamiana and their potential impact on virus resistance and gene silencing.
Materials and Methods
Plants and Viruses. N. benthamiana was grown as described (22). Purified TMV, U1 strain, and a mutant of the “masked” TMV strain, MIC1,3,6, were used (23, 24). TVCV and PVX inocula were progeny from infectious transcripts of cDNA clones described previously (25, 26). Sunn hemp mosaic virus (SHMV) and Fny strain of CMV were obtained from C. M. Deom (27) and M. Roossinck (Samuel Roberts Noble Foundation, Inc.), respectively.
Isolation of RdRP Genes from N. benthamiana and Medicago truncatula. Primers RP1 (5′-CACCATGCAAAGTTTATTTTTGTGGTCCAGA-3′) and RP2 (5′-GCCCGGAAAGTTTGCAGCATCATTGAAAGAAA-3′) were used to isolate RdRPs from N. benthamiana genomic DNA by PCR. The primers are identical or complementary to sequences conserved in genes encoding RdRPs from N. tabacum (GenBank accession no. AJ011576) and M. truncatula (S.-J.Y. and R.S.N., unpublished data). An amplified ≈1.2-kb fragment was used as template to prepare an RdRP-specific probe by random-primed labeling with 32P (Ready-To-Go DNA labeling beads, Amersham Pharmacia–Pharmacia Biotech). cDNA libraries from N. benthamiana sink leaves were screened for clones containing RdRP sequences with this probe. The 5′ sequence of clones containing RdRP sequences was determined by 5′-RACE with the gene-specific primer nbRdRPR1 (5′-CACAACACCTTTATATCCACCATAAC-3′) following manufacturer protocol (Smart RACE, CLONTECH).
An M. truncatula RdRP cDNA clone (MtRdRP1) was obtained through blastx analysis of an EST database representing a nodulated root M. truncatula cDNA library (28).
Transformation of N. benthamiana with MtRdRP1. A full-length cDNA fragment of MtRdRP1 was cloned into pRTL2 (29) previously digested with NcoI, blunted with Klenow, and digested with KpnI. Vector-derived sequence between the promoter and 5′ UTR was removed, and the 35S:MtRdRP1 sequence was released by PstI digestion and then ligated into the binary vector pCAMBIA 2300 (CAMBIA, Canberra, Australia) previously digested with PstI. The resulting plasmid, pCamtRdRP1, was electroporated into Agrobacterium tumefaciens (strain LBA4404) for leaf disk transformation following standard protocols (30) with minor modification. Specifically, shooting and rooting media were modified to contain 100 mM 1-naphthaleneacetic acid or β-indolylacetonitrile (equally effective).
Recombinant PVX Construction. Two cDNA fragments from the 5′ or 3′ portions of NbRdRP1m (5′, nucleotides 1200–2239; 3′, nucleotides 2600–3174) were ligated in sense or antisense orientation into pPVX201 (26) previously digested with SalI and made blunt with Klenow. N. benthamiana seedlings at the four- to six-leaf stage were inoculated by particle bombardment using a Helios gene gun system (Bio-Rad) as described (31).
Southern Analysis. Fifteen micrograms of genomic DNA from N. benthamiana leaves per reaction was digested with various enzymes that cut once near either the 5′ or 3′ border of the probed region. DNA separation, transfer, and membrane probing, with a 32P-labeled fragment of NbRdRP1m prepared by random-primed labeling, were performed by using standard protocols (32).
Northern Analysis. Total RNA was extracted from young leaves by using TRIzol Reagent (Invitrogen). Northern blot analysis was performed following standard protocols (32). Membranes were probed overnight with 32P-labeled DNA or RNA probes complementary to MtRdRP1, TMV, TVCV, or CMV (RNA 2) sequences. Random-primed labeling was used for DNA probe preparation, and the MAXIscript kit and procedure (Ambion, Austin, TX) was used for RNA probe preparation. cDNA representing TVCV (pTVCV50) was obtained from U. Melcher (Oklahoma State University, Stillwater) (25). cDNA representing RNA 2 of the Fny strain of CMV was obtained from M. Roossinck (33).
ELISAs. For double-antibody sandwich ELISAs, leaf tissue was ground in liquid nitrogen followed by the addition of 4 vol (vol/vol) of PBS buffer (0.14 M NaCl/10 mM phosphate buffer, pH 7.4/3 mM KCl). Virus detection was performed by using antibodies against PVX or CMV following the protocol provided by the antibody supplier (compound direct ELISA, Agdia, Elkhart, IN). For indirect ELISAs, ground leaf tissue was diluted with 4 vol of PBS buffer before binding to microtiter plates. Indirect ELISAs were performed by using antibody against TVCV, obtained from U. Melcher (34), and a procedure from a supplier (indirect ELISA, Agdia). Antibody dilution was 1:1,000. Washed plates containing the bound antigen–antibody complex were incubated with goat anti-rabbit IgG (Fc) conjugated with alkaline phosphatase (Promega).
Real-Time RT-PCR Analysis. Total RNA extracted from tissue was treated with DNase I, followed by phenol/chloroform extraction. First-strand cDNA was synthesized by using SuperScript RNaseH reverse transcriptase following manufacturer protocol (Invitrogen). Oligo(dT)12–18 was used as primer, and treated and extracted RNA (2–3 μg) was used as template. Primers specific to NbRdRP1m, MtRdRP1,or N. benthamiana EF1α were synthesized as specified by the equipment manufacturer (Applied Biosystems). Gene-specific probes were labeled with 6-carboxyfluorescein (FAM) reporter dye at their 5′ ends and tetramethylrhodamine (TAMRA) quencher dye at their 3′ ends. Probes and primers were: nbProb, 5′-FAM-TCCAGCCGATGGATTATACTCCAGCA-TAMRA-3′; nbPm5′,5′-TGTTGGGATCCAGACCTGGTT-3′; nbPm3′, 5′-TCAACTTCCTCAATTGTGACATCAT-3′; efProb, 5′-FAM-TCTGTTGAGATGCACCACGAAGCTCTTCAGGA-TAMRA-3′; efPm5′, 5′-TGGTGTCCTCAAGCCTGGTATGGTTGT-3′; efPm3′, 5′-ACGCTTGAGATCCTTAACCGCAACATTCTT-3′; mtProb, 5′-FAM-CTTTATTCTCCTACTTCAGATGATGCATATAACGTATACGAAG-TAMRA-3′; mtPm5′, 5′-CATTCACTTTATGCCATGACAATAAGACA-3′; and mtPm3′, 5′-TACAACTGCATGGAGTGAAATCTACT-3′. Purified plasmids or DNA fragments representing MtRdRP1, NbRdRP1m, and EF1α were serially diluted (100, 10, 1, and 0.1 pg/μl) for standard curve preparation. Two microliters of first-strand cDNA was added to the real-time PCR mixture containing Taqman universal master mix (Applied Biosystems), primers, and probe at concentrations specified by the manufacturer. PCRs then were performed by using an abi prism sds7000 under the following conditions: 50°C for 2 min (1 cycle), 95°C for 10 min (1 cycle), 95°C for 15 sec, 60°C for 1 min (40 cycles). The Ct, a cycle parameter showing detectable signal that had undergone an exponential amplification at 0.2 threshold, was converted into template concentration by using the standard curve. N. benthamiana EF1α mRNA was also quantified in cDNA samples to normalize the transcript levels of target genes.
Results
Isolation and Characterization of RdRP Sequences from N. benthamiana. We initiated our study of N. benthamiana sensitivity to virus infection by synthesizing primers within a conserved region from RdRPs transcribed in N. tabacum (NtRdRP1) (13) and L. esculentum (11) and two RdRPs transcribed in M. truncatula (S.-J.Y. and R.S.N., unpublished data). By using these primers and N. benthamiana genomic DNA as template, a PCR product of 1.2 kb was obtained and subjected to DNA sequence analysis. This PCR product contained a 72-nt insert, with consecutive in-frame stop codons, within the 5′ half of the RdRP ORF (Fig. 1 A and B). The lack of identifiable border motifs suggested that the insert was not an intron. This conclusion was supported through isolation and sequence analysis of a 2.6-kb cDNA fragment of the gene from an N. benthamiana leaf cDNA library. The cDNA contained the 72-nt insert. The N. benthamiana gene is referred to as NbRdRP1m (m = mutant). The 5′ sequence of NbRdRP1m was obtained by 5′-RACE using an NbRdRP1m-specific primer downstream of the 72-nt insertion. Sequences of seven independently derived RACE clones were identical to the previously cloned NbRdRP1m sequence within the 747-bp overlapping region analyzed (data not shown).
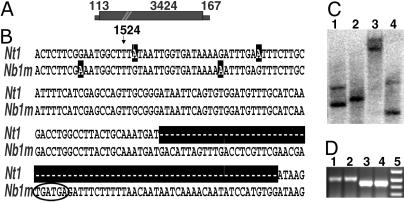
Fig. 1.
Analysis of the RdRP content of N. benthamiana. (A) Schematic figure of NbRdRP1m. The length of the ORF (large filled rectangle) and the 5′ and 3′ UTRs (small filled rectangles) are indicated, and the position of a 72-nt insert, beginning at nucleotide 1524, is indicated (cross bars). (B) Sequence alignment of a portion of N. tabacum NtRdRP1 (Nt1; GenBank accession no. AJ011576) with N. benthamiana NbRdRP1m (Nb1m; GenBank accession no. AY574374) showing the position of the 72-nt inserted sequence in NbRdRP1m (opposite the NtRdRP1 sequence marked with dashes). The tandem in-frame stop codons are circled, and nucleotides that differ between the two genes are highlighted. (C) RdRP copy number in N. benthamiana. Genomic DNA was digested with NheI (lane 1), EcoRV (lane 2), NdeI (lane 3), and XhoI (lane 4) and probed with a fragment of NbRdRP1m (nucleotides 1390–2412) representing a partially conserved region within plant RdRPs as determined by sequence alignment with clustalw. The restriction enzymes cleaved 3′ or 5′ of the probed sequence. (D) Analysis of RdRP mRNA sequences for the 72-nt insert. Total RNA from N. benthamiana (lanes 1 and 2) and N. tabacum (lanes 3 and 4) was amplified by using primers specific for NbRdRP1m and NtRdRP1. Lane 5 shows size-marker fragments representing 300, 400, 500, and 600 bp (bottom to top). Only the N. benthamiana samples contained a 72-nt insert.
The clone containing NbRdRP1m was 3,704 nt in length and would be predicted to encode a protein of 1,117 aa residues, not including the insert sequence (Fig. 1 A; GenBank accession no. AY574374). The presence of the consecutive UGA stop codons dictates the synthesis of a truncated protein of 470 aa. No other plant RdRP sequence reported to date contained the insert (LeRdRP1, GenBank accession no. Y10403; NtRdRP1, AJ011576; SDE1/SGS2, AF268093; AtRdRP1, AY148431; AtRdRP2, At4g11130; AtRdRP3, At2g19910; AtRdRP4, At2g19920; AtRdRP5, At2g19930). Except for the presence of the insert sequence, NbRdRP1m was 95% and 93% identical in nucleotide and amino acid sequence, respectively, to NtRdRP1 from N. tabacum.
Two RdRPs were identified in N. benthamiana by Southern analysis using a fragment of NbRdRP1m as probe (Fig. 1C). To determine whether the second gene expressed a transcript containing the 72-nt insert, RT-PCR was performed on RNA isolated from N. benthamiana or N. tabacum leaf tissue by using primers flanking the insert. Studies conducted with primers with identity to NbRdRP1m and NtRdRP1 amplified a fragment that contained the 72-nt insert from N. benthamiana but not from N. tabacum (Fig. 1D). An additional study was conducted to amplify RdRPs with less sequence identity to NbRdRP1m and NtRdRP1. The primers used in this experiment were degenerate for published sequences from all known transcribed plant RdRPs plus three unpublished but transcribed M. truncatula RdRP sequences (S.-J.Y. and R.S.N., unpublished data). Through this analysis a second RdRP sequence (187-nt fragment) was isolated from N. benthamiana leaf tissue that did not contain the insert and had higher sequence identity with SDE1/SGS2 RdRP (74%) than with NbRdRP1m (28%) (sequence not shown).
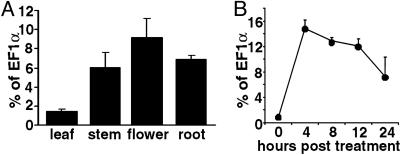
NbRdRP1m Expression. Transcripts of NbRdRP1m were difficult to detect by Northern blot analysis (data not shown). However, transcripts were readily detected by real-time RT-PCR analysis using leaf, stem, flower, and root tissues from N. benthamiana plants (Fig. 2A). NbRdRP1m RNA levels were estimated to be 0.09–0.009% of the total poly(A) RNA based on comparison with EF1α mRNA levels in L. esculentum tissues (Fig. 2 A and ref. 35). The presence of NbRdRP1m transcript indicated that NbRdRP1m was not a pseudogene.
Fig. 2.
Accumulation of NbRdRP1m transcript in N. benthamiana tissues and after treatment with SA. (A) Relative expression of NbRdRP1m in tissue harvested at early flowering. Expression levels were determined by real-time RT-PCR by using primers specific for NbRdRP1m and EF1α transcript. The expression of NbRdRP1m was normalized to EF1α transcript levels (percentage of EF1α, y axis). Bars represent standard deviations for three replicates. (B) NbRdRP1m transcript levels after SA treatment. Leaves were sprayed with a 2 mM SA solution at the eight-leaf stage. RNA was isolated from sprayed leaves and analyzed as described for A for NbRdRP1m transcript. Bars represent standard deviations for three replicates per time point.
When healthy N. benthamiana plants were sprayed with a 2-mM SA solution, we detected a 19-fold increase in NbRdRP1m transcript from leaf extracts 4 h after spraying (Fig. 2B). The level of transcript then decreased slowly but remained 7-fold higher than the untreated control 24 h posttreatment.
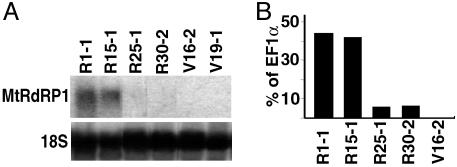
Expression of M. truncatula MtRdRP1 in N. benthamiana and the Response of Transgenic Plants to Virus Challenge. We hypothesized that NbRdRP1m reflected a loss-of-function mutation that explains the sensitivity of N. benthamiana to infection by certain viruses. To test this hypothesis, we attempted to complement the missing RdRP activity in N. benthamiana through constitutive overexpression of an NbRdRP1m ortholog from M. truncatula (MtRdRP1). MtRdRP1 has 62% identity with NbRdRP1m and is induced by SA treatment (S.-J.Y. and R.S.N., unpublished data). Individual transgenic lines that expressed or did not express MtRdRP1 transcripts (nonexpressing lines referred to as vector control) were developed and we used Northern and real-time RT-PCR analyses to identify lines that expressed the transgene at high and low levels (Fig. 3). The level of MtRdRP1 mRNA in transgenic plants expressing the gene at high levels was greater than that observed for the endogenous NbRdRP1m mRNA under all conditions tested (compare results shown in Figs. 2 and 3). Primary transformants expressing MtRdRP1 appeared normal by visual inspection.
Fig. 3.
MtRdRP1 transcript levels in transgenic N. benthamiana. Transcript levels from leaves of T0 plants transformed with a vector containing (lines designated R) or not containing (lines designated V) MtRdRP1. MtRdRP1 transcript levels were determined by Northern analysis using a probe complementary to MtRdRP1 (nucleotides 1–2135) (A) or real-time RT-PCR with gene-specific primers (conditions and controls as described for Fig. 2 A) (B). For the Northern analysis, lanes contained 20 μg of total RNA, and the same membrane was stripped and probed for 18S rRNA (18S). Each lane represents extract from independent plants.
Transgenic plants next were challenged with TMV to determine the extent of protection provided by MtRdRP1 expression. The U1 strain of TMV, a tobamovirus, causes severe mosaic symptoms on N. tabacum and the rapid death of N. benthamiana plants. Previously we isolated and characterized an attenuated strain of TMV, MIC1,3,6, that contains an attenuated suppressor of RNA silencing and induces milder symptoms on N. tabacum and delayed death of N. benthamiana compared with TMV U1 (24, 36). We challenged cuttings from the T0 transgenic plants with TMV MIC1,3,6 to determine the extent of protection afforded by the MtRdRP1 transgene against this virus. Developing leaves from both MtRdRP1-expressing (lines R1-1 and R15-1) and vector control (line V16-2) plants became chlorotic and rugose 5 days postinoculation (dpi) (data not shown). However, vector control plants sometimes displayed stem and leaf necrosis at this time, which was not observed on plants transformed with MtRdRP1. By 34 dpi, vector control plants were dead or near death, whereas MtRdRP1-expressing plants showed continued growth with mild yellowing and mosaic in young leaves (data not shown).
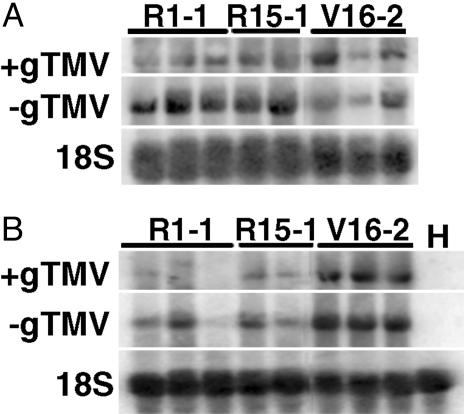
To determine whether the mild symptoms displayed by plants expressing MtRdRP1 were correlated with limited virus accumulation, we analyzed TMV MIC1,3,6 RNA accumulation in young leaves from inoculated plants (Fig. 4). At 6 dpi, the transgenic lines expressing high levels of MtRdRP1 transcript accumulated similar or greater levels of both + and - genomic viral RNA compared with the vector control line (Fig. 4A). By 13 dpi, however, the transgenic lines expressing MtRdRP1 transcript accumulated lower levels of both + and - genomic viral RNA compared with the vector control line (Fig. 4B). The appearance of protection correlated with the induction of native NbRdRP1m transcript. At 6 dpi, there was only a 1.5-fold increase in NbRdRP1m transcript in tissue from the inoculated vector control plants compared with the uninoculated vector control plants (data not shown), whereas at 13 dpi there was a 22-fold increase in transcript (percentage of EF1α transcript levels after real-time RT-PCR: 1.10 versus 0.05).
Fig. 4.
Viral RNA accumulation in T0 cuttings of N. benthamiana expressing MtRdRP1 after challenge with TMV MIC1,3,6. RNA was isolated from systemic leaves 6 (A)or13(B) dpi with TMV MIC1,3,6 (1 μg/ml). Total RNA (10 μg) from MtRdRP1-expressing (lines R1-1 and R15-1), vector control (line V16-2), or healthy (H) plants was hybridized with probe complementary to the TMV coat protein ORF or identical to the 5′ 1.5 kb of the TMV genome, respectively, for sense or minus-sense genomic viral RNA determinations (+gTMV or -gTMV). The same membrane was probed for 18S rRNA (18S) at each date. Each lane represents extract from one plant.
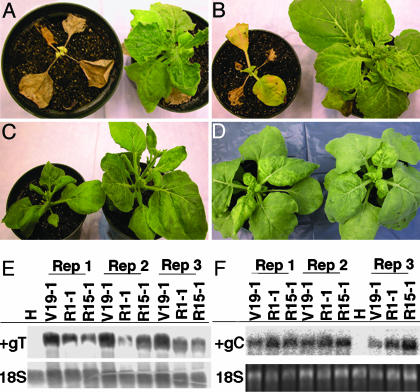
T1 progeny then were inoculated with TMV U1, SHMV, TVCV, CMV, or PVX to determine the extent of protection provided by MtRdRP1 expression. TVCV and SHMV are tobamoviruses distantly related to TMV, whereas CMV and PVX are members of different genera and, along with TVCV, were used previously in studies of plants with altered RdRP activities (9, 10, 13, 16). N. benthamiana plants expressing MtRdRP1 (lines R1-1 and R15-1) displayed fewer symptoms compared with plants from vector control lines after inoculation with TMV U1, SHMV, or TVCV (Fig. 5 A–C). Viral RNA and coat protein levels also were decreased in systemic tissue from MtRdRP1-expressing plants infected with TVCV (RNA, Fig. 5E; protein, relative coat protein levels in plants from lines R1-1 and R15-1, five plants per line, were 38% and 56%, respectively, of the vector control value 10 dpi). However, when plants were inoculated with CMV or PVX, no difference in symptoms was observed between MtRdRP1-expressing and the vector control plants (Fig. 5D and data not shown). In addition, CMV RNA accumulation in systemic tissue was similar between the plant lines (Fig. 5F), as were CMV and PVX coat protein levels (relative coat protein levels in plants from lines R1-1 and R15-1, eight plants per line, did not differ between lines and were 93% and 97%, respectively, of the vector control value 13 dpi).
Fig. 5.
Symptom suppression and decreased viral RNA accumulation displayed by T1 progeny of N. benthamiana expressing MtRdRP1 after challenge with TMV, SHMV, TVCV, or CMV. For A–D (images), the plant on the left did not contain MtRdRP1 (line V19-1), and the plant on the right expressed MtRdRP1 (line R1-1 for TMV, SHMV, or CMV and R15-1 for TVCV). (A) Symptoms at 21 dpi with TMV U1. (B) Symptoms at 21 dpi with SHMV. (C) Symptoms at 21 dpi with TVCV. (D) Symptoms at 14 dpi with CMV. (E) Accumulation of TVCV RNA in plants inoculated with TVCV. Leaves were harvested at 10 dpi, and total RNA (3 μg per sample) from MtRdRP1-expressing (lines R1-1 and R15-1), vector control (line V19-1), and healthy (H) plants was hybridized with a probe complementary to the 3′ end of the TVCV genome (nucleotides 5455–6311; +gT). The same membrane was probed for 18S rRNA (18S). Each group of three lanes represents results from developmentally matched plants. (F) Accumulation of CMV RNA in plants inoculated with CMV. Leaves were harvested at 13 dpi and total RNA (2 μg per sample) from MtRdRP1-expressing (lines R1-1 and R15-1), vector control (line V19-1), and healthy (H) plants was hybridized with a probe complementary to CMV RNA 2 (nucleotides 367–840; +gC). Ethidium-bromide-stained 18S rRNA (18S) was visualized on the membrane. Each group of three lanes represents results from developmentally matched plants.
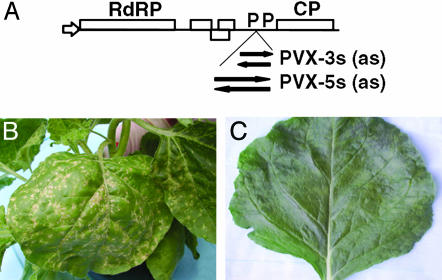
Virus-Induced Silencing of N. benthamiana RdRP Sequences and Its Influence on Disease. Virus-induced gene silencing (VIGS) is a gene-knockout method used to study host gene function quickly (e.g., ref. 37). PVX was used as a vector to express fragments of NbRdRP1m to study the disease phenotype induced during VIGS of this gene (Fig. 6A). When recombinant PVX constructs containing NbRdRP1m fragments in the sense orientation were biolistically introduced into wild-type N. benthamiana seedlings, severe symptoms of local necrosis in systemically infected leaves were displayed 10–20 dpi (e.g., Fig. 6B for PVX-3s). In contrast, PVX not containing the RdRP insert caused only mosaic symptoms in this tissue (Fig. 6C). No symptom differences were observed between plants infected with PVX carrying 5′ or 3′ sense or antisense inserts (data not shown). Endogenous NbRdRP1m transcript levels were decreased in young leaves of plants showing severe symptoms (data not shown). Thus, plants with decreased NbRdRP1m transcript displayed a severe disease symptom, suggesting that this or any other sufficiently related RdRP gene was necessary to control symptom development.
Fig. 6.
Symptoms induced on N. benthamiana after VIGS with NbRdRP1m. (A) Schematic diagram showing the insertion site within a PVX vector of two fragments of NbRdRP1m (5′, nucleotides 1200–2239; 3′, nucleotides 2600–3174) in either sense (PVX-5s and PVX-3s) or antisense (PVX-5as and PVX-3as) orientation. (B and C) Symptoms induced on leaves at 20 dpi with PVX containing an NbRdRP1m insert (PVX-3s) (B) or not containing an insert (PVX) (C). Symptoms were enhanced (leaf necrosis) when the RdRP sequence was present in PVX.
Discussion
N. benthamiana Contains a Natural Variant of an SA-Inducible RdRP. In this study we report that N. benthamiana contains an RdRP, NbRdRP1m, the transcript of which includes a unique 72-nt insert. The insert contains tandem in-frame stop codons that would cause the synthesis of a 470-aa protein, versus a 1,117-aa protein without the insert. This gene has high sequence similarity with NtRdRP1 from N. tabacum, a gene that provides resistance against TMV in that species (13). The truncated protein encoded from NbRdRP1m transcript is likely to be dysfunctional based on studies of an SA- and virus-inducible mutant RdRP gene from Arabidopsis, the transcript of which encodes a truncated RdRP (16). N. benthamiana is apparently a natural mutant for an RdRP gene family member resulting in the predicted loss of function of this gene.
NbRdRP1m may have been altered recently, because it is nearly identical to the NtRdRP1 sequence and its expression is induced by SA (Fig. 2B) and virus infection (Results). The relationship of NbRdRP1m with other RdRPs from Nicotiana species therefore is of interest to those studying the evolution of the Nicotiana genus. N. benthamiana is native to Australia (38) and most closely related to Nicotiana species found in Australia (39). N. tabacum originated in the Americas (40). Similar to N. benthamiana, other Nicotiana species from both the Americas (e.g., Nicotiana bigelovii and Nicotiana clevelandii) and Australia (e.g., Nicotiana occidentalis and Nicotiana cavicola) are susceptible to a wide range of viruses (20). A further understanding of the evolution of the Nicotiana species will be obtained by determining the penetration of the 72-nt insert within genus members.
Resistance to Tobamoviruses Was Enhanced in N. benthamiana. Through expression of MtRdRP1 from M. truncatula, an ortholog of NbRdRP1m, we increased the resistance of N. benthamiana against TMV, SHMV, and TVCV infection, all from the genus Tobamovirus (Figs. 4 and 5). These results indicate that the RdRP-mediated protection pathway in wild-type N. benthamiana is not saturated for tobamovirus challenge and suggest that a functional NbRdRP1m would aid in resisting tobamovirus infections.
We also observed that TMV MIC1,3,6 symptom induction and its accumulation was most reduced late after inoculation, a period coinciding with the 22-fold induction of the NbRdRP1m transcript during virus infection (13 dpi; Fig. 4 and Results). This is an important observation, linking the time after virus infection at which NbRdRP1m transcript was highly expressed with the inability to control virus accumulation and disease by the host. This correlation supports the conclusion that NbRdRP1m is either nonfunctional or diminished in activity. Additional support for this conclusion comes from a study conducted with Arabidopsis plants expressing or not expressing the putative functional ortholog of NbRdRP1m, AtRdRP1, in which the greatest difference in TMV-cg accumulation in the respective plants occurred late after inoculation (16). We speculate that MtRdRP1 functions to protect our transgenic plants from overwhelming virus accumulation late after inoculation when an active NbRdRP1m would normally function. In addition, because MtRdRP1 was driven by a 35S promoter and not a virus-inducible promoter, the absence of protection in the MtRdRP1-expressing plants early after inoculation may be explained by a requirement for a second virus-inducible host factor that functions in this pathway and accumulates only late after inoculation.
Resistance to Viruses Outside of the Tobamovirus Group Was Not Enhanced in N. benthamiana. TMV accumulation, but not CMV accumulation (especially locally), is controlled by SA treatment in N. tabacum (e.g., refs. 15, 17, and 19). In our transgenic plants expressing MtRdRP1, TMV but not CMV was limited in accumulation (Figs. 4 and 5). The lack of an effect by SA on CMV accumulation was linked to the presence of the CMV 2b silencing suppressor protein (18). Because MtRdRP1 was driven by the 35S promoter and not its endogenous SA-inducible promoter, our results could be considered to support the theory that this suppressor functions directly on the SA-inducible RdRP or downstream of this protein in this resistance pathway. However, these results also may be interpreted to indicate that an active SA-inducible NbRdRP1m does not function to control CMV accumulation. It is known that the CMV 2b suppressor prevents the accumulation of an SA-inducible alternative oxidase (18), an enzyme that, unlike NtRdRP1, responds to antimycin A treatment (18, 19). In addition, SA treatment inhibited TMV RNA accumulation equally well in N. tabacum plants expressing or not expressing NtRdRP1 (13). These findings suggest that SA-induced host resistance to viruses requires additional factors besides the SA-inducible RdRPs and that the CMV 2b suppressor may function at least partially by inhibiting this alternative SA-inducible host defense. Our transgenic plant lines may be used further to clarify the effect of SA on RdRP activity and virus accumulation.
When our transgenic plants were challenged with PVX, similar symptoms and systemic virus accumulation levels were observed in the MtRdRP1-expressing and vector-control plants. In other studies, PVX accumulation was inhibited 10-fold in SA-treated N. tabacum leaf disks (15); however, in N. tabacum leaves in which the SA-inducible NtRdRP1 transcript was downregulated, the greatest effect was to increase the systemic accumulation and not the local accumulation of the virus (13). The different findings in these studies may be because of the different methods of treatment and the multiple ways in which SA can affect pathogen accumulation. In our work, N. benthamiana was characterized as a natural knockout mutant for a specific SA-inducible RdRP, whereas antisense technology was used to down-regulate the expression of the N. tabacum SA-inducible RdRP (13). Because the entire coding region of NtRdRP1 was expressed within the antisense construct to down-regulate NtRdRP1 activity in N. tabacum, the potential exists that transcripts from additional members of the RdRP gene family within N. tabacum were down-regulated. In support of this hypothesis, we determined that expression in PVX of a portion of NbRdRP1m conserved within RdRP sequences across species led to greater susceptibility by N. benthamiana to PVX infection (Fig. 6). Thus, as for CMV, another N. benthamiana RdRP (possibly with greater identity to SGS2) may provide protection against PVX. However, we cannot dismiss the possibility that the truncated protein or the NbRdRP1m transcript may have residual activity against PVX.
RdRPs and Gene Silencing. It is known that N. benthamiana can silence RNA from viruses and transgenes (e.g., refs. 41 and 42), and thus the loss of NbRdRP1m activity does not prevent this species from regulating RNA expression through this pathway. The presence of a second RdRP in N. benthamiana with sequence similarity to NbRdRP1m was determined by Southern analysis (Fig. 1C). This RdRP either also contains the 72-nt insert, and likely would be inactive, or is sufficiently different in sequence from NbRdRP1m that it is not detected by RT-PCR using primers with identity to NbRdRP1m and NtRdRP1 (Fig. 1D). We identified a second RdRP with sequence similarity to SDE1/SGS2 by using degenerate primers. This RdRP does not have the 72-nt insert and may be responsible for silencing RNA from transgenes as suggested for the function of SDE1/SGS2 in Arabidopsis (9, 10).
What function might NtRdRP1, AtRdRP1, a functional NbRdRP1m, and other SA-inducible RdRPs have during VIGS? Host RdRPs have been proposed to be sensors that detect and modify aberrant RNAs into dsRNA for entry into the silencing pathway (e.g., ref. 43). SA-inducible RdRPs, such as an active NbRdRP1m, therefore might compete with SDE1/SGS2 RdRP and viral RdRPs for aberrant RNA substrate. Support for such a model comes from work in C. elegans, in which the loss of an RdRP (RRF-3) causes this organism to be hyperactive in RNA silencing (44). N. benthamiana repeatedly has been shown to produce easily observable phenotypes during VIGS with specific viruses (e.g., refs. 37 and 42). The absence of NbRdP1m activity may cause N. benthamiana to be hyperactive in RNA silencing through the increase in substrate (i.e., aberrant RNA) available to the SDE1-like RdRP. Inherent in this model is the assumption that products produced from SA-inducible RdRPs go to separate pools from products of the other RdRPs for later processing. This model has implications for those interested in determining gene function through VIGS, because it suggests that elimination of SA-inducible RdRPs will lead to plants hyperactive for VIGS and hypersensitive to infection by specific viruses.
Acknowledgments
We thank Dr. G. May for information on the M. truncatula EST database and RdRP sequences; T. Pierson and Dr. K. Palanichelvam for assistance on laboratory experiments; Drs. D. Baulcombe, C. M. Deom, U. Melcher, and M. Roossinck for viruses and cDNA clones; Drs. R. Dixon and X. Z. He for critical reading of the manuscript; A. Raney for text preparation; and D. Snelson and R. Bloomfield for figure preparation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RdRP, RNA-dependent RNA polymerase; CMV, Cucumber mosaic virus; TVCV, Turnip vein-clearing virus; SA, salicylic acid; TMV, Tobacco mosaic virus; PVX, Potato virus X; SHMV, Sunn hemp mosaic virus; dpi, days postinoculation; VIGS, virus-induced gene silencing.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY574374).
References
- 1.Baulcombe, D. (2002) Curr. Biol. 12, R82-R84. [DOI] [PubMed] [Google Scholar]
- 2.Nishikura, K. (2001) Cell 107, 415-418. [DOI] [PubMed] [Google Scholar]
- 3.Waterhouse, P. M., Wang, M.-B. & Lough, T. (2001) Nature 411, 834-842. [DOI] [PubMed] [Google Scholar]
- 4.Tijsterman, M., Ketting, R. F. & Plasterk, R. H. A. (2002) Annu. Rev. Genet. 36, 489-519. [DOI] [PubMed] [Google Scholar]
- 5.Cogoni, C. & Macino, G. (1999) Nature 399, 166-169. [DOI] [PubMed] [Google Scholar]
- 6.Smardon, A., Spoerke, J. M., Stacey, S. C., Klein, M. E., Mackin, N. & Main, E. M. (2000) Curr. Biol. 10, 169-178. [DOI] [PubMed] [Google Scholar]
- 7.Sijen, T., Fleenor, J., Simmer, F., Thijssen, K. L., Parrish, S., Timmon, L., Plasterk, R. H. A. & Fire, A. (2001) Cell 107, 465-476. [DOI] [PubMed] [Google Scholar]
- 8.Martens, H., Novotny, J., Oberstrass, J., Steck, T. L., Postlethwait, P. & Nellen, W. (2002) Mol. Biol. Cell 13, 445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalmay, T., Hamilton, A., Rudd, S., Angell, S. & Baulcombe, D. C. (2000) Cell 101, 543-553. [DOI] [PubMed] [Google Scholar]
- 10.Mourrain, P., Beclin, C., Elmayan, T., Feuerbach, F., Godon, C., Morel, J.-B., Jouette, D., Lacombe, A.-M., Nikic, S., Picault, N., et al. (2000) Cell 101, 533-542. [DOI] [PubMed] [Google Scholar]
- 11.Schiebel, W., Pelissier, T., Riedel, L., Thalmeir, S., Schiebel, R., Kempe, D., Lottspeich, F., Sanger, H. L. & Wassenegger, M. (1998) Plant Cell 10, 2087-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makeyev, E. V. & Bamford, D. H. (2002) Mol. Cell 10, 1417-1427. [DOI] [PubMed] [Google Scholar]
- 13.Xie, Z., Fan, B., Chen, C. & Chen, Z. (2001) Proc. Natl. Acad. Sci. USA 98, 6516-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chivasa, S., Murphy, A. M., Naylor, M. & Carr, J. P. (1997) Plant Cell 9, 547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naylor, M., Murphy, A. M., Berry, J. O. & Carr, J. P. (1998) Mol. Plant–Microbe Interact. 11, 860-868. [Google Scholar]
- 16.Yu, D., Fan, B., MacFarlane, S. A. & Chen, Z. (2003) Mol. Plant–Microbe Interact. 16, 206-216. [DOI] [PubMed] [Google Scholar]
- 17.Murphy, A. M. & Carr, J. P. (2002) Plant Physiol. 128, 552-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji, L.-H. & Ding, S.-W. (2001) Mol. Plant–Microbe Interact. 14, 715-724. [DOI] [PubMed] [Google Scholar]
- 19.Gilliland, A., Singh, D. P., Hayward, J. M., Moore, C. A., Murphy, A. M., York, C. J., Slator, J. & Carr, J. P. (2003) Plant Physiol. 132, 1518-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dijk, P., van Der Meer, F. A. & Piron, P. G. M. (1987) Neth. J. Plant Pathol. 93, 73-85. [Google Scholar]
- 21.Dawson, W. O. & Hilf, M. E. (1992) Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 527-555. [Google Scholar]
- 22.Cheng, N. H., Su, C. L., Carter, S. A. & Nelson, R. S. (2000) Plant J. 23, 349-362. [DOI] [PubMed] [Google Scholar]
- 23.Ding, X. S., Shintaku, M. H., Arnold, S. A. & Nelson, R. S. (1995) Mol. Plant–Microbe Interact. 8, 32-40. [Google Scholar]
- 24.Shintaku, M. H., Carter, S. A., Bao, Y. & Nelson, R. S. (1996) Virology 221, 218-225. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Y., Lartey, R. T., Hartson, S. D., Voss, T. C. & Melcher, U. (1999) Arch. Virol. 144, 957-971. [DOI] [PubMed] [Google Scholar]
- 26.Baulcombe, D. C., Chapman, S. & Santa Cruz, S. (1995) Plant J. 7, 1045-1053. [DOI] [PubMed] [Google Scholar]
- 27.Deom, C. M., He, X. Z., Beachy, R. N. & Weissinger, A. K. (1994) Virology 205, 198-209. [DOI] [PubMed] [Google Scholar]
- 28.Bell, C. J., Dixon, R. A., Farmer, A. D., Flores, R., Inman, J., Gonzales, R. A., Harrison, M. J., Paiva, N. L., Scott, A. D., Weller, J. W. & May, G. D. (2001) Nucleic Acids Res. 29, 114-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrington, J. C. & Freed, D. D. (1990) J. Virol. 64, 1590-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horsch, R. B., Fry, J. E., Hoffmann, N. L., Neidermeyer, J., Rogers, S. G. & Fraley, R. T. (1988) in Plant Molecular Biology Manual, eds. Gelvin, S. B., Schilperoort, R. A. & Verma, D. P. S. (Kluwer, Dordrecht, The Netherlands), pp. A5:1-9.
- 31.Yang, S. & Ravelonandro, M. (2002) Arch. Virol. 147, 2301-2312. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 33.Hord, M. J., Garciá, A., Villalobos, H., Rivera, C., Macaya, G. & Roossinck M. J. (2001) Plant Dis. 85, 952-954. [DOI] [PubMed] [Google Scholar]
- 34.Lartey, R. T., Hartson, S. D., Pennington, R. E., Sherwood, J. L. & Melcher, U. (1993) Plant Dis. 77, 21-24. [Google Scholar]
- 35.Pokalsky, A. R., Hiatt, W. R., Ridge, N., Rasmussen, R., Houck, C. M. & Shewmaker, C. K. (1989) Nucleic Acids Res. 17, 4661-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding, X. S., Liu, J., Cheng, N.-H., Folimonov, A., Hou, Y.-M., Bao, Y., Katagi, C., Carter, S. A. & Nelson, R. S. (2004) Mol. Plant–Microbe Interact., in press. [DOI] [PubMed]
- 37.Kumagai, M. H., Donson, J., Della-Cioppa, G., Harvey, D., Hanley, K. & Grill, L. K. (1995) Proc. Natl. Acad. Sci. USA 92, 1679-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burbidge, N. T. (1960) Aust. J. Bot. 8, 342-380. [Google Scholar]
- 39.Wheeler, M.-M. (1945) Proc. Natl. Acad. Sci. USA 31, 177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodspeed, T. H. (1954) in The Genus Nicotiana (Chronica Botanica, Waltham, MA), Vol. 16, pp. 49-57. [Google Scholar]
- 41.Ratcliff, F. G., MacFarlane, S. A. & Baulcombe, D. C. (1999) Plant Cell 11, 1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz, M. T., Voinnet, O. & Baulcombe, D. C. (1998) Plant Cell 10, 937-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamore, P. D. (2002) Science 296, 1265-1269. [DOI] [PubMed] [Google Scholar]
- 44.Simmer, F., Tijsterman, M., Parrish, S., Koushika, S. P., Nonet, M. L., Fire, A., Ahringer, J. & Plasterk, R. H. A. (2002) Curr. Biol. 12, 1317-1319. [DOI] [PubMed] [Google Scholar]